Abstract
Traumatic brain injury (TBI) induces cachexia and neuroinflammation which profoundly impact patient recovery. Adipokine genes such as leptin (ob), resistin (rstn) and fasting-induced adipose factor (fiaf) are implicated in energy metabolism and body weight control and are also associated with chronic low grade inflammation. Since central rstn and fiaf expression was increased following hypoxic/ischemic brain injury, we hypothesized that these genes would also be induced in the rat brain following TBI. Realtime RT-PCR detected a 2−2.5-fold increase in ob mRNA in the ipsilateral cortex and thalamus 12 h following lateral fluid percussion (FP)-induced brain injury. Fiaf mRNA was elevated 5−7.5-fold in cortex, hippocampus and thalamus, and modest increases were also detectable in the contralateral brain. Remarkably, rstn mRNA was elevated in ipsilateral (150-fold) and in contralateral (50-fold) hippocampus. To test whether these changes were part of an inflammatory response to TBI we also examined the effects of an intracerebral injection of lipopolysaccharide (LPS). We determined that central injection of LPS produced some, but not all, of the changes seen after TBI. For example, in contrast to the stimulatory influence of TBI, LPS had no effect on ob expression in any brain region, though fiaf and rstn mRNA levels were significantly elevated in both ipsi- and contralateral cortex. In conclusion: (a) brain-derived adipokines could be involved in the acute pathology of traumatic brain injury partly through modulation of central inflammatory responses, but also via leptin-mediated neuroprotective effects and (b) TBI-induced brain adipokines may induce the metabolic changes observed following neurotrauma.
Keywords: Leptin, Resistin, Fasting-induced adipose factor (FIAF), Inflammation, Lipopolysaccharide (LPS), Hypothalamus, Cortex
Traumatic brain injury (TBI) is currently one of the leading causes of injury-related death and results in memory loss and impaired motor function [7,18]. Moreover TBI patients are often hypophagic, hypercatabolic and the associated muscular atrophy can have detrimental consequences on patient outcome [17,25]. A period of increased energy expenditure and hyperthermia generally follows TBI, further compounding the underlying brain injury [17,25]. At present there are no therapeutic interventions capable of counteracting or repairing TBI-induced brain damage, despite extensive research that to date has focused on a variety of pharmacological strategies, including, for example, glutamate antagonists, endocannabinoids and free radical scavengers [22]. More recently, through microarray analysis, several genes have been implicated in the pathogenesis of TBI [7,15], though their individual roles and their suitability as therapeutic targets remain unclear.
The association of cachexia with TBI suggested the possible involvement of adipokines, such as leptin, that are well-known for their effects on appetite, bodyweight and energy metabolism [2,26]. An intriguing additional role for leptin is its stimulatory effects on brain protein synthesis in addition to its neuroprotective and trophic properties in the central nervous system (CNS) [1,8]. Leptin also protected against cell death in rat C6 glioblastoma cells and in the human SH-SY5Y neuroblastoma cell line [5,23]. Our laboratory demonstrated that the rat brain is a site of expression of several genes normally found in adipose tissue, including leptin (ob), resistin (rstn) and fasting-induced adipose factor (fiaf) [28]. We also detected differential increases in rstn and fiaf mRNA in the neonatal mouse brain following cerebral hypoxia/ischemia (H/I) [27]. Fiaf gene expression was significantly elevated in the ipsilateral cerebral cortex and hippocampus at 2 and 7 days post-injury, but returned to baseline values 21 days later. In contrast, rstn mRNA was not increased until 21 days post-H/I injury. FIAF is known to exert pro-angiogenic effects [13], and the time-course of fiaf upregulation in the neonatal mouse brain corresponds with that of cerebrovascular angiogenesis that occurs following focal brain ischemia in mice [11]. Thus, brain-derived adipokines could be involved in the pathology of brain injury and repair.
Given the neuroprotective properties of leptin [8], and that the rat brain expresses other adipokine genes in addition to leptin [28], we hypothesized that cerebral damage would induce the expression of these central adipokine genes as part of an acute neuroprotective mechanism that might also impact central energy metabolism following TBI. Experiments were undertaken to evaluate the expression of ob, fiaf and rstn mRNA using realtime RT-PCR 12 h following a lateral fluid percussion (FP) brain injury in adult rats [16]. Also, because of the known involvement of a central inflammatory response in TBI, a second series of experiments was undertaken to investigate the response of brain adipokines 12 h following the intracerebral injection of lipopolysaccharide (LPS).
The FP brain injury procedure was approved by the University of Pennsylvania's Animal Use and Care Committee and conformed to standards set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). The intracerebral injection procedure was approved by the Dalhousie University Committee on Laboratory Animals. For the TBI studies adult male Sprague-Dawley rats (350−400 g, Harlan, Indianapolis, IN) were anesthesitized with sodium pentobarbital (65 mg/kg) and underwent craniectomy followed by a lateral FP brain injury of moderate severity (2.92−3.17 atm) as previously described in detail [16]. Normothermia was maintained throughout all procedures and for 120 min after lateral FP injury. Animals were allowed to recover and were then returned to home cages. Sham-injured rats were anesthetized and surgically prepared in the exact same manner but did not receive FP brain injury. Animals were euthanized 12 h post-injury, and following decapitation, brains were removed quickly and a 3 mm coronal section was removed from the occipital–parietal level, which included the injury site over the left parietal cortex. First, the different brain regions were dissected out on the ipsilateral side (side of injury) on a cold, glass plate in the following order: (1) hippocampus, (2) cortex and (3) thalamus, followed by the contralateral sites. Sections were immediately placed in RNAlater® (Ambion, Austin, TX) and stored at room temperature before shipment to Halifax, Nova Scotia, for RNA isolation.
For intracerebral injection of LPS (from E. coli O26:B6; #L8274; Sigma, Oakville, ON) adult male Sprague-Dawley rats (Charles River Laboratories, St. Constant, Quebec; 275−300 g) were anesthetized using isoflurane USP (Abbott Laboratories, Montreal, QC) and placed in a Stoelting stereotaxic frame. Anaesthesia was maintained throughout the experiment by providing a 2−2.5% flow of isoflurane/oxygen. Rats were injected using coordinates from bregma that approximated the site of the fluid percussion injury: dorsal, 5 mm; lateral, 4 mm and ventral, 1.8 mm. Rats were slowly injected with either isotonic saline (5 μL) or the LPS solution (5 μL; 25 μg) using a Hamilton syringe over several seconds. The needle was left in place for a further 2 min, and slowly retracted before closing the incision with a wound clip. Xylocaine jelly (2%; Astra Pharma Inc.; Mississauga, ON) was applied and the rats were allowed to recover individually for 15 min prior to returning to their home cage where they were housed in pairs. Animals were closely monitored to confirm that there were no adverse effects from the surgery and tissues were collected 12 h later as described above.
Total RNA was isolated using the RNEasy mini kit following the DNase protocol (Qiagen, Mississauga, ON) and reverse transcribed (RT) and PCR amplified using the SuperScript™ III Platinum® Two-Step qRT-PCR Kit (Invitrogen) according to the manufacturer's protocol using specific primers and Taqman™ probes for rat leptin (ob), fiaf, resistin and cyclophilin (sequences available upon request). In brief, RNA (2 μg) was diluted to 16 μL and then heat denatured for 5 min at 70 °C. Samples were returned to ice prior to the addition of 20 μL of the 2× reverse transcription master mix and 4 μL of the SuperScript™ III RT enzyme master mix (Invitrogen). The RT reaction consisted of 10 min incubation at 25 °C, 45 min incubation at 42 °C, followed by a 5 min 85 °C termination step, and the resulting complementary DNA (cDNA; 40 μL) was stored at −20 °C. For PCR amplifications samples were amplified in duplicate and only one gene was analyzed per reaction. Individual PCR reactions consisted of a 2× Platinum® quantitative PCR SuperMix-UDG, 7 pmol of the sense and antisense primers, 1 pmol of the appropriate dual-labeled probe, 3 μL of cDNA, to a final volume of 33 μL in sterile water. Reactions were heated to 10 min at 95 °C, followed by 50 amplification cycles of 95 °C for 20 s and 60 °C for 60 s using a BioRad thermal cycler and an iQ realtime PCR detection system. A standard curve, that was prepared using a serial dilution of a reference sample, was included in each realtime run to correct for possible variations in product amplification. Relative copy numbers were obtained from standard curve values, and these were normalized to the values obtained for our house keeping gene, cyclophilin. Data are expressed as a percentage of the control ± S.E.M. It should be noted that no significant variations in the housekeeping gene were observed between groups when evaluated using either the threshold cycle (CT), or using the relative levels of expression, and this is consistent with previous reports [4]. Data from the ipsilateral and contralateral brain samples were analyzed independently using the Student's t-test with a significance level set at p < 0.05.
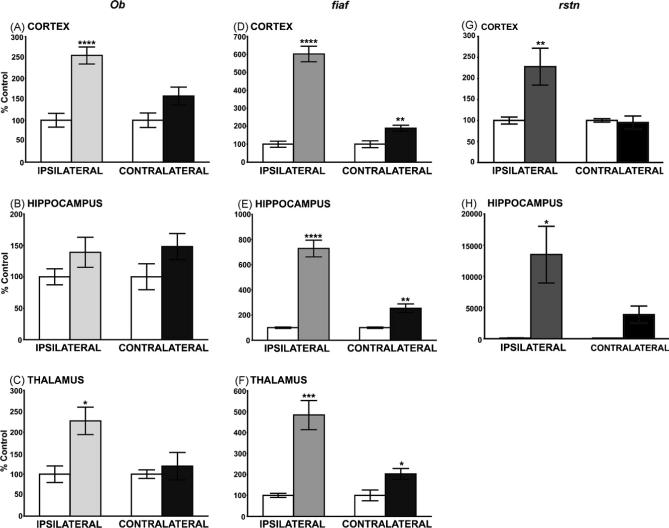
Twelve hours following brain injury, leptin mRNA levels were markedly increased in the ipsilateral cerebral cortex and thalamus (Fig. 1A and C; 2.5-fold, p < 0.0005 and 2-fold, p < 0.05, respectively), but not hippocampus, relative to sham controls. Note that leptin expression was not significantly affected in the contralateral cortex and thalamus. Large increases in fiaf gene expression were seen in the three brain regions ipsilateral to the injury (Fig. 1D–F; cortex [6-fold; p < 0.0001], hippocampus [7.5-fold; p < 0.0001] and thalamus [5-fold; p < 0.01] 12 h post-injury). In contrast to the leptin data we observed a significant, though smaller, increase in fiaf mRNA in the contralateral brain (Fig. 1D–F; cortex 89%, p < 0.01; hippocampus 155%, p < 0.01; thalamus 102%, p < 0.01). Significant increases in rstn mRNA were detected in the ipsilateral, but not the contralateral, cortex (128%; p < 0.01, Fig. 1G). Although rstn gene expression was very low in the thalamus (data not shown), hippocampal rstn mRNA levels were strikingly induced in both the ipsi and contralateral hippocampus (150-fold and 50-fold respectively; p < 0.05; Fig. 1H).
Fig. 1.
The effects of TBI (12 h) on ob, rstn and fiaf mRNA in various rat brain regions. (A–C) Leptin (ob) gene expression was significantly upregulated in the ipsilateral cortex (A, 2.5-fold) and thalamus (C, 2-fold) 12 h following lateral FP-induced brain injury. In contrast ob gene expression was unaffected in the hippocampus (B), or in the contralateral brain tissues. (D–F) Following TBI fiaf was significantly upregulated 6-fold, 7.5-fold and 5-fold in the ipsilateral cortex, hippocampus and thalamus respectively. Fiaf expression was also significantly upregulated in the contralateral tissues, but the increases were smaller (cortex 89%; thalamus 103%; hippocampus 150%). (G and H) TBI increased rstn mRNA in the ipsilateral cortex (G, 128%), but had no effects on the contralateral tissue. In contrast, rstn was detectable at low levels in the hippocampus of sham controls, but was strikingly induced following TBI in the ipsi- and contralateral hippocampus (H, 150-fold and 50-fold, respectively). Fiaf expression was undetectable in thalamic samples. Values are expressed as a percentage of the control ± S.E.M. obtained from duplicate experiments (N = 6−8; *p < 0.05, **p < 0.01 and ***p < 0.001, ****p < 0.0005). White bars represent sham, control rats; shaded bars indicate data from TBI rats.
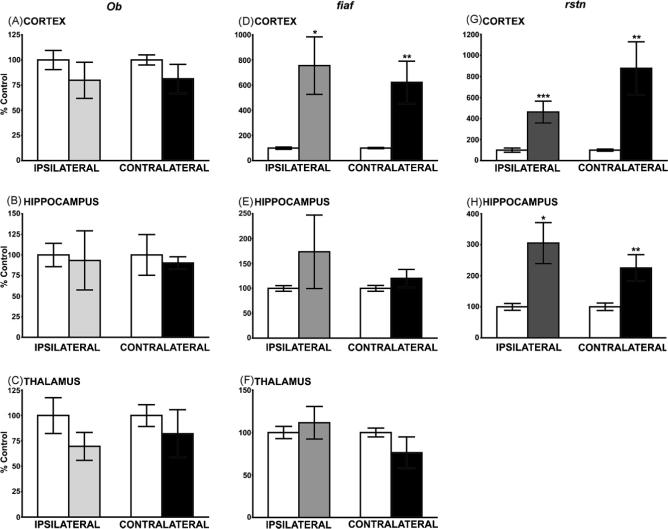
Data obtained from the intracerebral LPS injections revealed interesting differences in the response of adipokine genes to an inflammatory stimulus, compared to those seen after TBI. The most obvious change was the lack of response of ob in all three brain regions (Fig. 2A–C), compared to the significant increases seen following TBI (Fig. 1A and C). In contrast LPS induced large, significant increases in fiaf expression in both ipsi- and contralateral cortex (Fig. 2D; p < 0.02 and p < 0.01, respectively), but had no effect in hippocampus or thalamus (Fig. 2E and F). Note that fiaf expression was very sensitive to TBI in these two brain regions (Fig. 1E and F). The LPS-induced increase in rstn expression was similar to that resulting from TBI though the change was seen in both ipsi- and contralateral sites (Fig. 2G). No changes in body weight were observed in either group of rats.
Fig. 2.
The effects of intracerebral injection of LPS on ob, rstn and fiaf mRNA in various rat brain regions, measured 12 h post-injection. (A–C) The intracerebral injection of LPS had no effect on leptin (ob) gene expression in cortex, hippocampus or thalamus. (D–F) Following the central injection of LPS fiaf was upregulated in both the ipsilateral and contralateral cortex, but there was no effect in the thalamus or hippocampus. (G and H) LPS increased rstn mRNA in both the ipsilateral and contralateral cortex and hippocampus. Values are expressed as a percentage of the control ± S.E.M. obtained from duplicate experiments (N = 5−10; *p < 0.05, **p < 0.01 and ***p < 0.001). White bars represent sham, control rats; shaded bars indicate LPS-treated rats.
Our data show that TBI rapidly increases ob, fiaf and rstn gene expression in several regions of the adult rat brain. Although the largest changes occurred at the site of injury, significant increments in mRNA levels were also detected for fiaf and rstn in contralateral brain regions, relative to sham-operated controls. This was especially true for fiaf in contralateral cortex, thalamus and hippocampus, though rstn mRNA levels were also markedly increased in contralateral hippocampus. In contrast, elevations in ob mRNA were confined to ipsilateral cortex and thalamus, with no changes seen in ipsilateral or contralateral hippocampus. Taken together with our previous report, that hypoxic/ischemic insult in neonatal mouse brain stimulated fiaf and rstn gene expression [27], our current observations imply that the upregulation of centrally derived adipokine genes, such as ob, fiaf and rstn, could be a general response in the pathology of brain damage. In view of the known neuroprotective properties of leptin [8], central adipokines might contribute to local tissue repair and prevent further damage, though they may also lead to the altered metabolic status that is often associated with brain injury. For example rats and patients often experience loss of appetite and become hyperthermic following TBI, further exacerbating the initial neurotrauma [17,25]. Adipokines are known to modify appetite via a hypothalamic-dependent pathway [2]. For example the intracerebroventricular (i.c.v.) injection of leptin reduced appetite and increased energy expenditure in rodents, and more recently i.c.v. delivery of resistin acutely reduced food intake in rats [2]. Thus the increases in central adipokine gene expression that we describe could modulate energy expenditure and appetite. Future experiments designed to examine longer term (>12 h) changes in food intake, and adipokine gene expression in the hypothalamus, are required to investigate this possibility. In addition, since TBI compromises the integrity of the blood brain barrier (BBB), this may increase the brain's exposure to circulating factors of peripheral origin leading to further alterations in energy metabolism [7,16]. This might explain changes in gene expression that were detected in the contralateral, non-damaged, side of the brain; i.e., fiaf and rstn mRNA, but not ob mRNA were increased contralaterally, especially in the hippocampus. Other studies also found a significant damaging effect of TBI on contralateral hippocampal neurons as early as 10 min post-injury [6]. Using in vitro autoradiography Sihver et al. [24] demonstrated a marked reduction in binding affinity at cortical and hippocampal glutamate (NMDA) and GABAA receptors 12 h post-TBI. These neurochemical changes could also be the result of alterations in cerebral blood flow [21]. Whether glutamatergic neurons, or other neurotransmitter systems, are implicated in brain-derived adipokine gene expression is unknown. On the other hand, microarray analysis revealed that certain transcription factors, known to be involved in the regulation of adipokine genes, are rapidly increased in the rat following brain injury, most notably nuclear factor-κB (NF-κB) and hypoxia inducible factor-1α (HIF-1α) [7,15]. Resistin promoter analysis revealed the presence of numerous NF-κB binding sites, and activating NF-κB increased rstn gene expression in adipocytes [14]. In addition to increased hif-1α mRNA, the stability and transcriptional activity of HIF-1α would also be enhanced by hypoxic conditions that often result following brain injury [7]. HIF-1α is able to transactivate the leptin gene [10], and the FIAF promoter contains both NF-κB and HIF-1α response elements, suggesting they might be partially responsible for increasing adipokine expression following neurotrauma [3].
A further mechanism that may be at least partially responsible for TBI-induced increases in central adipokine gene expression is suggested by the results of our LPS experiments that were designed to produce an inflammatory response. Brain injury is known to increase local expression of several inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), that are capable of inducing adipokine gene expression [26]. However, an intracerebral injection of LPS produced some, but not all, of the changes seen after TBI. For example, in contrast to the stimulatory influence of TBI, LPS had no effect on ob expression in any brain region, though fiaf and rstn mRNA levels were significantly elevated in both ipsi- and contralateral cortex. This result suggests that fiaf and rstn expression, at least in cortex, were increased by a TBI-dependent inflammatory response, whereas ob mRNA levels are controlled by an alternative, perhaps neuroprotective, mechanism.
What purpose could increased adipokine gene expression serve in the injured brain? Leptin protected rat C6 glioblastoma cells, human neuroblastoma cells, and mouse cortical neurons from cell death [5,8,23], and leptin was also reported to be neuroprotective against ischemic damage in the mouse brain [29]. Leptin could assist in brain repair following injury through stimulatory effects on neuronal proteins such as growth associated protein 43 (GAP-43) [1], since there is evidence that axons injured by TBI may attempt to regenerate by up-regulating GAP-43 expression [9]. Our previous demonstration that brain fiaf expression is upregulated following a cerebral hypoxic/ischemic insult in neonatal mice indicates that increases in fiaf might be a common marker of brain damage, though it is difficult at this time to conclude whether elevated FIAF production is beneficial or deleterious to the outcome of cerebral damage. FIAF is reported to exert strong pro-angiogenic effects in ischemic renal carcinoma [13], and fiaf up-regulation is associated with cerebrovascular angiogenesis after focal brain ischemia in adult mice [11] or cortical cold injury in adult rats [19]. However a potentially damaging effect of FIAF following TBI could occur via the inhibition of lipoprotein lipase activity (LPL) [12], since elevated levels of LPL are thought to be neuroprotective [20]. The TBI-induced increase in brain rstn mRNA, measured at 12 h post-injury, is in marked contrast to the delayed elevation seen in hypoxic ischemic mouse brain (>7 days) [27]. This suggests that resistin, like FIAF, may participate in the acute responses to cerebral damage, possibly via the induction of inflammation, but this requires further investigation [14,26]. We conclude that important future investigations should focus not only on a more detailed time-course of the changes we describe, but on the determination of the levels and localization of adipokine proteins induced by TBI.
In summary, our data suggest that TBI-mediated increases in central adipokine gene expression could be associated with some of the clinical features of TBI, in particular neuroinflammation and cachexia. We speculate that brain-derived adipokines mediate their effects via an autocrine/paracrine mechanism to modulate local cytokine signaling and inflammatory responses following brain injury. Blocking local injury-induced increases in adipokine expression, perhaps through gene silencing techniques [28], could help resolve the individual roles of leptin, resistin and FIAF, in the pathology of brain injury. These genes may prove to be useful therapeutic targets in efforts to improve patient recovery following brain injury.
Acknowledgements
We are indebted to Diane Wilkinson and David LeBold for technical assistance.
This work was supported by the National Institutes of Health (Grants T32-NS043126 and T32-NR007106), CIHR/NSHRF, IWK Health Centre, UIMRF and Capital Health. RB is the recipient of a NSHRF Graduate Studentship and EU is a Dalhousie University Senior Clinical Scholar.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Qi Y, Singhal NS. Adipokines that link obesity and diabetes to the hypothalamus. Prog. Brain Res. 2006;153:155–174. doi: 10.1016/S0079-6123(06)53009-2. [DOI] [PubMed] [Google Scholar]
- 3.Belanger AJ, Lu H, Date T, Liu LX, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J. Mol. Cell. Cardiol. 2002;34:765–774. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- 4.Bond BC, Virley DJ, Cairns NJ, Hunter AJ, Moore GB, Moss SJ, Mudge AW, Walsh FS, Jazin E, Preece P. The quantification of gene expression in an animal model of brain ischaemia using TaqMan real-time RT-PCR. Brain Res. Mol. Brain Res. 2002;106:101–116. doi: 10.1016/s0169-328x(02)00417-5. [DOI] [PubMed] [Google Scholar]
- 5.Brown R, Morash B, Ur E, Wilkinson M. RNAi-mediated silencing of leptin gene expression increases cell death in C6 glioblastoma cells. Brain Res. Mol. Brain Res. 2005;139:357–360. doi: 10.1016/j.molbrainres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Carbonell WS, Grady MS. Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta Neuropathol. (Berl.) 1999;98:396–406. doi: 10.1007/s004010051100. [DOI] [PubMed] [Google Scholar]
- 7.Dash PK, Kobori N, Moore AN. A molecular description of brain trauma pathophysiology using microarray technology: an overview. Neurochem. Res. 2004;29:1275–1286. doi: 10.1023/b:nere.0000023614.30084.eb. [DOI] [PubMed] [Google Scholar]
- 8.Dicou E, Attoub S, Gressens P. Neuroprotective effects of leptin in vivo and in vitro. Neuroreport. 2001;12:3947–3951. doi: 10.1097/00001756-200112210-00019. [DOI] [PubMed] [Google Scholar]
- 9.Emery DL, Raghupathi R, Saatman KE, Fischer I, Grady MS, McIntosh TK. Bilateral growth-related protein expression suggests a transient increase in regenerative potential following brain trauma. J. Comp. Neurol. 2000;424:521–531. [PubMed] [Google Scholar]
- 10.Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J. Cereb. Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 12.Koster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 13.Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 2003;162:1521–1528. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu SC, Shieh WY, Chen CY, Hsu SC, Chen HL. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002;530:158–162. doi: 10.1016/s0014-5793(02)03450-6. [DOI] [PubMed] [Google Scholar]
- 15.Matzilevich DA, Rall JM, Moore AN, Grill RJ, Dash PK. High-density microarray analysis of hippocampal gene expression following experimental brain injury. J. Neurosci. Res. 2002;67:646–663. doi: 10.1002/jnr.10157. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 17.Moinard C, Neveux N, Royo N, Genthon C, Marchand-Verrecchia C, Plotkine M, Cynober L. Characterization of the alteration of nutritional state in brain injury induced by fluid percussion in rats. Intens. Care Med. 2005;31:281–288. doi: 10.1007/s00134-004-2489-9. [DOI] [PubMed] [Google Scholar]
- 18.Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, Graham DI, Stocchetti N, McIntosh TK. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Nag S. The blood-brain barrier and cerebral angiogenesis: lessons from the cold-injury model. Trends Mol. Med. 2002;8:38–44. doi: 10.1016/s1471-4914(01)02221-3. [DOI] [PubMed] [Google Scholar]
- 20.Paradis E, Clement S, Julien P, Ven Murthy MR. Lipoprotein lipase affects the survival and differentiation of neural cells exposed to very low density lipoprotein. J. Biol. Chem. 2003;278:9698–9705. doi: 10.1074/jbc.M208452200. [DOI] [PubMed] [Google Scholar]
- 21.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J. Neurotraum. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 22.Royo NC, Shimizu S, Schouten JW, Stover JF, McIntosh TK. Pharmacology of traumatic brain injury. Curr. Opin. Pharmacol. 2003;3:27–32. doi: 10.1016/s1471-4892(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 23.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Anti-apoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 24.Sihver S, Marklund N, Hillered L, Langstrom B, Watanabe Y, Bergstrom M. Changes in mACh, NMDA and GABA(A) receptor binding after lateral fluid-percussion injury: in vitro autoradiography of rat brain frozen sections. J. Neurochem. 2001;78:417–423. doi: 10.1046/j.1471-4159.2001.00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol. Dis. 2003;12:163–173. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 26.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 27.Wiesner G, Brown RE, Robertson GS, Imran SA, Ur E, Wilkinson M. Increased expression of the adipokine genes resistin and fasting-induced adipose factor in hypoxic/ischaemic mouse brain. Neuroreport. 2006;17:1195–1198. doi: 10.1097/01.wnr.0000224776.12647.ba. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson M, Brown R, Imran SA, Ur E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology. 2007;86:191–209. doi: 10.1159/000108635. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia; Program No. 759.15. 2006 Neuroscience Meeting Planner; Atlanta GA, Society for Neuroscience. 2006; [DOI] [PubMed] [Google Scholar]