Abstract
Objective Our studies aimed to measure the quality of antibody response to influenza vaccines in the elderly. The frequency of significant rise in hemagglutination inhibition (HAI) titer in the elderly is low and although annual vaccination reduces morbidity and mortality, better correlates of vaccine efficacy in the elderly are needed.
Methods We measured the amount and avidity of serum antibodies against native H3N2 influenza glycoproteins or denatured virus (unfoldons) in pre‐ and post‐vaccinated sera of 36 elderly subjects.
Results Eighty percent of subjects had high pre‐immunization antibody levels and only 13% showed ≥2fold increase after vaccination, but 33% showed ≥2fold increase in avidity. With increasing dosage there was a significant increase in avidity against unfoldons with 50% of subjects showing ≥2fold increase at the highest dose. Elderly subjects given subunit vaccine showed higher reactivity with unfoldons (78% of native) than younger subjects studied earlier who were given inactivated whole virus vaccine (19% of native).
Conclusion The clear inverse relationship between pre‐immunization antibody levels and antibody increase after vaccination implies that a major reason for the low frequency of antibody responses in elderly subjects is simply because they have high pre‐immunization antibody levels. Only low reactivity was observed with earlier viruses. The increased proportion and avidity of antibodies against unfoldons is of concern, as these are not protective, and vaccine developers need to be aware of the role of age or vaccine formulation in inducing anti‐unfoldon antibodies.
Keywords: Elderly, HA inhibition, influenza virus, native and denatured antigen, serum antibodies
Introduction
Influenza vaccine is recommended annually for prevention of influenza in people at high risk of complications and their contacts. Subunit influenza vaccines in the USA contain 15 μg of HA of each of three viruses per dose, but in elderly people this dose often fails to elicit significant antibody rise as measured by hemagglutination inhibition (HAI) titer. 1 Protection against influenza is thought to be correlated with serum antibody level and so an increase in HAI titer is considered a measure of response to vaccine. However, clinical data show a greater benefit of inactivated influenza vaccine to elderly individuals than is apparent from the low increase in HAI titer. 2 , 3 The standard HAI assay has a number of uncertainties, including underestimation of the true amounts of antibody. 4 The HAI test has been reported to be relatively insensitive for H5N1 avian influenza viruses 5 , 6 , 7 and to date there is not a good correlation between HAI, virus neutralization, and protection. 8 de Jong et al. 9 concluded that the HAI test is not ‘a functional test for measuring immunity and suffers from a number of drawbacks.’ The HAI test uses sialic acids on red blood cells as mimics of cellular receptors, and the problem seems to be that red cell sialic acids are not good receptor mimics for some viral strains. The HAI test for anti‐H5 antibodies is more sensitive when horse red cells are used instead of turkey or chicken cells, but the chemical differences in the sialic acids expressed are largely unknown. 10 Recent human H3N2 viruses have shown changes in binding specificity to red blood cells. 11 Both the H5 and H3 examples show that, although the basic sialic acid linkage specificity of NeuAcα2‐3Gal in avian viruses and NeuAcα2‐6Gal in human viruses is usually maintained, finer specificity differences in receptors are not mimicked by red cells and confound attempts to accurately measure neutralizing antibodies by HAI.
To investigate the utility of a more direct method of assessing vaccine response, we developed a sandwich ELISA method to separately measure antibodies against native glycoproteins, which are potentially protective, and antibodies against denatured glycoproteins or internal viral proteins (‘unfoldons’), 12 which are not protective. 13 , 14 , 15 , 16 , 17 , 18 Direct coating of virus onto microtiter plate wells results in virus disruption and denaturing some proportion of surface glycoproteins. Thus, direct virus coating will capture all anti‐influenza antibodies against native, denatured, and internal proteins. We think that specific measurement of antibodies against native glycoproteins (the ‘good’ antibodies) might be a better predictor of vaccine efficacy and/or protection than HAI titers, but as yet there are no data. Comparison of HA inhibiting antibodies with total binding antibodies may be helpful in resolving the lack of correlation between HAI and immune response. Measurements of bulk avidity of antibodies in elderly subjects might also be useful in finding a better correlate of vaccine efficacy.
It has often been noted that antibody response to vaccine in the elderly is considerably lower than in younger adults. 19 Keitel et al. 20 showed that a higher dosage of vaccine given to elderly subjects resulted in significantly higher serum HAI titers and a higher frequency of HAI increase. For our study we obtained pre‐ and post‐vaccination serum from 36 subjects from this trial to segregate and quantitate the antibody response. We determined the relative amounts of antibodies against native versus denatured viral particles, the level of cross‐reactivity with earlier strains and the overall avidity of anti‐influenza antibodies in the serum of each subject.
Materials and methods
Subjects and sera
The serum samples were obtained from a clinical trial at Baylor College of Medicine that was designed to evaluate the effects of increasing dosage levels of inactivated influenza vaccine among elderly subjects. Ambulatory individuals 65 years and older received a single intramuscular injection of the 2001–2002 formulation of trivalent inactivated subvirion influenza vaccine containing 15, 30, or 60 μg of hemagglutinin per strain or placebo. 20 The vaccine components were A/Panama/99 (H3N2), A/NewCaledonia/99 (H1N1), and B/Victoria/2000. Our studies focused only on antibodies against H3N2 viruses in sera of 36 subjects collected before and 1 month after vaccination. These sera were from 10 subjects in each vaccine dosage group, with varied HAI responses, and six subjects from the placebo/control group. The samples were blinded and coded before they left Baylor.
Viruses and cells
Influenza H3N2 viruses included in this study were vaccine strains A/Sydney/5/97, A/Panama/2007/99, and A/Wyoming/03/03 obtained from CDC, and A/Udorn/307/72 obtained from Dr. Ming Luo. The viruses were grown in embryonated chicken eggs and purified by sucrose gradient centrifugation. 21 Hemagglutinin titrations were made at 4°C, using human red blood cells. Viral protein was determined by the Bradford method (Bio‐Rad Protein Assay kit, Bio‐Rad, Hercules, CA, USA).
Preparation of red cell membrane extract
Packed human red cells were washed five times in phosphate‐buffered saline (PBS) and lyzed in cold water for 1 hour at 4°C. The resulting red cell ghosts were pelleted at 26 700 g for 20 minutes. Ghosts were washed three times or until washes were clear, in water with one drop of PBS added each time. The pellet was resuspended in water, aliquoted and stored at −20°C. For coating ELISA plates, aliquots were allowed to thaw and OG (n‐octyl‐β‐D‐glucopyranoside) was added to a concentration of 34 mM. After standing for 10 minutes at room temperature, the solubilized membranes were diluted 1:10 in PBS for coating the plates (∼200 μg protein per well).
ELISA to quantitate antibodies against native glycoproteins
Wells of a microtiter plate were coated with OG‐solubilized red cell membranes and incubated overnight at 4°C. Wells were washed four times with PBS, then virus (∼64 hemagglutinating units (HAU) per 50 μl in CaMg‐saline) was adsorbed to the coated wells overnight at 4°C or 1 hour at RT. After washing four times with PBS, nonspecific binding sites were blocked with 20% calf serum in PBS. The human serum samples were diluted 1:16 or 1:32 in 20% calf serum in PBS, and serial twofold dilutions of this were added to the wells and incubated at room temperature for 1 h. After washing in PBS, alkaline‐phosphatase‐conjugated goat anti‐human polyvalent immunoglobulin (α, γ and μ‐chain specific) secondary antibody (Sigma no. 3313, Sigma‐Aldrich, St. Louis, MO, USA) was bound for an hour, the wells washed free of unbound conjugate and p‐nitrophenyl phosphate substrate (Sigma 104) added. The color was developed at room temperature for 1 hour and the absorbance was read at 405 nm. We titrated all the reagents to maximize the signal to noise ratio of the assay (specific versus nonspecific binding) but we used high concentrations of both red cell membranes and virus to ensure maximum capture.
ELISA to measure antibodies against denatured proteins (unfoldons)
On a duplicate plate, virus was adsorbed to the red cell membrane‐coated wells as above so that the starting amount of virus was the same as for the native protein ELISA. After washing, the wells were treated with methanol for 45 minutes at 37°C and dried before adding serum samples to quantify antibodies against unfoldons. No native protein structures remain after the methanol treatment. The assay was validated for native and denatured states using a monoclonal antibody against native neuraminidase (NA) and an anti‐peptide antibody against denatured NA as described earlier. 13
Pre‐vaccination serum antibodies
The relative levels of pre‐immunization antibodies against Panama/99 and Udorn/72 were measured by adding a single dilution (1:32) of each of the 36 pre‐vaccine sera, in duplicate, to wells containing red cell membrane‐bound virus. Anti‐human secondary antibody was used as described above to quantify the pre‐existing serum antibodies.
Data analysis
Binding curves (absorbance at 405 nm versus μl serum in 50 μl binding mix) were subjected to curve fitting using Kaleidagraph software as described earlier. 13 The equation used for curve fitting is:
where A 405 is the absorbance reading, Amax is the relative concentration of antibody, P0 is the total antibody paratope concentration and Ka(app) is the apparent association constant.
In Kaleidagraph this equation was entered as:
where y = A 405, m 0 = P 0 = μl serum in 50 μl binding mix, m 1 = no serum blank, m 2 = A max and m 3 = 1/K d(app) = K a(app). A max is a relative measure of total antibody concentration and K a(app) gives the overall avidity.
The 72 serum samples (36 pre‐ and post‐immunization) were titrated with four viruses (A/Panama/2007/99, A/Sydney/5/97, A/Wyoming/03/03, and A/Udorn/307/72) and A max and K a(app) were obtained for each serum that showed significant reactivity under native and denatured conditions for each virus. The fold differences between the pre‐ and post‐vaccination sample were calculated for each subject. We also compared single point A 405 values between all subjects for a direct comparison of pre‐immunization antibody levels, native and denatured. The samples from the 36 subjects were processed together and repeated at least once, on a different day, and the results averaged.
Results
Pre‐immunization antibody levels
Individuals had been selected into the vaccine trial regardless of their immunization or influenza infection history, but 82% of participants reported receiving vaccine in the previous year. 20 As might be expected for elderly people who have lived through many influenza epidemics and who have been vaccinated in recent years, we found high pre‐immunization antibody levels (A 405 ≥ 0.4) against native A/Panama/99 in 80% of subjects (Table 1). No subject had this level of antibodies against Udorn/72. The average anti‐Udorn/72 antibody level was 43 ± 25% of the average antibody against Panama/99.
Table 1.
Serum response (as fold difference) in terms of hemagglutination inhibition (HAI) and to native and denatured red cell‐bound virus after vaccination, compared with the corresponding pre‐vaccination sample
| Subject | Dose (μg) | Fold HAI (log2)* Panama/99 | Native | Denatured | ||||
|---|---|---|---|---|---|---|---|---|
| Pre‐vac A 405 | Fold A max Panama/99 | Fold K a Panama/99 | Pre‐vac A 405 | Fold Amax Panama/99 | Fold K a Panama/99 | |||
| D | Placebo | 1 | 0.8 | 1.0 | 0.9 | 0.6 | 1.1 | 0.8 |
| H | Placebo | 1 | 0.4 | 0.9 | 1.1 | 0.4 | 1.0 | 0.8 |
| L | Placebo | 1 | 0.7 | 1.1 | 1.4 | 0.5 | 1.0 | 0.8 |
| P | Placebo | 1.8 | 0.4 | 1.0 | 0.9 | 0.3 | 0.8 | 1.1 |
| T | Placebo | 1 | 0.7 | 1.1 | 1.2 | 0.5 | 1.1 | 1.1 |
| W | Placebo | 1 | 0.4 | 1.0 | 0.8 | 0.4 | 1.1 | 1.2 |
| A | 15 | 1 | 0.8 | 1.0 | 1.2 | 0.8 | 1.2 | 1.0 |
| E | 15 | 1.3 | 0.9 | 1.2 | 1.9 | 0.7 | 1.2 | 2.1 |
| I | 15 | 1.3 | 0.7 | 1.1 | 1.1 | 0.7 | 1.1 | 1.1 |
| M | 15 | 1.5 | 0.4 | 1.5 | 0.6 | 0.3 | 2.0 | 0.5 |
| Q | 15 | 1 | 0.7 | 1.1 | 0.7 | 0.4 | 1.0 | 0.9 |
| U | 15 | 1.5 | 0.5 | 1.1 | 1.2 | 0.5 | 0.8 | 2.5 |
| X | 15 | 1 | 0.4 | 1.1 | 1.4 | 0.3 | 1.2 | 1.4 |
| AA | 15 | 2 | 0.9 | 1.2 | 2.2 | 0.9 | 1.2 | 2.3 |
| DD | 15 | 3.5 | 0.2 | 3.6 | 1.4 | 0.1 | 2.2 | 5.0 |
| GG | 15 | 1.3 | 0.4 | 1.4 | 2.6 | 0.3 | 1.8 | 2.5 |
| B | 30 | 1 | 0.4 | 1.1 | 1.0 | 0.3 | 1.4 | 0.9 |
| F | 30 | 1 | 0.3 | 2.3 | 0.4 | 0.1 | 1.7 | 0.9 |
| J | 30 | 1 | 0.4 | 1.2 | 1.0 | 0.2 | 1.8 | 0.7 |
| N | 30 | 1.5 | 0.6 | 1.2 | 1.5 | 0.6 | 0.8 | 1.9 |
| R | 30 | 1 | 0.7 | 1.0 | 2.1 | 0.6 | 0.9 | 3.6 |
| Y | 30 | 1 | 1.0 | 0.8 | 2.2 | 0.7 | 1.1 | 1.2 |
| BB | 30 | 1.4 | 0.6 | 1.1 | 1.3 | 0.5 | 1.1 | 1.5 |
| EE | 30 | 2.3 | 0.3 | 1.6 | 5.2 | 0.1 | 1.6 | 5.0 |
| HH | 30 | 1.6 | 0.2 | 1.6 | 3.4 | 0.1 | 2.7 | 3.2 |
| II | 30 | 2.3 | 0.3 | 1.5 | 1.1 | 0.2 | 1.4 | 2.2 |
| C | 60 | 0.9 | 0.8 | 1.0 | 1.1 | 0.5 | 1.4 | 0.8 |
| G | 60 | 1 | 0.5 | 1.3 | 2.0 | 0.3 | 1.0 | 2.7 |
| K | 60 | 1 | 0.4 | 1.1 | 1.4 | 0.4 | 1.0 | 1.5 |
| O | 60 | 1.3 | 0.6 | 1.0 | 2.4 | 0.6 | 0.9 | 2.8 |
| S | 60 | 2 | 0.5 | 1.5 | 3.2 | 0.5 | 1.2 | 3.9 |
| V | 60 | 1.3 | 0.6 | 1.3 | 2.9 | 0.4 | 1.0 | 4.0 |
| Z | 60 | 1.4 | 0.7 | 0.9 | 1.1 | 0.5 | 1.0 | 1.6 |
| CC | 60 | 1 | 0.9 | 0.9 | 0.9 | 0.8 | 1.0 | 1.3 |
| FF | 60 | 3 | 0.2 | 5.1 | 0.5 | 0.1 | 5.0 | 2.3 |
| JJ | 60 | 3 | 0.2 | 4.3 | 1.4 | 0.0 | 5.0 | 2.4 |
Pre‐immunization antibody levels against denatured Panama/99 were higher (average 78 ± 23% of native) compared with Udorn/72 (43% ± 34% of Panama/99), suggesting either an increased response toward unfoldons as the subjects aged, or that infection yields higher antibody levels against native proteins than the subunit vaccine. Healthy people were unlikely to have been vaccinated in the 1970s and the anti‐Udorn antibodies seen here likely resulted from infection.
Increase in antibody levels and overall avidity against native glycoproteins after vaccination
Response
Response toward native glycoproteins was classified as ‘good,’‘intermediate’ or ‘none’ according to the increase in A max as shown in Figure 1. A ‘good response’ was defined as more than twofold increase in A max together with A 405 ≥ 0.4 after vaccination, mostly observed when pre‐immunization serum antibody levels were low (Figure 1A). ‘No response’ was defined as low pre‐ and low post‐vaccination levels (Figure 1C), while ‘intermediate response’ was defined by high pre‐ and high post‐vaccination A max, indicating a capacity to respond (Figure 1B). Most of these elderly subjects showed intermediate responses and only 13% of all subjects showed a good response. However, the good response rate was 57% among those subjects with low pre‐immunization antibody levels (A 405 < 0.4) (Table 1).
Figure 1.
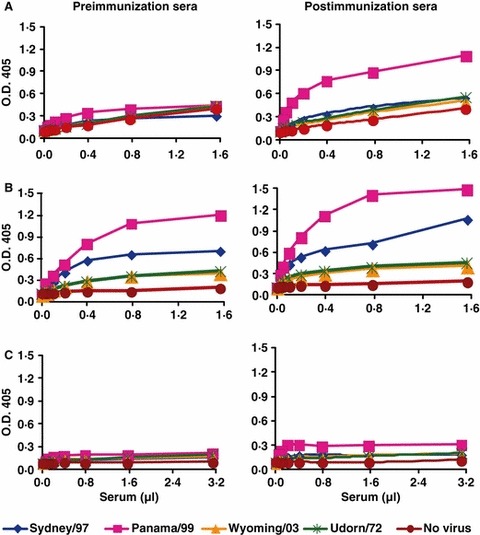
Examples of binding curves showing variable response toward native glycoproteins. The pre‐vaccination and post‐vaccination serum samples from three subjects are shown. In panel (A) the subject has very low pre‐immunization antibody levels and shows a good rise after vaccination. Panel (B) shows a subject with high levels of pre‐immunization antibodies and little increase on vaccination. The subject in panel (C) is a true non‐responder, with a very low level of pre‐immunization antibody and no increase after vaccination.
Rise in antibody levels and avidities
A clear inverse relationship between pre‐immunization antibody levels and fold increase in A max for the vaccinating virus was observed (Figure 2A), as was found in our earlier study. 13 No clear correlation was observed between pre‐immunization levels and increase in overall avidity but the increases in avidity were higher than those in A max (Figure 2B). Results were very similar for anti‐unfoldon antibody levels (Figure 2C,D). Comparing these results to the younger population studied earlier, 13 the average fold increase in anti‐native antibody against the vaccine strain (subunit vaccine) is 1.5‐fold in the elderly averaging over the 15, 30, and 60 μg dosages compared with 2.4‐fold in the younger group after the first vaccination with 15 μg (whole virus vaccine). An increase in K a was sometimes observed in the absence of increased antibody level (Figure 2A,B). Subjects whose pre‐immunization antibody levels were ≥0.4 absorbance units averaged 1.1‐fold increase in A max but a significantly higher 1.6‐fold increase in K a (P = 0.003).
Figure 2.
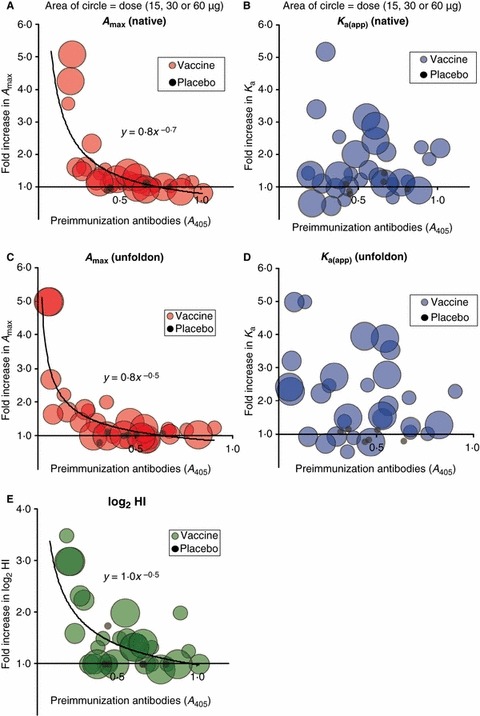
Relationships between pre‐immunization antibody levels, fold increase in antibodies, and vaccine dose. Panel (A) shows the fold increase in A max (post‐/pre‐immunization) determined by fitting the binding curve, plotted against initial antibody level (A 405) obtained by running all samples together for comparison, where the diameter of the circles reflects the dose of vaccine. Panel (B) shows fold increase in K a(app) plotted against pre‐immunization levels. In panels (C) and (D), the fold increase A max and K a of antibodies against denatured proteins are plotted against the pre‐immunization levels of anti‐unfoldon antibodies. Panel (E) shows the fold increase in hemagglutination inhibition determined previously 20 plotted against the pre‐immunization A 405 determined in this study.
Antibody levels and apparent avidity against unfoldons
Elderly subjects exhibited higher pre‐immunization serum antibody levels against denatured determinants than were observed in younger subjects. 13 The amount of anti‐unfoldon antibody in elderly subjects given subunit vaccine (78 ± 23% of anti‐native antibody for Panama/99) was almost four times that of the younger population given inactivated whole virus (19 ± 10%) but only slightly increased over younger subjects given subunit vaccine in a separate study (64%; J‐Q. Feng and G. M. Air, unpublished results). Antibodies against unfoldons increased an average 1.9‐fold for Panama/99 in the elderly group after vaccination (Figure 2C). As with antibodies against native proteins, avidity increase was higher (1.9‐fold) than increase in A max (1.1‐fold, P = 0.004) for those subjects with pre‐immunization levels of antibody ≥0.4 absorbance units (Figure 2D). Younger subjects did not show any significant increase in antibodies against denatured virus, even after repeated vaccination with inactivated whole virus vaccine 13 or when given subunit vaccine.
Cross‐reactivity
Various cross‐reactivity patterns with earlier and later viruses were observed in pre‐immunization sera. In most cases the reactivity with Sydney/97 mirrored that with Panama/99. There was no significant reactivity of pre‐immunization sera with the later strain Wyoming/03. Reactivity with native Udorn/72 averaged 43% of the antibody level against native Panama/99. An increase in antibodies against Panama/99, the vaccine strain, was often accompanied by an increase against Sydney/97, but there was almost no increase in antibodies against Wyoming/03, a later strain. Ten percent of subjects showed a twofold increase in antibodies against Udorn/72 after vaccination with Panama/99, but in these the antibody level remained low, resulting in large errors in the fold increase, and there was a much greater increase in antibodies against Panama/99. We interpret this as no significant increase in antibodies against Udorn/72.
Dose response
Increases in average A max and K a(app) of anti‐native antibodies were observed with increasing dosage, but these were not significant (P = 0.46 and 0.48, respectively) because so few subjects showed any increase. Both A max and avidity increased with increasing dosage more against unfoldons than against native proteins (Figure 3) although again not statistically significant (P = 0.48 and 0.16, respectively, comparing 15 μg with 60 μg dosage). There was an increase in avidity of antibodies against unfoldons (P = 0.10) at the highest dosage (60 μg) when compared with the regular 15 μg dosage level; for A max the difference was not significant (P = 0.25).
Figure 3.
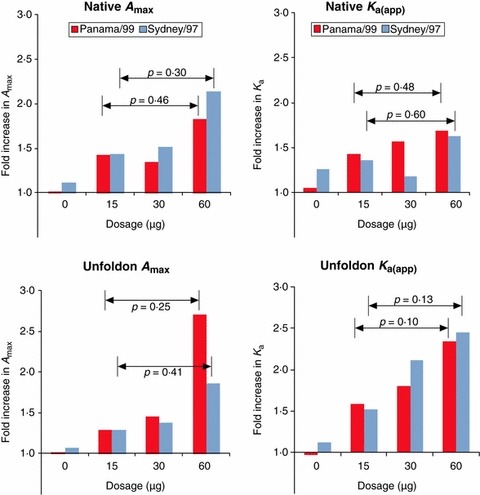
Effect of increasing dosage of vaccine on A max and K a(app) of antibodies against Panama/99 and Sydney/97. The average fold increase in anti‐native and anti‐unfoldon serum antibody levels and avidity after vaccination with increasing dosages of vaccine is compared. The P‐values from the Student’s t‐test comparing the 15 μg dosage with the highest dosage (60 μg) are shown for each virus. There are increasing trends with dosage for each parameter, but only the avidity of unfoldon antibodies significantly increases with dosage. Each vaccine dosage group has 10 subjects, and the control group has six subjects.
Discussion
Rationale and standardization
The sandwich ELISA method was developed using fetuin as a capturing agent, 13 but recent H3N2 influenza viruses have changed receptor specificity and were not retained on fetuin. Recent viruses bind tightly to human red blood cells and we first designed a triple‐layer ELISA in which lectin from Sambucus nigra is bound to the wells and used to capture glycoconjugates containing α2‐6 sialic acid linkages from a detergent extract of human red blood cell ghosts. Virus particles then bind to the exposed α2‐6 sialic acids. Unexpectedly, we found strong nonspecific binding between virus and lectin, which was not reduced after neuraminidase treatment of virus, so instead we coated the plates directly with the red cell membrane extract and this efficiently captured virus in the native state. Then each serum sample was titrated to obtain a binding curve to distinguish the effects of increased avidity from the effects of increased antibody levels. We wanted to compare the cross‐reactivity of serum antibodies against newer and older H3N2 viruses; however, they have differing receptor specificities resulting in different affinities for red cell membranes. 11 This might lead to different amounts of virus being captured. We coated with ∼64 HAU per well to ensure that all viruses were maximally bound.
Pre‐vaccinated serum antibody level determines antibody rise against native antigen after vaccination
Our earlier study showed a clear inverse relationship between pre‐immunization antibody levels and increase after vaccination. 13 This study of elderly subjects also shows this inverse correlation, but with 80% subjects exhibiting high pre‐immunization levels against native proteins, far more than in the younger population studied earlier. 13 The persistence of vaccine‐ or infection‐induced antibody is consistent with other reports. 2 , 22 , 23
Thus, the well‐known lack of antibody rise after vaccination of elderly subjects, with no statistically significant differences between elderly vaccinees and controls (placebo), 19 , 24 is largely due to the high pre‐immunization antibody levels in elderly subjects. In our study, only 13% subjects showed more than twofold increase in A max, and all of these had low pre‐existing antibody levels (Figure 2A).
Conformation dependence: native versus denatured antigens
As neutralizing antibodies against influenza are conformation‐dependent, 15 the ratio of antibody reactivity to native versus denatured viral antigens should reflect the protective potential of antibodies. The elderly population studied here exhibited approximately four times higher antibodies against denatured antigens than the younger group 13 and showed more increase in avidity than in amount after vaccination. Younger subjects given subunit vaccine had approximately three times higher antibodies against unfoldons than a similar group given whole virus vaccine, indicating that subunit vaccine induces more antibodies against unfoldons than whole virus vaccine. A high dosage of vaccine increased the avidity of antibodies against unfoldons more than those against native proteins in the elderly (Figure 3). The higher fold increase in antibodies against unfoldons in elderly subjects compared with the younger group given a similar subunit vaccine suggests that the elderly have an enhanced response to denatured protein. This might reflect a memory response to conserved linear epitopes, as conformational epitopes on HA and NA are variable and so would be expected to show less memory response. However, the subjects showed little cross‐reactivity to denatured epitopes on Udorn/72, despite the sequence conservation. Therefore, it seems that there might be more processing of antigen in elderly people, leading to a biased response toward unfoldons that is specific to the vaccine and not due to recall.
Comparison with HAI
The HAI responses in this vaccine trial were reported earlier and the conclusion was that higher pre‐immunization antibody levels in elderly subjects lead to a higher seroprotection rate and lower seroconversion rate. Overall 80–90% of subjects achieved protective antibody levels against the H3N2 virus. 20 A review by Goodwin et al. 19 showed that for the H3 antigen, the young had a 62% seroconversion rate and 84% seroprotection rate (the percentage of subjects with HAI antibody titer ≥40 after vaccination), while institutionalized elderly had a 65% seroconversion rate and 80% seroprotection rate. In our subset of 36 subjects, 53% were seroprotected before vaccination (titers ≥32 HAU) and 44% subjects seroconverted after vaccination as indicated by HAI.
As in our previous study, 13 we did not see a direct correlation between fold increase in HAI titers and fold increase in serum antibody level or avidity. Among the elderly subjects, 13% had ≥2fold increase in A max while 33% showed ≥2fold increase in avidity. The increase in HAI (23% of our subset of subjects had ≥2fold increase in log2 HAI titer, Figure 2E) therefore appears to be derived from a combination of increase in antibody amount and increase in avidity. Subjects with the highest increase in HAI titers had the lowest pre‐immunization antibody levels, in accord with the increase in antibody amount (Figure 2).
Cross‐reactivity and original antigenic sin
‘Original antigenic sin’ is the concept that the level of antibody which is specific to the first influenza strain experienced continues to rise throughout life through boosts from each subsequent infection or vaccination with viruses of the same subtype. 25 We reasoned that by 1972 most people would have been exposed to an H3N2 virus and therefore we used Udorn/72 to represent the early H3 strains rather than using the 1968 strain itself. There is a high degree of cross‐reactivity between Aichi/68 and Udorn/72, while there was much less between these and the next drift strain, Victoria/3/75 (data from CDC). In our studies we saw increases in antibodies against Panama/99 and the closely‐related Sydney/97 but only marginal increase, and in only 10% of subjects, against Udorn/72. We also observed higher pre‐immunization antibody levels against Panama/99 and Sydney/97, compared with Udorn/72, in these elderly subjects, indicating recent vaccination history or influenza exposure. Thus, our data suggest that the serum antibodies are specific, cross‐reactive only to closely related strains, with no sign of original antigenic sin. Brokstad et al. 26 observed a stronger immune response (in terms of HAI) to previous H3N2 strains in young healthy subjects than against vaccine virus, which they thought was a recall of H3N2 priming to common antigenic determinants. 27 These antibodies were non‐neutralizing. We did not observe this phenomenon in our study, again emphasizing the difference in assay system. Haaheim has discussed evidence that antibodies generated under the mechanism of original antigenic sin may not have any major epidemiological relevance 28 and perhaps the HAI test over‐emphasizes these.
Dose response
There have been several studies to determine whether increasing the dosage can increase the effectiveness of vaccination in the elderly. 1 , 20 , 29 Palache et al. 30 reported that higher vaccine dosages (20 and 60 μg) resulted in significant antibody response against influenza B but not against A/Taiwan/1/86 (H1N1) or A/Sichuan/2/87 (H3N2). Goodwin et al. 19 concluded from other studies that there is no indication that increasing the dosage of antigen increases the response to the vaccine in the elderly, but the number of subjects was low. Increased dosage was reported to be more effective in case of H5 vaccine 31 and for elderly subjects given baculovirus‐expressed trivalent HA vaccine. 32
The samples we are using were from the study by Keitel et al., 20 who found that increasing dosages of vaccine (0, 15, 30, 60 μg) in elderly subjects increased the geometric mean serum HAI titers against H3N2 virus to 43, 86, 91, and 125, respectively. Frequencies of antibody responses nearly doubled after the 60 μg dosage and the percentage of participants showing a fourfold increase in HAI increased with dosage, although only 10% of subjects had low initial HAI titers and showed a significant increase.
Our measurement of antibodies showed an increase in level and/or avidity of antibodies against native Panama/99 as the dosage of vaccine increased, but this was accompanied by a higher proportion of antibodies with higher avidities against unfoldons (Figure 3).
Conclusions
Subjects with low pre‐immunization levels showed a significant increase in serum antibody levels after vaccination, while the 80% of subjects with high pre‐immunization antibody levels showed little increase in amount but significantly greater increase in avidity (Figure 2). There was a significant trend toward an increased proportion of antibodies to denatured antigens with increasing dosage of vaccine (Figure 3), especially in avidity, even though there were only 10 subjects given each dose. In the context of a vaccine, antibodies against unfoldons represent wasted resources and vaccines that need to be given at high dosages may need to be adjuvanted in a way that minimizes denaturation or degradation. An additional purification step could also be added during vaccine manufacture to remove denatured antigen.
The sandwich ELISA method segregates the response in terms of antibodies against native glycoproteins versus unfoldons. The inverse correlation between pre‐immunization serum antibody levels and fold increase in A max is maintained in the elderly, the relationship approximating y = x −0.5 where y is fold increase in A max and x is pre‐immunization antibody level.
The subjects in this study were not followed to assess protection, but we are participating in an ongoing study to determine if our direct measurement of antibodies against native glycoproteins versus unfoldons is a better correlate of protection than HAI or neutralizing antibody titers.
Author contribution
All authors contributed to the design or data analysis of the study, the writing of the article, and approval of the final version.
Conflict of interest
WAK has received funding for research from Protein Sciences, Inc.
Acknowledgements
This study was supported in part by grant R01‐AI50933 from the National Institute of Allergy and Infectious Diseases. The vaccine trial was supported by contracts N01‐AI65298 and N01‐AI25465 from NIAID. We thank Shelly Gulati and Helga Veeraprame for growth and purification of viruses. Informed consent was obtained from the subjects at the start of the vaccine trial according to protocols approved by Baylor College of Medicine and NIAID review boards. Transfer of the samples to Oklahoma and the analyses described here were approved by the Institutional Review Boards of Baylor College of Medicine and University of Oklahoma Health Sciences Center according to guidelines from both institutions and the US Department of Health and Human Services.
References
- 1. Arden NH, Patriarca PA, Lui KJ et al. Safety and immunogenicity of a 45‐microgram supplemental dose of inactivated split‐virus influenza B vaccine in the elderly. J Infect Dis 1986;153:805–806. [DOI] [PubMed] [Google Scholar]
- 2. Beyer WE, Palache AM, Sprenger MJ et al. Effects of repeated annual influenza vaccination on vaccine sero‐response in young and elderly adults. Vaccine 1996;14:1331–1339. [DOI] [PubMed] [Google Scholar]
- 3. Jefferson T, Rivetti D, Rivetti A et al. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005;366:1165–1174. [DOI] [PubMed] [Google Scholar]
- 4. Nauta JJ, De Bruijn IA. On the bias in HI titers and how to reduce it. Vaccine 2006;24:6645–6646. [DOI] [PubMed] [Google Scholar]
- 5. Nicholson KG, Colegate AE, Podda A et al. Safety and antigenicity of non‐adjuvanted and MF59‐adjuvanted influenza A/Duck/Singapore/97 (H5 N3) vaccine: a randomised trial of two potential vaccines against H5 N1 influenza. Lancet 2001;357:1937–1943. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz B, Gellin B. Vaccination strategies for an influenza pandemic. J Infect Dis 2005;191:1207–1209. [DOI] [PubMed] [Google Scholar]
- 7. Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti‐H5 responses in human sera by HI using horse erythrocytes following MF59‐adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res 2004;103:91–95. [DOI] [PubMed] [Google Scholar]
- 8. Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross‐protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5 N1) virus in ferrets. J Infect Dis 2006;194:1040–1043. [DOI] [PubMed] [Google Scholar]
- 9. De Jong JC, Palache AM, Beyer WE et al. Haemagglutination‐inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 10. Harvey R, Martin AC, Zambon M, Barclay WS. Restrictions to the adaptation of influenza a virus h5 hemagglutinin to the human host. J Virol 2004;78:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gulati U, Wu W, Gulati S et al. Mismatched hemagglutinin and neuraminidase specificities in recent human H3 N2 influenza viruses. Virology 2005;339:12–20. [DOI] [PubMed] [Google Scholar]
- 12. Laver WG, Air GM, Webster RG, Smith‐Gill SJ. Epitopes on protein antigens: misconceptions and realities. Cell 1990;61:553–556. [DOI] [PubMed] [Google Scholar]
- 13. Gulati U, Kumari K, Wu W, Keitel WA, Air GM. Amount and avidity of serum antibodies against native glycoproteins and denatured virus after repeated influenza whole‐virus vaccination. Vaccine 2005;23:1414–1425. [DOI] [PubMed] [Google Scholar]
- 14. Haigwood NL, Nara PL, Brooks E et al. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad‐spectrum neutralizing antibodies in baboons. J Virol 1992;66:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knossow M, Gaudier M, Douglas A et al. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology 2002;302:294–298. [DOI] [PubMed] [Google Scholar]
- 16. Nestorowicz A, Tregear GW, Southwell CN et al. Antibodies elicited by influenza virus hemagglutinin fail to bind to synthetic peptides representing putative antigenic sites. Mol Immunol 1985;22:145–154. [DOI] [PubMed] [Google Scholar]
- 17. Van Regenmortel MH. The conformational specificity of viral epitopes. FEMS Microbiol Lett 1992;79:483–487. [DOI] [PubMed] [Google Scholar]
- 18. Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol 1990;8:737–771. [DOI] [PubMed] [Google Scholar]
- 19. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24:1159–1169. [DOI] [PubMed] [Google Scholar]
- 20. Keitel WA, Atmar RL, Cate TR et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006;166:1121–1127. [DOI] [PubMed] [Google Scholar]
- 21. Laver WG. Purification of influenza virus; in Habel K, Salzman NP. (eds): Fundamental Techniques in Virology. New York: Academic Press, 1969. [Google Scholar]
- 22. Hirota Y, Kaji M, Ide S, Goto S, Oka T. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine 1996;14:1597–1602. [DOI] [PubMed] [Google Scholar]
- 23. Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997;15:1114–1122. [DOI] [PubMed] [Google Scholar]
- 24. De Bruijn IA, Remarque EJ, Beyer WE et al. Annually repeated influenza vaccination improves humoral responses to several influenza virus strains in healthy elderly. Vaccine 1997;15:1323–1329. [DOI] [PubMed] [Google Scholar]
- 25. Francis T Jr, Davenport FM, Hennessy AV. A serological recapitulation of human infection with different strains of influenza virus. Trans Assoc Am Physicians 1953;66:231–239. [PubMed] [Google Scholar]
- 26. Brokstad KA, Cox RJ, Major D, Wood JM, Haaheim LR. Cross‐reaction but no avidity change of the serum antibody response after influenza vaccination. Vaccine 1995;13:1522–1528. [DOI] [PubMed] [Google Scholar]
- 27. Kuo YC, Oxford JS, Schild GC. Immunological studies with the HA1 and HA2 polypeptides of influenza A virus haemagglutinin. Exp Cell Biol 1978;46:338–354. [DOI] [PubMed] [Google Scholar]
- 28. Haaheim LR. Original antigenic sin. A confounding issue? Dev Biol (Basel) 2003;115:49–53. [PubMed] [Google Scholar]
- 29. Palache AM, Beyer WE, Luchters G et al. Influenza vaccines: the effect of vaccine dose on antibody response in primed populations during the ongoing interpandemic period. A review of the literature. Vaccine 1993;11:892–908. [DOI] [PubMed] [Google Scholar]
- 30. Palache AM, Beyer WE, Sprenger MJ et al. Antibody response after influenza immunization with various vaccine doses: a double‐blind, placebo‐controlled, multi‐centre, dose‐response study in elderly nursing‐home residents and young volunteers. Vaccine 1993;11:3–9. [DOI] [PubMed] [Google Scholar]
- 31. Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006;354:1343–1351. [DOI] [PubMed] [Google Scholar]
- 32. Treanor JJ, Schiff GM, Couch RB et al. Dose‐related safety and immunogenicity of a trivalent baculovirus‐expressed influenza‐virus hemagglutinin vaccine in elderly adults. J Infect Dis 2006;193:1223–1228. [DOI] [PubMed] [Google Scholar]


