Abstract
The predictive value of acute gut-associated lymphoid tissue (GALT) CD4+ T cell depletion in lentiviral infections was assessed by comparing three animal models illustrative of the outcomes of SIV infection: pathogenic infection (SIVsmm infection of rhesus macaques (Rh)), persistent nonprogressive infection (SIVagm infection of African green monkeys (AGM)), and transient, controlled infection (SIVagm infection of Rh). Massive acute depletion of GALT CD4+ T cells was a common feature of acute SIV infection in all three models. The outcome of this mucosal CD4+ T cell depletion, however, differed substantially between the three models: in SIVsmm-infected Rh, the acute GALT CD4+ T cell depletion was persistent and continued with disease progression; in SIVagm, intestinal CD4+ T cells were partially restored during chronic infection in the context of normal levels of apoptosis and immune activation and absence of damage to the mucosal immunologic barrier; in SIVagm-infected Rh, complete control of viral replication resulted in restoration of the mucosal barrier and immune restoration. Therefore, our data support a revised paradigm wherein severe GALT CD4+ T cell depletion during acute pathogenic HIV and SIV infections of humans and Rh is necessary but neither sufficient nor predictive of disease progression, with levels of immune activation, proliferation and apoptosis being key factors involved in determining progression to AIDS.
Primate lentiviral infections lead to one of three potential outcomes: pathogenic infection with progression to AIDS, as in humans infected with HIV and rhesus macaques (Rh)3 infected with SIVmac (1); lack of disease progression despite persistent viral replication, as in the natural African non-human primate hosts of SIVs (2–10); or transient SIV replication and clearance upon cross-species transmission in partially permissive hosts (11–14).
Pathogenic HIV and SIV infections are characterized by invariable progression to AIDS in a variable time frame (1). The hallmarks of pathogenic infection are: 1) massive, continuous viral replication (15–17), with viral load set-point being predictive for the duration of progression to AIDS (18 –20); 2) continuous depletion of CD4+ T cells in peripheral blood (21, 22) that is more pronounced at mucosal sites (23–28); and 3) high levels of immune activation (29, 30), the magnitude of which has been reported to be predictive of disease progression (29, 30). The interaction among these factors cripples the immune system and eventually results in severe immune deficiency and death (21, 22, 31).
SIV infections in their natural African non-human primate hosts are characterized by 1) active viral replication, with set-point levels similar, or even higher than those reported in pathogenic infection (2–10); 2) transient depletion of peripheral CD4+ T cells during primary infection that rebound to preinfection levels during the chronic stage (3, 4, 7, 9, 10, 32, 33); and 3) transient and moderate increases in immune activation and proliferation during acute infection, with return to baseline levels during the chronic phase (2–10). Altogether, the action of all these factors results in an active persistent infection, which generally has no deleterious consequences on the natural hosts of SIV.
Lentiviruses have a high propensity for cross-species transmission, the outcome of which can vary widely; some of the virus strains naturally infecting African species induce pathogenic infection in new species (34, 35). Human lentiviruses originated from cross-species transmission of SIVs, with HIV-1 deriving from the chimpanzee virus SIVcpz (36), and HIV-2 from the sooty mangabey virus SIVsmm (37). Accidental or experimental transmission of SIVsmm to Rh in primate centers in the United States resulted in the emergence of highly pathogenic SIVmac and SIVsmm (38 – 41). It is very likely that, to establish as a pathogen in the new host, the cross-species transmitted lentivirus needs to adapt to the new host, most probably through serial passages (41, 42). In the majority of cases, cross-species transmission of SIVs does not result in persistent infection. Infection control was reported for Rh infected with viruses that naturally infect African green monkeys (AGMs, SIVagm) (11), l'hoesti monkeys (SIVlhoest) (12), or red-capped mangabeys (SIVrcm) (13). In all these instances, virus isolation was successful only during acute infection and the exposed animals survived for long periods of time with no clinical or biological sign of AIDS (11–13). The mechanisms of viral control in these infections are, to date, unknown. Finally, some of the cross-species transmission events are completely controlled by host-restriction factors, such as APOBEC or Trim-5α (43– 45). Thus, exposure of Rh to restricted viruses does not result in viral replication, as in Rh exposed to HIV-1 (14).
A series of recent studies on viral replication and CD4+ T cell depletion at the mucosal sites in pathogenic SIV and HIV infections showed that viral replication results in a rapid and profound depletion of intestinal CD4+ T cells (23–28), which was reported to be critical for disease progression (21, 22). However, the prognostic significance of this acute mucosal depletion of CD4+ T cells is unknown. Moreover, it was recently shown that the rate of CD4+ T cell decline in blood is only minimally predicted by HIV RNA levels in untreated persons. Other factors, as yet undefined, likely drive CD4+ T cell losses in HIV infection (46).
To understand the relationship between viral virulence and intestinal CD4+ T cell depletion, we examined the dynamics of SIV replication and that of gut-associated lymphoid tissue (GALT) CD4+ T cells in two animal models of AIDS nonprogression illustrative for two potential outcomes of lentiviral infection: persistent nonprogressive SIVagm.sab infection of sabaeus AGMs and controlled SIVagm infection of Rh. We compared the dynamics of these infections with that of pathogenic SIVmac/smm infection in Rh.
We report in this study that pathogenic, nonpathogenic, and controlled lentiviral infections resulted in a similar degree of mucosal CD4+ T cell depletion during primary infection, the amount of which was correlated with viral loads. We also show that acute GALT CD4+ T cell depletion was not predictive of the outcome of infection, and that partial restoration of CD4+ T cells was observed in both nonprogressing models: in SIVagm-infected AGMs GALT CD4+ T cell restoration occurred in the presence of a significant viral replication, whereas in SIVagm-infected Rh it occurred in the context of complete control of viral replication. Conversely, in Rh infected with SIVmac/smm, persistent viral replication, excessive immune activation, and proliferation were associated with continuous GALT CD4+ T cell loss and progression to AIDS.
Materials and Methods
Animals, infection, and samples
For the study of nonprogressive SIV infection, we included 12 Caribbean AGMs (Chlorocebus sabaeus) and three Rh (Macaca mulatta). Animals received plasma equivalent to 300 tissue culture-infective dose (TCID)50 of SIVagm.sab92018 (3). Pathogenic controls were four SIVsmm-infected Rh inoculated with plasma equivalent to 300 TCID50 of SIVsmm923 that progressed to AIDS in 10 mo postinfection (p.i.). Four negative controls were also included to investigate the role of experimental procedures (e.g., i.v. inoculation, frequent anesthesia, repeated intestinal biopsy) on GALT CD4+ T cell counts. The negative controls were Rh inoculated with 300 TCID50 HIV-1 group N strain YBF30. Similar to other HIV-1 strains, HIV-1 group N does not replicate in Rh (44). Animals were housed and handled at the Tulane National Primate Research Center in accordance with American Association for Accreditation of Laboratory Animal Care, Guide for the Care and Use of Laboratory Animals (U.S. Public Health Service) and the Animal Welfare Act. Tulane University Institutional Animal Care and Use Committee approved all protocols and procedures for these studies.
Tissue sampling
Blood and tissue samples (superficial lymph nodes (LNs), bronchoalveolar lavage (BAL), intestinal biopsy, and intestinal resections) were collected as reported (3, 23, 47). Plasma and PBMC and mononuclear cells from the intestine, BAL, and LNs were isolated as described previously (3, 23).
Viral quantification
Plasma viral loads were quantified as described previously (3, 33). For tissue quantification, viral RNA was extracted from 5 × 105−106 cells from intestine and BAL with RNeasy (Qiagen), and viral loads were quantified as described elsewhere (3). Simultaneous quantification of 18S ribosomal RNA (ribosomal RNA control reagent kit; PerkinElmer) normalized the RNA input from cells (48). Assay sensitivity was 100 RNA copies/105 cells.
Abs and flow cytometry
Whole blood-, intestine-, and LN-derived mononuclear cells were stained for flow cytometry as described previously (3, 47). Monoclonal Abs used were: CD3-FITC or CD3-PerCP; CD20-PE; CD8-PerCP or CD8-PE; CD4-allophycocyanin or CD4-PerCP; HLA-DR-PerCP; CD95-FITC or CD95-allophycocyanin; CD28-allophycocyanin or CD28-PE; CD69-allophycocyanin or CD69-FITC; Ki-67-FITC; and annexin V-PE (BD Biosciences). All Abs were validated and titrated using AGM and Rh PBMC. Samples were stained for Ki-67 and apoptosis using Ki-67 R-PE-conjugated mouse anti-human mAb set and annexin V (PE Apoptosis Detection kit I; BD Pharmingen) as per the manufacturer's instructions. Apoptotic CD4+ T cells were defined by annexin V and 7-aminoactinomycin D (7-AAD) as annexin V+7-AAD−, whereas the necrotic CD4+ T cells were defined as annexin V+7-AAD+. Data were acquired with a FACSCalibur flow cytometer (BD Immunocytometry Systems) and analyzed with CellQuest software (BD Biosciences). CD4+ and CD8+ T cell percentages were obtained by first gating on lymphocytes, then on CD3+ T cells. Memory, activation, proliferation, and apoptosis markers were determined by gating on lymphocytes, then on CD3+ T cells and finally on CD4+CD3+ or CD8+CD3+ T cells.
Immunohistochemical staining and in situ hybridization (ISH)
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissues using an avidin-biotin complex HRP technique (Vectastain Elite ABC kit; Vector Laboratories) and either mouse monoclonal anti-human CD4 (NCL-CD4−1F6; Novocastra) or rabbit polyclonal activated caspase 3 (Abcam). For the double-fluorescent labeling with CD4 and activated caspase 3, the secondary Abs were goat anti-mouse Alexa Fluor 568 and goat anti-rabbit Alexa Fluor 488. For SIV ISH, sections were subjected to high-temperature unmasking, treated with 0.2 N HCl, and hybridized overnight at 45°C with either sense or antisense SIVagm digoxigenin UTP-labeled riboprobe, blocked with normal sheep serum, incubated with sheep anti-digoxigenin alkaline phosphatase and incubated with NBT/chloride-5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics) (single staining) or either Ferangi blue (Biocare Medical) or HNPP Fluorescent Detection set (Roche) (double labels). SIVagm-infected cell phenotype was determined after ISH by incubating sections with: rabbit anti-human CD3 (DakoCytomation); mouse anti-human macrophage (HAM56; DakoCytomation); mouse anti-human Ki-67 (DakoCytomation); mouse anti-human CD45RA (Caltag Laboratories) followed by the appropriate goat anti-mouse or goat anti-rabbit Abs labeled with Alexa Fluor 488. Avidin-biotin complex method (Vectastain Elite ABC kit) and 3-amino-9-ethylcarbazole (DakoCytomation) were also used to detect HAM56. Negative controls included an antisense probe with uninfected tissues, a sense probe with infected tissues, an antisense probe with infected tissues and anti-rabbit or anti-mouse secondary Abs only.
CD4+ T cells were manually counted in the lamina propria of normal and SIVagm-infected AGM and Rh. At least 10 ×40 fields were counted per animal and per time point. Results were reported as the mean CD4+ T cells per power field.
LPS levels
These assays were done as previously described (31). Plasma samples were diluted 5-fold with endotoxin-free water and then heated to 70°C for 10 min to inactivate plasma proteins. Plasma LPS was then quantified with a commercially available Limulus amebocyte assay (Cambrex), according to manufacturer's protocol. Each sample was run in duplicate.
Statistical analysis
The difference between Rh and AGM intestinal CD4+ T cell depletion and rebound rates was tested by assessing the significance of the interaction term between those groups and the slope of change in a linear mixed effects model (49). This approach allows the most effective use of the data, with direct comparison of the slopes in the two groups. Considering the result of each monkey as a random sample from the general population, either SIVagm-infected Rh vs SIVagm-infected AGMs; or SIVagm-infected monkeys vs SIVsmm-infected Rh were compared. The same methodology was used to test the relationship between CD4+ T cell depletion/rebound and the other variables (apoptosis, necrosis, immune activation and proliferation). For these tests, it was assumed that the full covariate conditional mean assumption was verified (50). Statistical tests were performed in S-Plus 2000 (Insightful) and R (Comprehensive R-Archive Network at http://CRAN.R-project.org/).
Results
To investigate the predictive value of GALT CD4+ T cell depletion on the outcome of SIV infection, we have compared a pathogenic model, Rh infected with SIVsmm, with two animal models for nonprogression: AGMs and Rh infected with SIVagm. sab92018 (4, 32, 33). SIVagm.sab naturally infects sabaeus AGMs from Senegal (32). Similar to other SIVagm.sab strains (51), strain SIVagm.sab92018 is dual tropic, using both CCR5 and CXCR4 coreceptors (4). However, during our previous studies we have shown that the dynamics of SIVagm.sab infection in sabaeus monkeys do not differ from those of other CCR5-tropic SIV infections in their natural hosts (2, 4, 33).
SIVagm.sab replication in AGMs and Rh and a comparison with SIVsmm replication in Rh
SIVagm.sab92018 replicated actively in both AGMs and Rh during primary infection, reaching peak plasma viral loads by day 10 postinoculation (Fig. 1a). Peak viral loads were not significantly different between SIVagm-infected Rh and AGMs (median of log viral load = 7.41 vs 7.44, respectively, p > 0.5). These SIVagm.sab replication profiles during acute infection were essentially identical with those observed in SIVsmm-infected Rh (median of log viral loads = 7.53) (Fig. 1a). In both SIVagm-infected AGMs and SIVsmm-infected Rh, the postpeak decline of plasma viral loads was followed by establishment of a set-point viral load of ≈105 copies/ml after day 42 p.i. (Fig. 1a). No significant differences in viral loads between the two species were seen during the first 100 days p.i. (p > 0.05) (Fig. 1a). Then, viral replication showed divergent patterns, with maintenance of the set-point levels during the follow-up in AGMs and constant increase in viral replication and progression to AIDS in Rh infected with SIVsmm (Fig. 1a). The postpeak viral load decline in SIVagm-infected Rh continued until the viral load became undetectable, around day 72 p.i. (Fig. 1a). Negative-nested PCRs of different SIV genomic regions on RNA and DNA from serial samples of plasma, PBMC, or intestinal lymphocytes confirmed that Rh completely controlled SIVagm (data not shown). Anti-SIVagm Ab titers decreased significantly in Rh at late time points, also suggesting SIVagm control in Rh (data not shown).
FIGURE 1.
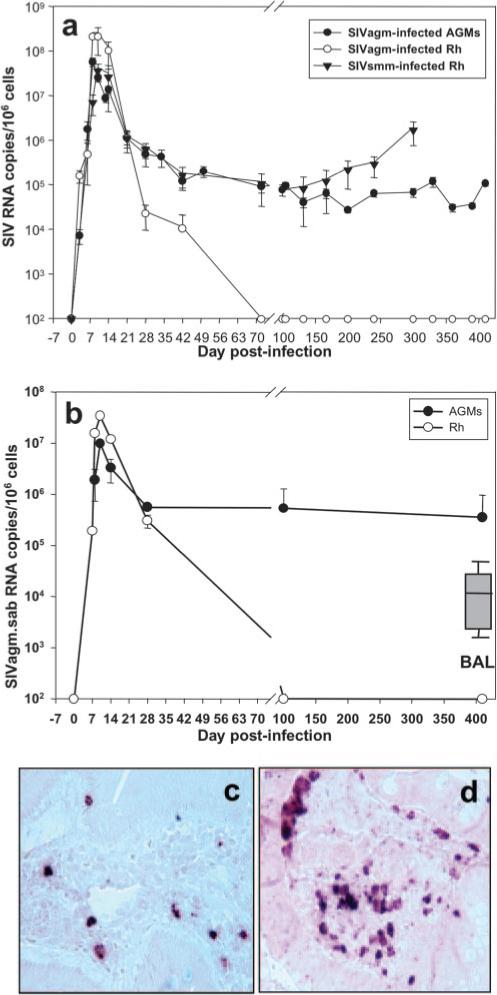
A comparison of viral dynamics during acute and chronic pathogenic SIVsmm infection of Rh and SIVagm infection of AGMs and Rh. a, No significant difference between the three models during acute infection was shown. Important differences in viral replication were observed during the chronic phase: in SIVsmm-infected Rh, plasma viral load showed significant increases with the progression to AIDS, starting from day 100 p.i. In SIVagm-infected AGMs, set-point viral loads were maintained during the follow-up. In SIVagm-infected Rh, plasma viral load became undetectable at day 72 p.i. SIVagm RNA dynamics in the intestine paralleled that of plasma viral load. b, Intestinal viral loads were maintained in SIVagm-infected AGMs, whereas in Rh, SIVagm RNA became undetectable by day 100 p.i. BAL viral loads quantified during chronic infection in AGMs were lower than those from intestine (insert). The detection limit of the assay was 102 copies/ml of plasma and 102 copies per 105 cells. Plots represent the average viral loads from the animals in each study group. Vertical lines represent SD. ISH showed that SIVagm replication is lower in SIVagm-infected AGMs (c) compared with SIVagm-infected Rh (d), in agreement with real-time PCR quantification and with the lower number of target cells in AGMs (47).
RNA viral load dynamics in the intestine was only done in SIVagm-infected AGMs and Rh (Fig. 1b). In both species, intestinal viral load dynamics paralleled those observed in AGM and Rh plasma, with peak viral loads slightly lower than in plasma (Fig. 1b). Peak viral load in the intestine were lower in AGMs than in Rh, and these results were confirmed by ISH (Fig. 1c, AGM, and d, Rh). During the chronic phase of infection of AGMs, SIVagm viral loads in the intestinal biopsies were consistently high (∼5 × 105 SIVagm RNA copies/106 cells) (Fig. 1b). In SIVagm-infected Rh, RNA loads in intestinal cells became undetectable by day 100 p.i. when peripheral viral loads were also undetectable (Fig. 1b). In AGMs, SIVagm production was also documented in BALs during the chronic phase of SIVagm infection in AGMs (Fig. 1b, insert). Viral loads in BAL were lower than those observed in the intestine.
These data showed that, although very active viral replication occurred during acute infection in all the three models, the patterns of SIV dynamics diverged considerably during chronic infection: 1) persistent viral replication and increases in viral loads in SIVsmm-infected Rh that progressed to AIDS, 2) persistent but stable viral levels in nonprogressive SIVagm-infected AGMs, and 3) control of viral replication to below detection in SIVagm-infected Rh.
CD4+ T cell dynamics
We studied the CD4+ T cell dynamics in peripheral blood, LNs, and intestine in all three models (Figs. 2 and 3). Blood CD4+ T cell dynamics were consistent with the patterns of viral replication. In all models, high SIV replication observed during acute infection was accompanied by a significant peripheral CD4+ T cell depletion after the viral load peak. In SIVagm-infected AGMs and Rh, CD4+ T cell counts rebounded to near preinfection levels during the chronic phase (3). In SIVsmm-infected Rh, after an initial rebound, CD4+ T cell counts declined continuously with the disease progression (data not shown). Similar dynamic patterns were observed in the LNs of SIVagm-infected AGMs and Rh. CD4+ T cell depletion in the LNs persisted longer than in peripheral blood (up to 200 days p.i.), but restoration was almost complete in both species at later time points, as determined by flow cytometry (3) and immunohistochemistry staining on serial LN samples (Fig. 2).
FIGURE 2.
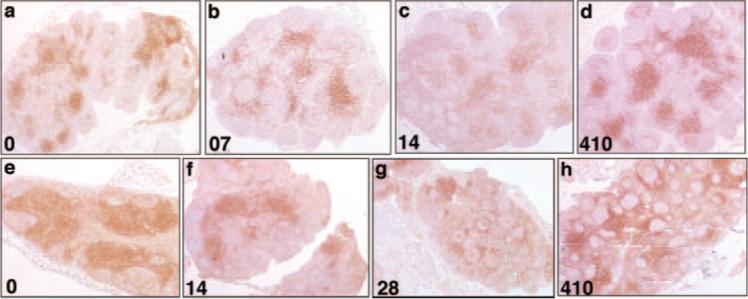
Immunohistochemistry for CD4 confirmed the loss of CD4+ cells in the SIVagm-infected AGM LNs. Loss was demonstrated by the paucity of CD4+ T cells in LNs at day 7 p.i. (b), and day 14 p.i. (c), compared with either preinfection (a) or day 410 p.i. (d), when CD4+ T cells had recovered despite persistent viremia. SIVagm-infected Rh showed rapid depletion of LN CD4+ T cells during acute infection. Immunohistochemistry for CD4 confirms the loss of CD4+ cells in the LNs by demonstrating the paucity of CD4+ T cells at day 14 p.i. (f) and 28 p.i. (g), compared with either preinfection (e) or day 410 (h), when CD4+ T cells had recovered. Original magnification, ×50.
FIGURE 3.
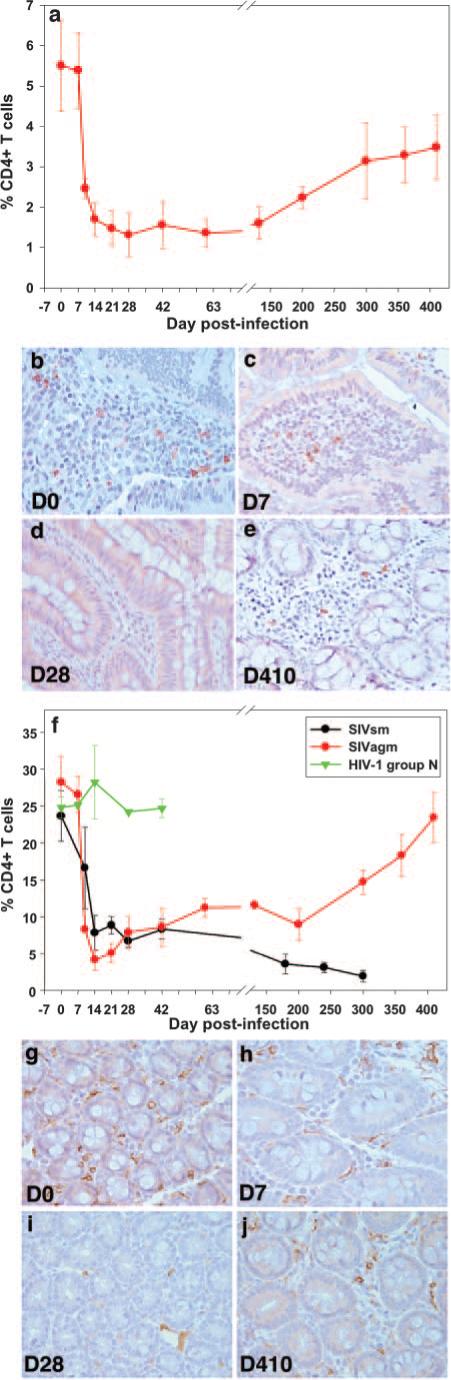
Longitudinal flow cytometric analysis of the percentage of intestinal CD4+ T cells in SIVagm-infected AGMs inoculated with SIVagm. A similar magnitude of CD4+ T cell depletion in the intestine (a) was shown as in pathogenic infection (f). Plots represent mean percentages of CD4+ T cells from animals in each group. Vertical lines with cross marks represent the SEM. Immunohistochemistry for CD4 confirms the loss of CD4+ cells in the gut by demonstrating the paucity of CD4+ T cells in lamina propria at day 7 p.i. (c), and day 28 p.i. (d), compared with either preinfection (b) or day 410 p.i. (e) when CD4+ T cells had recovered despite persistent viremia. Rh inoculated with SIVagm (red line) showed rapid and profound depletion of intestinal CD4+ T cells during acute infection (f) of the same order of magnitude as Rh infected with SIVsm (black line) (f). Controls inoculated with HIV-1 group N showed no change in CD4+ T cells (green line) (f). Vertical lines represent SEM. Immunohistochemistry for CD4 in macaques infected with SIVagm confirms the loss of CD4+ cells in the gut by demonstrating the paucity of CD4+ T cells in the lamina propria at day 7 p.i. (h) and 28 p.i. (i) compared with either preinfection (g) or day 410 (j), when CD4+ T cells recover. Original magnification, ×400.
In the intestine, a dramatic acute CD4+ T cell depletion occurred in all models of SIV infection at the immune effector sites, with the majority of lamina propria CD4+ T cells being depleted by days 14−28 p.i. in SIVagm-infected AGMs (Fig. 3a) and Rh (Fig. 3f, red), as well as in SIVsmm pathogenic infection of Rh (Fig. 3f, black). Linear mixed-effects analysis showed no statistical difference in the rate of depletion of CD4+ T cells over the first month of infection between SIVagm-infected AGM and Rh (slope = −0.066/day vs −0.073/day, respectively, p = 0.76) or between SIVagm-and SIVsmm-infected animals (slope = −0.068/ day vs −0.044/day, respectively, p = 0.18). Because the percentages of CD4+ T cells in the intestine are normally much lower in AGMs than in Rh (Fig. 3, a and f), we compared the percentage of decrease in CD4+ T cells from preinfection levels in each animal. In AGMs, 60−95% of the GALT CD4+ T cells were depleted during primary SIVagm infection, whereas Rh lost 70−95% of the CD4+ T cell population during both SIVagm and SIVsmm infection. Thus, there was no statistical difference in the percentage of CD4+ T cell depletion in GALT after 1 mo of infection (p = 0.42). In Rh infected with SIVagm, the magnitude of this depletion was higher than in pathogenic SIVsmm infection of Rh (Fig. 2f) probably because of the dual tropism of SIVagm.sab. The magnitude of GALT CD4+ T cell depletion in SIVagm-infected AGMs and Rh, which are both nonpathogenic infections, was therefore of the same order of magnitude as the CD4+ T cell depletion that occurred in pathogenic SIVsmm infections of Rh (Fig. 3f), which was in the same range of magnitude as previously reported (27).
To confirm that this CD4+ T cell loss was not an artifact due to plasma inoculation, frequent sampling, or anesthesia, we analyzed the GALT CD4+ T cell dynamics in Rh exposed to HIV-1 group N, a virus completely restricted in macaques (44). In the absence of HIV-1 group N replication, no impact from sampling and anesthesia was observed in the GALT CD4+ T cells of this negative control (Fig. 3f, green).
Finally, to further investigate the magnitude of GALT CD4+ T cell depletion in nonprogressive infections, we performed immunohistochemistry staining on serial intestinal resection samples from SIVagm-infected AGMs and Rh. Immunohistochemical staining confirmed the marked GALT CD4+ loss during primary SIVagm infection, with the nadir at day 14−28 p.i. (Fig. 2, b–d, AGMs and g–i, Rh). Thus, in AGMs, the 23 ± 7 CD4+ cells/×40 field observed at the baseline (Fig. 3b), were depleted to 7 ± 2 CD4+ cells/×40 field at day 7 p.i. (Fig. 3c), with a nadir of 1 ± 0.78 CD4+ cells/×40 field at day 28 p.i. (Fig. 3d). In Rh, the 164 ± 84 CD4+ cells/×40 field observed at the baseline (Fig. 3g) were depleted to 62 ± 19 CD4+ cells/×40 field at day 7 p.i. (Fig. 3h), with a nadir of 11 ± 3 CD4+ cells/×40 field at day 28 p.i. (Fig. 3i).
The level of CD4+ T cell depletion in the AGMs intestine far exceeded CCR5 expression in these cells, which is normally low in African species (47). The loss of intestinal CD4+ T cells in Rh also exceeded the percentage of CD4+ T cells expressing CCR5, consistent with our previous in vitro data, indicating that the SIVagm.sab92018 strain uses both CCR5 and CXCR4 coreceptors (3). However, this observation is supported in another model of natural nonprogressive SIV infection: sooty mangabeys infected with SIVsmm, a CCR5-tropic virus (52), experienced the same massive GALT CD4+ T cell depletion, exceeding the percentage of CD4+ T cells expressing CCR5 (53). Thus, it is possible that some CCR5+ memory T cells may not express enough receptor for flow cytometry detection, as hypothesized in macaques (27).
Therefore, we concluded that, similar to the dynamics of viral loads, the acute phase of pathogenic (23–28), nonpathogenic, and controlled SIV infections has a very similar effect on GALT CD4+ T cells, in terms of both rate and magnitude of depletion.
During the chronic phase of infection, however, remarkably different dynamic patterns were observed between pathogenic and nonpathogenic infections. A significant recovery of the intestinal CD4+ T cell depletion in GALT was observed from day 90 p.i. (p < 0.0001) in both AGMs and Rh infected with SIVagm, with rates that were not significantly different between AGM and Rh (slope = 0.0019/day vs 0.0027/day, respectively, p = 0.36). By the end of the follow up, at day 410 p.i., the CD4+ T cell levels were 35−75% of baseline in AGMs and 45−60% in Rh infected with SIVagm (Fig. 3, a and f). Immunohistochemical staining confirmed recovery of GALT CD4+ T cells. Thus, at day 410 p.i., CD4+ cells were 9 ± 2/×40 field in AGMs (Fig. 3e) and 79 ± 6/×40 field in Rh (Fig. 3j).
It should be noted that the context of restoration differed substantially between Rh, where SIVagm replication was controlled, and AGMs where a significant viral burden, higher than viral loads observed in humans infected with HIV-1 (20), persisted throughout the follow-up (Fig. 1, a and b). Conversely, in SIVsmm-infected Rh, the CD4+ T cell depletion was rapid, sustained and progressive, with very low levels of GALT CD4+ T cells at the time of AIDS (Fig. 2f). Statistical analyses showed a significantly different slope of change in CD4+ T cells in SIVsmm-infected Rh after day 31 p.i. when compared with the two nonprogressive, SIVagm-infected models (slope = 0.0039/day vs −0.0023/day, respectively, p < 0.0001).
To conclude, our data showed that common patterns of viral replication during the acute infection have similar impact on the acute dynamics of mucosal CD4+ T cells; however, these early events were not predictive for the outcome of lentiviral infection during the chronic phase of infection.
Phenotyping the SIVagm-infected cells
We analyzed in detail the phenotype of SIVagm-infected cells by combined ISH and immunohistochemical stainings and demonstrated that SIVagm share the same target cells as SIVmac (28), which suggested that the in vivo biology of these two viruses is very similar. Our results showed that SIVagm replicates predominantly in lymphocytes in both intestine (Fig. 4, a and b) and LNs (data not shown).
FIGURE 4.
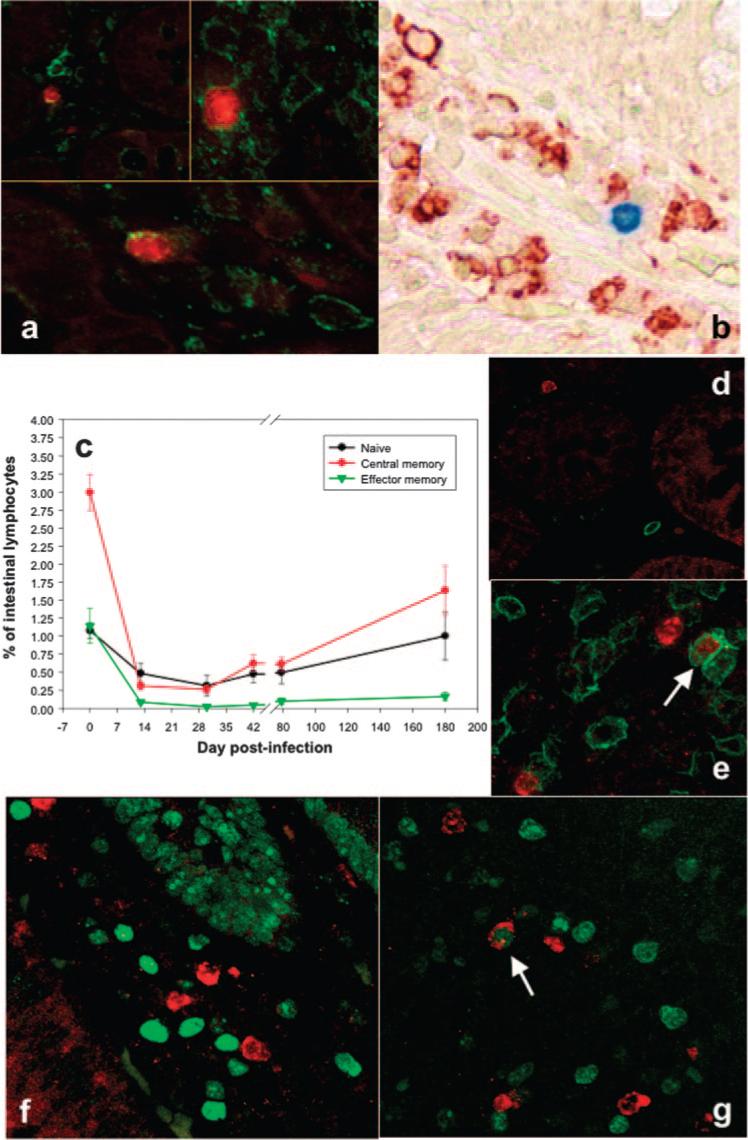
Phenotyping of SIVagm-infected cells. Combined ISH (SIV) and immunohistochemistry staining (T lymphocytes-CD3 and macrophages-HAM56) at day 10 p.i. showed that SIVagm (red) replicated mainly in T lymphocytes (green) (a), with no macrophages (red-HAM56, immunohistochemical staining, blue-SIV, ISH) being infected at the peak viral production (b) in the intestine. c, All CD4+ T cell subsets, naive, central memory, and effector memory CD4+ T cells, were depleted in the intestine of SIVagm-infected AGMs, as determined by flow cytometry. The major depletion is observed in the memory CD4+ T cell compartment. Naive cells are defined as CD3+CD4+CD28+CD95−, central memory cells are defined as CD3+CD4+CD28+CD95+, and effector memory cells are defined as CD3+CD4+CD28− CD95+. Combined ISH (SIV-red) and immunohistochemical staining (CD45RA-green) confirmed that the majority of infected cells in the lamina propria (d) and Peyer's patches (e) display a memory phenotype (lack of colocalization between the virus and CD45RA+ cells). Only a few naive cells are infected (double positive for SIV and CD45RA), shown by white arrow in e. The majority of SIVagm-infected cells (red) do not express Ki-67 (green) in lamina propria (f), similar to SIVmac-infected Rh (28). Few SIV+ cells coexpressed Ki-67 in the Peyer's patches (white arrow) (g). Original magnification: ×200 (a, top left); ×400 (a, top right); ×400 (a, bottom); ×400 (b, e, f, and g); and ×200 (d).
Phenotyping of GALT CD4+ T cell subpopulations was done to determine the dynamics of naive (defined as CD3+CD4+CD28+CD95−), central memory (defined as CD3+CD4+CD28+CD95+), and effector memory (defined as CD3+CD4+CD28−CD95+) cells (54). Our results showed that all three CD4+ T cell subpopulations were depleted during acute SIVagm infection in AGMs (Fig. 4c), with memory CD4+ T cells being the most affected population. As previously reported for pathogenic SIVmac infection (24), depletion of effector memory CD4+ T cells was not restored during the chronic phase of SIVagm infection in AGMs, the GALT CD4+ T cell restoration observed at later time points being due to increases in naive and central memory CD4+ T cells (Fig. 4c). These flow cytometry results were confirmed by in situ data that showed, at the peak of viral replication, that both memory CD45RA− and naive CD45RA+ cells may be infected in lamina propria (Fig. 4d) and Peyer's patches (Fig. 4e). Moreover, at the viral peak, SIVagm-infected cells rarely express Ki-67 (Fig. 4, f and g), similar to SIVmac-infected cells (28).
Differences in the mechanism of CD4+ T cell depletion between SIVagm-infected AGMs and Rh
We then sought to investigate the mechanism of GALT CD4+ T cell depletion in AGMs and Rh infected with SIVagm by examining levels of apoptosis vs necrosis of intestinal CD4+ T cells. This study was done by flow cytometry using annexin V and 7-AAD markers and by immunohistochemical staining using activated caspase 3. In AGMs, during primary infection we observed a 3- to 4-fold increase in necrotic cells and no increase in apoptosis (Fig. 5a). The dynamics of GALT-associated CD4+ T cell depletion (Fig. 3a) correlated strongly with necrosis (p = 0.0057), but not apoptosis (p = 0.57) (Fig. 5a). Because the annexin V+7-AAD+ cell population can include some late apoptotic cells, as well as necrotic cells, we sought to confirm the lack of increase of apoptosis by immunohistochemical staining using activated caspase 3. Immunohistochemical staining performed on serial samples from SIVagm-infected AGMs showed no increase in activated caspase 3 expression (data not shown). At the peak of viral load, the high number of SIVagm-infected cells (Fig. 5c) exceeded the number of apoptotic cells (Fig. 5d). Moreover, apoptotic cells were located in the germinal centers (B cell areas), whereas SIV-infected cells were located in interfollicular areas (T cell areas) in Peyer's patches (Fig. 5d). Finally, CD4 and activated caspase 3 did not colocalize (Fig. 5, e and f). Altogether, these data suggest that, in contrast to SIVmac-infected Rh (28), the contribution of apoptosis to CD4+ T cell destruction in SIVagm-infected AGMs is minor, and may explain why partial immune restoration in the intestine of AGMs occurred in the presence of active viral replication at later time points.
FIGURE 5.
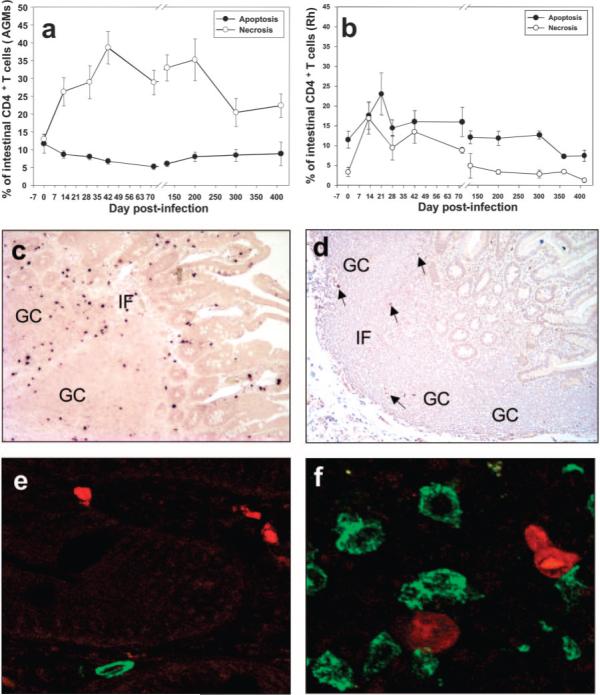
Mechanism of CD4+ T cell depletion in SIVagm infection. No significant contribution of apoptosis to intestinal CD4+ T cells was observed in SIVagm-infected AGMs (a) and transient increases in apoptosis occurred in SIVagm-infected Rh (b). a, Significant increase in necrotic cells, paralleling CD4+ T cell depletion suggests that the initial CD4+ T cell depletion in AGMs is caused by direct viral lysis. b, Apoptosis levels increased only in Rh and remained elevated even after viral clearance, paralleling the dynamics of T cell activation. Plots represent mean percentages of apoptotic and necrotic CD4+ T cells from the animals in each study group. Vertical lines represent the SEM. In AGMs, SIVagm replication in lamina propria and Peyer's patches (c) exceeded the amount of apoptotic cells (d). Infected cells were located in the interfollicular (IF) area (T cell zone), whereas apoptotic cells were more frequent in the germinal centers (GC) (B cell zone). Arrows point to apoptotic cells. Dual CD4 (green) and activated caspase 3 (red) staining showed nocolocalization of CD4+ and apoptotic cells in either lamina propria (e) or Peyer's patches (f).
In SIVagm-infected Rh, significant correlations between CD4+ T cell depletion with either apoptosis (p = 0.45) or necrosis (p = 0.10) were not observed, probably as a consequence of the fact that both were increased during primary SIVagm infection (Fig. 5b). However, increased apoptosis and necrosis in Rh persisted after the viral load was undetectable, suggesting that both are significant contributors to the delay in CD4+ T cell repopulation after viral clearance.
Differences in immune activation and T cell proliferation levels between pathogenic and nonpathogenic infections
Significant differences in the patterns of immune activation, as assessed by HLA-DR and CD69 expression on CD4+ and CD8+ T cells, were observed between pathogenic and nonpathogenic infections. Thus, in Rh infected with SIVsmm that progressed to AIDS, both CD4+ and CD8+ T cells showed progressively increasing levels of immune activation during infection (Fig. 6a), which is in agreement with previous reports (22, 30). Similarly, the proliferating compartments of CD4+ and CD8+ T cells showed significant and constant increases with the progression to AIDS, similar to what was previously reported (24) (Fig. 7a).
FIGURE 6.
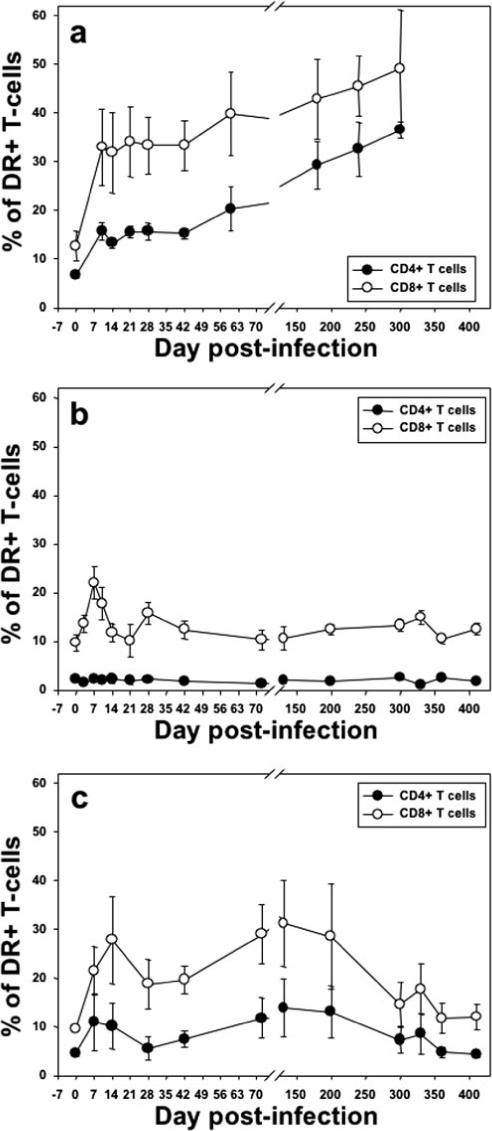
Comparative dynamics of peripheral CD4+ and CD8+ T cell activation (DR). Unlike SIVagm infections in AGMs (b), pathogenic SIVsmm infection in Rh showed continuous increases in immune activation that correlated with progression to AIDS (a). The number represents the percentage of CD3+CD4+ and CD3+CD8+ T cells. c, SIVagm infection of Rh induced intermediate and transient levels of activation of both CD3+CD4+ and CD3+ CD8+ T cells. Plots represent the average expression for the animals in each study group. Vertical lines represent the SEM.
FIGURE 7.
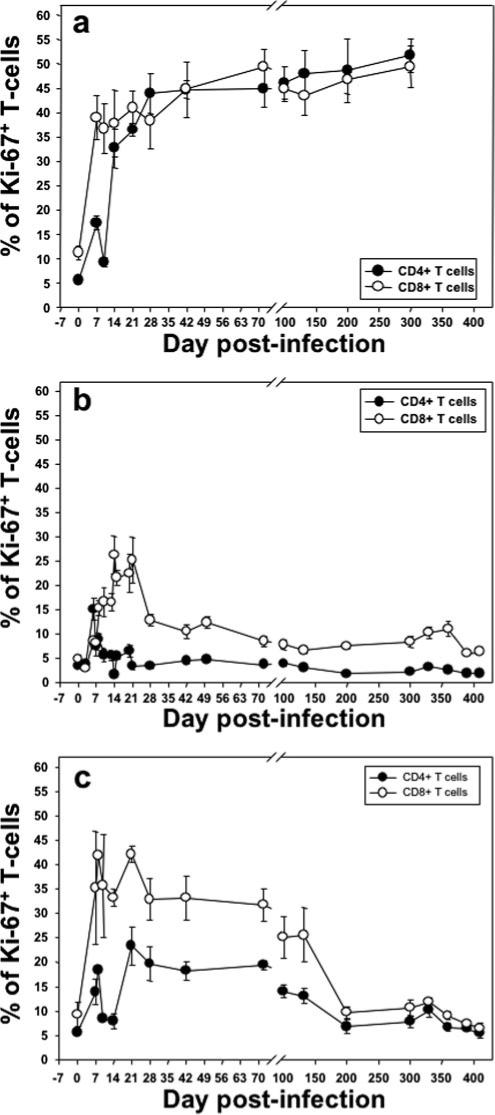
Comparative dynamics of peripheral CD3+CD4+ and CD3+ CD8+ T cell proliferation (Ki-67). Percentages are shown in SIVsmm-infected Rh (a), SIVagm-infected AGMs (b), and SIVagm-infected Rh (c). Unlike SIVagm infections in AGMs (b), pathogenic SIVsm infection in Rh showed sustained increases in cell proliferation that correlated with progression to AIDS (a). Data represent percentage of CD3+CD4+ and CD3+ CD8+ T cells. c, SIVagm infection of Rh induced intermediate and transient levels of cell proliferation of both CD3+CD4+ and CD3+CD8+ T cells. Plots represent the average expression for the animals in each study group. Vertical lines represent the SEM.
Conversely, in SIVagm-infected AGMs, there was no significant increase in activation levels of the CD4+ T cells in either blood (p = 0.55) (Fig. 6b) or intestine (data not shown). Only CD8+ T cells showed an early increase in activation (p = 0.02, for the first month p.i.), which returned to baseline levels after the set-point of plasma viral load (Fig. 6b and data not shown). Moreover, CD4+ and CD8+ T cell proliferation (Ki-67) increased in the blood only during acute infection and the initial stages of chronic infection in SIVagm-infected AGMs (p = 0.006 and p = 0.0005, for Ki-67+ CD4+ and CD8+ T cells, respectively, for the first month p.i.) (Fig. 7b).
In contrast to SIVagm-infected AGMs, SIVagm-infected Rh had a more significant activation of CD8+ and, more importantly, of CD4+ T cells (p = 0.0005) (Fig. 6c). CD8+ T cell activation persisted longer in Rh than in AGMs; the levels of DR+ CD8+ T cells being statistically increased for the first 4 mo p.i. (p = 0.028) (Fig. 6c), with levels returning to baseline after day 200 p.i. (Fig. 6c). Similarly, CD4+ T cell proliferation remained elevated longer in Rh than in AGMs (Fig. 7, b and c). Overall, SIVagm-infected Rh displayed intermediate levels of T cell activation and proliferation between pathogenic SIVsmm Rh infection and persistant SIVagm infection of AGMs (Figs. 6 and 7). No significant correlation (p = 0.68) between proliferation dynamics and GALT CD4+ T cell recovery was found in AGMs and Rh infected with SIVagm.
Lack of immune activation during chronic SIVagm infection of AGMs is partly due to maintenance/restoration of mucosal immunologic barrier
Persistent immune activation was recently reported to be a consequence of deterioration of gastrointestinal immunologic barrier by massive HIV/SIV replication resulting in microbial translocation from the gastrointestinal tract (21, 31). We therefore investigated the dynamics of the plasma levels of LPS as a marker of loss of mucosal integrity and microbial translocation. In AGMs, there were no appreciable increases in LPS (Fig. 8a) and all levels were within the ranges observed previously in uninfected humans, Rh and sooty mangabeys (31). A “blip” in plasma LPS was observed during the chronic phase of infection in one AGM following perforation of the small intestine during an endoscopic biopsy (Fig. 8a).
FIGURE 8.
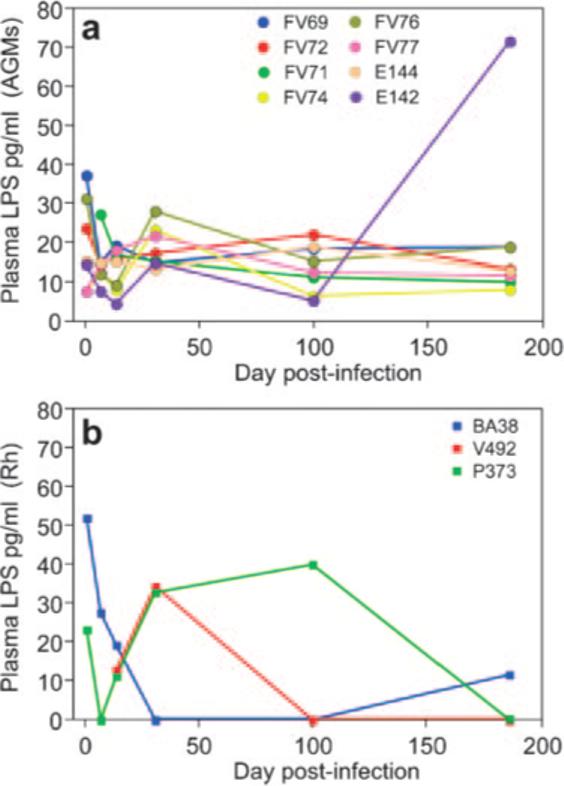
Longitudinal analysis of the plasma levels of LPS in SIVagm-infected models. Eight SIVagm-infected AGMs (a) and three SIVagm-infected Rh (b) are shown. No increase in the plasma LPS levels was observed in AGMs, with the exception of a blip in AGM EI42 following perforation of the small intestine during an endoscopic biopsy. Only transient increase was observed in SIVagm-infected Rh. Then, with the control of viral replication, the LPS levels returned to normal.
In Rh, the massive SIVagm replication during the primary infection, higher levels of immune activation and increase in apoptosis levels resulted in some damage to their intestinal immunologic barrier, as illustrated by the transient increase in plasma LPS (Fig. 8b). However, these LPS levels became undetectable after the control of infection.
Discussion
In this study, we investigated the mechanisms and predictive value for progression to AIDS of acute mucosal CD4+ T cell depletion, by comparing three representative models for the outcome of SIV infection, i.e., pathogenic (SIVsm infection of Rh), persistent non-progressive (SIVagm infection of AGMs), and controlled, nonprogressive (SIVagm infection of Rh) infection.
Pathogenic SIV and HIV infections are known to cause rapid and profound loss of memory CD4+ T cells, which reside predominantly in mucosal sites (23–28, 55). The magnitude of this depletion is thought to be critical for disease progression. However, given the fact that massive CD4+ T cell depletion occurs within weeks, it is not clear why progression to AIDS only occurs after a prolonged incubation period.
Our study does not conflict with prior observations that massive mucosal CD4+ T cell depletion is common in pathogenic infections. However, our data extend the current knowledge by showing that GALT CD4+ T cell depletion is a common feature of pathogenic, nonpathogenic, or controlled infections and therefore we concluded that, although mucosal CD4+ T cell depletion is necessary for progression to AIDS, acute depletion has no predictive value for the disease progression in HIV/SIV infection. Our results show that although viral replication sets the virus-host interplay early after infection, the outcome is determined in the postacute stage. Therefore, our data extend the paradigm of AIDS as a “tale of two infections” (56): a destructive acute phase with massive viral depletion of CD4+ memory T cells, which we show in this study to be common for all lentiviral infections, and a chronic phase that differs between models. In pathogenic infections, the immune system, crippled by acute infection slowly dies (56). In both other potential outcomes of lentiviral infection, persistent, nonprogressive infection in AGMs and controlled cross-species transmitted infection to Rh, the mucosal immune system, although experiencing severe mucosal CD4+ T cell depletion during acute infection, does not progress to “exhaustion” during the chronic phase. In contrast to pathogenic HIV/SIV infections, in nonprogressive infections the CD4+ T cell population maintains and/or regenerates itself in concert with control of disease progression.
One may argue that natural hosts of SIVs developed ways to cope with SIV infection by adapting to be less dependent on GALT CD4+ T cells to maintain function, as demonstrated by the typical low levels of CD4+ T cells in uninfected AGMs (3, 4). However, we show in this study that resistance to AIDS is not intrinsic to African monkeys and that CD4+ T cell depletion in the intestinal tract during acute infection is indistinguishable between Rh infected with SIVsmm and SIVagm; yet Rh infected with SIVagm recover despite evidence for functional compromise at the intestinal immunologic barrier (transient elevation of plasma LPS).
The major question raised by our data on SIVagm-infected AGMs is why natural hosts of SIV, unlike humans, are generally resistant to AIDS despite such dramatic immunologic injury during primary infection and persistent viral replication. The answer is likely that, after thousands of years of host/virus coadaptation (47), natural hosts of SIVs (AGMs, sooty mangabeys, or man-drills) effectively limit T cell immune activation and proliferation (3, 5–7, 10).
Studies on SIVmac infection in Rh have shown that during acute infection, there is a strong correlation between CD4+ T cell depletion and increases in the apoptosis of CD4+ T cells (28). As the number of SIV-infected cells was much lower than the number of depleted CD4+ T cells, it was concluded that both direct virus killing and an increase in apoptosis are responsible for the destruction of mucosal CD4+ T cells in pathogenic SIV infection (27, 28, 57).
However, in this study we report that apoptosis is not a major mechanism behind mucosal CD4+ T cell depletion in AGMs and provide four lines of evidence to support this conclusion: 1) no increase in apoptosis was observed during SIVagm infection in AGMs; 2) at the peak of viral load, the high number of SIVagm-infected cells exceeded the number of apoptotic cells; 3) apoptotic cells were located in different anatomic sites than SIV-infected cells; and 4) CD4 and activated caspase 3 did not colocalize.
In Rh infected with SIVagm, there was a concomitant increase in apoptosis and cellular necrosis from viral lysis, both contributing to the CD4+ T cell depletion, similar to what was previously reported in SIVmac-infected Rh (28). However, with the control of viral replication and return to baseline levels of the T cell immune activation in Rh infected with SIVagm, there was a significant reduction in both apoptosis and necrosis during the follow-up, which eventually resulted in CD4+ T cell recovery.
The lack of increase in apoptosis of CD4+ T cells observed in AGMs is probably essential for mucosal CD4+ T cell recovery during the chronic infection. We believe that the fact that cell destruction only occurs through viral lysis can explain why we observe significant recovery of CD4+ T cells during chronic SIVagm infection of AGMs even in the presence of a sustained viral load.
Previous studies have shown that the dynamics of programmed cell death in pathogenic and nonpathogenic SIV infections closely correlates with the levels of immune activation (3, 5, 7, 8, 10, 33). In this model, by comparing pathogenic, nonpathogenic, and controlled infection we showed that the most consistent difference between progressive and nonprogressive infection relies on the levels of chronic immune activation, thus confirming previous results by us and others (3, 5, 7, 8, 10, 33). In pathogenic HIV and SIV infection, the levels of immune activation constantly increased with the progression to AIDS (22, 30, 58) and previous studies reported that an increased immune activation is predictive for disease progression (22, 30, 58). This chronic immune activation is deleterious because activated CD4+ T cells serve as highly fertile targets for SIV infection and therefore activation will result in an increased viral replication (22). Moreover, persistent immune activation also create a proinflammatory environment that consolidate the damages induced by direct virus action, and was associated with an increase in apoptosis, followed by excessive CD4+ T cell proliferation in an attempt of the T lymphocyte compartment to restore the overall T cell homeostasis in a blind fashion (59). In this context, the lack of immune activation in African non-human primates might be the key feature that enables the natural SIV hosts to avoid disease progression.
During a previous study, we have reported that in SIVagm-infected AGMs, an anti-inflammatory milieu is rapidly established to prevent immune activation (33). Our results suggested that in AGMs, early increase of TGF-β may participate in the generation of T regulatory cells. This in turn releases IL-10, another potent anti-inflammatory cytokine, and altogether these may result in very low and transient T cell immune activation in SIVagm-infected AGMs (33).
We report another potential mechanism of avoiding excessive immune activation during the chronic SIV infection in nonprogressing hosts. It was recently shown that during chronic pathogenic HIV and SIV infection in humans and macaques, there is a significant microbial translocation from the intestine as a consequence of significant disruption of the mucosal immunologic barrier resulting from acute lentiviral infection (31). We have investigated the dynamics of microbial translocation in both AGMs and Rh infected with SIVagm by measuring the plasma levels of LPS (31). We report that in AGMs there is no increase in the LPS levels, which suggests that AGMs maintain the integrity of the mucosal barrier after acute SIVagm infection. The mechanism behind maintaining the integrity of the mucosal barrier may rely on the rapid anti-inflammatory responses installed in AGMs within days after SIVagm infection (33).
The SIVagm-infected Rh model convincingly shows that if viral replication is effectively controlled, the effects of mucosal injury associated with acute SIV infection can be mitigated. In SIVagm-infected Rh, after the complete control of viral replication, immune activation returned to preinfection levels. The integrity of the mucosal barrier was likely maintained, as reflected in the plasma LPS levels, which remained low in the chronic phase of infection.
In all, our results suggest that massive intestinal CD4+ T cell depletion does not necessarily lead to disease progression and that mucosal barrier and CD4+ T cell restoration is possible in circumstances in which inflammation is controlled. Thus, therapeutic approaches to control immune activation should be considered with the currently available antiretroviral therapies to slow disease progression in HIV-infected individuals.
Acknowledgments
We thank Vanessa M. Hirsch, Amitinder Kaur, Michaela Muller-Trutwin, Sue VandeWoude, Eugen Carasevici, Catalin Filipeanu, and Donald Sodora for discussion; Mary Barnes, Crystal Stoulig, Joseph Barbercheck, Chris Monjure, Anders C. Carter, Mary Jane Dodd, Janel LeBlanc, and Kelsi Rasmussen for technical assistance; Division of Veterinary Medicine of the Tulane National Primate Research Center for animal care; and Robin Rodriguez for help in preparing figures.
Footnotes
This work was supported by Grants RO1 AI064066 and R21AI069935 (to I.P.), R01 AI49080 (to R.V.), R01 AI065325 and P20 RR020159 (to C.A.), RR06555 and AI28433 (to A.S.P.), RR18745 (to R.M.R.), and P51 RR000164 (to Tulane National Primate Research Center) from the National Institute of Allergy and Infectious Diseases and from the National Center for Research Resources. A part of this work was done under the auspices of contract DE-AC52-06NA25396 of the U.S. Department of Energy.
Abbreviations used in this paper: Rh, rhesus macaque; GALT, gut-associated lymphoid tissue; AGM, African green monkey; ISH, in situ hybridization; TCID, tissue culture-infective dose; p.i., postinfection; BAL, bronchoalveolar lavage; LN, lymph node; 7-AAD, 7-aminoactinomycin D.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 2.Pandrea I, Silvestri G, Onanga R, Veazey RS, Marx PA, Hirsch VM, Apetrei C. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J. Med. Primatol. 2006;35:194–201. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 3.Pandrea I, Apetrei C, Dufour J, Dillon N, Barbercheck J, Metzger M, Jacquelin B, Bohm R, Marx PA, Barre-Sinoussi F, Hirsch VM, Muller-Trutwin MC, Lackner AA, Veazey R. Simian immunodeficiency virus (SIV) SIVagm.sab infection of Caribbean African green monkeys: new model of the study of SIV pathogenesis in natural hosts. J. Virol. 2006;80:4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandrea I, Kornfeld C, Ploquin MJ-I, Apetrei C, Faye A, Rouquet P, Roques P, Simon F, Barré-Sinoussi F, Müller-Trutwin MC, Diop OM. Impact of viral factors on very early in vivo replication profiles in SIVagm-infected African green monkeys. J. Virol. 2005;79:6249–6259. doi: 10.1128/JVI.79.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandrea I, Onanga R, Kornfeld C, Rouquet P, Bourry O, Clifford S, Telfer PT, Abernethy K, White LT, Ngari P, et al. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology. 2003;317:119–127. doi: 10.1016/j.virol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J. Med. Primatol. 2005;34:243–252. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 7.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 9.Onanga R, Kornfeld C, Pandrea I, Estaquier J, Souquiere S, Rouquet P, Mavoungou VP, Bourry O, M'Boup S, Barre-Sinoussi F, et al. High levels of viral replication contrast with only transient changes in CD4+ and CD8+ cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 2002;76:10256–10263. doi: 10.1128/JVI.76.20.10256-10263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onanga R, Souquiere S, Makuwa M, Mouinga-Ondeme A, Simon F, Apetrei C, Roques P. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx). J. Virol. 2006;80:3303–3309. doi: 10.1128/JVI.80.7.3301-3309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson PR, Goldstein S, London WT, Fomsgaard A, Hirsch VM. Molecular clones of SIVsm and SIVagm: experimental infection of macaques and African green monkeys. J. Med. Primatol. 1990;19:279–286. [PubMed] [Google Scholar]
- 12.Hirsch VM, Campbell BJ, Bailes E, Goeken R, Brown C, Elkins WR, Axthelm M, Murphey-Corb M, Sharp PM. Characterization of a novel simian immunodeficiency virus (SIV) from l'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J. Virol. 1999;73:1036–1045. doi: 10.1128/jvi.73.2.1036-1045.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, Makuwa M, Lee F, Gettie A, Russo C, Marx PA. SIVrcm infection of macaques. J. Med. Primatol. 1998;27:94–98. doi: 10.1111/j.1600-0684.1998.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 14.Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, Morton WR, Katze MG. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992;257:103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- 15.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 16.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 17.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 18.Mellors JW. Viral-load tests provide valuable answers. Sci. Am. 1998;279:90–93. doi: 10.1038/scientificamerican0798-90. [DOI] [PubMed] [Google Scholar]
- 19.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Mellors JW, Rinaldo CR, Jr., Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 21.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 22.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 23.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 24.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr., Wang C, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 29.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 30.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999;179:859–87. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 31.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Lambotte O, Altmann D, Blazar BR, et al. Microbial trans-location is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 32.Diop OM, Gueye A, Dias-Tavares M, Kornfeld C, Faye A, Ave P, Huerre M, Corbet S, Barre-Sinoussi F, Muller-Trutwin MC. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 2000;74:7538–7547. doi: 10.1128/jvi.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VandeWoude S, Apetrei C. Going wild: Lessons from T-lympho-tropic naturally occurring lentiviruses. Clin. Microbiol. Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon S, Pandrea I, Dunham R, Apetrei C, Silvestri G. The call of the wild: what can be learned from studies of SIV infection of natural hosts? In: Leitner T, Foley B, Hahn B, Marx P, McCutchan F, Mellors J, Wolinsky S, Korber B, editors. HIV Sequence Compendium 2004. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; Los Alamos.: 2005. p. 2. [Google Scholar]
- 36.Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 37.Hirsch VM, Dapolito G, McGann C, Olmsted RA, Purcell RH, Johnson PR. Molecular cloning of SIV from sooty mangabey monkeys. J. Med. Primatol. 1989;18:279–285. [PubMed] [Google Scholar]
- 38.Murphey-Corb M, Martin LN, Rangan SR, Baskin GB, Gormus BJ, Wolf RH, Andes WA, West M, Montelaro RC. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 39.Mansfield KG, Lerche NW, Gardner MB, Lackner AA. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J. Med. Primatol. 1995;24:116–122. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 40.Apetrei C, Kaur A, Lerche NW, Metzger M, Pandrea I, Hardcastle J, Fakelstein S, Bohm R, Kohler J, Traina-Dorge V, et al. Molecular epidemiology of SIVsm in US primate centers unravels the origin of SIVmac and SIVstm. J. Virol. 2005;79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apetrei C, Lerche NW, Pandrea I, Gormus B, Metzger M, Silvestri G, Kaur A, Bohm R, Robertson DL, Hardcastle J, et al. Kuru experiments triggered the emergence of pathogenic SIVmac. AIDS. 2006;21:317–321. doi: 10.1097/01.aids.0000206498.71041.0e. [DOI] [PubMed] [Google Scholar]
- 42.Marx PA, Alcabes PG, Drucker E. Serial human passage of simian immunodeficiency virus by unsterile injections and the emergence of epidemic human immunodeficiency virus in Africa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:911–920. doi: 10.1098/rstb.2001.0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song B, Javanbakht H, Perron M, Park DH, Stremlau M, Sodroski J. Retrovirus restriction by TRIM5α variants from Old World and New World primates. J. Virol. 2005;79:3930–3937. doi: 10.1128/JVI.79.7.3930-3937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 45.Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau NR. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. J. Am. Med. Assoc. 2006;296:14980–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 47.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gueye A, Diop OM, Ploquin MJ, Kornfeld C, Faye A, Cumont MC, Hurtrel B, Barre-Sinoussi F, Muller-Trutwin MC. Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. J. Med. Primatol. 2004;33:83–97. doi: 10.1111/j.1600-0684.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- 49.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-Plus. Springer-Verlag; New York: 2002. [Google Scholar]
- 50.Diggle PJ, Heagerty P, Laing K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; Oxford, U.K: 2002. [Google Scholar]
- 51.Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gautam R, Carter AC, Katz N, Butler IF, Barnes M, Hasegawa A, Ratterree M, Silvestri G, Marx PA, Hirsch VM, et al. In vitro characterization of primary SIVsmm isolates belonging to different lineages: in vitro growth on rhesus macaque cells is not predictive for in vivo replication in rhesus macaques. Virology. 2007;362:257–270. doi: 10.1016/j.virol.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon S, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free SIV-infected sooty mangabeys. J. Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 55.Smit-McBride Z, Mattapallil JJ, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picker LJ, Watkins DI. HIV pathogenesis: the first cut is the deepest. Nat. Immunol. 2005;6:430–432. doi: 10.1038/ni0505-430. [DOI] [PubMed] [Google Scholar]
- 57.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 59.Staprans SI, Feinberg MB. The roles of nonhuman primates in the preclinical evaluation of candidate AIDS vaccines. Expert Rev. Vaccines. 2004;3:5–32. doi: 10.1586/14760584.3.4.s5. [DOI] [PubMed] [Google Scholar]


