Summary
Introduction
Iron deficiency is widespread in the developing world and is especially common in young children who live on the Indian subcontinent. Supplementation with iron and folic acid alleviates severe anaemia and enhances neurodevelopment in deficient populations, but little is known about the risks of mortality and morbidity associated with supplementation.
Methods
We did a community-based, cluster-randomised, double-masked, placebo-controlled, 2• 2 factorial trial in children aged 1−36 months and residing in southern Nepal. We randomly assigned children daily oral supplementation to age 36 months with: iron (12·5 mg) and folic acid (50 • g; n=8337), zinc alone (10 mg), iron, folic acid, and zinc (n=9230), or placebo (n=8683); children aged 1−11 months received half the dose. Our primary outcome measure was all-cause mortality, and our secondary outcome measures included cause-specific mortality and incidence and severity of diarrhoea, dysentery, and acute respiratory illness. Analyses were by intention to treat. This study is registered at clinicaltrials.gov, number NCT00109551.
Findings
The iron and folic acid-containing groups of the study were stopped early in November, 2003, on the recommendation of the data and safety monitoring board; mortality in these groups did not differ from placebo and there was low power to detect positive or negative effects by the time enrolment was completed. We continued to enrol children to the placebo and zinc alone groups. 25 490 children participated and analyses are based on 29 097·3 person-years of follow-up. There was no difference in mortality between the groups who took iron and folic acid without or with zinc when compared with placebo (HR 1·03, 95% CI 0·78−1·37, and 1·00, 0·74−1·34, respectively). There were no significant differences in the attack rates for diarrhoea, dysentery, or respiratory infections between groups, although all the relative risks except one indicated modest, non-significant protective effects.
Interpretation
Daily supplementation of young children in southern Nepal with iron and folic acid with or without zinc has no effect on their risk of death, but might protect against diarrhoea, dysentery, and acute respiratory illness.
Introduction
Although substantial progress has been made over the past 20 years in improving child survival in developing countries, millions of children still die every year from preventable causes such as diarrhoea, pneumonia, and undernutrition.1 Findings of an analysis of risk factors for child mortality done for WHO's Global Burden of Disease Project suggest that underweight and micronutrient deficiencies are the leading underlying causes of child death in the developing world.2–5 In Nepal, for example, despite a well functioning vitamin A supplementation programme, the mortality rate in children younger than age 5 years (under-5 mortality) still exceeds 90 per 1000 livebirths.6
Iron and zinc deficiencies are common in children in developing countries.7–9 In the central plains of Nepal, an area without great malaria transmission, more than half of infants are anaemic (haemoglobin concentration • 110 g/L) by 12 weeks of age.10 Furthermore, national survey data indicate that more than three-quarters of preschool children are anaemic, with more than 3% severely anaemic (haemoglobin concentration • 70 g/L).11 Although there are few data on zinc deficiency in children in this population, dietary intake of zinc is low and serum concentrations of zinc in women of reproductive age suggest that most are zinc deficient.12
Iron deficiency and iron-deficiency associated anaemia are associated with impaired cognitive and motor development, slow growth, and decreased appetite in young children; improvements have been noted in some, but not all, populations after supplementation with iron.13–19 The role of iron-deficiency anaemia and the effect of iron supplementation on the incidence and severity of common morbidities of childhood are controversial and probably vary by setting and disorder.20,21 There is a similar lack of consensus with respect to the effect of iron-deficiency anaemia, and its correction through iron supplementation, on child mortality, although most agree that very severe anaemia is life threatening.22
Results of work done over the past 5 years indicate that zinc deficiency is an important risk factor for diarrhoea and acute respiratory infections in young children, that zinc supplementation is efficacious as a therapeutic adjuvant to oral rehydration for diarrhoea and might prevent serious respiratory illness, and that zinc supplementation of premature infants might reduce their risk of mortality.5,23–25 Whether universal zinc supplementation can reduce mortality rates in preschool children in populations where zinc deficiency is endemic, however, is unknown.
Our aim, therefore, was to ascertain whether supplementation of young children with iron and folic acid with or without zinc can improve child survival in a population with high rates of anaemia associated with iron deficiency and zinc deficiency. This report focuses on the effect of supplementation with iron and folic acid, with or without zinc, on mortality and morbidity. The effects of zinc alone will be reported separately when that portion of the trial is complete.
Methods
Participants
Between October, 2001, and November, 2003, we did a community-based, placebo-controlled, randomised, 2• 2 factorial trial in the southern plains of Nepal (Nepal Nutrition Intervention Project, Sarlahi-4 [NNIPS-4]). The study population consisted of children 1−36 months of age who lived in households in the NNIPS catchment area of 30 Village Development Committees (VDCs; formerly called panchayats) in Sarlahi District along the border with Bihar State in northern India. This area is part of the flood plain of the Ganges river and its tributaries that drain from the Himalayas to the Bay of Bengal. It is typical of much of northern India and large parts of western Bangladesh and northern Pakistan. The participating VDCs are similar to others in the district with a predominance of traditional, rural, Hindu culture. The population is composed mostly of peasant farmers or labourers and their families and is considered a poor area even in Nepal; three-quarters of the population lives below the poverty line established by the government of Nepal.26 Every VDC is divided administratively into nine wards by the government. We have further divided these 270 wards into 426 sectors, to enable one local female community worker to visit all of the houses in her sector within a 20-h week. The number of sectors in each ward varies with the total population of the ward and the geographical layout. All of the sectors, and the villages within the sectors, have been mapped and the houses numbered. All children aged 1−35 months and living in households in the study area during the baseline enrolment round were eligible. In addition, all children born into households in the study area were eligible once 1 month old if that house was their primary residence.
We obtained oral informed consent on a community basis during meetings with community leaders. We also obtained verbal informed consent at the household level from the parents of eligible children. Occasionally, parents who initially refused to allow their children to be enrolled changed their minds during the follow-up phase of the study. We then enrolled these children. The Nepal Health Research Council, the Committee on Human Research of the Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA, and the Institutional Review Board of Cornell University, Ithaca, NY, USA, provided ethical approval.
Procedures
We randomised children by sector, stratified by geographic area and in blocks of four, to receive one tablet daily (or half a tablet if • 1 year old), containing: iron (12·5 mg) and folic acid (50 • g), iron and folic acid plus zinc, or placebo. The sweet, vanilla flavoured supplements were specially formulated and manufactured as dispersible tablets by Nutriset (Malaunay, France) in conjunction with the Department of Child and Adolescent Health and Development at WHO, and were packaged in blister packs of seven tablets. The foil backing on the blister pack was imprinted with the treatment code. All children older than 6 months also received vitamin A as part of a national programme or, if missed, by study staff: those aged 12 months or older were given 200 000 IU of vitamin A every 6 months and those aged 6−12 months were given 100 000 IU.
We obtained data at the community, household, and individual level. Community-level data on the presence of economic, education, and health facilities were obtained in interviews with community leaders done just before the start of enrolment. Household-level data on socioeconomic status, health indicators, household structure, and housing material were obtained during the full population census also done before enrolment. All households that entered the trial for the first time because of a recent birth had their household interview at the time of child enrolment. On an individual level, study staff visited the children twice a week. They gave them a tablet directly and left enough tablets with the child's mother or caregiver to cover the daily doses until the next visit. Older children usually ate the tablet directly and mothers were instructed to dissolve the tablet in clean water or breastmilk for younger children. Study staff assessed compliance every week during their biweekly dosing and vital status assessment visits. The total number of tablets consumed by the child in the preceding week was recorded for every child. Investigators, study staff, and participants were unaware of assigned treatments. The Department of Child and Adolescent Health and Development at WHO, Geneva, Switzerland, kept the treatment assignment codes.
To confirm that the supplements used were active, we selected and tested the iron and zinc status of a sample of children aged 24 months or older after 12 months of follow-up. A blood sample was taken from participating children and the haemoglobin concentration measured with the HemoCue haemoglobinometer (HemoCue, Angelhom, Sweden). The remaining blood was centrifuged and the serum separated, collected, stored at • 10°C in Nepal, and then shipped in liquid nitrogen to Baltimore, MD, USA, where it was stored at • 70°C until analysis was undertaken. Serum ferritin was assessed with an ELISA assay (DELFIA system by Wallac, Gaithersburg, MD, USA).
Our primary outcome was all-cause mortality. The community workers recorded any deaths during their weekly visits to the participating families. The causes of death were determined by two physicians by independent review of information obtained by verbal autopsy with members of the immediate family after an appropriate period of mourning. If there was disagreement about the cause of death, a consensus meeting was held. Secondary outcomes included cause-specific mortality and the incidence and severity of diarrhoea, dysentery, and acute respiratory illness in two subsamples of 1200 children who were enrolled in the main trial and were younger than 24 months of age; we followed-up the first sample for 12 months and the second from enrolment in February, 2003, until the iron and folic acid-containing groups of the study were stopped in mid-November, 2003. Children in the morbidity subsamples were visited weekly. Mothers were asked about the onset and length of specific signs and symptoms for every day of the preceding week. The morbidities assessed included cough, fever, difficult or rapid breathing, diarrhoea, and dysentery. Visits to health-care providers in the previous week were also recorded. We diagnosed diarrhoea in individuals who produced four or more loose watery stools per day for one or more consecutive days, and persistent diarrhoea in those with watery stools for longer than 14 days. We defined dysentery as diarrhoea with blood or mucus in the stool on at least 1 day. We separated episodes of diarrhoea and dysentery by at least 3 symptom-free days. We diagnosed acute respiratory infection as one or more consecutive days of fever, cough, and difficulty breathing (all three symptoms had to be present on at least 1 day during the episode) with a minimum of 7 days between episodes.
Statistical analysis
To detect a reduction of 20% in overall mortality in the active treatment group with 80% power, a 2-sided type I error of 5%, a design effect of 1·23, accounting for 10% loss to follow-up, and based on the assumption of no interaction of iron, folic acid, and zinc on mortality, we calculated that approximately 24 000 person-years in every row (column) of the marginal comparisons—ie, iron and folic acid versus placebo or zinc versus placebo—were required. However, at the second meeting of the data and safety monitoring board about 14 months into recruitment, we noted that the mortality rate of children was much lower than originally expected. We therefore recalculated the sample size estimates. The final number needed was calculated at about 33 000 person-years in every row (column) of the marginal comparisons.
Analyses were by intention to treat. Children who migrated out of the study area or who refused further participation were censored at the time they left the study. We used SAS (version 8) and STATA (version 8.0) for statistical analyses. We compared treatment groups by baseline household, maternal, and child characteristics to assess imbalance after randomisation. We adjusted estimates of standard error to account for the clustered randomisation, using the generalised estimating equations approach.27 We used two approaches to assess the effect of treatment on mortality: the first estimated the incidence density of mortality with person-time as the denominator of the observed rates; and the second used survival analysis techniques such as Kaplan-Meier survival curves. We used Cox proportional hazard models to adjust for potentially confounding factors imbalanced in the treatment groups and to model potential effect modification. We estimated standard errors of the relative risks from proportional hazards models, using robust variance estimation to account for the clustered randomisation.28 We calculated incidence density rates of diarrhoea, dysentery, and acute respiratory infections with days during episodes excluded from the denominator of person-years at risk.
An independent data and safety monitoring board that included a paediatrician, a biostatistician, and the Member Secretary of the Nepal Health Research Council was formed and met four times to review the protocol and the data for safety and efficacy. There was no pre-established statistical stopping rule adopted by the data and safety monitoring board. However, detailed analysis was done only if the statistical evidence for treatment differences in mortality reached a p value of 0·2 or lower. At the fourth meeting in July, 2003, the board recommended that the iron and folic acid-containing groups of the trial be stopped because there was no evidence of a beneficial effect and the statistical power to detect significant differences in mortality between the treatment groups would be small by the time study recruitment and follow-up were completed. They concluded that further recruitment efforts would be better expended on increasing the zinc versus placebo groups of the trial. These groups will continue enrolment and follow-up in 2006. The ethical committees approved this decision and the protocol change was made in mid-November 2003.
So that the investigators did not become aware of treatment allocation, we gave a data file to an independent systems analyst who replaced the individual identifiers with a new, random set of identification numbers, filed the linked old and new identification information in a secure location, and then replaced the treatment codes with the actual treatment received. Here, we report the primary and secondary outcomes and their differences between the groups who were taking placebo, iron and folic acid, and iron and folic acid with zinc. The investigators, staff, and participants remain masked to the actual treatment codes.
This study is registered at clinicaltrials.gov, number NCT00109551.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
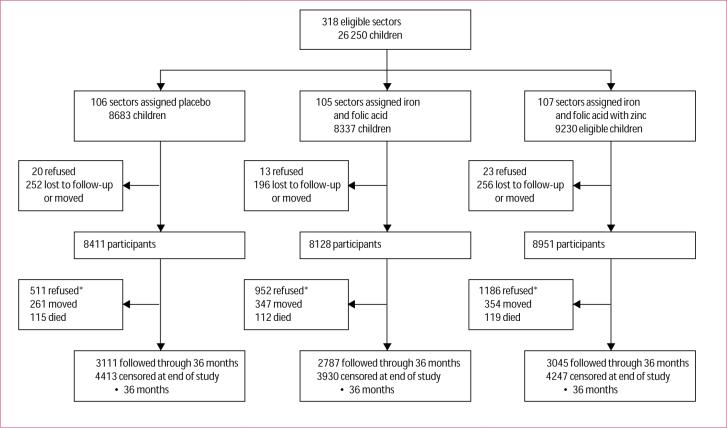
Figure 1 shows the trial profile. In 318 sectors, we approached the households of 26 250 eligible children. Less than 1% of parents refused to permit their children to participate and 3% moved or were lost between initial recruitment and enrolment. Parents of 25 490 (97%) children agreed to participate. Once enrolled and dosed, 2649 children (10%) subsequently refused to continue to participate. The baseline characteristics of those enrolled were similar across the three groups (table 1). We enrolled slightly more boys than girls, which was probably an indication of the young age distribution at enrolment; almost half of all children were aged 1−5 months when they entered the study. About two-thirds of families were Madeshi (ethnic group originally from north India) and about one-third Pahadis (ethnic group originally from the hill areas of Nepal). Only about 15% of families were from the higher castes, including Brahmins and Chetris. Between a fifth and a quarter of women and about half of men in participating households were literate at a basic level. Three-quarters of the fathers were either farmers or labourers and about half of families owned farmland. One or more children younger than age 5 years had died previous to the study in about a quarter of families. Only about 12% of households had electricity; ownership of other items was similar across treatment groups (table 1).
Figure 1. Trial profile.
*People who refused in these boxes began taking supplements but then decided to discontinue them at some point during follow-up. All were alive at time they discontinued and were censored.
Table 1.
Baseline characteristics
| |
Placebo (n=8411) |
Iron and folic acid (n=8128) |
Iron and folic acid with zinc (n=8951) |
|---|---|---|---|
| Age (months) | |||
| 1−5 | 3978 (47%) | 3814 (47%) | 4249 (48%) |
| 6−11 | 961 (11%) | 966 (12%) | 1017 (11%) |
| 12−23 | 1758 (21%) | 1784 (22%) | 1900 (21%) |
| 24−35 | 1714 (20%) | 1564 (19%) | 1785 (20%) |
| Sex | |||
| Male | 4239 (50%) | 4244 (52%) | 4568 (51%) |
| Female | 4172 (50%) | 3884 (48%) | 4383 (49%) |
| Ethnic group (n=142 missing) | |||
| Pahadi | 2798 (33%) | 2951 (37%) | 3150 (35%) |
| Madeshi | 5577 (67%) | 5127 (64%) | 5745 (65%) |
| Caste (n=120 missing) | |||
| Brahmin | 724 (9%) | 508 (6%) | 689 (8%) |
| Chetri | 618 (7%) | 753 (9%) | 608 (7%) |
| Vaiysha | 4914 (59%) | 5370 (66%) | 5960 (67%) |
| Shudra | 1151 (14%) | 991 (12%) | 1067 (12%) |
| Muslim or other (not included above) | 972 (12%) | 465 (6%) | 580 (7%) |
| Paternal literacy (n=149 missing) | |||
| Yes | 4454 (53%) | 4474 (56%) | 4736 (53%) |
| Maternal literacy (n=122 missing) | |||
| Yes | 1957 (23%) | 2034 (25%) | 2030 (23%) |
| Paternal occupation (n=204 missing) | |||
| Farmer | 3896 (47%) | 3782 (47%) | 3923 (44%) |
| Labourer | 2362 (28%) | 2383 (30%) | 2670 (30%) |
| Business man | 1144 (14%) | 904 (11%) | 1205 (14%) |
| Private service | 602 (7%) | 676 (8%) | 662 (8%) |
| Government | 247 (3%) | 220 (3%) | 287 (3%) |
| None | 106 (1%) | 76 (1%) | 141 (2%) |
| Previous child mortality (n=82 missing) | |||
| Rate/1000 livebirths | 88·0 | 82·6 | 87·3 |
| • 1 previous child death | 2139 (26%) | 1936 (24%) | 2277 (26%) |
| Electricity in house (n=121 missing) | |||
| Yes | 1922 (23%) | 1958 (24%) | 2045 (23%) |
| Ownership | |||
| Bicycle (n=119 missing) | 4015 (48%) | 3939 (49%) | 4213 (47%) |
| Cart (n=119 missing) | 989 (12%) | 929 (12%) | 901 (10%) |
| Radio (n=123 missing) | 2412 (29%) | 2167 (27%) | 2335 (26%) |
| Television (n=137 missing) | 1430 (17%) | 1425 (18%) | 1563 (18%) |
| (n=220 missing) | |||
| Non-paddy farm land (n=217 missing) | 3801 (46%) | 3440 (43%) | 3867 (44%) |
| Cattle (n=120 missing) | 5139 (61%) | 4827 (60%) | 5279 (59%) |
| Goats (n=126 missing) | 3912 (47%) | 3831 (47%) | 4030 (45%) |
| Paddy farm land | 4027 (48%) | 3941 (49%) | 4142 (47%) |
| House wall material (n=118 missing) | |||
| None | 132 (2%) | 137 (2%) | 128 (1%) |
| Thatch | 2103 (25%) | 2220 (27%) | 2486 (28%) |
| Mud | 4717 (56%) | 4223 (52%) | 4774 (54%) |
| Wood | 292 (4%) | 313 (4%) | 261 (3%) |
| Concrete | 1135 (14%) | 1196 (15%) | 1255 (14%) |
| House roof material (n=119 missing) | |||
| None | 37 (• 1%) | 18 (• 1%) | 69 (• 1%) |
| Thatch | 1513 (18%) | 1304 (16%) | 1688 (19%) |
| Tile or tin | 6480 (77%) | 6441 (80%) | 6784 (76%) |
| Concrete | 349 (4%) | 326 (4%) | 362 (4%) |
| Latrine at house (n=155 missing) | |||
| Yes | 1026 (12%) | 1031 (13%) | 1085 (12%) |
| Water source (n=121 missing) | |||
| Tube well | 6978 (83%) | 6530 (81%) | 7094 (80%) |
| Ring well | 1143 (14%) | 1391 (17%) | 1578 (18%) |
| Other | 257 (3%) | 167 (2%) | 231 (3%) |
| Mid—upper arm circumference (cm) (n=504 missing) | |||
| Mean (SD) | 11·95 (1·98) | 11·95 (1·97) | 12·01 (1·98) |
| Median (IQR) | 12·4 (3·20) | 12·4 (3·20) | 12·4 (3·20) |
| • 11·5 | 3099 (38%) | 2955 (37%) | 3248 (37%) |
| 11·5−12·4 | 1171 (14%) | 1158 (15%) | 1223 (14%) |
| 12·5−13·4 | 1774 (22%) | 1824 (23%) | 1874 (21%) |
| • 13·5 | 2177 (27%) | 2035 (26%) | 2448 (28%) |
Data are number (%) unless otherwise indicated.
In a subsample, 12 months after the start of supplementation, concentrations of both haemoglobin and serum ferritin were significantly higher in the groups taking iron and folic acid than in the placebo group, indicating that the supplements contained bioavailable iron (table 2). Although 6% (95% CI 2·6−10·7) of children in the placebo group had severe anaemia (haemoglobin • 70 g/L), only 1% (0·1−4·6) of those in the iron and folic acid group and 3% (1·1−5·8) of those taking iron and folic acid with zinc were severely anaemic. We noted similar differences for moderate anaemia (haemoglobin 70−90 g/L). Overall rates of anaemia were 62% (48·2−69·7) in the placebo group versus 45% (37·7−53·4) and 48% (37·5, 56·2) in the groups taking iron and folic acid without and with zinc, respectively. Serum ferritin concentrations were significantly higher in the groups supplemented with iron and folic acid. The group taking iron and folic acid with zinc had a smaller difference in serum ferritin concentrations when compared with placebo than did the group taking only iron and folic acid. The prevalence of iron-deficiency anaemia was 84% (65−92) lower in the group taking iron and folic acid and 53% (18−73) lower in the group taking iron and folic acid with zinc than in the placebo group (table 2).
Table 2.
12-month post-supplementation iron status indicators by treatment group (substudy)
| |
Placebo |
Iron and folic acid |
Iron and folic acid with zinc |
|---|---|---|---|
| Haemoglobin (g/L) | |||
| Number assessed | 187 | 152 | 182 |
| Mean (SD) | 10·31 (1·71) | 11·11 (1·18) | 10·96 (1·61) |
| Difference (95% CI)* | Ref | 0·71 | 0·59 |
| (0·34−1·09) | (0·10−1·08) | ||
| • 70 | 11 (6%) | 1 (1%) | 5 (3%) |
| 70−89 | 18 (10%) | 5 (3%) | 14 (8%) |
| 90−110 | 87 (47%) | 63 (42%) | 68 (37%) |
| • 110 | 71 (38%) | 83 (55%) | 95 (52%) |
| p* | Ref | 0·007 | 0·037 |
| Serum ferritin (• g/L) | |||
| Number assessed | 159 | 146 | 164 |
| Median (IQR) | 19·58 (24·54) | 53·57 (47·12) | 35·92 (31·01) |
| Difference (95% CI)* | Ref | 34·25 | 13·07 |
| (22·82−45·69) | (2·35−23·79) | ||
| • 12 | 45 (28%) | 7 (5%) | 22 (13%) |
| Relative prevalence | Ref | 0·17 | 0·49 |
| (95% CI) | (0·08−0·36) | (0·29−0·83) | |
| Iron-deficiency anaemia† | |||
| Number (%) affected | 42 (26%) | 6 (4%) | 20 (12%)‡ |
| Relative prevalence | Ref | 0·16 | 0·47 |
| (95% CI) | (0·08−0·35) | (0·27−0·82) |
Difference between active treatment and placebo.
Haemoglobin• 110 g/L and serum ferritin• 12• g/L.
Comparison of iron and folic acid with and without zinc: p=0·0039.
There was no difference in all-cause mortality by treatment group, with relative risks (95% CIs) of 1·03 (0·78−1·37) and 1·00 (0·74−1·34) without and with zinc, respectively (table 3, figure 2). There was no evidence for interaction of treatment group with sex, ethnic group, a previous child death in the family, and baseline mid–upper arm circumference (table 3). We noted a non-significant, monotonic decline in the relative risk with increasing age for both treatment groups compared with placebo but the strength of evidence for an interaction was weak (test for interaction: iron and folic acid vs placebo p=0·86; iron and folic acid with zinc vs placebo p=0·85; table 3).
Table 3.
Mortality by treatment group
|
Placebo |
Iron and folic acid |
Iron and folic acid with zinc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Person-years |
Deaths |
Rate/1000 person-years |
Person-years |
Deaths |
Rate/1000 person-years |
HR (95% Cl) |
Person-years |
Deaths |
Rate/1000 person-years |
HR (95% Cl) |
| Sex | |||||||||||
| Male | 4909·0 | 52 | 10·59 | 4827·5 | 41 | 8·49 | 0·80 (0·52−1·22) | 5153·7 | 48 | 9·31 | 0·88 (0·58−1·31) |
| Female | 4889·5 | 63 | 12·88 | 4383·2 | 71 | 16·20 | 1·25 (0·87−1·79) | 4934·2 | 71 | 14·39 | 1·11 (0·76−1·61) |
| Age (months) | |||||||||||
| 1−5 | 1282·7 | 28 | 21·83 | 1211·5 | 34 | 28·07 | 1·28 (0·79−2·08) | 1353·0 | 36 | 27·35 | 1·21 (0·74−1·98) |
| 6−11 | 1720·0 | 24 | 14·00 | 1612·3 | 24 | 14·89 | 1·06 (0·59−1·92) | 1791·0 | 27 | 15·08 | 1·08 (0·62−1·88) |
| 12−23 | 3429·2 | 37 | 10·79 | 3247·2 | 34 | 10·47 | 0·97 (0·57−1·64) | 3546·4 | 34 | 9·59 | 0·89 (0·53−1·48) |
| 24−36 | 3367·2 | 26 | 7·72 | 3140·2 | 20 | 6·37 | 0·82 (0·45−1·51) | 3397·7 | 22 | 6·47 | 0·83 (0·46−1·51) |
| Ethnic group (n=142 missing; 2 deaths) | |||||||||||
| Pahadi | 3108·7 | 25 | 8·04 | 3252·8 | 25 | 7·69 | 0·95 (0·52−1·74) | 3394·7 | 32 | 9·43 | 1·16 (0·66−2·05) |
| Madeshi | 6651·8 | 89 | 13·38 | 5922·2 | 86 | 14·52 | 1·08 (0·79−1·48) | 6656·6 | 87 | 13·07 | 0·97 (0·69−1·37) |
| History of child death (n=80 missing; 4 deaths) | |||||||||||
| No | 7204·0 | 68 | 9·44 | 6977·5 | 73 | 10·46 | 1·10 (0·78−1·55) | 7414·8 | 71 | 9·58 | 1·01 (0·71−1·42) |
| Yes | 2593·7 | 46 | 18·12 | 2233·0 | 38 | 17·02 | 0·96 (0·59−1·54) | 2670·5 | 46 | 18·00 | 0·96 (0·60−1·56) |
| Mid-upper arm circumference (cm) (n=504 missing; 3 deaths) | |||||||||||
| • 11·5 | 3425·3 | 72 | 21·02 | 3098·9 | 77 | 24·85 | 1·17 (0·84−1·63) | 3426·3 | 69 | 20·14 | 0·95 (0·67−1·34) |
| 11·5−12·4 | 1679·0 | 19 | 11·32 | 1604·8 | 11 | 6·85 | 0·60 (0·30−1·20) | 1688·8 | 19 | 11·25 | 0·99 (0·53−1·85) |
| 12·5−13·4 | 2292·5 | 11 | 4·80 | 2309·4 | 11 | 4·76 | 0·99 (0·37−2·63) | 2384·5 | 14 | 5·87 | 1·22 (0·52−2·86) |
| • 13·5 | 2244·7 | 12 | 5·35 | 2070·9 | 12 | 5·79 | 1·08 (0·48−2·44) | 2444·9 | 16 | 6·54 | 1·22 (0·57−2·62) |
| Total | 9798·6 | 115 | 11·74 | 9210·7 | 112 | 12·16 | 1·03 (0·78−1·37) | 10088·0 | 119 | 11·80 | 1·00 (0·74−1·34) |
There was little evidence of interactions as all statistical tests had p values • 0·10.
Figure 2.
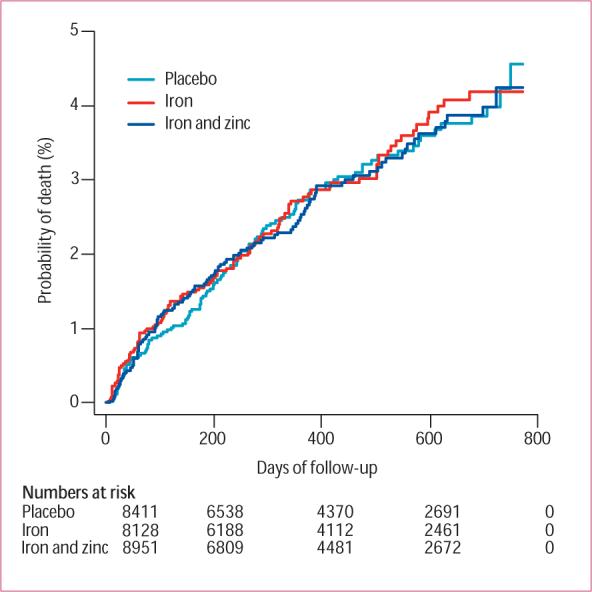
Cumulative mortality by treatment group
The most common causes of death in this population were diarrhoea and acute lower respiratory illness (table 4). Cause-specific mortality analysis did not indicate significant differences by treatment group except for an elevated risk of death from “other infections” in the group taking iron and folic acid versus placebo (table 4). Although the CIs for the point estimate of the relative risk of 3·58 (1·05−13·52) were above 1·00, the difference was based on only ten and three deaths in the iron and folic acid and placebo groups, respectively.
Table 4.
Cause-specific mortality by treatment group
|
Placebo (person-years=9762.4) |
Iron and folic acid (person-years=9190.8) |
Iron and folic acid with zinc (person-years=10 075.7) |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Deaths |
Rate/1000 person-years |
Deaths |
Rate/1000 person-years |
HR (95% CI) |
Deaths |
Rate/1000 person-years |
HR (95% CI) |
| Acute lower respiratory illness | 29 | 2.97 | 24 | 2.61 | 0.88 (0.50−1.46) | 24 | 2.38 | 0.80 (0.45−1.34) |
| Diarrhoea | 21 | 2.15 | 24 | 2.61 | 1.21 (0.66−2.11) | 29 | 2.88 | 1.34 (0.74−2.28) |
| Dysentery | 12 | 1.23 | 11 | 1.20 | 0.98 (0.42−2.14) | 9 | 0.89 | 0.72 (0.30−1.68) |
| Malnutrition | 9 | 0.92 | 9 | 0.98 | 1.10 (0.46−2.81) | 8 | 0.79 | 0.86 (0.37−2.38) |
| Sudden infant death syndrome | 10 | 1.02 | 7 | 0.76 | 0.75 (0.25−1.69) | 4 | 0.40 | 0.39 (0.11−1.08) |
| Injuries | 5 | 0.51 | 1 | 0.11 | 0.22 (0.02−1.76) | 6 | 0.60 | 1.18 (0.35−3.71) |
| Other infections* | 3 | 0.31 | 10 | 1.11 | 3.58 (1.05−13.52) | 4 | 0.40 | 1.29 (0.28−5.62) |
| Other† | 0 | .. | 5 | 0.44 | .. | 0 | .. | .. |
| Uncertain or missing | 26 | 2.66 | 21 | 2.28 | 0.86 | 35 | 3.47 | 1.30 |
Sepsis (14), hepatitis (1), meningitis (1), and gastrointestinal infections (1).
Premature birth (1), congenital heart defects (1), rabies (1), retinoblastoma (1), and other miscellaneous (1).
There were no significant differences in the incidence of diarrhoea, persistent diarrhoea, dysentery, or acute lower respiratory illness between the iron and folic acid and placebo groups, although all the relative risks were below 1·0 (table 5). We noted a similar pattern for those taking iron and folic acid with zinc versus placebo. There were no significant differences in the attack rates, but all the relative risks, except for persistent diarrhoea, were below 1·0.
Table 5.
Morbidity incidence rates by treatment group
| |
Placebo |
Iron and folic acid |
Iron and folic acid with zinc |
|---|---|---|---|
| Diarrhoea | |||
| Episodes | 1327 | 1355 | 1286 |
| Person-years | 341.5 | 352.8 | 368.0 |
| Rate/child per year | 3.89 | 3.84 | 3.50 |
| RR (95% CI) | Ref | 0.94 (0.84−1.05) | 0.91 (0.81−1.01) |
| Median (IQR) duration (days) | 2 (1−3) | 2 (1−3) | 2 (1−3) |
| Persistent diarrhoea | |||
| Episodes | 24 | 19 | 27 |
| Person-years | 350.6 | 362.2 | 376.4 |
| Rate/child per year | 0.069 | 0.053 | 0.072 |
| RR (95% CI) | Ref | 0.74 (0.38−1.44) | 1.04 (0.56−1.91) |
| Median (IQR) duration (days) | 18 (16−24) | 17 (16−19) | 18 (16−22) |
| Dysentery | |||
| Episodes | 187 | 166 | 164 |
| Person-years | 349.6 | 361.4 | 376.0 |
| Rate/child per year | 0.53 | 0.46 | 0.44 |
| RR (95% CI) | Ref | 0.78 (0.57−1.09) | 0.85 (0.62−1.17) |
| Median (IQR) duration (days) | 2 (1−3) | 2 (1−3) | 2 (1−3) |
| Acute lower respiratory infection | |||
| Episodes | 481 | 482 | 461 |
| Person-years | 347.6 | 358.6 | 373.3 |
| Rate/child per year | 1.38 | 1.34 | 1.23 |
| RR (95% CI) | Ref | 0.92 (0.77−1.09) | 0.91 (0.76−1.08) |
| Median (IQR) duration (days) | 3 (2−4) | 3 (2−4) | 3 (2−4) |
Discussion
Our findings indicate that daily supplementation with iron and folic acid with or without zinc has no effect on risk of mortality in this population of young Nepali children. This lack of effect cannot be explained by a lack of iron deficiency in this population, since we noted significant differences in concentrations of haemoglobin and serum ferritin between the active treatment and placebo groups after about 12 months of supplementation. There was some indication that the addition of zinc to the iron and folic acid regimen reduced either absorption or mobilisation of iron. Although we have not yet analysed the zinc concentrations by treatment group to remain masked to the zinc and placebo treatment assignments, findings of previous studies29 in this population indicate that zinc deficiency is endemic. Selection bias is also unlikely to have played a part in these findings, since participation rates exceeded 96% at enrolment for all three treatment groups.
A small proportion of the children in the treatment groups moved out of the study area or were lost to follow-up. All of these children were alive at the time they were censored for this analysis. If there had been differences in the mortality rates of these children by treatment group, our estimates of treatment effect might have been biased. Although these children might have had a different overall risk of mortality than those that we followed-up, we think it is unlikely that this selection would be different by treatment group.
About twice the proportion of those who took iron and folic acid compared with placebo refused to continue with supplementation at some point. This finding suggests that some children detected an unpleasant taste in the active supplements. However, similar to the issue of migration, bias in our estimates of treatment effects would require differential mortality risk by treatment groups between those who refused and those who continued to participate. All three treatment groups had similar characteristics at baseline. Therefore, random imbalance in predictors of child mortality is also an implausible explanation for our results.
Our hypothesis was that supplementation with iron and folic acid in this population with a high risk of iron-deficiency anaemia would prevent the most severe forms of anaemia and, thereby, reduce the mortality risk of these young children. Supplementation was associated with a lower prevalence of severe and moderate anaemia, but the rates of morbidity and mortality did not differ between treatment and placebo groups. Limitations in iron status are not the only nutritional deficiency affecting response to infectious morbidity and mortality in this setting. Iron deficiency in this population becomes progressively more severe with age, doubling in prevalence from mid-infancy to the second year of life.30 An alternative explanation for our results is that many of the deaths occurred in infants, who were not yet sufficiently iron deficient to benefit from supplementation on this outcome (mortality). This notion is consistent with the decreasing (but non-significant) trend in the relative risk associated with supplementation with iron and folic acid as age increased beyond 12 months. Finally, the prevalence of protein–energy malnutrition in this population is high and deficiencies in other micronutrients probably exist despite the high coverage of vitamin A supplementation. The protective effect of iron supplementation, if mediated through its effect on anaemia, could depend on adequate levels of other dietary constituents to translate to observable differences in morbidity and mortality.
We noted no significant effect of either treatment on the incidence of common morbidities in this population. However, all relative risks except one were in the direction of a protective effect, and these effects for iron and folic acid with zinc on rates of diarrhoea, dysentery, and acute respiratory illness are consistent with findings of previous research.31 The definitions based on maternal report in our study were perhaps not specific enough to differentiate those children with serious illness from those with more routine morbid episodes. Alternatively, the combination of iron and folic acid with zinc might not be as efficacious as that of zinc alone for these outcomes. The independent effect of supplementation with zinc alone on morbidity, mortality, child development, and growth is still being assessed in the ongoing portions of this trial.
Results of some previous studies have indicated that children given iron supplements are at increased risk of illness.32 In this trial, there was no evidence for such an adverse effect or of an increased risk in mortality. Despite the lack of effect in this study, universal supplementation with iron and folic acid might still be warranted because of their potential role in motor and cognitive development. However, results from a parallel trial in Zanzibar,33 showing an adverse effect of this regimen on hospital admission and death, suggest that decisions about the routine use of prophylactic iron and folic acid supplementation in young children should consider the disease patterns in the population and the availability and use of treatment services for common infectious disease.
Acknowledgments
This study was done by the Center for Human Nutrition and the Sight and Life Institute in the Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA, with grants from the National Institutes of Health, Bethesda, MD, USA (HD 38753), the Bill and Melinda Gates Foundation, Seattle, Washington, DC, USA (810−2054), and a Cooperative Agreement between Johns Hopkins University and the Office of Health and Nutrition, US Agency for International Development, Washington, DC, USA (HRN-A-00−97−00015−00). We thank the members of the data and safety monitoring board:, Michael Hambidge, University of Colorado, Denver, CO, USA), William Blackwelder, Rockville, MD, USA, and Anil Mishra, Nepal Health Research Council, Kathmandu, Nepal.
Footnotes
Contributors J M Tielsch, R J Stoltzfus, and J Katz made primary contributions to the design and undertaking of the study, analysis and interpretation of results, and writing of this manuscript. S K Khatry, S C LeClerq, and S Shresta helped to do the fieldwork and controlled the quality of the trial. L C Mullany contributed to quality control, and to data management and analysis. R Adhikari participated in review of the verbal autopsies and in interpretation of the results. R E Black contributed to the development and design of the trial and to the interpretation of the study results.
Conflict of interest statement We declare that we have no conflict of interest.
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 2.Fishman S, Caulfield LE, de Onis M, et al. Childhood and maternal underweight. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. [Google Scholar]
- 3.Rice AL, West KP, Black RE. Vitamin A deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. [Google Scholar]
- 4.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. [Google Scholar]
- 5.Caulfield L, Black RE. Zinc deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization; Geneva: 2004. [Google Scholar]
- 6.Ministry of Health (Nepal), New ERA, ORC Macro . Nepal demographic and health survey, 2001. Ministry of Health,; Calverton: 2002. [Google Scholar]
- 7.Stoltzfus RJ, Dreyfuss ML. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. International Life Sciences Institute Press; Washington: 1998. [Google Scholar]
- 8.Ramakrishnan U, Huffman SL. Multiple micronutrient malnutrition: what can be done? In: Semba RD, Bloem MW, editors. Nutrition and health in developing countries. Humana Press; Totowa: 2001. [Google Scholar]
- 9.United Nations Administrative Coordinating Committee, Subcommittee on Nutrition . Fourth report on the world nutrition situation. UNACC/SCN; Geneva: 2000. [Google Scholar]
- 10.Dreyfuss ML, Shrestha JB, Khatry SK, et al. The prevalence of anemia among pregnant and lactating women, and among their infants in Sarlahi District. J Nepal Med Assn. 1997;35:234–40. [Google Scholar]
- 11.Child Health Division, Ministry of Health, HMG, Nepal . Nepal micronutrient status survey 1998. Ministry of Health; Kathmandu: 1998. [Google Scholar]
- 12.Jiang T, Christian P, Khatry SK, WE L, West KP., Jr Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr. 2005;135:1106–12. doi: 10.1093/jn/135.5.1106. [DOI] [PubMed] [Google Scholar]
- 13.Stoltzfus RJ, Chwaya HM, Montresor A, et al. Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr. 2004;134:348–56. doi: 10.1093/jn/134.2.348. [DOI] [PubMed] [Google Scholar]
- 14.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, et al. Effects of iron supplementation and anhelminthic treatment on motor and language development of Zanzibari preschool children. BMJ. 2001;323:1–8. doi: 10.1136/bmj.323.7326.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:51–61. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 16.Lind T, Lonnerdal B, Stenlund H, et al. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr. 2004;80:729–36. doi: 10.1093/ajcn/80.3.729. [DOI] [PubMed] [Google Scholar]
- 17.Pollitt E. The developmental and probabilistic nature of the functional consequences of iron-deficiency anemia in children. J Nutr. 2001;131:669S–75S. doi: 10.1093/jn/131.2.669S. [DOI] [PubMed] [Google Scholar]
- 18.Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. J Nutr. 1994;124:645–54. doi: 10.1093/jn/124.5.645. [DOI] [PubMed] [Google Scholar]
- 19.Black MM, Baqui AH, Zaman K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;80:903–10. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- 20.Gera T, Sachdev HPS. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ. 2002;325:1142–44. doi: 10.1136/bmj.325.7373.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar AH. Malaria. In: Semba RD, Bloem MW, editors. Nutrition and health in developing countries. Humana Press; Totowa: 2001. [Google Scholar]
- 22.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131(suppl):636S–45S. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 23.Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003;133(suppl):1485S–89S. doi: 10.1093/jn/133.5.1485S. [DOI] [PubMed] [Google Scholar]
- 24.Baqui AH, Zaman K, Persson LA, et al. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhea and acute lower respiratory infection in Bangladeshi infants. J Nutr. 2003;133:4150–57. doi: 10.1093/jn/133.12.4150. [DOI] [PubMed] [Google Scholar]
- 25.Sazawal S, Black RE, Menon VP, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics. 2001;108:1280–86. doi: 10.1542/peds.108.6.1280. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan EK, LeClerq SC, Khatry SK, et al. A window to child health in the terai. NNIPS monograph number 1. Nepal Nutrition Intervention Project-Sarlahi; Kathmandu: 1999. [Google Scholar]
- 27.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 28.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assn. 1989;84:1074–78. [Google Scholar]
- 29.Christian P, Khatry SK, Yamini S, et al. Zinc supplementation might potentiate the effect of vitamin A in restoring night vision in pregnant Nepalese women. Am J Clin Nutr. 2001;73:1045–51. doi: 10.1093/ajcn/73.6.1045. [DOI] [PubMed] [Google Scholar]
- 30.Siegel E. Anemia, motor development, and cognition: a randomized trial of iron-folic acid and/or zinc supplementation in young nepali children; Ph.D. thesis; Department of International Health, Johns Hopkins University. 2004. [Google Scholar]
- 31.Bhutta ZA, Black RE, Brown KH, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–33S. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 33.Sazawal S, Black RE, Ramsan M, et al. Effect of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]