Abstract
Background
Brain imaging studies have revealed anatomical anomalies in the brains of individuals with Tourette syndrome (TS). Prefrontal regions have been found to be larger and the corpus callosum (CC) area smaller in children and young adults with TS compared with healthy control subjects, and these anatomical features have been understood to reflect neural plasticity that helps to attenuate the severity of tics.
Method
CC white matter connectivity, as measured by the Fractional Anisotropy (FA) index from diffusion tensor images, was assessed in 20 clinically well-defined boys with Tourette syndrome and 20 age- and gender-matched controls.
Results
The hypothesis that children with TS would show reduced measures of connectivity in CC fibers was confirmed for all subregions of the CC. There was no significant interaction of TS and region. Reductions in FA in CC regions may reflect either fewer interhemispheric fibers or reduced axonal myelination. FA values did not correlate significantly with the severity of tic symptoms. Group differences in measures of connectivity did not seem to be attributable to the presence of comorbid ADHD or OCD, to medication exposure, or group differences in IQ.
Conclusion
Our findings of a reduced interhemispheral white matter connectivity add to the understanding of neural connectivity and plasticity in the brains of children who have TS.
Keywords: Tourette syndrome, brain development, brain imaging
Tourette syndrome (TS) is characterized by the presence of chronic motor and phonic tics that fluctuate in severity (ICD 10/DSM IV)(American Psychiatric Association, 1994). The phenotypic spectrum of tics can range from simple movements or sounds to more complex and debilitating motor behaviors and vocalizations. Recent studies confirm the importance of genetic factors that predispose individuals to developing TS (Abelson et al., 2005; Pauls, 2003). Moreover, a genetically mediated relationship between TS and obsessive-compulsive disorder (OCD) has been established through family-genetic (Eapen, Pauls, & Robertson, 1993; Pauls, Leckman, Towbin, Zahner, & Cohen, 1986; Pauls, Raymond, Stevenson, & Leckman, 1991) and molecular genetic studies (Cuker, State, King, Davis, & Ward, 2004; State et al., 2003). TS children in clinical and epidemiological settings have high rates of comorbid OCD (Termine et al., 2006); attention-deficit/hyperactivity disorder (ADHD) and depression (Kurlan et al., 2002) are also commonly seen. The typical natural history of tic symptoms in TS is an increase in severity from childhood to early adolescence and, in most cases, an attenuation during or after puberty (Leckman et al., 1998; Pappert, Goetz, Louis, Blasucci, & Leurgans, 2003). More than 40% of all children with TS are free of tics by the age of 18 (Leckman et al., 1998). This natural history suggests that the brain develops mechanisms during adolescence that help to modulate the severity of tics (Spessot, Plessen, & Peterson, 2004).
Recent structural and functional brain imaging studies have improved our understanding of brain adaptation and neural plasticity (Pascual-Leone, Amedi, Fregni, & Merabet, 2005). In TS, reports of abnormalities in brain morphology have focused on the basal ganglia as an important part of cortico-striato-thalamo-cortical (CSTC) circuits (Alexander, DeLong, & Strick, 1986), which are implicated in the underlying pathophysiology of TS (Peterson et al., 1993, 1998, 2003). Anatomical abnormalities of the basal ganglia in childhood have also been found to predict the severity of tics in early adulthood (Bloch et al., 2005). Cortical regions, on the other hand, seem to play an important role in the modulation and suppression of tics (Fredericksen et al., 2002; Peterson et al., 1998; Peterson, Staib et al., 2001; Serrien, Orth, Evans, Lees, & Brown, 2005). Investigations of cortical volume in children and adults with TS (Peterson, Staib et al., 2001) have revealed larger dorsal prefrontal cortex (DPFC) volumes in children with TS compared with control children, and the magnitude of this volumetric enlargement was associated with fewer tic symptoms. Adults with persistent TS, however, did not show this enlargement of prefrontal volumes, and in fact their volumes were smaller than in control adults, suggesting that their prefrontal cortices did not adapt successfully to the presence of tics during adolescence. In light of prior anatomical studies demonstrating a normal reduction in prefrontal gray matter density during childhood and adolescence (Sowell et al., 2003), we have speculated that the normal regressive processes of childhood and adolescence, including the pruning of axons, dendrites, and synapses in prefrontal regions, are slowed in individuals who successfully adapt to the presence of tics, presumably because these brain regions are activated prominently (Peterson et al., 1998) by the frequent need for children to suppress tics in social situations.
The overall midsagittal cross-sectional area of the corpus callosum (CC) has been reported to be smaller in subjects with TS (Peterson et al., 1994; Plessen et al., 2004) and to be correlated inversely in children with both symptom severity and prefrontal volumes (Plessen et al., 2004). These studies have thus suggested that interhemispheric axons in children with TS, together with prefrontal cortices, successfully reorganize in order to enhance regulatory control of motor and phonic tics. This interpretation is consistent with the known functional characteristics of the CC, which involves primarily transfer of information between the two hemispheres, as well as modulation of attention (Hugdahl, 1998), and inhibition of cortical activity (Boroojerdi, Topper, Foltys, & Meincke, 1999) and plastic reorganization of the brain (Werhahn, Mortensen, Kaelin-Lang, Boroojerdi, & Cohen, 2002). Transcallosal inhibition presumably involves GABAergic inhibitory interneurons, as the CC itself consists of glutamatergic excitatory fibers (Carr & Sesack, 1998; Conti & Manzoni, 1994).
Interhemispheric fiber tracts within the CC can be studied directly using diffusion tensor imaging (DTI), a magnetic resonance imaging (MRI)-based technique that measures the constrained diffusion of water, or the degree of anisotropy, within neuronal tissue (Ramnani, Behrens, Penny, & Matthews, 2004). It has been shown that DTI reliably detects white matter tracts and can be regarded as a tool to map connectivity between cortical regions (Basser, Mattiello, & LeBihan, 1994; Basser, Pajevic, Pierpaoli, Duda, & Aldroubi, 2000; Pierpaoli et al., 2001). The parameter used most widely to characterize the degree of anistropy and degree of structural organization in the brain, particularly in clinical applications of DTI, is Fractional Anisotropy (FA), an index that ranges from 0 (isotropic diffusion, characteristic of the diffusion of unconstrained water within CSF) to 1 (complete anisotropic diffusion and maximal organization of tissue)(Hasan, Alexander, & Narayana, 2004). The FA has been shown to correlate with histological markers of myelination in newborns, toddlers, and adults (Wimberger et al., 1995; Huppi et al., 1998; Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999), which likely contributes to the increasing speed of neural transmission with advancing development (Paus et al., 1999). Based on our prior report of a smaller CC area in children with TS (Plessen et al., 2004), the present study investigated the hypothesis that a smaller CC in TS subjects is the consequence of either fewer interhemispheric fibers or reduced myelination, which should result in reduced FA values within the CC. By further subdividing the CC into five anatomically discrete regions, we investigated differences in the FA index for different CC regions and possible interactions with a diagnosis of TS.
Methods
Subjects
TS subjects were recruited from the Department of Child and Adolescent Psychiatry at the Haukeland University Hospital, University of Bergen, Norway and from outpatient clinics in the greater Bergen area in the western part of Norway. All met DSM-IV criteria for a diagnosis of TS (American Psychiatric Association, 1994). Healthy control children (HC) were recruited by randomly contacting local schools in the same geographic area. The controls were matched for age and gender with the patient group. Written informed consent was obtained from all participants and the study was approved by Regional Committee for Medical Research Ethics, West-Norway.
Exclusion criteria for the control group included a lifetime history of Tic Disorder, OCD, ADHD, or a current DSM-IV Axis 1 disorder. Additional exclusion criteria for both groups were epilepsy, head trauma with loss of consciousness, former or present substance abuse, or an IQ below 70, as measured with the WISC-III (Wechsler, 1996).
Psychiatric diagnoses were established using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (Kaufman et al., 1997) and a best-estimate consensus procedure that considered all available study materials (Leckman, Sholomskas, Thompson, Belanger, & Weissman, 1982). OCD symptoms were quantified using the Children's Yale-Brown Obsessive Compulsive Scale (Goodman et al., 1989; Scahill et al., 1997), and the severity of tics was rated with Yale Global Tic Severity Scale (YGTSS) (Leckman et al., 1989). Socioeconomic status (SES) was estimated by measuring the parental level of education in four categories, dependent on their school and higher education (JAACAP, 2005).
Sample size was limited to the first 20 subjects in each group who were referred consecutively to the study and who met the criteria for inclusion. Only two girls with TS were referred to the study, and they experienced severe tics during the scanning session that produced imaging artifacts requiring exclusion of these subjects. Thus, the reported data consist of two male groups: 20 TS and 20 HC boys, 9 to 17 years of age. The groups were of comparable age (TS = 13.6 years, ±1.9; HC = 13.4 years, ±2.4; t = −.3; p = .77) and SES. The diagnostic interview revealed that five of the subjects in the TS group in addition had combined-type ADHD, four others had comorbid OCD, and none had both conditions. Participants were right-handed as measured by the Edinburgh handedness inventory (Oldfield, 1971), except for two left-handed individuals in each group.
At the time of MRI scanning, nine subjects in the TS group were taking medication, either neuroleptics (n = 4), alpha agonists (n = 2), selective serotonin uptake inhibitors (n = 1), or stimulants (n = 2). No subjects were on combinations of medications. HC subjects were free of any psychotropic medications. Tic severity at the time of scan in the TS group was 11.4 ± 2.9 for motor and 9.2 ± 3.4 for phonic tics, and at for lifetime-worst ever was 15.5 ± 5.1 for motor and 13.7 ± 5.5 for phonic tics (possible range 0 to 25 in each category). The groups differed in verbal IQ (TS = 94.4 ± 11.4; HC = 104.4 ± 10.5; t = 2.9; p < .006), performance IQ (TS = 95.6 ± 10,8; HC = 106.1 ± 12,1; t = 2.9; p < .006), and full-scale IQ (TS= 94.5 ± 10.2; HC = 105.7 ± 9.2; t = 3.6; p < .001).
MRI scanning and image-analysis
MR-images were acquired on a Siemens Symphony, 1.5 Tesla scanner. Head positioning was standardized using canthomeatal landmarks. T1-weighted, sagittal 3D volume MPRage images were acquired for all subjects, with repetition time (TR) = 1910 ms, echo time (TE) = 3.93 ms, flip angle (FA) (α) = 4°, image matrix = 256 × 192, field of view (FOV) = 256 mm, slice thickness = 1 mm with 176 contiguous slices. The DTI data were acquired with single shot spin echo EPI sequence in axial and in sagittal acquisition, with TR = 4000 ms, TE = 96 ms, 128 × 128 matrix and 240 mm FOV, at a nominal resolution of 1.875 × 1.875 × 4.0 mm3. The diffusion weighting was b = 1000 s/mm2 in 6 noncollinear directions (8 averaged means per slice), in addition to one reference (b = 0) image per slice. We focused on acquiring data of high quality using behavioral relaxation techniques and eventually repeating series containing movement artifacts.
MR image analysis for overall CC area, using the T1-weighted MPRage images, was performed using Analyze 6.0 software (Rochester Minnesota) on a Linux Workstation. Raters were blind to subject characteristics and group assignments. Each MR dataset was realigned to the midsagittal slice, which was identified using standard midline landmarks (callosal sulcus, cerebral aqueduct, pineal gland, peaked roof of the fourth ventricle, and minimal gray matter in the interhemispheric fissure), to minimize variability in CC size between subjects that may have been caused by individual differences in head positioning during scanning (Rauch & Jinkins, 1996). The midsagittal slice was magnified eightfold, and the CC contour was segmented automatically, using an isointensity contour function, which was then manually edited.
DTI data analysis
In-house software was used for preprocessing of the DTI data, which included correction of images for eddy current distortions (Haselgrove & Moore, 1996), and interpolation to a 256 × 256 matrix. After tensor diagonalization and calculation, the eigenvector maps and FA index were estimated. The CC endpoints at the tip of the rostrum and the tip of the splenium were selected using local curvature maxima. To compare FA values for CC subregions that carry fiber tracts belonging to different cortical regions, the CC was subdivided into seven segments according to a previously published method (Witelson, 1989), except for summing the two most anterior and two most posterior regions (Luders et al., 2005) (see Figure 1). Also, to enhance the stability of FA measures further, mean FA values from the CC regions (Moeller et al., 2005) from three midsagittal slices (midsagittal and the two adjacent parasagittal slices 4 mm bilaterally off the midline) were summed into a single midsagittal FA index, after assuring a normal distribution by visual inspection. Table 1 shows mean values and SD for FA measures for each group.
Figure 1.
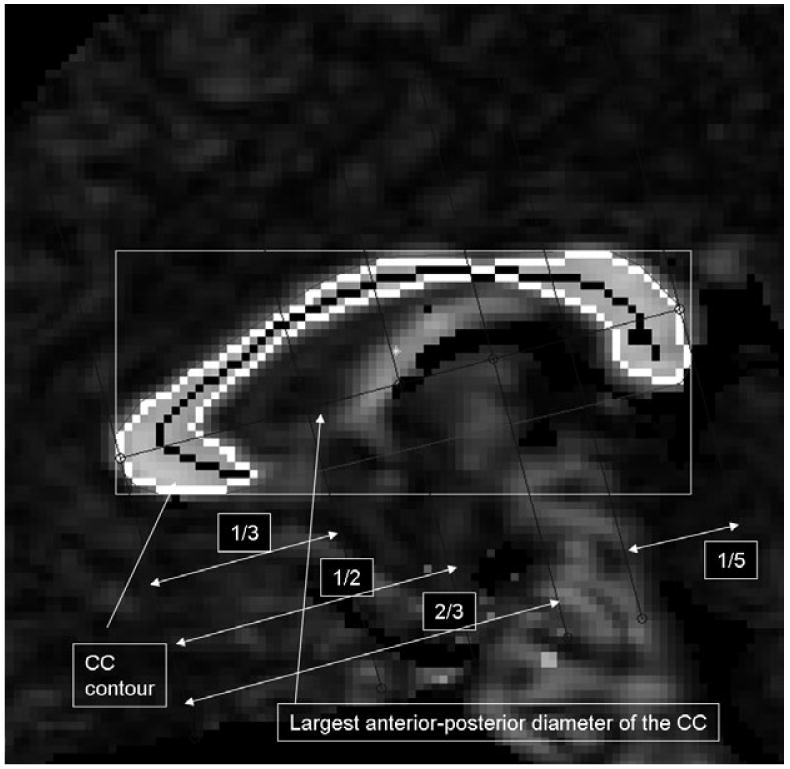
Corpus callosum divisions were made by identifying the most anterior and most posterior point of the CC. The five regions were delineated by calculating the fifth, thirds and half of the largest anterior-posterior diameter
Table 1.
MR measures. Mean corpus callosum fractional anisotropy by region and group and overall CC area
| TS (SD) n = 20 | HC (SD) n = 20 | |
|---|---|---|
| FA Region 1 | .797 (.02) | .815 (.03) |
| FA Region 2 | .801 (.02) | .817 (.02) |
| FA Region 3 | .806 (.02) | .825 (.03) |
| FA Region 4 | .787 (.03) | .812 (.03) |
| FA Region 5 | .803 (.02) | .818 (.03) |
| CC area (mm2) | 641.67 (85.8) | 661.84 (114.7) |
SD = standard deviation; FA = fractional anisotropy index.
The main analyses reported here were complemented by a co-registration and spatial normalization of the DTI images, which were intended to confirm findings based on FA values averaged across all voxels in each CC subregion. We further confirmed our findings of differences in average FA values between groups through a comparison of FA values at each pixel of the CC in a common template space. To compute group-average FA values at all voxels, we coregistered, normalized, and reoriented the DT image of each subject into the common space by a two-step procedure. In the first step, we coregistered and nonlinearly warped the DT image of each subject to its anatomical MR image (Xu, Mori, Shen, van Zijl, & Davatzikos, 2003). In the second step, we coregistered and normalized the MR image of each subject to the reference image (Bansal, Staib, Whiteman, Wang, & Peterson, 2005). The reference image was an anatomical MR image of a randomly chosen single health subject. We concatenated the two nonlinear deformations estimated in the two steps and used the concatenated deformation to coregister and normalize the DT image of each subject into the template space. Tensors were correctly reoriented into the template space by using the method of Procrustean estimation (Xu et al., 2003). Once DT images for each subject had been normalized and reoriented into the common template space, we computed the average FA values at each pixel in the template space. We then displayed and compared visually the gray-scale encoded average FA values for the two groups.
Statistical analyses
All statistical procedures were performed in SAS v. 8.2 (SAS Institute inc., Cary NC) or SPSS v. 13 (SPSS, 1999). The a priori hypothesis was tested using a mixed-model regression analysis (PROC MIXED) with repeated measures over a spatial domain (the five regions of the CC) with a first order autoregressive (AR1) model of the covariance structure. The model included the within-subjects factors ‘CC region’ with 5 levels. ‘Diagnosis’ (TS or controls) was a between-subjects factor. ‘Age’ was included as a covariate in the model.
In addition to the independent variables described above, all two and three-way interactions of ‘group’ (TS and HC), ‘region’, and ‘age’ were tested. Interactions that were not statistically significant were hierarchically eliminated via backward stepwise regression, with the constraint that the model at each step had to be hierarchically well formulated (i.e., all possible lower order terms had to be included in the model, regardless of their statistical significance) (Morrell, Pearson, & Brant, 1997). Whole-brain volume was not included in the model, as it has been shown that FA values are not influenced by brain volume (Schulte, Sullivan, Muller-Oehring, Adalsteinsson, & Pfefferbaum, 2005).
The hypothesized regional changes in FA values between groups and across regions were tested with assessment of the statistical significance of the ‘group-by-region’ term in the model. Whether these regional differences varied with age was tested with the group-by-region-by-age, 3-way interaction term. All tests for significance were of the 2-sided type and were thresholded at .05.
Potential confounds
In order assess the effects of comorbidity and medication on our findings in the TS group, the analysis was repeated while restricted to all subjects with TS, excluding those with comorbid ADHD (remaining n = 15) or OCD (remaining n = 16), and excluding those on medication (remaining n = 11), compared with the original control sample (n = 20). Because the TS and HC groups significantly differed in IQ, this also was considered as a covariate in the mixed model.
Association with symptom severity
Correlations of FA values with symptom severity in the TS group were investigated by calculating the Pearson correlation coefficient r for each CC subregion, and by visual inspection of scatterplots of FA values and symptom severity. Partial correlations were performed by using age and IQ as a covariate.
Results
The test for fixed effects in a mixed model revealed a significant main effect of group (F1,38 = 7.60; p < .009), demonstrating the hypothesized differences in FA values for the CC in individuals with TS compared to controls (see Table 2).
Table 2.
Hypothesis testing. The final models used in the SAS PROC MIXED regression analyses after backward step-wise elimination. The models at each step were hierarchically well formulated. All terms not retained in the analyses were excluded at a p > .10
| All subjects n = 40 | ADHD excluded n = 35 | OCD excluded n = 36 | Medication-free only n = 31 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | NDF | DDF | F | p | DDF | F | p | DDF | F | p | DDF | F | p |
| Region | 4 | 155 | 9.74 | <.001 | 135 | 8.04 | .001 | 140 | 8.77 | <.001 | 119 | 7.20 | <.001 |
| TS | 1 | 38 | 7.60 | .009 | 33 | 6.64 | .015 | 35 | 6.41 | .016 | 29 | 9.39 | .005 |
| Age | 1 | 38 | 2.17 | .150 | 33 | .98 | .330 | 35 | 2.97 | .094 | 29 | 1.73 | .199 |
NDF = numerator degrees of freedom; DDF = denominator degrees of freedom; F = F statistic for Type III sum of squares; p = probability values; TS = group (TS or HC).
Other covariates in the final model were region of the CC (F4,155 = 9.74; p < .001) and age (F1,38 = 2.17; p = .15). Age of the subjects was conservatively retained in the model, due to biologic plausibility. Group differences were not affected whether age was retained in the model or not. No significant interaction between TS and subregion of the CC (at the point of elimination n.s.) was detected, indicating that the group influence on FA values did not vary according to CC subregion (see also Figure 2). Also, the group-by-region-by-age interaction was not significant (at the point of elimination n.s.), indicating that the findings were stable across the age range of children studied.
Figure 2.
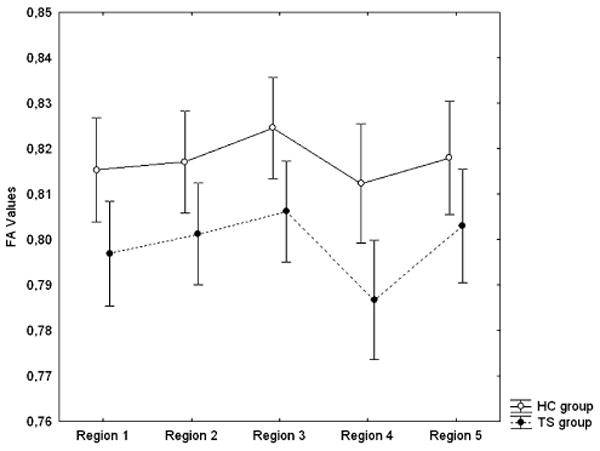
FA values for the five CC regions; TS and HC group separately
Post hoc analyses
A post-hoc assessment of the origin of this difference between groups, using a test of differences in least-square means, indicated that FA values in the TS group were lower than in controls (mean FA .80 vs. .82; t38 = 2.76; p < .009).
Potential confounding variables
The model remained stable, even when subjects with comorbid diagnoses of ADHD (n = 5) and OCD (n = 4) and those taking medication (n = 9) were excluded from the analyses subsequently (Table 2). Also IQ as a covariate did not show a significant influence on FA values, whether verbal (at the point of elimination n.s.), performance (at the point of elimination n.s.) or full-scale IQ (at the point of elimination n.s.). Moreover, when excluding subjects with comorbid ADHD, verbal IQ no longer differed significantly between the two groups, whereas the finding of lower FA values in the TS group remained stable (see Table 3).
Table 3.
T-test IQ measure comparisons between TS and HC groups. The various subscales of the WISC-III are provided for several subsamples of the participating subjects (i.e., excluding subjects with ADHD (n = 5), subjects with OCD (n = 4), and all subjects receiving medication (n = 9))
| TS vs. HC (n= 40) | TS(ADHD excluded) vs. HC (n = 35) | TS (OCD excluded) vs. HC (n = 36) | TS (Not medicated) vs. HC (n = 31) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | t-value | p | df | t-value | p | df | t-value | p | df | t-value | p | |
| Verbal IQ | 38 | 2.89 | .006 | 33 | 1.89 | n.s. | 34 | 2.33 | n.s. | 29 | 1.56 | n.s. |
| Performance IQ | 38 | 2.91 | .006 | 33 | 2.17 | .038 | 34 | 2.81 | .008 | 29 | 1.71 | n.s. |
| Full-scale IQ | 38 | 3.63 | .001 | 33 | 2.74 | .010 | 34 | 3.08 | .004 | 29 | 2.16 | .039 |
| Verbal Comprehension Index | 36 | 2.58 | .014 | 31 | 1.62 | n.s. | 31 | 2.03 | n.s. | 27 | 1.28 | n.s. |
| Perceptual Organisation Index | 36 | 1.69 | n.s. | 31 | 1.33 | n.s. | 31 | 1.57 | n.s. | 27 | .81 | n.s. |
| Freedom From Distractability Index | 36 | 1.03 | n.s. | 31 | .60 | n.s. | 31 | .58 | n.s. | 27 | .27 | n.s. |
| Processing Speed Index | 36 | 2.41 | .021 | 31 | 1.98 | n.s. | 31 | 1.85 | n.s. | 27 | 1.43 | n.s. |
Associations with tic severity
Correlations of tic severity with FA values in each of the five CC regions were not significant (region 1 r = 0.06; region 2 r = 0.25; region 3 r = 0.07; region 4 r = 0.28; region 5 r = −0.14). However, symptom severity did correlate positively, even if not at a level of statistical significance, with FA values in the four anterior CC subregions (see Figure 3).
Figure 3.
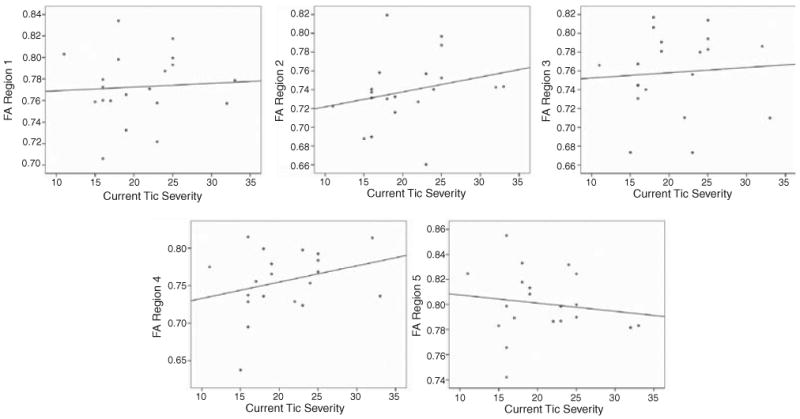
Correlation FA values with symptom severity of current motor symptoms in the TS group
Coregistration and normalization of DTI images
In normalized space, FA maps for the TS group showed smaller FA values in the CC (visualized as lower image intensity within the CC in Figure 4), and hence confirmed the findings derived using the mean values without prior normalization.
Figure 4.
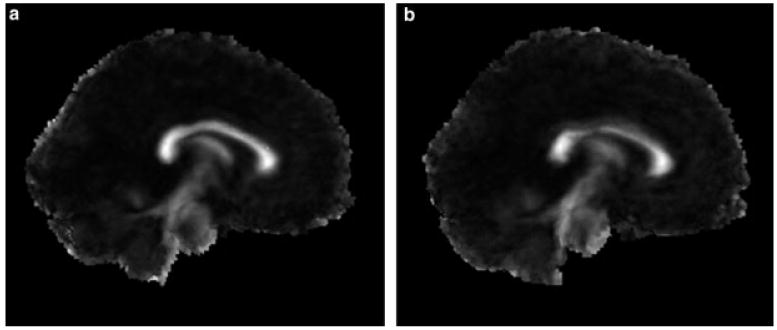
Co-registered DTI and anatomical images for the HC (a) and TS (b) group. The averaged CC FA map is shown on the co-registred midsagittal slice and intensities in white matter are visualized
To visualize FA differences further, fiber-tracking was performed by placing one region of interest in the anterior CC of one randomly chosen individual in each group (Figure 5).
Figure 5.
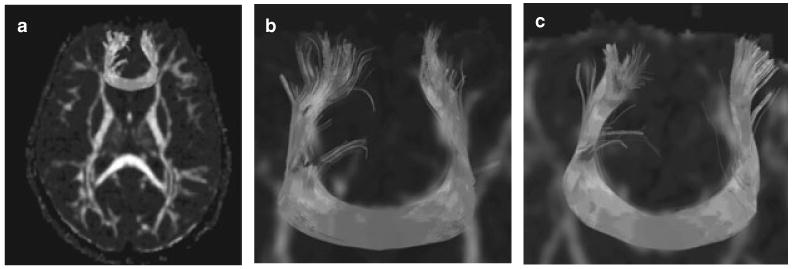
Fibre-tracking in two representative children, (a) a healthy child; (b) a child with TS. The seeds for fiber tracking were placed on an axial slice within the CC
Discussion
White matter microstructure in TS children showed less directional organization (lower FA values) compared with control children, within all subregions of the CC. Correlations of FA values with measures of current tic severity were not significant. Reduction of FA values in the TS group confirmed our a priori hypothesis. We verified findings of lower FA values by also presenting group FA values in the CC with the DTI maps spatially normalized to a template (Figure 4). Lower FA values in the TS group likely represent a reduction of the number of axons, reduction in their degree of myelination, or a disruption of their structural organization within the white matter of the CC. It is not possible to determine the underlying biological ultrastructure of the group difference with the present investigation. However, given extensive prior evidence for the presence of compensatory neuroplasticity within prefrontal cortices of children with TS, we suspect that the most likely cause of reduced FA values in the CC in TS children is the presence of fewer callosal axons connecting the cerebral hemispheres, although we cannot exclude other explanations at this point. Fewer interhemispheric axons would likely reduce transcallosal inhibition of cortical neurons and thereby enhance prefrontal functioning and ultimately support frontostriatal control of tic symptoms in children with TS. Although the neurotransmitter specifications of most transcallosal axons are glutamatergic and therefore excitatory, the CC exerts primarily inhibitory effects on cortical functioning that seem to be mediated by synapses of CC axons onto GABAergic inhibitory interneurons within cortical gray matter (Chen, 2004). Fewer transcallosal axons presumably provide less inhibitory influence on prefrontal cortices, and therefore would enhance the net output of prefrontal cortices which are thought to regulate tic behaviors. Also, in healthy individuals there is growing evidence for a high degree of interhemispheric inhibition of voluntary movements, possibly to counteract the production of mirror movements, yet it still remains to be determined which cortical regions contribute to the interhemispheric inhibitory interactions (Duque et al., 2005). The importance of inhibitory processes for neuroplasticity has been recognized lately (Kandler, 2004). This model is consistent with prior reports of cortical hyperexcitability in children with TS, which presumably is attributable to a reduction in cortical GABAergic interneurons (Ziemann, Paulus, & Rothenberger, 1997). Thus the CC seems to be a component of a larger compensatory neuroregulatory system in TS involving prefrontal control on motor activity within the basal ganglia portions of CSTC circuits.
These postulated neuroplastic effects within the CC and frontal cortices are presumably mediated through altered rates of pruning during CNS development. Pruning of axons within the CC and of axons, dendrites, and synapses within prefrontal cortices is a prominent feature of normal CNS development (Hua & Smith, 2004). Given their smaller CCs that seemingly contain fewer axons, normal axonal pruning is likely accelerated in children with TS. Their larger prefrontal cortices, an exaggeration of the higher prefrontal gray matter density present in younger compared with older healthy children (Sowell et al., 2003), suggests that normal pruning in frontal cortices is reduced in children with TS. These alterations in rates of pruning during development likely reflect the effects of an activity-dependent plasticity that is induced by the intense activation of prefrontal cortices that accompanies the suppression of tic behaviors (Peterson et al., 1998), a frequent and nearly ubiquitous occurrence for most children with TS. Another possible model to understand a reduction of interhemispheric connectivity could be the direct involvement of CC fibers in tic suppression. Glutamatergic neurons within the CC do not only mediate inhibitory functions, thus we cannot exclude that a lack of modulatory excitatory impulses to the motor cortices directly may be involved in tic suppression. The fact that FA reduction is found in all regions of the CC could also point to the involvement of the CC in tic generation as opposed to a local compensatory phenomenon. A recent DTI study (Huang et al., 2005), however, suggests that cortical fibers originating from one cortical region spread widely along the midsagittal CC axis. Especially frontal fibers spread from the most anterior region of the CC along the whole callosal body. Hence, the reduction of FA values along all regions may still be contributed to compensatory mechanisms primarily located in the prefrontal cortices.
It should, however, be noted that almost all the cited studies, including the present study, dealing with brain morphology in TS had a cross-sectional study design, which only allows limited conclusions concerning developmental trajectories and cannot infer about causation or about the temporal sequencing in the development (Kraemer, Yesavage, Taylor, & Kupfer, 2000; Peterson, 2003). The suggested models should thus be further tested in longitudinal studies. Moreover, the integration of multimodal MR imaging techniques in the same sample, e.g., the inclusion of volumetric prefrontal and basal ganglia together with DTI measurements and eventually functional MRI studies in larger samples, would help to further confirm or refute the suggested models.
Although the cross-sectional areas of the CC did not differ significantly between TS and HC groups in this sample, overall average size of the CC was smaller in the TS group, consistent with our findings in a much larger sample of TS children. There was no significant group-by-region interaction that pointed to any of the CC regions being specifically involved in the TS condition.
Correlations of FA values with symptom severity were not statistically significant. Positive associations of FA values with current tic severity in two CC regions tend to support our hypothesis that lower FA values in the TS group reflect a compensatory, neuroplastic response that helps to modulate tic severity. The absence of significant correlations of FA values with age may attest to the stability of the observed findings in the examined age range.
Although IQ measures were significantly lower in the TS children, covariance analyses provided evidence that IQ differences across groups were not responsible for the lower FA value in the TS group. When excluding subjects with comorbid ADHD, verbal IQ was no longer significantly different between the TS and HC groups, consistent with prior evidence that comorbidity with ADHD entails academic difficulties in individuals with TS (Abwender et al., 1996; Erenberg, Cruse, & Rothner, 1987; Peterson, Pine, Cohen, & Brook, 2001; Schuerholz, Baumgardner, Singer, Reiss, & Denckla, 1996).
Comorbidity with ADHD or OCD, and medication use did not seem to contribute to group differences in FA values. When these effects were controlled statistically, and when subjects with these conditions were separately excluded from the statistical analyses, findings of significantly lower FA values in the TS group persisted, despite the loss of statistical power that inclusion of fewer subjects entailed (see Table 2).
This is, to our knowledge, the first DTI investigation of individuals with TS. It confirms the hypothesized reduction in anatomical connectivity of the CC in children with TS. Reduced connectivity in the CC provides further supporting evidence for the presence of neuroplastic responses in the CC and other regions in the brains of these children, presumably in the service of enhancing prefrontal functioning and the regulation of tic behaviors.
Acknowledgments
We thank Roger Barndon, Martin Ystad, Anne Øfsthus and Liv Heldal for their technical assistance and Alf Inge Smievoll for his radiological expertise. This work was supported by the Center for Child and Adolescent Mental Health, University of Bergen, Norway, the Dedichsen fond and the Nyquist legat and in part by NIHM grants K02–74677 and MH06831 to Bradley Peterson and grants from the Research Council of Norway, Oslo and from the Health Authority of Western Norway to Kenneth Hugdahl.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, Davis NR, Ercan-Sencicek AG, Guez DH, Spertus JA, Leckman JF, Dure LSt, Kurlan R, Singer HS, Gilbert DL, Farhi A, Louvi A, Lifton RP, Sestan N, State MW. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- Abwender DA, Como PG, Kurlan R, Parry K, Fett KA, Cui L, Plumb S, Deeley C. School problems in Tourette's syndrome. Archives of Neurology. 1996;53:509–511. doi: 10.1001/archneur.1996.00550060051016. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: 1994. [Google Scholar]
- Bansal R, Staib LH, Whiteman R, Wang YM, Peterson BS. ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage. 2005;24:150–162. doi: 10.1016/j.neuroimage.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, James F, Zhu H, Peterson BS, Bradley S. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Topper R, Foltys H, Meincke U. Transcallosal inhibition and motor conduction studies in patients with schizophrenia using transcranial magnetic stimulation. British Journal of Psychiatry. 1999;175:375–379. doi: 10.1192/bjp.175.4.375. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Callosal terminals in the rat prefrontal cortex: Synaptic targets and association with GABA-immunoreactive structures. Synapse. 1998;29:193–205. doi: 10.1002/(SICI)1098-2396(199807)29:3<193::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Experimental Brain Research. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Conti F, Manzoni T. The neurotransmitters and postsynaptic actions of callosally projecting neurons. Behavioral Brain Research. 1994;64:37–53. doi: 10.1016/0166-4328(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Cuker A, State MW, King RA, Davis N, Ward DC. Candidate locus for Gilles de la Tourette syndrome/obsessive compulsive disorder/chronic tic disorder at 18q22. American Journal of Medical Genetics A. 2004;130:37–39. doi: 10.1002/ajmg.a.30066. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cerebral Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Eapen V, Pauls DL, Robertson MM. Evidence for autosomal dominant transmission in Tourette's syndrome. United Kingdom cohort study. British Journal of Psychiatry. 1993;162:593–596. doi: 10.1192/bjp.162.5.593. [DOI] [PubMed] [Google Scholar]
- Erenberg G, Cruse RP, Rothner AD. The natural history of Tourette syndrome: A follow-up study. Annals of Neurology. 1987;22:383–385. doi: 10.1002/ana.410220317. [DOI] [PubMed] [Google Scholar]
- Fredericksen K, Cutting L, Kates W, Mostofsky S, Singer H, Cooper K, Lanham D, Denckla M, Kaufmann W. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58:85–89. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- Goodman W, Price L, Rasmussen S, Mazure C, Fleischmann R, Hill C, Heninger G, Charney D. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Alexander AL, Narayana PA. Does fractional anisotropy have better noise immunity characteristics than relative anisotropy in diffusion tensor MRI? An analytical approach. Magnetic Resonance in Medicine. 2004;51:413–417. doi: 10.1002/mrm.10682. [DOI] [PubMed] [Google Scholar]
- Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magnetic Resonance in Medicine. 1996;36:960–964. doi: 10.1002/mrm.1910360620. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: Application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. The corpus callosum: More than a passive ‘corpus’. Behavioral and Brain Sciences. 1998;21:335. [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric Research. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Instructions for the authors. Journal of the American Academy of Child and Adolescent Psychiatry. 2005 Retrieved from http://www.jaacap.com.
- Kandler K. Activity-dependent organization of inhibitory circuits: Lessons from the auditory system. Current Opinion in Neurobiology. 2004;14:96–104. doi: 10.1016/j.conb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy Childern Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neurology. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, Eapen S, McDermott MP. The behavioral spectrum of tic disorders: A community-based study. Neurology. 2002;59:414–420. doi: 10.1212/wnl.59.3.414. [DOI] [PubMed] [Google Scholar]
- Leckman J, Riddle M, Hardin M, Ort S, Swartz K, Stevenson J, Cohen D. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman J, Sholomskas D, Thompson W, Belanger A, Weissman M. Best estimate of lifetime psychiatric diagnosis: A methodological study. Archives of General Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS. Course of tic severity in Tourette syndrome: The first two decades. Pediatrics. 1998;102(1 Pt 1):14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cerebral Cortex. 2005;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. American Statistician. 1997;51:338–343. [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pappert EJ, Goetz CG, Louis ED, Blasucci L, Leurgans S. Objective assessments of longitudinal outcome in Gilles de la Tourette's syndrome. Neurology. 2003;61:936–940. doi: 10.1212/01.wnl.0000086370.10186.7c. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annual Review of Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pauls DL. An update on the genetics of Gilles de la Tourette syndrome. Journal of Psychosomatic Research. 2003;55:7–12. doi: 10.1016/s0022-3999(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Leckman JF, Towbin KE, Zahner GE, Cohen DJ. A possible genetic relationship exists between Tourette's syndrome and obsessive-compulsive disorder. Psychopharmacological Bulletin. 1986;22:730–733. [PubMed] [Google Scholar]
- Pauls DL, Raymond CL, Stevenson JM, Leckman JF. A family study of Gilles de la Tourette syndrome. American Journal of Human Genetics. 1991;48:154–163. [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Development and Psychopathology. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman J, Duncan J, Wetzles R, Riddle M, Hardin M, Cohen D. Corpus callosum morphology from magnetic resonance images in Tourette's syndrome. Psychiatry Research. 1994;55:85–99. doi: 10.1016/0925-4927(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Pine DS, Cohen P, Brook JS. Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders in an epidemiological sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:685–695. doi: 10.1097/00004583-200106000-00014. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, Leckman JF. Reduced basal ganglia volumes in Tourette's syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43:941–949. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of General Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Archives of General Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Archives of General Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13(6 Pt 1):1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, Leckman JF, Bansal R, Peterson BS. Altered interhemispheric connectivity in individuals with Tourette's disorder. American Journal of Psychiatry. 2004;161:2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Penny W, Matthews PM. New approaches for exploring anatomical and functional connectivity in the human brain. Biological Psychiatry. 2004;56:613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rauch R, Jinkins J. Variability of corpus callosal area measurements from midsagittal MR images: Effect of subject placement within the scanner. American Journal of Neuroradiology. 1996;17:27–28. [PMC free article] [PubMed] [Google Scholar]
- Scahill L, Riddle M, McSwiggin-Hardin M, Ort S, King R, Goodman W, Cicchetti D, Leckman J. Children's Yale-Brown Obsessive Compulsive Scale: Reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Schuerholz LJ, Baumgardner TL, Singer HS, Reiss AL, Denckla MB. Neuropsychological status of children with Tourette's syndrome with and without attention deficit hyperactivity disorder. Neurology. 1996;46:958–965. doi: 10.1212/wnl.46.4.958. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: A diffusion tensor imaging study. Cerebral Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Orth M, Evans AH, Lees AJ, Brown P. Motor inhibition in patients with Gilles de la Tourette syndrome: Functional activation patterns as revealed by EEG coherence. Brain. 2005;128(Pt 1):116–125. doi: 10.1093/brain/awh318. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spessot AL, Plessen KJ, Peterson BS. Neuroimaging of developmental psychopathologies: The importance of self-regulatory and neuroplastic processes in adolescence. Annals of the New York Academy of Science. 2004;1021:86–104. doi: 10.1196/annals.1308.010. [DOI] [PubMed] [Google Scholar]
- SPSS, I. SPSS Base 10.0 for Windows User's Guide. Chicago IL: SPSS Inc.; 1999. [Google Scholar]
- State MW, Greally JM, Cuker A, Bowers PN, Henegariu O, Morgan TM, Gunel M, DiLuna M, King RA, Nelson C, Donovan A, Anderson GM, Leckman JF, Hawkins T, Pauls DL, Lifton RP, Ward DC. Epigenetic abnormalities associated with a chromosome 18(q21–q22) inversion and a Gilles de la Tourette syndrome phenotype. Proceedings of the National Academy of Science USA. 2003;100:4684–4689. doi: 10.1073/pnas.0730775100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine C, Balottin U, Rossi G, Maisano F, Salini S, Nardo RD, Lanzi G. Psychopathology in children and adolescents with Tourette's syndrome: A controlled study. Brain Development. 2006;28:69–75. doi: 10.1016/j.braindev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-III Manual. Canadian Supplement. Toronto: Psychological Corporation; 1996. [Google Scholar]
- Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125:1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of ‘premyelination’ by diffusion-weighted MRI. Journal of Computer Assisted Tomography. 1995;19:28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Xu D, Mori S, Shen D, van Zijl PC, Davatzikos C. Spatial normalization of diffusion tensor fields. Magnetic Resonance in Medicine. 2003;50:175–182. doi: 10.1002/mrm.10489. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: Evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]


