Abstract
Objective
To compare the behavioral features and to investigate the neuroanatomic correlates of behavioral dysfunction in anatomically defined temporal and frontal variants of frontotemporal dementia (tvFTD and fvFTD).
Methods
Volumetric measurements of the frontal, anterior temporal, ventromedial frontal cortical (VMFC), and amygdala regions were made in 51 patients with FTD and 20 normal control subjects, as well as 22 patients with Alzheimer disease (AD) who were used as dementia controls. FTD patients were classified as fvFTD or tvFTD based on the relative degree of frontal and anterior temporal volume loss compared with controls. Behavioral symptoms, cerebral volumes, and the relationship between them were examined across groups.
Results
Both variants of FTD showed significant increases in rates of elation, disinhibition, and aberrant motor behavior compared with AD. The fvFTD group also showed more anxiety, apathy, and eating disorders, and tvFTD showed a higher prevalence of sleep disturbances than AD. The only behaviors that differed significantly between fvFTD and tvFTD were apathy, greater in fvFTD, and sleep disorders, more frequent in tvFTD. FvFTD was associated with greater frontal atrophy and tvFTD was associated with more temporal and amygdala atrophy compared with AD, but both groups showed significant atrophy in the VMFC compared with AD, which was not associated with VMFC atrophy. In FTD, the presence of many of the behavioral disorders was associated with decreased volume in right-hemispheric regions.
Conclusion
FvFTD and tvFTD show many similarities in behavior, which appear to be associated with damage to right frontal and temporal structures.
Frontotemporal dementia (FTD) is a clinically and anatomically heterogeneous disorder, and frontally predominant (frontal variant of FTD [fvFTD]) and temporally predominant (temporal variant of FTD [tvFTD]) subtypes have been described.1–3 Typically, tvFTD is defined by the presence of deficits in language and semantic knowledge, whereas fvFTD is defined by its behavioral features. However, recent studies suggest that both of these clinical-anatomic syndromes are associated with behavioral disturbances.4–6 Determination of the differences and similarities in behavior in these two variants could help to elucidate the roles of the frontal and anterior temporal lobes in the regulation of behavior. The previous studies examining behavior in anatomic variants of FTD were limited because they included clinical features in their patient classification strategy and did not directly examine behavioral-anatomic relationships.
The work presented here had two goals: 1) to compare the behavioral abnormalities in fvFTD and tvFTD using strictly anatomic criteria to define the comparison groups and 2) to examine the association between regional cerebral volumes and behavioral abnormalities. Patients with Alzheimer disease (AD) were included as a control group. Anatomic regions of interest were chosen based on previous work indicating that fvFTD and tvFTD both affect brain regions putatively involved in the regulation of behavior including the anterior temporal neocortex, amygdala, and the ventromedial frontal cortex.6–9
Methods
Subjects
Patients
Fifty-one patients with FTD (31 men, 20 women, mean age 63.1 years) and 22 patients with AD or mild cognitive impairment (MCI) (10 men, 12 women, mean age 67.4 years), matched for Mini-Mental State Examination (MMSE) score to the FTD group, were recruited from among patients evaluated at the University of California at San Francisco (UCSF) Memory and Aging Center. The most recent criteria for FTD described three clinical variants of FTD, referred to as FTD, semantic dementia, and progressive nonfluent aphasia (PA).10 Patients were included in this study if they met clinical criteria for FTD or semantic dementia. Cases of PA were excluded from this analysis, due to its overlap with AD.11,12 Twenty-seven patients were diagnosed with semantic dementia, and 24 with FTD. The diagnosis of AD was based on National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria.13 The patients with MCI had significant memory complaints confirmed by an informant and objective impairment in memory based on neuropsychological testing.14 Both subsequently converted to AD.
All patients were initially evaluated by a neurologist (B.L.M. or H.J.R.) and a nurse and underwent a neuropsychological evaluation including the MMSE,15 verbal memory assessment (California Verbal Learning Test–Mental Status version [CVLT-MS]16), nonverbal memory assessment (copy of modified version of the Rey-Osterrieth figure with a 10-minute free recall delay trial), language assessment (15-item Boston Naming Test,17 comprehension of seven syntactically complex commands and questions, repetition of three phonemically complex phrases), and visuospatial function (copy of modified Rey-Osterrieth figure). Assessment of frontal lobe functioning included tests of verbal (semantic—animals/1 minute, phonemic—D-words/1 minute) and nonverbal fluency (Trial 1 of the Design Fluency subtest of the Delis-Kaplan Executive Functions Scale18), a visuo-motor set-shifting and sequencing task (a modified version of the Trails B test19), backwards digit span, the Stroop interference task,20 written reproduction of alternating “m”s and “n”s, and assessment of echopraxic errors, or the response to two verbal commands given with a conflicting visual cue (e.g., asking the patient to point to the ceiling while extending one’s hand as if to shake), to assess inhibition of overlearned responses. A frontal lobe composite score was created, which was the sum of rule violations on the fluency tasks, errors on the modified trials task and Stroop task, echopraxic errors, and degree of perseveration on alternating “m”s and “n”s (scale of 0 to 2).
Neuroimaging control subjects
Twenty subjects (10 men, 10 women, mean age 66.8 ± 8.4 years) were chosen from among a group of subjects enrolled in ongoing neuroimaging research in the San Francisco Veterans Administration hospital to match the patient groups in age. The neuroimaging control subjects had no history of neurologic or psychiatric disorders, and had no evidence of focal disease or subcortical white matter ischemic changes on MRI.
Identification of behavioral abnormalities
Data from the Neuropsychiatric Inventory (NPI) were analyzed.21 This is a validated, caregiver-based behavioral rating system developed for the assessment of dementia that evaluates the presence or absence, severity (rated 1 to 3, 3 being most severe), and frequency (rated 1 to 4, 4 being most frequent) of 12 major behavioral disorders, including delusions, hallucinations, aggression/agitation, depression, anxiety, elation/euphoria, apathy, disinhibition, irritability/lability, aberrant motor behavior, sleep disturbances, and eating disorders. In each patient where symptoms are endorsed, specific subquestions explore the details of the behavior.
Acquisition of MRI and cerebral volumes
MRI scanning
Structural MR imaging was accomplished using a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ), a standard quadrature head coil, and previously described sequences6,8 to obtain 1) scout views of the brain for positioning subsequent MRI slices, 2) proton density and T2-weighted MRI, and 3) T1-weighted (magnetization-prepared rapid gradient echo) images of entire brain.
Tissue segmentation
Subject brains were first segmented into gray matter, white matter, and CSF using previously described methods,22 and further separated manually by a trained operator into cortical and subcortical gray matter, ventricular and sulcal CSF, and normal white matter and white matter lesions.
Cerebral volume measurements
Volumes were obtained for the right and left frontal, anterior temporal, and ventromedial frontal cortex (VMFC), and the amygdala using methods described previously8 (figure), with one change: the anterior border for the ventromedial frontal cortex was designated as the most anterior extent of the temporal fossa, as opposed to the temporal lobe in previous analyses. This avoids artifactual dependence of ventro-medial frontal cortex volume on temporal lobe volume.
Figure.
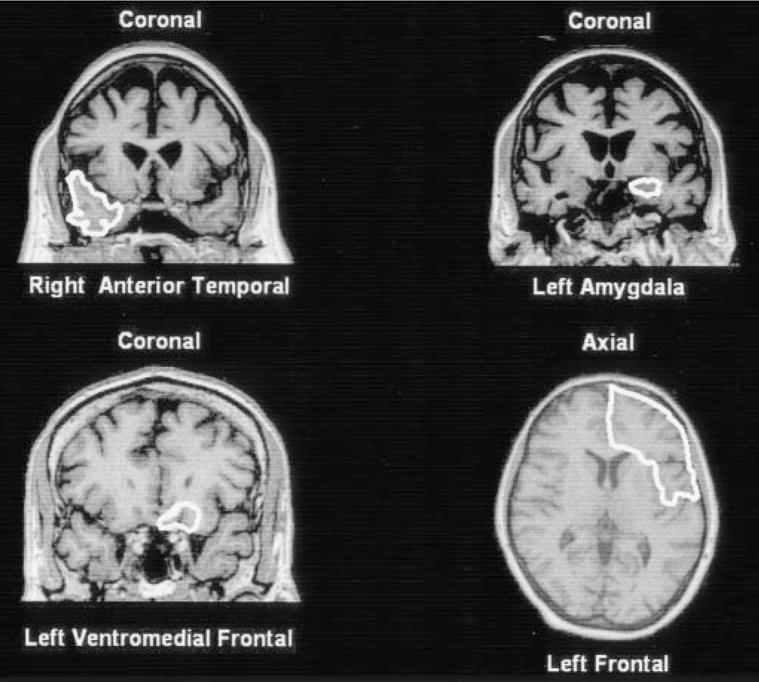
Depiction of region-of-interest markings on representative slices for the four regions measured. MR images are coronal and axial T1-weighted magnetization-prepared rapid gradient echo images in a normal control.
Identification of fvFTD and tvFTD patients
The frontal and anterior temporal volumes for each patient were compared to the corresponding regions in the control group, and a z-score was created for each region in each patient. Patients were designated as fvFTD or tvFTD patients based on whichever region had the larger (more negative) mean z-score, provided that the z-score for the frontal and temporal regions in that patient differed by at least 0.5.
Data analysis
Cortical volumes and neuropsychological results were compared across subject groups using one-way analysis of variance (ANOVA), with post hoc pairwise comparisons and Bonferroni correction for multiple comparisons. The proportion of patients that showed each behavioral disorder was compared across patient groups using χ2 analysis. In addition, an index of severity was created for each behavioral variable by multiplying the frequency and severity scores (creating a frequency by severity product), as has been done in the past using the NPI.23 This score was compared between the fvFTD and tvFTD groups for each disorder using the Mann-Whitney test. For this frequency by severity product analysis, only the patients who showed evidence of a particular behavioral abnormality were included. Clinical-anatomic relationships were examined by comparing regional volumes in patients with and without behavioral disorders, using unpaired t-tests. Statistical analysis was accomplished using the SPSS software package (version 10.1 for Windows, SPSS Inc., Chicago, IL).
The study was approved by the UCSF committee on human research. All subjects or their surrogates provided informed consent before participating.
Results
Results of anatomic classification process
Anatomic designation of clinical subtypes
Twenty-seven of the 51 patients enrolled in this study were diagnosed with semantic dementia, and 24 with FTD. One patient with FTD and one with semantic dementia had nearly identical mean frontal and temporal z-scores and were excluded, leaving 49 FTD patients for further analysis. Twenty-three of the 26 remaining patients with a clinical diagnosis of semantic dementia were designated as tvFTD (88%) and 3 as fvFTD, whereas 20 of the 23 remaining patients with a clinical diagnosis of FTD were designated as fvFTD (87%) and 3 as tvFTD. Thus, there were 26 tvFTD and 23 fvFTD patients. For the temporal group, the mean z-score was − 1.11 in the frontal lobes and − 3.31 in the temporal lobes. For the frontal group, the mean z-score was − 3.18 in the frontal lobes and − 1.11 in the temporal lobes.
Neuropsychological and behavioral analysis
Group demographics and neuropsychology
Demographic and neuropsychological data for the three patient groups are shown in table 1. There were no significant differences between the groups in age, MMSE, or sex distribution. The AD group showed the greatest impairment in memory, with verbal memory significantly worse than fvFTD and visual memory significantly worse than tvFTD. The fvFTD score on the frontal composite measure was elevated relative to both other patient groups. The tvFTD group showed a significant naming impairment, but no other deficits, when compared with the other patient groups.
Table 1.
Neuropsychological test results for AD patients, fvFTD patients, and tvFTD patients
| Characteristics and tests | Overall ANOVA | AD | fvFTD | tvFTD |
|---|---|---|---|---|
| Age, y | F(2,68) = 1.90 | 67.4 (10.0) | 62.3 (9.0) | 65.3 (9.2) |
| Males/females | 10/12 | 12/11 | 16/10 | |
| MMSE, max = 30 | F(2,68) = 0.7 | 20.9 (5.4) | 20.8 (8.7) | 24.1 (4.2) |
| CVLT-MS, max = 9 | ||||
| Trial 4 | F(2,55) = 1.57 | 4.8 (1.9) | 3.7 (2.8) | 4.3 (1.7) |
| 10′ Free recall | F(2,55) = 3.79† | 0.7 (1.2) | 2.6 (2.5)‡ | 1.7 (2.4) |
| Modified Rey-Osterrieth Delay, max = 17 | F(2,60) = 6.03§ | 2.0 (2.1) | 5.4 (4.6) | 7.3 (5.8)* |
| Digit Span Backwards | F(2,57) = 10.84§ | 3.8 (1.3) | 2.6 (1.8) | 5.0 (0.8)¶ |
| Modified Trails, no. lines/min | F(2,56) = 7.67§ | 7.5 (5.5) | 5.8 (5.4) | 11.9 (4.5)* |
| Stroop, no. correct/min | F(2,50) = 1.7 | 19.6 (11.8) | 21.5 (17.5) | 28.9 (17.4) |
| Frontal composite score | F(2,44) = 6.35§ | 5.0 (4.2) | 11.6 (10.6)‡ | 3.4 (2.6) ¶ |
| Design Fluency | F(2,60) = 3.23† | 4.9 (3.2) | 4.2 (3.9) | 7.4 (3.8) |
| Phonemic Fluency | F(2,59) = 3.51† | 9.3 (5.3) | 5.1 (5.9)‡ | 7.5 (3.0) |
| Semantic Fluency | F(2,60) = 2.22 | 9.3 (4.3) | 5.7 (7.0) | 6.5 (4.4) |
| Abbreviated BNT, max = 15 | F(2,60) = 22.37§ | 11.2 (3.5) | 9.8 (4.3) | 4.8 (3.8)*¶ |
| Sentence Comprehension, max = 7 | F(2,56) = 1.37 | 6.0 (1.3) | 5.6 (1.4) | 5.3 (2.2) |
| Phrase Repetition, max = 3 | F(2,58) = 0.22 | 2.5 (0.8) | 2.4 (1.0) | 2.4 (0.9) |
| Modified Rey-Osterrieth Copy, max = 17 | F(2,62) = 4.69† | 13.9 (3.5) | 12.8 (4.7) | 15.7 (3.0)|| |
| Calculations, max = 5 | F(2,59) = 3.77† | 3.7 (1.3) | 2.8 (2.0) | 4.3 (0.9)|| |
Values are mean (SD).
p < 0.01 vs AD.
p < 0.05 Across all groups.
p < 0.05 vs AD.
p < 0.01 Across all groups.
p < 0.01 vs fvFTD.
p < 0.05 vs fvFTD.
AD = Alzheimer disease; fvFTD = frontal variant of frontotemporal dementia; tvFTD = temporal variant of FTD; ANOVA = analysis of variance; MMSE = Mini-Mental State Examination; CVLT-MS = California Verbal Learning Test–Mental Status; BNT = Boston Naming Test.
Behavioral analysis
The proportion of patients with evidence for each behavioral disorder included in the NPI was compared across the three patient groups (AD, fvFTD, and tvFTD; table 2). As expected, there were significant differences between FTD and AD. When compared with AD, both the tvFTD and fvFTD groups showed higher proportions of patients with elation/euphoria, disinhibition, and aberrant motor behavior. In addition, the tvFTD group showed a higher prevalence of sleep disorders than AD and the fvFTD group showed more apathy, anxiety, and eating disorders than AD (eating disorders appeared to be more prevalent, p = 0.063, in tvFTD compared with AD).
Table 2.
Number of patients (%) in each diagnostic group with behavioral abnormalities on the NPI
| Feature | AD | fvFTD | tvFTD |
|---|---|---|---|
| Delusions | 1 (5) | 5 (22) | 5 (19) |
| Hallucinations | 0 | 3 (13) | 0 |
| Agitation | 7 (32) | 11 (47) | 14 (52) |
| Depression | 6 (27) | 6 (26) | 12 (44) |
| Anxiety | 6 (27) | 13 (56)* | 11 (41) |
| Elation | 1 (5) | 9 (39)† | 10 (37)† |
| Apathy | 8 (36) | 22 (96)ठ| 13 (48) |
| Disinhibition | 4 (18) | 17 (73)† | 20 (74)‡ |
| Irritability | 9 (41) | 8 (35) | 9 (33) |
| Aberrant motor behavior | 3 (14) | 16 (69)‡ | 14 (52)† |
| Sleep disorders | 4 (18) | 6 (26)¶ | 14 (52)* |
| Eating disorders | 6 (27) | 17 (74)† | 14 (52) |
p < 0.05 Compared with AD.
p < 0.01 Compared with AD.
p < 0.001 Compared with AD.
p < 0.001 Compared with tvFTLD.
p < 0.05 Compared with tvFTLD.
NPI = Neuropsychiatric Inventory; AD = Alzheimer disease; fvFTD = frontal variant of frontotemporal dementia; tvFTD = temporal variant of FTD.
When the two variants of FTD were directly compared apathy was more prevalent and showed a higher frequency by severity product in fvFTD, and sleep disturbances were more prevalent in tvFTD.
When the six cases that did not show the expected distribution of atrophy based on their clinical diagnosis were removed, apathy was still more prevalent in fvFTD and sleep disorders more prevalent in tvFTD. In addition, the tvFTD group now appeared to have a higher proportion of patients with depression (55% in tvFTD versus 25% in fvFTD, p = 0.051). There was no difference in the severity of depression across groups. Individual data for this group of six indicated behavioral problems in all cases (disinhibition and eating disorders in all, apathy in five out of six; see table E-1 at www.neurology.org), with no specific pattern across the individuals.
Qualitative analysis of behavior across groups
In order to understand whether the differences in behavior seen across groups represented qualitative, as well as quantitative differences, we examined the specific subquestions endorsed in the behavioral categories where the groups showed differences. There appeared to be no significant patterns differentiating the patient groups in terms of the specific features of the behavioral disorders in each group. However, it was remarkable that of the 14 caregivers of patients with tvFTD who noted sleep disorders, none reported any problems with early awakening.
Volumetric analyses
TvFTD showed a reduction in bilateral anterior temporal but not frontal volumes, compared with fvFTD, and fvFTD showed a reduction in bilateral frontal but not temporal volumes compared with tvFTD (table 3). In the tvFTD group, the left temporal volume was slightly smaller than the right, and in the fvFTD group, the right frontal volume was slightly smaller than the left (p < 0.05, paired t-tests, for both regions). In each group, more than half the patients had significant atrophy in both the frontal and temporal regions, as defined by volumes more than 1.5 standard deviations below control mean in one or both hemispheres (15 out of 23 fvFTD, 16 out of 26 tvFTD). In the tvFTD group, 18 cases showed more left than right-sided atrophy, and 8 cases showed more right than left-sided atrophy.
Table 3.
Cerebral volumes across groups
| Region | Controls | AD | fvFTD | tvFTD |
|---|---|---|---|---|
| R frontal | 99.96 (6.02) | 93.4 (8.44)* | 78.94 (9.62)†‡ | 94.71 (6.52)§ |
| L frontal | 100.60 (6.11) | 92.71 (6.63)† | 81.42 (8.34)†‡ | 92.36 (7.68)†§ |
| R atem | 13.86 (2.13) | 12.42 (2.38) | 11.37 (3.16)* | 7.87 (3.09)†‡§ |
| L atem | 13.81 (2.07) | 12.39 (2.89) | 11.61 (2.91)* | 6.45 (1.56)†‡§ |
| R vmfc | 4.15 (0.7) | 4.21 (0.57) | 3.43 (0.8)†‡ | 3.36 (0.75)†‡ |
| L vmfc | 4.07 (0.62) | 3.92 (0.61) | 3.38 (0.76)† | 3.11 (0.66)†‡ |
| R amygdala | 2.5 (0.4) | 1.94 (0.35)† | 2.11 (0.43)† | 1.51 (0.31)†‡§ |
| L amygdala | 2.3 (0.49) | 1.74 (0.34)† | 1.84 (0.39)† | 1.22 (0.26)†‡§ |
Values are volume, cc3 (SD). Volumes reduced in one group compared with all three other groups are italicized.
p < 0.05 vs controls.
p < 0.01 vs controls.
p < 0.05 vs AD.
p < 0.01 vs fvFTD.
AD = Alzheimer disease; fvFTD = frontal variant of frontotemporal dementia; tvFTD = temporal variant of FTD; atem = anterior temporal; vmfc = ventromedial frontal cortex.
The six cases that did not show the expected distribution of atrophy based on their clinical diagnosis were examined individually (see table E-2 at www.neurology.org). None of these cases was pure, in that each case showed significant atrophy (greater than 1.5 standard deviations below control mean) in both the temporal and frontal lobes. Atrophy was more prominent on the left in four out of six cases (two fvFTD and two tvFTD patients).
Frontal and temporal volumes versus AD
The designation of patients into anatomic groups based on comparison with a control group did not guarantee that these designations would be apparent in comparison with the AD group. Because the AD group was used as the control for the behavioral analysis, frontal and temporal volumes were compared across the three patient groups (see table 3). ANOVA demonstrated main effects for both regions bilaterally. Post hoc testing demonstrated that the tvFTD group showed a reduction in temporal lobe cortical volumes, but not frontal volumes, compared with AD. Conversely, the fvFTD group showed a reduction in frontal lobe cortical volumes, but not temporal volumes, compared to AD. Thus, even relative to AD, the two groups could be described as predominantly frontal or temporal.
VMFC and amygdala volumes across groups
ANOVA demonstrated main effects for both regions bilaterally. In the VMFC, the tvFTD group showed a reduction bilaterally compared with AD and controls. The fvFTD group showed a reduction on the right compared with AD, although the volume reduction was nearly significant on the left as well (right: p < 0.01, left: p = 0.054). Compared with controls, the fvFTD group showed a significant reduction bilaterally. The AD group showed no significant reduction in VMFC volume compared with controls. There was no difference in VMFC volume between the fvFTD and tvFTD groups. In the amygdala all three groups showed reductions compared with controls. However, only the tvFTD group showed reductions in amygdala volumes compared with the other patient groups.
Behavioral-anatomic associations
Because hallucinations and delusions occurred rarely, if at all, for all three patient groups, these variables were not analyzed further. We examined the anatomic associations for the remaining variables where no significant differences were found between the two FTD groups (agitation, depression, anxiety, elation, disinhibition, irritability, aberrant motor behavior, and eating disorders). Because it is possible that several variables would relate to atrophy in the same anatomic structures, we performed a principal components analysis with varimax rotation on the frequency by severity product data to extract factors representing combinations of variables. Using these eight variables, aberrant motor behavior loaded nearly equally on two factors and was dropped from the analysis because it was not unique to one factor. Three unique factors, accounting for 64% of the variance, were extracted using the remaining seven variables. The first factor was most heavily loaded on agitation and irritability, the second on depression, anxiety, and eating disorders, and the third on disinhibition and elation.
These factors were used to collapse related variables, such that if a patient was rated as showing any variable on a factor, the patient was defined as having that factor present. For each factor, the FTD group was split according to the presence of that factor, and all volumes of interest were compared across groups (using unpaired t-tests, p < 0.05 threshold, one-tailed). No significant differences were seen when the FTD group was divided according to the presence of factor 1. The presence of factor 2 was associated with decreased volume in the right frontal region. The presence of factor 3 was associated with decreased volume in the right anterior temporal and right and left VMFC regions.
In order to understand which variables were contributing to the anatomic differences associated with the factors, the FTD group was split according to the presence of each behavioral disorder, and the volumes of interest were compared across groups. No significant differences were seen when the FTD group was divided according to the presence of either component of factor 1. Within factor 2, there was no anatomic reduction associated with anxiety, but depression was associated with decreased volume in the right amygdala and right anterior temporal cortex and eating disorders with decreased volume in the right frontal cortex and right VMFC. For factor 3 components, there was no anatomic association with elation; however, disinhibition was associated with decreased volume in the right amygdala, right VMFC, and the right anterior temporal cortex.
Discussion
The primary goal of this study was to compare the frequency of different types of behavioral disorders in the two major anatomic variants of FTD: fvFTD and tvFTD. The two variants showed overlap in several behavioral domains, including elation, disinhibition, and aberrant motor behavior. When fvFTD and tvFTD were directly compared, fvFTD was associated with more apathy and tvFTD showed a higher prevalence of sleep disturbances and a trend indicating a higher prevalence of depression. The presence of behavioral abnormalities in FTD was associated with decreased gray matter volume in several right hemispheric structures.
The behavioral findings from this study are concordant with the major findings from previous work (see table E-3 at www.neurology.org for a summary). In all studies, more differences were found between FTD as a group and AD than between anatomic variants of FTD. However, in the current study tvFTD and fvFTD also showed specific differences in behavior, most prominently in the domain of apathy, which was more prevalent in fvFTD. Previous studies demonstrated an association between anterior cingulate hypoperfusion24 and plaque burden25 and the severity of apathy in AD. FvFTD may be associated with a higher degree of atrophy in the anterior cingulate region than tvFTD, which may account for severe apathy in fvFTD.6 The second behavioral difference between the FTD subtypes was the increased prevalence of sleep disorders in tvFTD compared with fvFTD. Although two previous studies have demonstrated abnormal sleep patterns in FTD compared with controls26 and AD,27 no previous work has examined the patterns of sleep disruption in different subtypes of FTD. Notably, of the 14 patients with tvFTD and sleep disorders, none had difficulties with early morning awakening. Whether this type of disorientation is less common in tvFTD than in other dementia syndromes remains to be seen. The third behavioral difference between anatomic variants was in the domain of depression. Our study suggested increased depression in the tvFTD group, a finding reflected in previous studies.4 Limbic structures hit in FTD, including the amygdala, orbital frontal region, and anterior cingulate region, play a major role in depression.28 Amygdala volume has been shown to be decreased in patients with recurrent depression29 and it is notable that the tvFTD group showed significantly reduced amygdala volumes compared with all other groups, suggesting that depressive symptoms in FTD may be related to amygdala dysfunction. Another factor that may explain this finding is self-awareness. Compared with AD and fvFTD patients, tvFTD patients have better insight into some of their symptoms, usually their language symptoms, which in turn could contribute to their expressing more depressed thoughts.
Prior studies of behavior in FTD have identified differences between the fvFTD and tvFTD that were not seen in our study. As shown in table E-3 (available at www.neurology.org), each of the studies looking at behavior in FTD used a different measure, making them difficult to compare directly with our work. Our tvFTD group may have had a higher proportion of patients with right-sided disease than groups studied by previous investigators,4 which may account for a higher proportion of patients with disinhibition.
With respect to behavioral-anatomic correlations, the results obtained here support previous observations in FTD and focal neurologic injury. Prior studies have indicated that social behavioral problems are more likely in FTD when frontotemporal dysfunction is more prominent on the right.1,30,31 Furthermore, recent studies of patients with focal cerebral injury demonstrated that damage to the right, but not left VMFC is associated with impaired social functioning.32 The mechanism by which right-sided cerebral damage leads to behavioral disorders is not completely understood, but the phenomenon may relate to the functional organization of emotional processing in the brain. Lesion studies indicate that the right hemisphere is important for the comprehension of emotional stimuli33 and impairment in emotional processing in tvFTD is highly correlated with atrophy in the right amygdala and VMFC.8 The one region of atrophy common to fvFTD and tvFTD was in the VMFC, confirming prior work.6 However, the behavioral-anatomic analysis demonstrated that other regions in addition to VMFC were associated with specific behavioral problems, and some behaviors were associated with decreased volume in other structures, but not VMFC.
The FTD and AD groups in this analysis were matched for MMSE and age. MMSE is not an ideal parameter for matching, because it is relatively insensitive to the executive deficits and behavioral abnormalities seen in FTD.34,35 However, the fact that each patient group showed neuropsychological impairment in specific domains relative to the other two groups (AD: memory, semantic dementia: naming, FTD: frontal score) suggests that the behavioral findings are not directly related to severity. Furthermore, recent work has demonstrated that, although FTD can be associated with a longer disease duration and more functional impairment compared with an AD group with the same MMSE, this is not the case for semantic dementia.36 Thus, disease severity could potentially account for more severe behavioral impairment in FTD/fvFTD relative to AD, but not in semantic dementia/tvFTD. Despite the potential difference in severity between fvFTD and tvFTD, tvFTD did show some behaviors that were more severe.
This study differed from related previous work in that FTD patients were anatomically classified according to objective criteria. Notably, the concordance between the clinical and anatomic classification schemes was very high, although not perfect. Future studies attempting to understand behavioral abnormalities in FTD and their relationship to anatomic injury should incorporate quantitative measurement of cerebral injury whenever possible.
Acknowledgments
Supported by the John Douglas French Foundation for Alzheimer’s research, the McBean Foundation, the Sandler Foundation, the Doris Duke Charitable Foundation, National Institute on Aging (NIA) grants 1K08AG020760-01, AG10129, P50-AG05142, and AG16570, the State of California Alzheimer’s Disease Research Center of California (ARCC) grant 01-154-20, and NIH grant M01 RR00079 (UCSF General Clinical Research Center).
Footnotes
Additional material related to this article can be found on the Neurology Web site. Go to www.neurology.org and scroll down the Table of Contents for the March 9 issue to find the title link for this article.
References
- 1.Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt 6):1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- 2.Gregory CA, Serra-Mestres J, Hodges JR. Early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychologic tests? Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:128–135. [PubMed] [Google Scholar]
- 3.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–2284. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 4.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Cummings JL, Duchen LW. Kluver-Bucy syndrome in Pick disease: clinical and pathologic correlations. Neurology. 1981;31:1415–1422. doi: 10.1212/wnl.31.11.1415. [DOI] [PubMed] [Google Scholar]
- 8.Rosen HJ, Perry RJ, Murphy J, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125:2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- 9.Boccardi M, Pennanen C, Laakso MP, et al. Amygdaloid atrophy in frontotemporal dementia and Alzheimer’s disease. Neurosci Lett. 2002;335:139–143. doi: 10.1016/s0304-3940(02)01169-2. [DOI] [PubMed] [Google Scholar]
- 10.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 11.Greene JD, Patterson K, Xuereb J, Hodges JR. Alzheimer disease and nonfluent progressive aphasia. Arch Neurol. 1996;53:1072–1078. doi: 10.1001/archneur.1996.00550100158027. [DOI] [PubMed] [Google Scholar]
- 12.Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the mental state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 17.Kaplan E, Goodglass H, Wintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 18.Wecker NS, Kramer JH, Wisniewski A, Delis DC, Kaplan E. Age effects on executive ability. Neuropsychology. 2000;14:409–414. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- 19.Reitan RM. Validity of the Trailmaking Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 20.Golden C. Stroop Color and Word Test: manual for clinical and experimental uses. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 21.Cummings JL. The Neuropsychiatric Inventory. Assessing psychopathology in dementia patients. Neurology. 1997;48(5 suppl 6):S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe JL, Amend D, Schuff N, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol. 1997;18:115–123. [PMC free article] [PubMed] [Google Scholar]
- 23.Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions. Arch Neurol. 1996;53:687–690. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- 24.Craig AH, Cummings JL, Fairbanks L, et al. Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neurol. 1996;53:1116–1120. doi: 10.1001/archneur.1996.00550110056012. [DOI] [PubMed] [Google Scholar]
- 25.Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49:355–361. [PubMed] [Google Scholar]
- 26.Autret A, Lucas B, Mondon K, et al. Sleep and brain lesions: a critical review of the literature and additional new cases. Neurophysiol Clin. 2001;31:356–375. doi: 10.1016/s0987-7053(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 27.Harper DG, Stopa EG, McKee AC, et al. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry. 2001;58:353–360. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- 28.Drevets WC. Functional anatomical abnormalities in limbic and pre-frontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 29.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 30.Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4:204–213. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]
- 31.Mychack P, Kramer JH, Boone KB, Miller BL. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56(11 suppl 4):S11–15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- 32.Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- 33.Bowers D, Blonder LX, Feinberg T, Heilman KM. Differential impact of right and left hemisphere lesions on facial emotion and object imagery. Brain. 1991;114(Pt 6):2593–2609. doi: 10.1093/brain/114.6.2593. [DOI] [PubMed] [Google Scholar]
- 34.Miller BL, Cummings JL, Villanueva-Meyer J, et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology. 1991;41:1374–1382. doi: 10.1212/wnl.41.9.1374. [DOI] [PubMed] [Google Scholar]
- 35.Gregory CA, Hodges JR. Clinical features of frontal lobe dementia in comparison to Alzheimer’s disease. J Neural Transm. 1996;47:103–123. doi: 10.1007/978-3-7091-6892-9_6. [DOI] [PubMed] [Google Scholar]
- 36.Rosen HJ, Narvaez JM, Hallam B, et al. The functional impact of frontal lobe dysfunction: a comparison of neuropsychological (MMSE) and functional (CDR) estimates of severity in FTD and AD. 56th annual meeting of the American Academy of Neurology; Honolulu, HI. 2003. [Google Scholar]


