Abstract
Purpose
To investigate the circadian regulation and acute illumination effects on the expression and secretion of retinoschisin from vertebrate retinas.
Methods
Retinas were studied on the second day of constant darkness (DD) after several days of entrainment to 12-hour light/12-hour dark (LD) cycles in ovo or in vitro. Quantitative real-time PCR and Western immunoblotting were used to examine the mRNA and protein expressions of retinoschisin at different circadian time points. Pharmacologic treatments in whole retina and dissociated retinal cell cultures were used to investigate the cellular mechanisms underlying the circadian regulation of retinoschisin content and secretion. Different illumination conditions were given to examine changes in retinoschisin content in association with acute light/dark adaptation.
Results
The mRNA level, protein expression, and secretion of retinoschisin were under circadian control, all of which were higher at night and lower during the day. The Ras, MAP kinase Erk, CaMKII pathway served as part of the circadian output regulating the rhythmicity of retinoschisin. Blockage of L-type VGCCs dampened the retinoschisin rhythm, but inhibition of L-type VGCCs did not completely abolish the secretion of retinoschisin. The protein expression of retinoschisin also responded to acute illumination changes.
Conclusions
The mRNA and protein expression, as well as retinoschisin secretion, are under circadian control. L-type VGCCs play a role in the circadian regulation of retinoschisin, but the molecular mechanism underlying retinoschisin secretion does not depend on L-type VGCCs. Protein expression of retinoschisin in response to acute illumination changes depends on previous light exposure experience.
Retinoschisin is a 224-amino acid protein secreted mainly from retina photoreceptors.1,2 Mutations in genes encoding retinoschisin cause X-linked retinoschisis (XLRS), which is a leading cause of macular degeneration in juvenile male patients,3–5 affecting worldwide populations ranging from 1:5000 to 1:25,0004 and commonly leading to vision loss.4–6 Retinoschisin is a secreted octameric complex consisting of identical subunits linked by disulfide bonds.1,7 Each subunit contains a single discoidin domain that has been implicated in cellular adhesion or cell-cell interactions, which may function to maintain cellular organization and synaptic structure of the retina.1,3,8,9 The Rs1 knockout mouse has a retinal phenotype closely resembling human XLRS with a highly disorganized retina.10 Replacement of the RS1 gene leads to improvement in retinal function and morphology,11,12 indicating a role for retinoschisin in the development and maintenance of retinal architecture. However, in adult animals, the concentration of retinoschisin remains high in the photoreceptor layer,13 and recent evidence shows that the pineal glands in rodents and humans also express retinoschisin.14 Although RS1 mutations cause structural delamination of the neural retinal layers in mice, the pineal gland structures are still intact in these mutant animals.14 Thus, the functions of retinoschisin in adult animals and humans remain unclear, and the mechanism of retinoschisin secretion is not completely understood.
Photoreceptors are nonspiking neurons, and their synapses mediate the continuous release of neurotransmitters, which is an L-type voltage-gated calcium channel (VGCC)-dependent process.15 In retina photoreceptors, the synthesis and release of melatonin are under circadian control, and they are also L-type VGCC dependent.16,17 Because retinoschisin is secreted from photoreceptors and the mechanisms underlying its secretion are not known, we tested whether the secretion of retinoschisin could also be an L-type VGCC-dependent process. Circadian oscillators in the retina provide a mechanism for visual systems to initiate more sustained adaptive changes in ambient illumination throughout the day.18,19 Circadian oscillators in photoreceptors are endogenous and able to function independently in the absence of other retinal inputs.20–22 Importantly, photoreceptors are more sensitive to intense light damage at night than during the day, even in animals that have been maintained in constant darkness for several days after circadian light-dark cycle entrainment.23 We have shown that the L-type VGCCs in chick cone photoreceptors are under circadian control.24 mRNA levels, protein expression, and currents of the L-type VGCCs are greater when measured during the subjective night than during the subjective day.24 Because retinoschisin is present in retinas and pineal glands,14 there is a possible circadian regulation of retinoschisin or a role of retinoschisin in the circadian regulation of retina physiology.
Here, we report that mRNA levels, protein expression, and secretion of retinoschisin—all which are high at night and low during the day—are under circadian control. The Ras, Erk, CaMKII pathway is part of the output pathway that regulates the circadian expression of retinoschisin. The retinoschisin rhythm is concurrent with the circadian rhythm of L-type VGCCs,24 and the L-type VGCC inhibitor, nitrendipine, abolishes the rhythms of retinoschisin. The result indicates a relationship between L-type VGCCs and retinoschisin, in which the circadian rhythms of retinoschisin total cellular content levels and secretion are VGCC dependent. In addition, acute changes in illumination affect the protein expression of retinoschisin in the chick retina, which depends on prior light exposure experience. This result implies that the oscillations seen in retinoschisin are truly circadian in nature, not simply a response to bright light. To our knowledge, this study is the first to elucidate the circadian regulation of retinoschisin.
Materials and Methods
Circadian Entrainment and Cell Culture
Fertilized eggs (Gallus gallus) were obtained from the Poultry Science Department, Texas A&M University (College Station, TX). Chick embryos from embryonic day (E) 11 were entrained to 12-hour light/12-hour dark (LD) cycles at 39°C in ovo. Zeitgeber time (ZT) 0 was designated as the time lights turned on, and ZT 12 was designated as the time lights turned off. After in ovo LD entrainment for 6 days, eggs were kept in constant darkness (DD) for another day. On the second day of DD, retinas were dissected out for biochemistry or molecular biology analysis at various circadian times (CT) of the day. In some experiments, on the last day of LD entrainment, retina cells were dissociated, cultured in DD (39°C, 5% CO2), as described previously,21,25 and used for biochemistry and molecular biology assays on the second day of DD. To quantify retinoschisin secretion, whole retinas were cultured in 300 μL medium and maintained in DD. On the second day of DD, the culture media and the whole retinas were harvested separately for analysis of secreted and cellular retinoschisin.
Acute Light and Dark Adaptation
To study acute light or dark adaptation, chick embryos were exposed to light (at ZT 16) or darkness (at ZT 4) for 0, 5, 15, 30, 45, 60, and 120 minutes after several days of LD entrainment. In some experiments, chick embryos were exposed to the light for various lengths on the second day of DD at CT 4 (subjective day) or CT 16 (subjective night). The retinas were excised at different exposure times and subjected to Western immunoblotting.
Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction
Total RNA from intact chick retinas was collected using a RNA isolation kit (Qiagen, Valencia, CA), and 500 ng total RNA from each sample was used to quantify the expression of chick retinoschisin and chick β-actin (loading control) mRNA by Q-PCR using the one-step RT-PCR kit (TaqMan; Applied Biosystems, Foster City, CA) and an ABI Prism 7500 Sequence Detection System (Applied Biosystems). Forward and reverse primers for chick β-actin are listed in Ko et al.25,26 Forward and reverse primers for retinoschisin were 5′ GGGTTGGAGCAGCTGATCTG3′ and 5′ CAGAGTGCCCCTACCACAAG3′, respectively, and they were designed based on the sequence reported in GenBank (accession number AI438129). All primers and probes were made by Applied Biosystems. Accumulation of PCR products was detected directly by monitoring the increase in fluorescence from the reporter dye. Data are expressed as the ratio of retinoschisin to β-actin. All measurements were repeated four times.
Western Immunoblotting Analysis
The procedure has been described in detail previously.21 Briefly, cultured retinal cells were washed in ice-cold PBS and lysed in RIPA buffer. In some experiments, intact retinas were homogenized in RIPA buffer. Samples were mixed with 2 × Laemmli sample buffer, separated on 10% SDS-PAGE gels, and transferred to nitrocellulose membranes. Primary antibodies used in the studies were a polyclonal antibody specific for retinoschisin (generated in the laboratory of DT) and a polyclonal antibody insensitive to the phosphorylation state of Erk (loading control; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were visualized using anti–rabbit secondary antibodies conjugated to HRP and an ECL detection system (Pierce, Rockford, IL). The ratio of retinoschisin to total Erk in each sample was determined by densitometry using Scion Image (National Institutes of Health, Bethesda, MD). All measurements were repeated four to six times. Manumycin A, KN-92, and KN-93 were obtained from Calbiochem (San Diego, CA); PD 98059 was obtained from AG Scientific (San Diego, CA); nitrendipine was obtained from Tocris (Ellisville, Missouri).
Statistical Analysis
All of the data were presented as mean ± SE. Student’s t-test or one-way ANOVA followed by Tukey post hoc test for unbalanced n were used for statistical analysis. P < 0.05 was regarded as significant.
Results
Circadian Expression of Retinoschisin in Chick Retinas
We set out to determine whether there was a circadian rhythm in the mRNA expression of retinoschisin in chick retina. On the second day of DD after 6 days of LD entrainment in ovo, retinas were excised at six different circadian time points (CT 0, 4, 8, 12, 16, and 20) and were prepared for quantitative real-time RT-PCR. We found that levels of retinoschisin mRNAs were under circadian control peaking at CT 12, which was significantly different from other circadian time points (CT 0, 16, and 20; P < 0.05; Fig. 1a).
Figure 1.
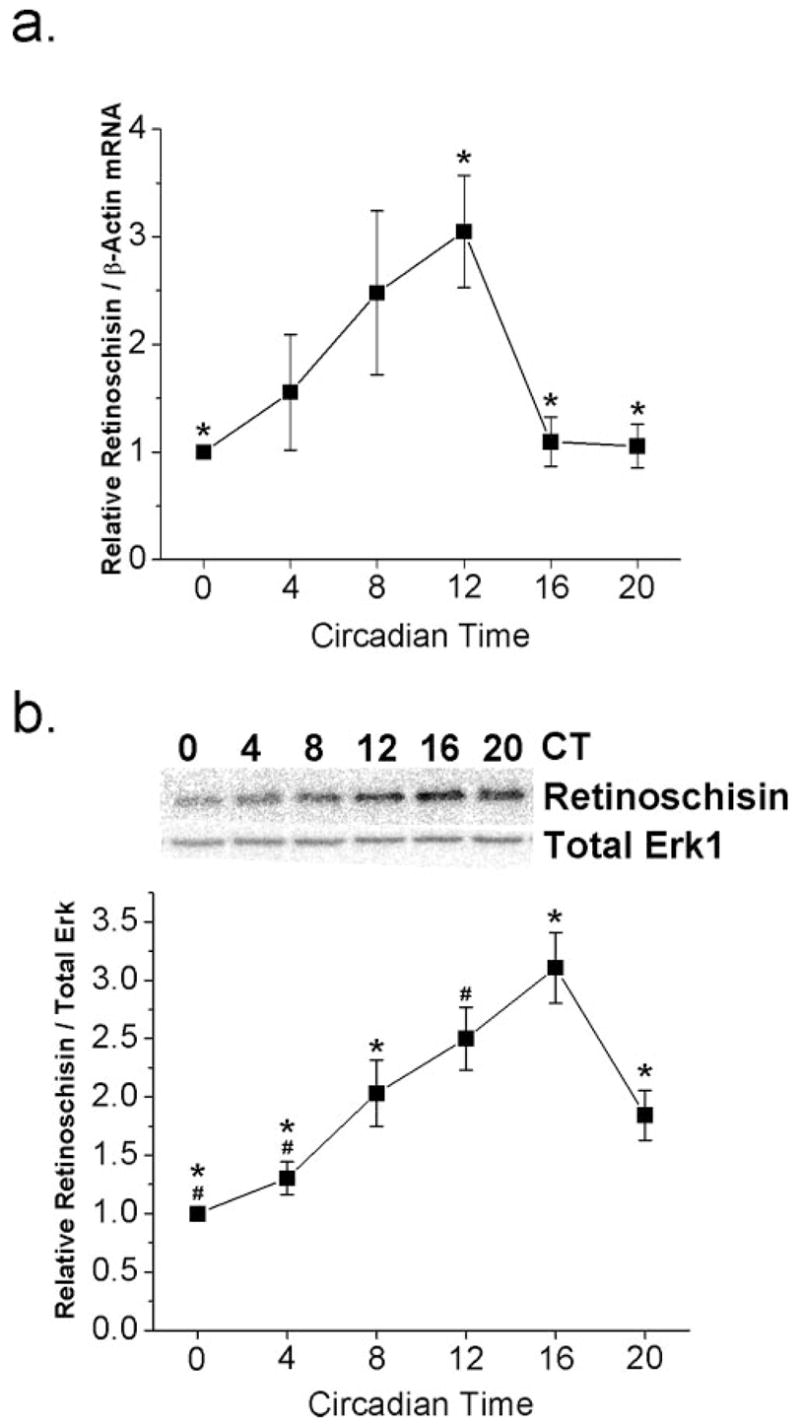
Circadian rhythm in the mRNA and protein expression of retinoschisin. Chick embryos (E11) were entrained under LD cycles for 6 days and kept in DD. On the second day of DD, retinas were taken out every 4 hours at six different circadian time (CT) points—CT 0, 4, 8, 12, 16, and 20—and were processed for quantitative real time RT-PCR or immunoblotting assays for retinoschisin and total Erk (loading control). (a) Retinoschisin mRNA peaked at CT 12, which was significantly different from CT 0, 16, and 20 (*P < 0.05 using ANOVA with Tukey post hoc test). n = 4 for each circadian time point. (b) Protein expression of retinoschisin peaked at CT 16. n = 3 or 4 for each circadian time point. *Significant difference at CT 16 compared with CT 0, 4, 8, and 20. #Significant difference at CT 12 compared with CT 0 and CT 8 using ANOVA with Tukey post hoc test; P < 0.05.
Because retinoschisin mRNA levels were rhythmic, we subsequently examined whether protein expression was under circadian control. On the second day of DD, retinas were harvested for Western immunoblot analysis for the protein expression of retinoschisin (Fig. 1b). The cellular content of retinoschisin protein in the whole retina was higher during the subjective night and lower during the subjective day. Protein expression peaked at CT 16 and was significantly different from CT 0, 4, 8, and 20 (P < 0.05), whereas the protein level at CT 12 was significantly different from that at CT 0 and CT 4 (P < 0.05). These results showed that there was a circadian regulation of retinoschisin protein expression in the chick retina, and the peak expression of retinoschisin protein was 4 hours later than its mRNA expression. We next examined the cellular mechanisms underlying the circadian regulation of retinoschisin.
Circadian Outputs to Regulate Retinoschisin Rhythms Partially through Ras, MAP Kinase Erk, and CaMKII
Previously, Ko et al.21,25 showed that the small GTPase Ras, MAP kinase Erk, and calcium-calmodulin kinase II (CaMKII) are part of the circadian output pathway regulating the rhythmicity of cGMP-gated cation channels. This Ras-Erk-CaMKII pathway also serves as part of the circadian output to control VGCC rhythmicity.24 We developed whole retina cultures and associated retinal cell cultures to investigate whether the circadian regulation of retinoschisin was also through the same output pathway. Protein expression of retinoschisin in the chick retina is rhythmic (Fig. 1); therefore, we first examined whether the secretion of retinoschisin was subject to circadian control. After LD entrainment in ovo, both retinas from one embryo were cultured in one well of a 24-well plate containing 300 μL medium and were kept in DD. On the second day of DD, the media and whole retina tissue were harvested separately for Western immunoblotting of retinoschisin at CT 5 (subjective day) and CT 17 (subjective night). We found that the secretion of retinoschisin was under circadian control in whole retina cultures (Figs. 2a, 3a). The content of retinoschisin in the media (secreted; Fig. 2a, control) and in the whole retina (whole retina; Fig. 2b, control) was high in the subjective night (CT 17) and low in the subjective day (CT 5). The retinoschisin content collected from the dissociated retinal cell cultures also displayed a similar circadian rhythmicity (Fig. 2c, control). On the second day of DD, cultured whole retinas or dissociated retinal cells were treated with the MEK inhibitor PD98059 (50 μM) for 2 hours at CT 3 and CT 15. The culture medium from the whole retina cultures, whole retina tissues, and dissociated retinal cells were harvested at CT 5 and 17 for Western immunoblotting. By inhibiting MEK, PD98059 prevents the activation of Erk, which is phosphorylated and activated by MEK. PD98059 decreased the protein content of retinoschisin secreted in the medium (Fig. 2a), whole retina tissues (Fig. 2b), and dissociated retinal cell cultures (Fig. 2c). Similarly, treatment with the Ras inhibitor manumycin A (1 μM) or the CaMKII inhibitor KN-93 (10 μM) for 2 hours decreased the amount of retinoschisin secreted in the medium (Fig. 2a), in whole retina tissue (Fig. 2b), and in dissociated retinal cell cultures (Fig. 2c) during the subjective night (CT 17) but not the subjective day (CT 5). Treatment with KN-92 (10 μM), the inactive analog of KN-93, had no effect on retinoschisin protein expression (Fig. 2). Thus, the Ras-Erk-CaMKII pathway serves as a common circadian output pathway in chick retina to regulate ion channels and other molecules, including retinoschisin. Given that the secretion of retinoschisin was under circadian control, we next examined the cellular mechanism governing the circadian regulation of retinoschisin secretion.
Figure 2.
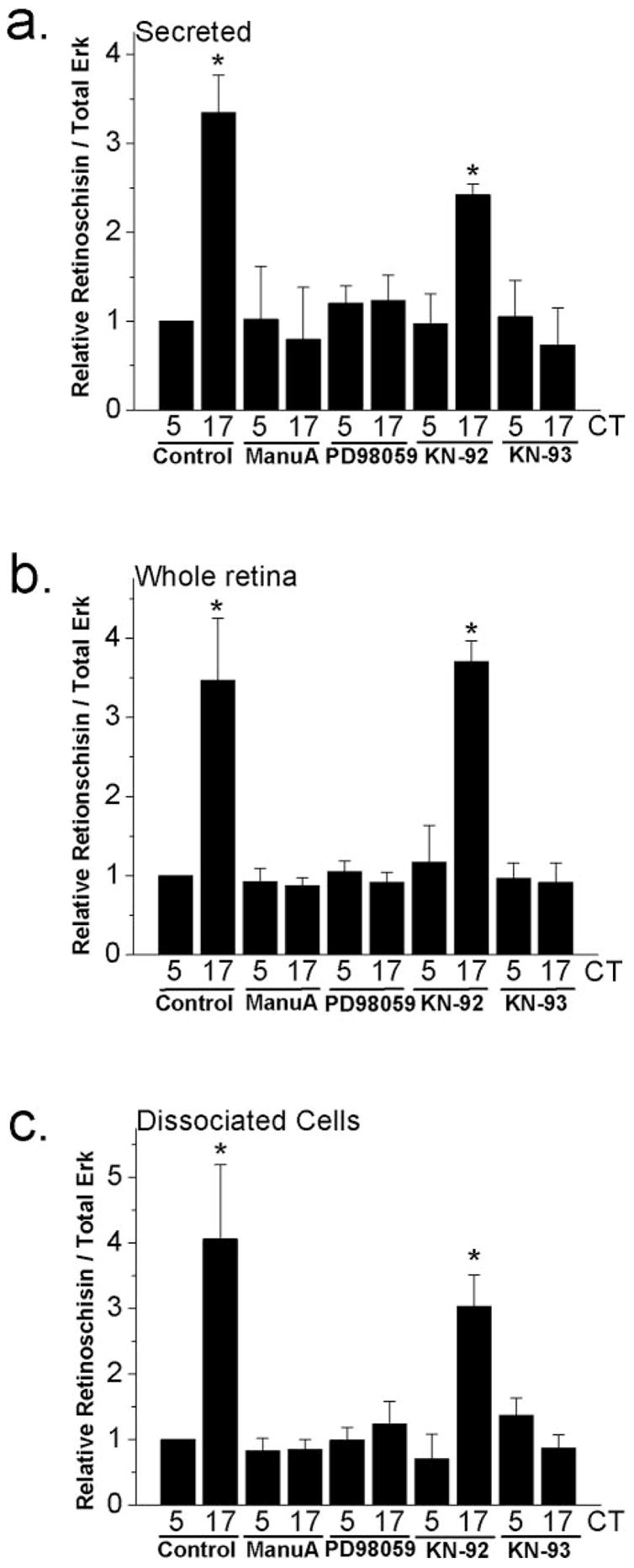
Circadian regulation of retinoschisin content and secretion is through Ras, MAPK (Erk), and CaMKII. After LD entrainment for 6 days, both retinas from each embryo (E16) were dissected, cultured in 300 μL medium, and kept in DD for 2 days. On the second day of DD, culture media and retinal tissue were harvested separately at CT 5 (subjective day) and CT 17 (subjective night) after 2 hours of different treatments. (a) Retinoschisin secreted in the media was significantly higher at CT 17 than at CT 5 (controls). Treatment with a Ras inhibitor manumycin A (ManuA; 1 μM) or a MEK1 inhibitor PD98059 (50 μM) in whole retina cultures for 2 hours decreased the secreted retinoschisin amount in the media during the subjective night (CT 17) but had no effect during the subjective day (CT 5). Treatment with a CaMKII inhibitor KN-93 (10 μM) also dampened the circadian regulation of retinoschisin secretion, whereas KN-92 (10 μM), the inactive analog of KN-93, had no effect on the circadian control of retinoschisin secretion. (b) Retinoschisin expressed in the whole retina showed a pattern similar to that secreted in the medium after different treatments. (c) Retinoschisin in dissociated retinal cell cultures also showed a pattern similar to that in secreted medium and whole retinas after different treatments. n = 3 or 4 in each group. Comparisons were made between CT 5 and CT 17 using Student’s t-test (*P < 0.05).
Figure 3.
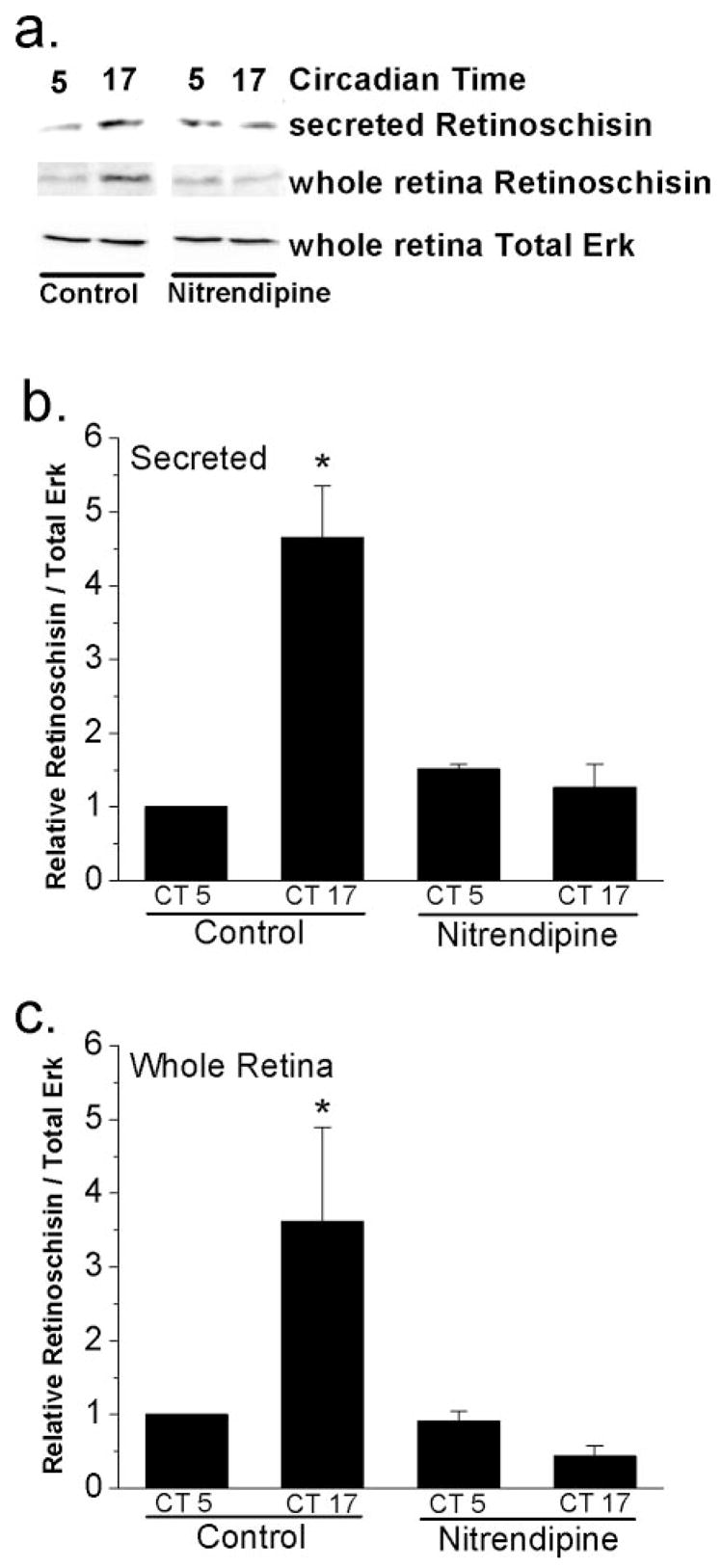
L-type VGCCs regulate the circadian control of retinoschisin secretion and content. The culture media and retinal tissue from whole retina cultures were harvested at CT 5 (subjective day) and CT 17 (subjective night) for immunoblotting assays. (a) Immunoblots show the secreted retinoschisin in the media, protein content of retinoschisin in the whole retina, and loading control (whole retina total Erk). Treatment with an L-type VGCC blocker, nitrendipine (3 μM), for 2 hours decreased the secreted retinoschisin and the protein content of retinoschisin in the whole retina during the subjective night (CT 17) but not subjective day (CT 5). (b) Secretion of retinoschisin was under circadian control, which was significantly higher during the subjective night (CT 17) than during the subjective day (CT 5). Nitrendipine treatment abolished the circadian rhythms of secreted retinoschisin in the media. (c) Similar results were observed in retinoschisin protein expression in cultured whole retinas. n = 3 or 4 in each group. Comparisons were made between CT 5 and CT 17 using Student’s t-test (*P < 0.05).
L-Type VGCCs Regulating Retinoschisin Secretion
Synaptic vesicle-mediated neurotransmitter release is an L-type VGCC-dependent process in retinal photoreceptors.15 In the chick retina, L-type VGCCs are responsible for the synthesis and release of melatonin from photoreceptors,16,17 even though the secretion of melatonin is not a synaptic vesicle-dependent process.27 The cellular mechanisms underlying the secretion of melatonin27 are poorly understood, and those of retinoschisin are completely unknown. Previously, we showed that the L-type VGCCs in chick cone photoreceptors are under circadian control.24 The mRNA levels, protein expression, and currents of the L-type VGCCs are greater when measured during the subjective night than during the subjective day.24 Interestingly, the circadian expression of retinoschisin shown in this study (Fig. 1) is concurrent with the L-type VGCC rhythm.24 Hence, we tested whether the L-type VGCCs were a part of the circadian output to regulate the synthesis and secretion of retinoschisin. Treatment with nitrendipine (3 μM), a specific L-type VGCC inhibitor, for 2 hours at CT 3 and CT 15 abolished the circadian rhythms of retinoschisin content secreted in the medium (Figs. 3a, 3b) and in whole retinas (Figs. 3a, 3c). However, nitrendipine did not completely block the secretion of retinoschisin; we still detected secreted retinoschisin in the media in the presence of nitrendipine in whole retina cultures in the subjective day (CT 5) and in the subjective night (CT 17). In addition, nitrendipine treatment for 2 hours did not alter retinoschisin mRNA expression (data not shown).
Acute Changes in Illumination Affecting Protein Levels of Retinoschisin Depending on Previous Light-Dark Entrainment
The circadian oscillators in the retina provide a mechanism for visual systems to initiate more sustained adaptive changes throughout the day because visual systems have to anticipate the large daily changes in ambient illumination.18,19 For example, the magnitude of light pulse-induced photoreceptor disc shedding in rat retinas is under circadian control.28 At the same time, retinas are capable of undergoing rapid morphologic and physiologic changes in response to acute changes in illumination.29–31 In dark-adapted retinas, exposure to light for 30 minutes is enough to cause changes in protein expression, in which it causes an increase in dihydroxyphenylalanine32 and a decrease in arylalkylamine N-acetyltransferase (AA-NAT) protein and activity.33 Therefore, it is possible that acute changes in illumination might have effects on the protein expression of retinoschisin in the retina. In this study, E18 chick embryos from various preconditions were exposed to light or dark for different periods of time (0 – 120 minutes). In LD-entrained E18 embryos, exposure to complete darkness at ZT 4 (4 hours after lights on) had no effect on the protein expression of retinoschisin (Fig. 4a), whereas exposure to light at ZT 16 (4 hours after lights off) for 15 minutes significantly increased protein expression of retinoschisin (Fig. 4b; P < 0.05 compared with all other time periods). Brief light exposure for 5 minutes at CT 4 (on the second day of DD) transiently decreased retinoschisin content, but light exposure for 30 minutes at CT 4 significantly increased protein expression of retinoschisin (Fig. 4c; P < 0.05, 30 minutes vs. 0 and 15 minutes). Although brief light exposure at CT 16 (on the second day of DD) for various periods of time had no effect on the protein expression of retinoschisin (Fig. 4d), exposure to light for 60 to 120 minutes significantly decreased the protein expression of retinoschisin in E18 chick embryos that had never been exposed to light (Fig. 4e; P < 0.05, 60 minutes vs. 0, 5, 15 minutes; 120 minutes vs. 0, 5, 15 minutes). Therefore, the protein expression of retinoschisin in response to acute illumination changes depends on previous light-dark entrainment.
Figure 4.
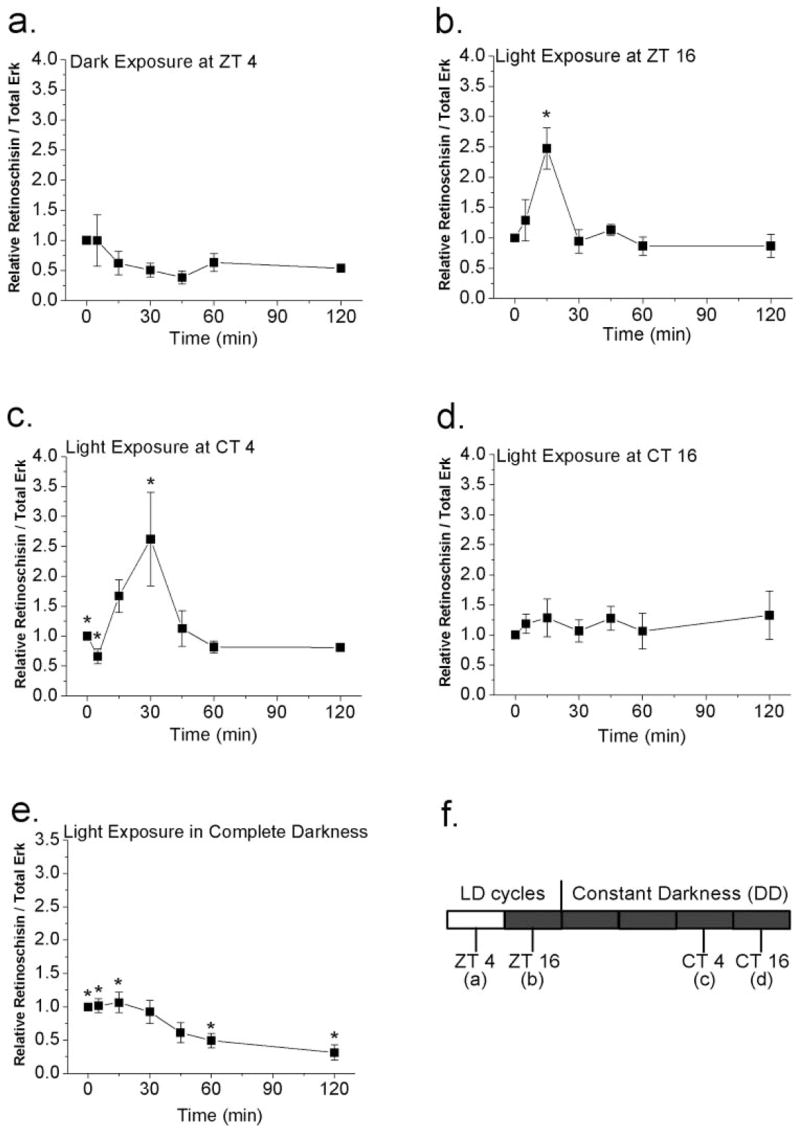
Acute changes in illumination affect protein levels of retinoschisin. (a, b) E18 chick embryos in different preconditions were exposed to light or dark briefly for 0, 5, 15, 30, 45, 60, and 120 minutes. E18 embryos were under LD cycles for 7 days. (a) At ZT 4 (4 hours after lights on), brief exposure to darkness of various lengths of time did not affect the retinoschisin content. (b) At ZT 16 (4 hours after lights off), exposure to light for 15 minutes significantly increased the protein content of retinoschisin compared with other exposure time periods (0, 5, 30, 45, 60, 120 minutes). *P < 0.05. (c, d) E18 chick embryos, entrained under LD cycles for 5 days and kept in DD for 2 days, were exposed to the light on the second day of DD at CT 4 (c) or CT 16 (d) for 0, 5, 15, 30, 45, 60, and 120 minutes. (c) At CT 4 (subjective day), brief exposure to light for 30 minutes significantly increased retinoschisin compared with 0- and 5-minute light exposure (*P < 0.05). (d) At CT 16 (subjective night), acute light exposure did not change the retinoschisin content. (e) In E18 embryos that had been kept in complete darkness without any light experience, exposure to light for 60 or 120 minutes significantly decreased the protein expression of retinoschisin compared with 0-, 5-, and 15-minute light exposure (*P < 0.05). n = 5 to 6 for each time point. ANOVA with Tukey post hoc test was used to compare the retinoschisin protein level at different exposure time periods. (f) Different experimental paradigms from (a) to (d).
Discussion
Our study indicates a circadian regulation of the expression and secretion of retinoschisin in the chick retina. Protein expression and secretion of retinoschisin were higher during the subjective night and lower during the subjective day, and the highest mRNA expression was 4 hours more advanced than the peak of protein expression. The mRNA and protein rhythms of retinoschisin have the same patterns in constant darkness (DD) as in LD cycles (data not shown). In addition, we found that Ras, MAP kinase Erk, and CaMKII were part of the circadian output pathway regulating the rhythmicity of retinoschisin. This Ras-MAP kinase-CaMKII pathway also regulates the affinity rhythm of cGMP-gated ion channels to cGMP21,25 and the circadian expression of L-type VGCCs.24 Therefore, the Ras-MAP kinase-CaMKII pathway may serve as the “universal” output pathway in regulating photoreceptor physiology.
Interestingly, the rhythmicity of retinoschisin was concurrent with the circadian rhythms of L-type VGCC currents. Blockage of L-type VGCCs dampened the retinoschisin rhythm but did not completely block the secretion of retinoschisin. By contrast, the release of melatonin is an L-type VGCC-dependent process. Nitrendipine, an L-type VGCC blocker, inhibits the melatonin synthesis enzyme arylalkylamine N-acetyltransferase, and it also blocks the release of melatonin in chick retina photoreceptors.16,17 Hence, L-type VGCCs played a role in the circadian regulation of retinoschisin content and secretion, but the molecular mechanism underlying retinoschisin secretion did not entirely depend on L-type VGCCs. Even though inhibition of L-type VGCCs did not completely inhibit the secretion of retinoschisin, we cannot rule out the possibility that the secretion of retinoschisin could be a calcium-dependent process.
Retinoschisin is known to serve as an anchor protein in maintaining the architecture of the retina synapses, especially around the photoreceptor synapses.1,10,13 Normally, upon secretion, retinoschisin interacts with proteins and phospholipids at the surfaces of photoreceptor membranes of the inner segments and the outer plexiform layer,34,35 and retinoschisin forms a stabilizing scaffold as a multimolecular complex for retinal synapses.36 The retinas of patients with human X-linked retinoschisis (XLRS) display macular atrophy.4,37 The electroretinogram (ERG) recordings from XLRS patients show that the synaptic currents between photoreceptors and bipolar cells (b-wave) are significantly altered, and the cone-driven ERG responses are more severely affected than rod-driven responses.4,37 In retinoschisin-deficient mice, the number of photoreceptors decreases with photoreceptor displacement,10 and the extracellular space increases in the region of photoreceptor ribbon synapses.10 Replacement of the RS1 gene in retinoschisin-knockout mice leads to an improvement in retinal structure and function.11,12 Hence, retinoschisin is believed to play an important role in maintaining the proper architecture of the retina during development.1,10 However, the protein expression of retinoschisin remains high throughout adulthood, and there is an especially heavy concentration of retinoschisin along the inner segments and synaptic regions of photoreceptors.13 Therefore, retinoschisin could have functions other than maintaining retinal architecture during development.
Photoreceptors undergo daily cycling changes in retinomotor movement of inner segments,29,38,39 outer segment disc shedding and membrane renewal,28,40,41 morphologic changes at synaptic ribbons,42,43 gene expression,44–46 and functional properties of ion channels,21,24 among other photoreceptor activities in vertebrates. At photoreceptor synapses, the length and the shape of the photoreceptor synaptic ribbons change over 24-hour daily cycles in mice.42,43 The number of synaptic ribbons in photoreceptor terminals of fish retinas also changes on a circadian cycle.47 All of the evidence described above points to the circadian control of synaptic plasticity in the retina in vivo. The circadian expression of retinoschisin presented here supports the notion that retinoschisin plays an important role in daily photoreceptor synaptic plasticity and in maintaining photoreceptor stability during inner segment retinomotor movement and outer segment renewal during 24-hour cycles.
The ultrastructure and the length of synaptic ribbons are under circadian control but also respond to illumination.42,43,48 The ribbons form protrusions and release them into the cytoplasm within 30 to 60 minutes after lights on; the reverse occurs within 30 minutes after lights off.48 In a similar fashion, retinoschisin can respond to acute illumination changes, but this response depends on previous light exposure experience. We found that brief light exposure caused an increase of retinoschisin at night (ZT 16) under LD cycles and during the subjective day (at CT 4, in DD), whereas acute light exposure failed to elicit a transient increase of retinoschisin during the subjective night (at CT 16) and in embryos that were never exposed to the light before. Hence, retinoschisin plays an important role in the circadian regulation of photoreceptor physiology and function, and it may also participate in the circadian-dependent, and illumination-dependent, synaptic plasticity at ribbon synapses.
Acknowledgments
Supported by start-up funds from Texas A&M University and by National Institutes of Health Grant RO1EY017452 (GY-PK).
The authors thank Tao Wang for assistance in making the retinoschisin antibody.
Footnotes
Disclosure: M.L. Ko, None; Y. Liu, None; L. Shi, None; D. Trump, None; G.Y.-P. Ko, None
References
- 1.Reid SN, Yamashita C, Farber DB. Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and Müller cells. J Neurosci. 2003;23:6030–6040. doi: 10.1523/JNEUROSCI.23-14-06030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grayson C, Reid SN, Ellis JA, et al. Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri-Rb1 cells. Hum Mol Genet. 2000;9:1873–1879. doi: 10.1093/hmg/9.12.1873. [DOI] [PubMed] [Google Scholar]
- 3.Molday RS. Focus on molecules: retinoschisin (RS1) Exp Eye Res. 2007;84:227–228. doi: 10.1016/j.exer.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Jr, Donoso LA. X-linked retinoschisis: a clinical and molecular genetic review. Surv Ophthalmol. 2004;49:214–230. doi: 10.1016/j.survophthal.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Sikkink SK, Biswas S, Parry NR, Stanga PE, Trump D. X-linked retinoschisis: an update. J Med Genet. 2007;44:225–232. doi: 10.1136/jmg.2006.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimenides D, George ND, Yates JR, et al. X-linked retinoschisis: clinical phenotype and RS1 genotype in 86 UK patients. J Med Genet. 2005;42:e35. doi: 10.1136/jmg.2004.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu WW, Molday RS. Defective discoidin domain structure, subunit assembly, and endoplasmic reticulum processing of retinoschisin are primary mechanisms responsible for X-linked retinoschisis. J Biol Chem. 2003;278:28139–28146. doi: 10.1074/jbc.M302464200. [DOI] [PubMed] [Google Scholar]
- 8.Wu WW, Wong JP, Kast J, Molday RS. RS1, a discoidin domain-containing retinal cell adhesion protein associated with X-linked retinoschisis, exists as a novel disulfide-linked octamer. J Biol Chem. 2005;280:10721–10730. doi: 10.1074/jbc.M413117200. [DOI] [PubMed] [Google Scholar]
- 9.Molday LL, Hicks D, Sauer CG, Weber BH, Molday RS. Expression of X-linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Invest Ophthalmol Vis Sci. 2001;42:816–825. [PubMed] [Google Scholar]
- 10.Weber BH, Schrewe H, Molday LL, et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci USA. 2002;99:6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min SH, Molday LL, Seeliger MW, et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol Ther. 2005;12:644–651. doi: 10.1016/j.ymthe.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Y, Takada Y, Kjellstrom S, et al. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004;45:3279–3285. doi: 10.1167/iovs.04-0576. [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Fariss RN, Tanikawa A, et al. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest Ophthalmol Vis Sci. 2004;45:3302–3312. doi: 10.1167/iovs.04-0156. [DOI] [PubMed] [Google Scholar]
- 14.Takada Y, Fariss RN, Muller M, Bush RA, Rushing EJ, Sieving PA. Retinoschisin expression and localization in rodent and human pineal and consequences of mouse RS1 gene knockout. Mol Vis. 2006;12:1108–1116. [PubMed] [Google Scholar]
- 15.Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova TN, Iuvone PM. Melatonin synthesis in retina: circadian regulation of arylalkylamine N-acetyltransferase activity in cultured photoreceptor cells of embryonic chicken retina. Brain Res. 2003;973:56–63. doi: 10.1016/s0006-8993(03)02540-x. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: a mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–103. doi: 10.1016/j.brainres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Retinal Eye Res. 1995;14:267–291. [Google Scholar]
- 19.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- 20.Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 21.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones: Erk MAP kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 22.Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993;601:303–307. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem Photobiol. 2002;75:547–553. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Ko ML, Liu Y, Dryer SE, Ko GY. The expression of L-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J Neurochem. 2007;103:784–792. doi: 10.1111/j.1471-4159.2007.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated channels of vertebrate cone photoreceptors: role of cAMP and Ras. J Neurosci. 2004;24:1296–1304. doi: 10.1523/JNEUROSCI.3560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko GY, Ko ML, Dryer SE. Circadian phase-dependent modulation of cGMP-gated channels of cone photoreceptors by dopamine and D2 agonist. J Neurosci. 2003;23:3145–3153. doi: 10.1523/JNEUROSCI.23-08-03145.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota T, Kagiwada S, Kasahara T, Okano T, Murata M, Fukada Y. Effect of brefeldin A on melatonin secretion of chick pineal cells. J Biochem (Tokyo) 2001;129:51–59. doi: 10.1093/oxfordjournals.jbchem.a002836. [DOI] [PubMed] [Google Scholar]
- 28.Reme C, Wirz-Justice A, Rhyner A, Hofmann S. Circadian rhythm in the light response of rat retinal disk-shedding and autophagy. Brain Res. 1986;369:356–360. doi: 10.1016/0006-8993(86)90550-0. [DOI] [PubMed] [Google Scholar]
- 29.Burnside B. Light and circadian regulation of retinomotor movement. Prog Brain Res. 2001;131:477–485. doi: 10.1016/s0079-6123(01)31038-5. [DOI] [PubMed] [Google Scholar]
- 30.Burkhardt DA. Light adaptation and contrast in the outer retina. Prog Brain Res. 2001;131:407–418. doi: 10.1016/s0079-6123(01)31033-6. [DOI] [PubMed] [Google Scholar]
- 31.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–4249. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iuvone PM, Brown AD, Haque R, et al. Retinal melatonin production: role of proteasomal proteolysis in circadian and photic control of arylalkylamine N-acetyltransferase. Invest Ophthalmol Vis Sci. 2002;43:564–572. [PubMed] [Google Scholar]
- 34.Molday LL, Wu WW, Molday RS. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J Biol Chem. 2007;282:32792–32801. doi: 10.1074/jbc.M706321200. [DOI] [PubMed] [Google Scholar]
- 35.Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Invest Ophthalmol Vis Sci. 2007;48:991–1000. doi: 10.1167/iovs.06-0915. [DOI] [PubMed] [Google Scholar]
- 36.Steiner-Champliaud MF, Sahel J, Hicks D. Retinoschisin forms a multi-molecular complex with extracellular matrix and cytoplasmic proteins: interactions with β2 laminin and αB-crystallin. Mol Vis. 2006;12:892–901. [PubMed] [Google Scholar]
- 37.Bradshaw K, Allen L, Trump D, Hardcastle A, George N, Moore A. A comparison of ERG abnormalities in XLRS and XLCSNB. Doc Ophthalmol. 2004;108:135–145. doi: 10.1023/b:doop.0000036786.22179.44. [DOI] [PubMed] [Google Scholar]
- 38.Pierce ME, Besharse JC. Circadian regulation of retinomotor movements, I: interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menger GJ, Koke JR, Cahill GM. Diurnal and circadian retinomotor movements in zebrafish. Vis Neurosci. 2005;22:203–209. doi: 10.1017/S0952523805222083. [DOI] [PubMed] [Google Scholar]
- 40.LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- 41.Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- 42.Adly MA, Spiwoks-Becker I, Vollrath L. Ultrastructural changes of photoreceptor synaptic ribbons in relation to time of day and illumination. Invest Ophthalmol Vis Sci. 1999;40:2165–2172. [PubMed] [Google Scholar]
- 43.Balkema GW, Cusick K, Nguyen TH. Diurnal variation in synaptic ribbon length and visual threshold. Vis Neurosci. 2001;18:789–797. doi: 10.1017/s0952523801185123. [DOI] [PubMed] [Google Scholar]
- 44.Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- 45.Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- 46.Haque R, Chaurasia SS, Wessel JH, 3rd, Iuvone PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13:2247–2251. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- 47.Allwardt BA, Lall AB, Brockerhoff SE, Dowling JE. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J Neurosci. 2001;21:2330–2342. doi: 10.1523/JNEUROSCI.21-07-02330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur J Neurosci. 2004;19:1559–1571. doi: 10.1111/j.1460-9568.2004.03198.x. [DOI] [PubMed] [Google Scholar]


