Abstract
Objective
To examine the effects of human immunodeficiency virus (HIV) on central nervous system (CNS) function in patients receiving antiretroviral therapy (ART) who have suppressed viral loads.
Methods
Event-related brain potentials (ERPs) were recorded from 15 virally suppressed HIV patients and 15 age-, sex-, and education-matched controls while they performed a 3-stimulus auditory oddball task. The amplitude and latency of the P3a, P3b, and early auditory components were examined in HIV patients and controls.
Results
Virally suppressed HIV patients on ART were more depressed than controls, as determined by the Beck Depression Inventory (BDI). After controlling for the effects of depression, HIV patients had smaller P2, P3a, and P3b amplitudes and longer P3a and P3b latency than control subjects. BDI scores correlated positively with N1 latency in HIV patients and negatively with P3b amplitude in all subjects.
Conclusions
These electrophysiological results suggest that, even in the absence of detectable levels of HIV in the peripheral blood, viral replication persists in the CNS and continues to cause disease in HIV patients on ART.
Keywords: Human immunodeficiency virus, P3a, P3b, Viral load, Antiretroviral therapy
1. Introduction
Human immunodeficiency virus (HIV) can enter the central nervous system (CNS) early after the initial seroconversion reaction. Prior to the introduction of antiretroviral therapy (ART), numerous studies reported CNS damage and cognitive decline in patients infected with HIV (Becker et al., 1997; Bornstein et al., 1993; Grant et al., 1987; Heaton et al., 1995; Levy et al., 1987; Miller et al., 1990; Navia and Price, 1986; White et al., 1995; Wilkie et al., 1990). Several event-related brain potential (ERP) studies have documented significant HIV-related reductions in the P300 (or P3b) (Ollo et al., 1991; Polich et al., 2000; Schroeder et al., 1994). Because the P3b is thought to reflect neural activity related to attention and working memory processes (Donchin et al., 1986; Johnson, 1988; Polich, 1998), these results substantiated HIV’s impact on the CNS and cognition.
The advent ART has dramatically reduced the incidence and severity of opportunistic infections associated with HIV. However, relatively few ERP studies have reexamined the effects of HIV on CNS function since the widespread use of ART. One longitudinal study compared P3b latency in HIV patients receiving zidovudine mono-therapy with HIV patients not taking any medication. After 2 years P3b latency decreased significantly in HIV patients on treatment while it increased significantly in untreated patients (Evers et al., 1998). Recently, this group has extended these findings to HIV patients receiving highly active antiretroviral therapy (HAART) in a 1 year prospective study (Husstedt et al., 2002). In a cross-sectional study of HIV patients on antiretroviral medication, Polich and Basho (2002) reported that patients had smaller P3a but not P3b components than control subjects. The authors interpreted these results as suggesting that the P3a, an ERP component believed to index rapid, involuntary attentional shift towards an unexpected, task-irrelevant stimulus (Courchesne et al., 1975), may be more sensitive than the P3b to HIV’s effects on CNS function in the era of ART.
Most currently available antiretroviral regimens have low levels of blood–brain barrier penetration. Therefore, the CNS may be a ‘sanctuary’ for HIV (Bukrinsky et al., 1991; Schrager and D’Souza, 1998). Moreover, there is evidence that viral replication can persist in the CNS and continue to cause disease even in the absence of detectable levels of HIV in the peripheral blood (Chun et al., 2000; Schrager and D’Souza, 1998). Therefore, the goal of the current study is to use ERPs to examine CNS function in HIV patients on ART who have undetectable plasma viral loads. We tested the following hypotheses: (1) virally suppressed HIV patients on ART have reduced P3a amplitude; (2) comparable P3b amplitude; and (3) comparable P3a and P3b latencies relative to seronegative control subjects.
2. Materials and methods
2.1. Subjects
Table 1 summarizes the clinical and demographic characteristics of the study samples. Fifteen HIV-positive patients and 15 age-, sex-, and education-matched, ELISA-verified seronegative control subjects were studied. All HIV patients had been taking antiretroviral medication for at least 3 months prior to participating in the study and had been seropositive for an average of 12.9 ± 4.9 years. Specific antiretroviral medications varied; however, all patients were taking drug regimens that included a protease inhibitor plus two nucleoside analog reverse transcriptase inhibitors or a protease inhibitor plus a nucleoside analog reverse transcriptase inhibitor plus a non-nucleoside reverse transcriptase inhibitor. None of the HIV patients had a current severe acute illness or detectable levels of plasma viral loads (less than 50 copies/ml). All subjects were screened to exclude those with a history of psychiatric and neurological disorders and substance dependence. All subjects gave written informed consent approved by the Institutional Review Boards of the San Francisco Department of Veterans Affairs Medical Center and the University of California, San Francisco. All subjects received monetary compensation for their participation.
Table 1.
Demographic and clinical characteristics of control and HIV groups
| Control (n = 15)
|
HIV (n = 15)
|
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 44.1 | 8.0 | 28–54 | 44.5 | 7.6 | 25–54 |
| Education (years) | 15.6 | 1.8 | 13–18 | 15.1 | 1.7 | 12–18 |
| Time since seroconversion (years) | – | – | – | 12.9 | 4.9 | 4–19 |
| Beck depression inventory* | 8.2 | 4.5 | 2–16 | 12.7 | 6.4 | 4–26 |
| CD4 (cells/mm3)** | 834.3 | 206.4 | 539–1194 | 440.5 | 202.8 | 204–1008 |
| Viral load (copies/ml) | – | – | – | < 50 | – | – |
Controls vs. HIV:
P < 0.05,
P < 0.0001.
2.2. Procedure
The experiments were conduced in a sound attenuated room. Binaural audiometric thresholds were determined at 1000 Hz for each subject using a method of limits procedure. Auditory stimuli were subsequently presented binaurally through stereo headphones 55 dB above each subject’s audiometric threshold.
Computer-generated tones (52 ms in duration; 5 ms onset and offset envelopes) served as the target and standard stimuli for the auditory oddball task. The standard stimulus was a 1000 Hz tone occurring with 70% frequency. The target was a 2000 Hz tone occurring with 15% frequency. Sounds digitized from a sound effects tape served as the infrequent non-target stimuli. These occurred with 15% frequency. The inter-stimulus interval varied randomly from 1.8 to 2.0 s.
Throughout the experiment, subjects looked at a centrally displayed fixation point on a computer monitor approximately 1 m from their eyes. Subjects were instructed to press a button with the index finger of their dominant hand as quickly as possible whenever they heard the target stimulus (i.e. 2000 Hz tone). Before the experiment, subjects were trained on 15 trial runs to ensure accurate identification of the target stimuli. Subsequently, all subjects received 250 trials.
Electroencephalographic (EEG) activity was recorded using an Electro-Cap with 64 tin electrodes placed according to the extended 10–20 International System. Sixty-four scalp EEG channels and 4 electro-occulographic electrodes, placed at the outer canthus and supraorbitally to the left eye, were referenced to linked earlobes. The impedance for all sites was kept below 10 kΩ or less. The EEG was amplified (bandpass 0.1–100 Hz) and digitized (250 Hz/channel) continuously with a Neuroscan acquisition system, together with condition codes at the onset of each stimulus. Subsequently, peak amplitudes of the components of interest were measured relative to a 200 ms pre-stimulus baseline.
Trials contaminated by eye movements, excessive peak-to-peak deflections (greater than 90 μV), or amplifier blocking were rejected automatically from the averaged data. Trials with ‘correctable’ blinks (i.e. uncontaminated by other artifacts) were corrected by means of a regression analysis in combination with artifact averaging method described by Semlitsch et al. (1986) and included in the relevant averages. Because data from many frontal and temporal electrodes were contaminated by excessive muscle activity and/or ‘uncorrectable’ eye blinks, we selected data from 28 electrodes (AF3, AF4, F3, Fz, F4, FC5, FC1, FCz, FC2, FC6, T3, C3, Cz, C4, T4, CP5, CP1, CPz, CP2, CP6, T5, P3, Pz, P4, T6, O1, POz, O2) that provided representative coverage of the brain and have commonly been reported in other ERP studies for further analyses. ERP averages for each trial with a valid response were computed for individual subjects. Group averages were also computed for controls and HIV patients. The averaging epoch started 200 ms before stimulus onset and ended 760 ms after stimulus presentation.
2.3. Statistical analysis
Average ERPs were analyzed with a semiautomatic peak detection program. P1, N1, and P2 components were identified from ERPs to standard stimuli and defined as the largest positive or the largest negative peak in the intervals 30–60, 75–150, and 150–270 ms post-stimulus onset, respectively. The P3a was identified from ERPs to infrequent non-target stimuli and defined as the largest positive peak 270–400 ms post-stimulus onset. The P3b was identified from ERPs to the target stimuli and defined as the largest positive peak 325–550 ms post-stimulus onset. The time windows were determined by inspecting individual averages and group superaverages. The peak amplitudes (μV) were measured relative to a 200 ms pre-stimulus baseline and the peak latencies (ms) were measured from the time of stimulus onset.
In light of the finding that depressive disorders may be more common among people with HIV disease (Komiti et al., 2003; Valente, 2003), we assessed depressive symptomatology with the Beck Depression Inventory (BDI; Beck, 1967; Beck et al., 1961). Initial analyses revealed significant group differences in BDI scores. Because previous studies have reported reduced P300 amplitudes in depressed patients (Blackburn et al., 1990; Bruder et al., 1991; Gangadharar et al., 1993; Hasenne et al., 1996; Urretavizcaya et al., 2003), we used BDI scores as a covariate in analyses of the ERP and behavioral data. We also correlated ERP and behavioral results with BDI scores using Spearman’s rank correlational analyses.
The amplitudes and latencies of the P1, N1, and P2 components were subjected to repeated measures analysis of covariance (ANCOVA) with BDI scores as covariate, group as the between-subject factor, and midline electrodes (Fz, FCz, Cz, CPz, Pz, POz) as the within-subject factor. The amplitudes and latencies of the P3a and P3b were organized into 5 regional groupings: frontal (AF3, AF4, F3, Fz, F4), central (FC1, FC2, FCz, FC5, FC6, C3, Cz, C4), parietal (CP1, CPz, CP2, P3, Pz, P4), temporal (CP5, CP6, T3, T4, T5, T6), and occipital (POz, O1, O2). These were then subjected to repeated measures ANCOVA with BDI scores as covariate, group as a between-subject factor, and electrode as a within-subject factor. For all effects with two or more degrees of freedom in the numerator, the Greenhouse–Geisser correction was applied. The corrected P values and uncorrected degrees of freedom are reported. Accuracy, false alarms, and reaction time for correct responses on the oddball task were analyzed with MANCOVAs, again with BDI scores as a covariate. Significance was set at P < 0.05.
3. Results
3.1. Behavior
Table 2 summarizes the behavioral data for the auditory oddball task. There were no significant differences in response time (P = 0.81), percent error (P = 0.28) or percent false positives (P = 0.26 for standard and P = 0.20 non-target stimuli) between patients and controls.
Table 2.
Behavioral performance on auditory oddball task
| Control | HIV | |
|---|---|---|
| Response time to target stimuli (ms) | 463.3 (59.0) | 446.1 (89.8) |
| Error rate (%) | 0.9 (1.6) | 2.0 (3.2) |
| False positives (%) | ||
| Standard | 0.2 (0.3) | 0.5 (0.7) |
| Non-target | 1.0 (2.1) | 1.1 (1.7) |
Values are mean (sd)
3.2. Electrophysiology
3.2.1. Standard stimuli
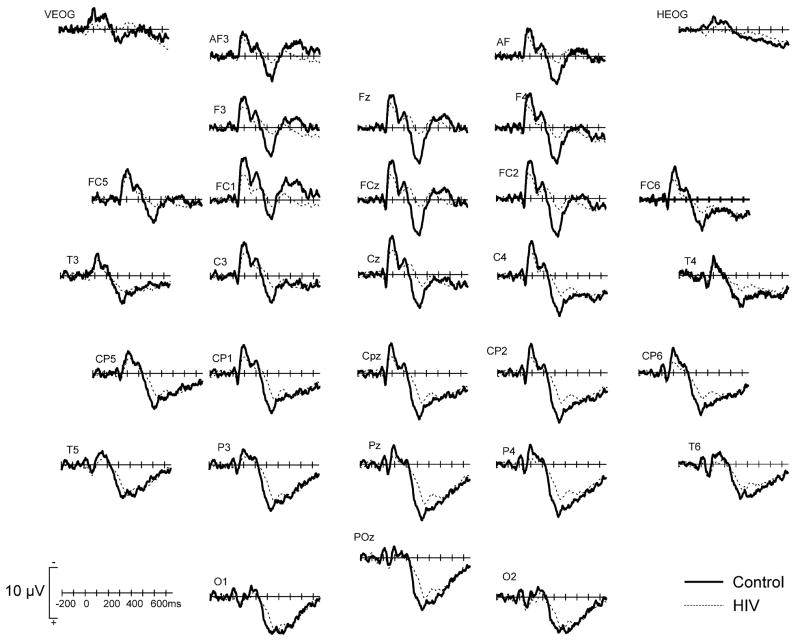
Grand averaged waveforms of the ERPs elicited by standard stimuli are shown at 6 midline electrodes in Fig. 1. Prominent P1, N1, and P2 components characterized these waveforms. The mean (and SD) amplitude and latency these components, averaged across 6 midline electrodes (Fz, FCz, Cz, CPz, Pz, POz), are presented in Table 3. Both groups generated P1 components of comparable amplitude (P > 0.6) and latency (P = 0.7). There was no significant group effect for N1 amplitude (P > 0.2) or latency (P > 0.6). However, there was a significant main effect of electrode (F5,135 = 16.25, P < 0.0001) due to the fact that the N1 was maximal at Cz and smaller at posterior sites. P2 amplitude was significantly larger in controls than HIV patients (group effect: F1,27 = 4.56, P = 0.04) but there was no significant group effect for P2 latency.
Fig. 1.
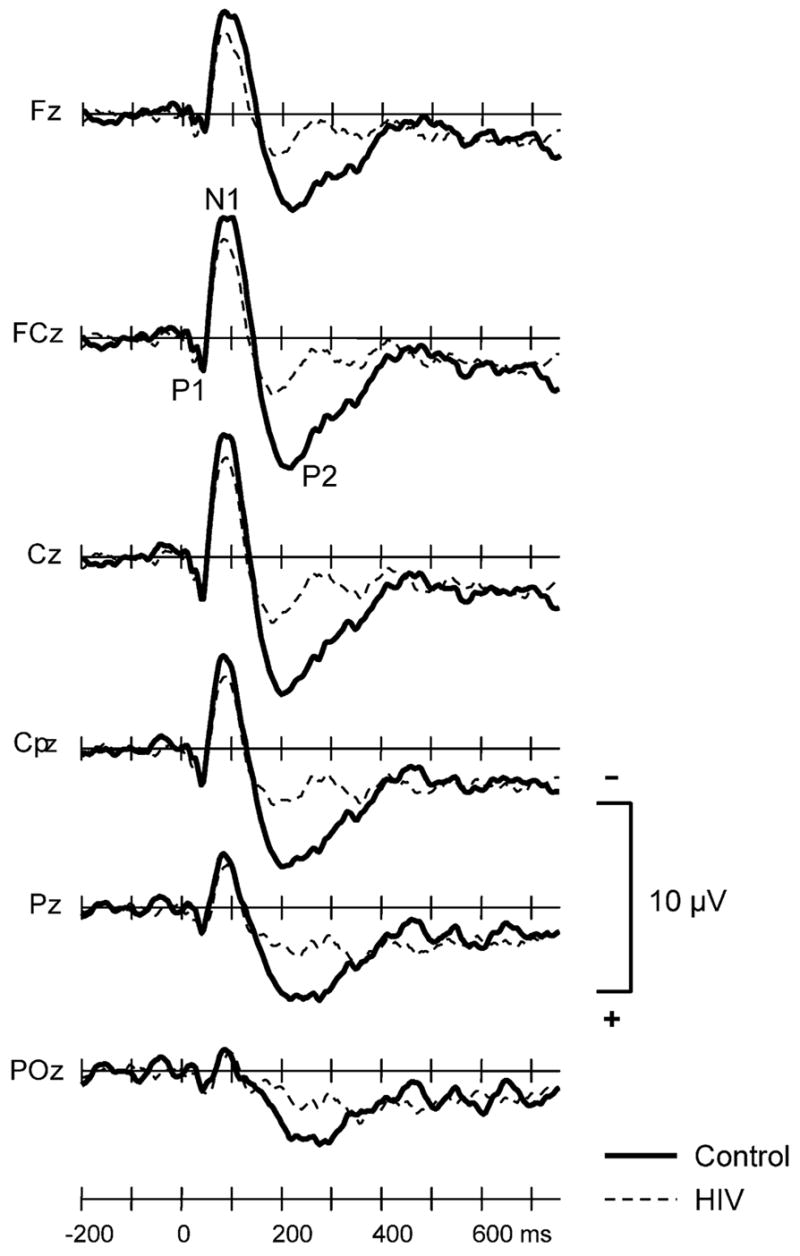
Grand averaged ERPs to the standard stimuli for controls (thick solid line) and HIV + patients (thin dashed line) at 6 midline electrodes.
Table 3.
Mean (SD) amplitude and latency of P1, N1, and P2 components for control and HIV
| Control | HIV | P-valuea | |
|---|---|---|---|
| P1 | |||
| Amplitude (mV) | 2.23 (3.22) | 2.31 (1.68) | NS |
| Latency (ms) | 44.4 (7.5) | 43.0 (7.2) | NS |
| N1 | |||
| Amplitude (mV) | −6.50 (4.02) | −4.43 (2.8) | NS |
| Latency (ms) | 90.9 (13.8) | 94.0 (11.3) | NS |
| P2 | |||
| Amplitude (mV) | 6.31 (3.52) | 3.57 (2.95) | 0.04 |
| Latency (ms) | 205.2 (13.7) | 197.9 (15.1) | NS |
Mean amplitude averaged across 6 midline electrodes.
With BDI as covariate.
3.2.2. Infrequent non-target stimuli
The mean (and SD) amplitude and latency of the P3a and P3b components at 6 midline electrodes are presented in Table 4. Results of the repeated measures ANCOVA for the P3a and P3b are summarized in Table 5. Grand averaged waveforms of ERPs elicited by the infrequent, non-target stimuli at 28 electrode sites are presented in Fig. 2.
Table 4.
Mean (SD) P3a and P3b amplitude (μV) and latency (ms) for control and HIV
| P3a amplitude
|
P3a latency
|
P3b amplitude
|
P3b latency
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | HIV | P† | Control | HIV | P† | Control | HIV | P† | Control | HIV | P† | |
| Fz | 13.76 (4.94) | 6.31 (4.76) | <0.01 | 311.2 (36.1) | 320.5 (22.9) | NS | 9.49 (4.21) | 3.21 (3.99) | <0.01 | 330.1 (28.4) | 355.2 (49.7) | NS |
| FCz | 16.03 (6.14) | 6.86 (5.38) | <0.01 | 308.5 (35.7) | 325.1 (20.3) | NS | 10.18 (4.95) | 3.41 (4.70) | <0.01 | 332.8 (32.1) | 357.9 (50.8) | NS |
| Cz | 16.11 (6.82) | 7.48 (4.60) | <0.01 | 313.1 (35.3) | 328.8 (19.7) | NS | 9.96 (5.54) | 4.22 (5.14) | <0.05 | 343.7 (35.2) | 401.1 (70.3) | < 0.05 |
| CPz | 15.65 (6.95) | 7.89 (4.48) | <0.01 | 318.0 (37.1) | 328.8 (19.7) | <0.05 | 12.24 (3.95) | 7.72 (5.90) | <0.05 | 368.8 (58.6) | 406.7 (60.4) | NS |
| Pz | 15.23 (6.96) | 8.01 (4.05) | <0.05 | 310.7 (33.1) | 330.7 (19.6) | <0.05 | 13.71 (3.24) | 9.59 (6.22) | NS | 361.6 (39.0) | 397.6 (58.6) | < 0.05 |
| POz | 13.38 (6.76) | 7.23 (4.00) | <0.05 | 304.0 (34.1) | 329.1 (17.8) | <0.05 | 12.69 (2.67) | 9.85 (6.03) | NS | 369.6 (50.5) | 389.1 (55.3) | NS |
With BDI as covariate.
Table 5.
Summary of F-ratios from repeated measures ANCOVA for P3a and P3b amplitude
| P3a | P3b | |
|---|---|---|
| Frontal electrodes | ||
| Group | F1,27 = 6.51** | F1,27 = 5.97* |
| Electrode | F4,108 = 16.34*** | F4,108 = 3.99* |
| Group × electrode | F4,108 = 4.36** | F4,108 = 4.29** |
| Central electrodes | ||
| Group | F1,27 = 9.76** | F1,27 = 3.71* |
| Electrode | F7,189 = 4.92** | F7,189 = 1.54 |
| Group × electrode | F7,189 = 3.93** | F7,189 = 1.15 |
| Parietal electrodes | ||
| Group | F1,27 = 7.96** | F1,27 = 4.53* |
| Electrode | F5,135 = 2.93* | F5,135 = 3.61* |
| Group × electrode | F5,135 = 3.88** | F5,135 = 1.81 |
| Temporal electrodes | ||
| Group | F1,27 = 3.80 | F1,27 = 1.78 |
| Electrode | F5,135 = 5.72*** | F5,135 = 1.25 |
| Group × electrode | F5,135 = 2.02 | F5,135 = 0.61 |
| Occipital electrodes | ||
| Group | F1,27 = 3.11 | F1,27 = 0.34 |
| Electrode | F2,54 = 10.35*** | F2,54 = 3.06 |
| Group × electrode | F2,54 = 8.83** | F2,54 = 4.28* |
P < 0.05,
P ≤ 0.01,
P ≤ 0.001 with BDI as covariate.
Fig. 2.
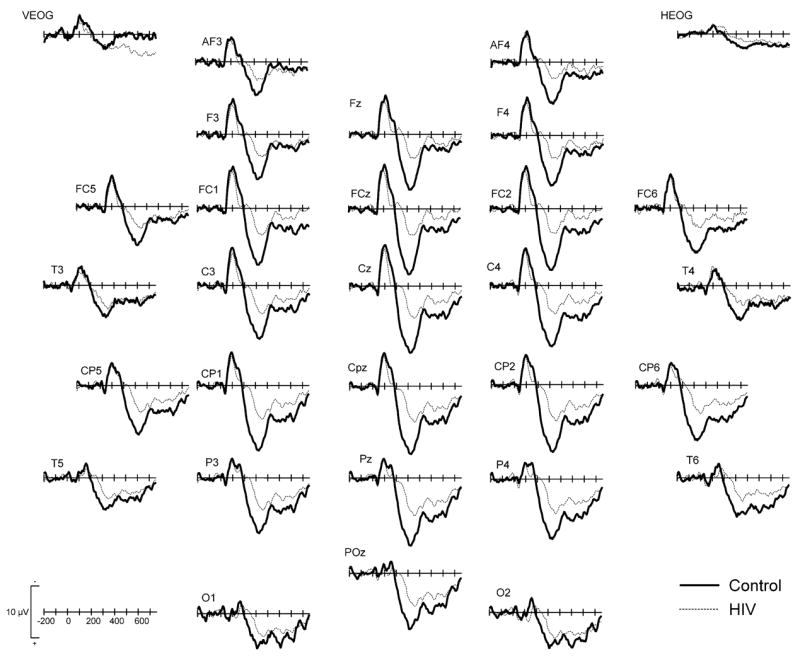
Grand averaged ERPs to the infrequent, non-target stimuli for controls (thick solid line) and HIV + patients (thin dashed line) at 28 electrodes.
There were main group effects for P3a amplitude over frontal, central, and parietal regions because the P3a was smaller in HIV patients relative to controls (Tables 4 and 5). There were also main effects of electrode over frontal, central, parietal, and occipital regions because P3a amplitude was largest at midline sites (e.g. the P3a was maximal at Fz in the frontal group, at FCz and Cz in the central group, etc). There were significant group × electrode interactions at frontal, central, parietal, and occipital regions because the difference in amplitude across electrodes was more pronounced in controls compared to HIV patients. Finally, there were significant main group effects for P3a latency over parietal (F1,27 = 5.15, P = 0.03) and occipital regions (F1,27 = 7.98, P < 0.01), and a marginally significant group effects over frontal (F1,27 = 3.19, P = 0.09) and central (F1,27 = 4.22, P = 0.05) regions because P3a latency was longer in HIV patients than controls.
3.2.3. Frequent target stimuli
Grand averaged waveforms of ERPs elicited by the target stimuli at 28 electrode sites are presented in Fig. 3. There were main group effects for P3b amplitude over frontal, central, and parietal regions because the P3b was smaller in HIV patients relative to controls (Tables 4 and 5). There were main electrode effects over frontal and parietal regions because P3b amplitude was largest at Fz and Pz. There were significant interactions between group × electrode over frontal and occipital regions because the difference in amplitude across electrodes in these regions were more pronounced in controls than HIV patients. Lastly, there was a significant group effect for P3b latency over the central region (F1,27 = 6.30, P = 0.02) because P3b latency was longer in HIV patients than controls.
Fig. 3.
Grand averaged ERPs to correctly detected frequent, target stimuli for controls (thick solid line) and HIV + patients (thin dashed line) at 28 electrodes.
3.2.4. Relationship between BDI scores and ERPs
We examined the relationship between BDI scores and ERP amplitudes and latencies. The Spearman rank correlation coefficients are summarized in Table 6. In HIV patients, but not in controls, N1 latency correlated positively with BDI scores over several fronto-central electrodes. In both patients and controls, P3b amplitude correlated negatively with BDI scores over multiple frontal electrodes.
Table 6.
Spearman’s rank correlation coefficients between BDI scores N1 latency and P3b amplitude at fronto-central electrodes
| Electrode | N1 latency
|
P3b amplitude
|
||
|---|---|---|---|---|
| Control | HIV | Control | HIV | |
| AF3 | 0.51 | 0.56 | −0.56* | −0.52* |
| AF4 | 0.09 | 0.00 | −0.69** | −0.70** |
| F3 | 0.39 | 0.35 | −0.52* | −0.45 |
| Fz | 0.14 | 0.31 | −0.52* | −0.45 |
| F4 | 0.13 | 0.34 | −0.52* | −0.62* |
| FC5 | 0.27 | 0.54* | −0.47 | −0.41 |
| FC1 | 0.31 | 0.66** | −0.22 | −0.46 |
| FCz | 0.10 | 0.52* | −0.33 | −0.39 |
| FC2 | 0.19 | 0.58* | −0.37 | −0.47 |
| FC5 | 0.01 | 0.42 | −0.14 | −0.60* |
P < 0.05,
P < 0.01.
4. Discussion
The main finding of this study is that virally suppressed HIV patients on ART had impaired CNS function, as detected by ERPs. Specifically, virally suppressed HIV patients on ART generated smaller P2, P3a, and P3b amplitudes and longer P3a and P3b latencies than controls. We also found HIV patients more depressed than controls, and that depression in HIV patients correlated with increased N1 latency and decreased P3b amplitude.
The first finding of this study is that virally suppressed HIV patients receiving ART generated smaller P2 components than seronegative control subjects. There is evidence that the P2 ERP component may be related to attention. Johnson (1989) observed larger P2 amplitudes in a reaction time task compared to a simple counting task, suggesting that the P2 may be sensitive to tasks that require more attention. Larger P2 amplitudes have also been associated with phasic changes in attention. For example, Holcomb et al. (1986) found that P2 amplitude was larger to stimuli that captured more attention. Thus, virally suppressed HIV patients on ART may have generated smaller P2 components than controls because HIV affected the patients’ ability to attend to the stimuli. Evidence corroborating this comes from the slightly higher error rates and false positives to standard and non-target stimuli in HIV patients relative to controls.
The second finding of this study is that virally suppressed HIV patients on ART had smaller P3a amplitude and longer P3a latency than control subjects. This is consistent with the findings of Polich and Basho (2002). Lesion (Knight, 1984) and intracranial ERP studies in pre-surgical epilepsy patients (Halgren et al., 1995a) have shown that the frontal lobe contributes to the scalp-recorded P3a. Thus, HIV may have affected P3a amplitude and latency via its negative impact on the frontostriatal system (Barker et al., 1995; Kure et al., 1990; Power et al., 1993; Rottenberg et al., 1987). The basal ganglia contain some of the brain’s highest viral burden as well as HIV-infected macrophages and multinucleated giant cells during active HIV infection (Kure et al., 1990). Because outputs from the basal ganglia are transmitted to regions of the frontal lobe via the frontostriatal system (Goldman-Rakic and Porrino, 1985; Matelli et al., 1989; Schell and Strick, 1984), this may be one mechanism by which HIV reduces P3a amplitude and prolongs P3a latency.
The third finding of this study is that virally suppressed HIV patients on ART generated smaller P3b components than control subjects. Prior to the widespread use of ART, many ERP studies reported similar findings (Ollo et al., 1991; Polich et al., 2000; Schroeder et al., 1994). However, Polich and Basho (2002) recently found no reliable P3b amplitude differences between HIV patients on antiviral medication and control subjects. One reason for the discrepant findings may be different patient populations. Nine patients in the present study had AIDS and 5 had CD4 nadirs that were lower than 20. Although, there were no significant correlations between P3a or P3b amplitude and CD4 nadir and no significant differences between P3a and P3b amplitudes in HIV patients with or without AIDS, it is possible that our patients’ CNS were more severely compromised by long-term HIV-infection than the patients studied by Polich and Basho. Polich and Basho did not provide information about the duration of HIV disease in their HIV patients; however, the average length of infection of the patients in the current study was 12.9 years. Polich and Basho speculated that they might not have observed any significant P3b amplitude differences between HIV patients and controls because antiviral medication had improved attention and memory functions thought to underlie P3b generation in the HIV patients. Therefore, another possible reason for the discrepant findings may be that the HIV patients in the present study had poorer attention and memory functions, possibly due to long-term HIV-infection, than the HIV patients studied by Polich and Basho. This explanation is in accordance with our interpretation of the HIV-related P2 amplitude reduction. Lastly, task/stimuli differences may also account for the discrepant findings as the relative perceptual distinctiveness among stimuli in the oddball task can determine P3a and P3b amplitudes (Katayama and Polich, 1998). The current study employed a 3-stimulus oddball task with highly discrepant non-target stimuli (sound effect noises) and easily distinguished target and standard stimuli. In contrast, Polich and Basho utilized a 3-stimulus oddball task with difficult target/stimulus discrimination. However, it is noteworthy that Polich and Basho also failed to detect significant P3b amplitude differences between HIV patients and controls with a 2-stimulus oddball task with easily discriminated target/standard stimuli.
Lesions studies have shown that damage to the temporo-parietal junction including posterior hippocampus, posterior temporal plane, superior temporal sulcus, anterior and medial temporal lobe all result in significant reductions of the scalp-recorded P3b (Knight, 1996; Knight et al., 1989; Onofrj et al., 1992; Verleger et al., 1994). Intracranial recording studies have also implicated similar regions in generation of the scalp-P3b (Halgren et al., 1995a,b; McCarthy et al., 1989; Smith et al., 1990). Thus, HIV-associated atrophy in temporal limbic gray matter and posterior cortex (Aylward et al., 1995; Jernigan et al., 1993; Patel et al., 2002) may have contributed to P3b amplitude reductions and P3b latency increases in HIV patients in the current study.
It is interesting to note that, despite generating smaller amplitude and longer latency P3b components than controls, there were no significant differences in the response time to target stimuli between patients and controls. This is consistent with the idea that P3b latency is unrelated to response selection processes (McCarthy and Donchin, 1981; Pfefferbaum et al., 1986) and therefore independent of behavioral relation time (Duncan-Johnson, 1981; Verleger, 1997). It is also consistent with growing literature documenting the longitudinal benefit provided by potent ART for neuropsychological function, particularly psycho-motor processing speed, in patients with HIV (Ferrando et al., 2003).
Consistent with previous studies that have found depressive disorders common among people infected with HIV (Komiti et al., 2003; Valente, 2003), HIV patients in the present study had higher BDI scores than control subjects. Furthermore, BDI scores correlated positively with N1 latency in HIV patients and negatively with P3b amplitude in all subjects. Other reports have noted significant relationships between depression and ERP measures (Blackburn et al., 1990; Bruder et al., 1991; Gangadharar et al., 1993; Hasenne et al., 1996; Urretavizcaya et al., 2003). Although none of the HIV patients in the present study were clinically depressed, the tendency for more depressed HIV patients to generate longer latency N1 and smaller amplitude P3b components may be related to the alteration of information processing in melancholic patients during depressive episode.
In conclusion, the current data suggest that there are disturbances in the cognitive potentials of virally suppressed HIV patients on ART. The current P2 findings suggest that there may be decreased arousal in HIV patients relative to seronegative controls. With regard to cognitive activity involved in stimulus evaluation, discrimination, and decision-making processes, the reduced P3a and P3b amplitudes are suggestive of cognitive decline in HIV patients, in accordance with previous reports in the literature (Polich et al., 2000; Schroeder et al., 1994). One interpretation of these results is that, even in the absence of detectable levels of HIV in the peripheral blood, viral replication persists in the CNS and continues to cause disease in HIV patients on ART. An alternative explanation is that alterations in the CNS structures of HIV patients on ART occurred after the initial virus impact. The cross-sectional nature of this study, and the significant group differences in BDI scores, make it difficult to distinguish between these alternatives and whether the ERP differences observed between HIV patients and controls reflect factors in the patients’ medical history preceding the onset of HIV-infection and/or the adverse effects of ART. Future longitudinal studies will be able to better resolve this issue. Nevertheless, these findings highlight the importance of developing antiretroviral regimens with better levels of blood–brain barrier penetration.
Acknowledgments
This work was funded by the NIAAA program project, P01 AA11493. The authors would like to thank Ms Erin Clevenger for assistance with data acquisition and analysis. This manuscript is dedicated to the memory of Linda Rogers, PhD (1946–2003).
References
- Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry. 1995;152:987–94. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195:58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- Beck AJ. Depression: clinical, experimental and theoretical aspects. New York: Harper & Row; 1967. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Erbaugh JK. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:567–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanchez J, Dew MA, Lopez OL, Dorst SK, Banks G. Neuropsychological abnormalities among HIV-infected individuals in a community-based sample. Neuropsychology. 1997;11:592–601. doi: 10.1037//0894-4105.11.4.592. [DOI] [PubMed] [Google Scholar]
- Blackburn IM, Roxborough HM, Muir WJ, Glaus M, Blackwood DHR. Perceptual and psychological dysfunction in depression. Psychol Med. 1990;20:95–103. doi: 10.1017/s003329170001326x. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Nasrallah HA, Para MF, Whitacre CC, Rosenberger P, Fass RJ. Neuropsychological performance in symptomatic and asymptomatic HIV infection. AIDS. 1993;7:519–24. doi: 10.1097/00002030-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Toewy JP, Stewart JW, Friedman D, Tenke C, Quitkin FM. Event-related potentials in depression: influence of task, stimulus hemifield and clinical features on P3 latency. Bio Psychiatry. 1991;30:233–46. doi: 10.1016/0006-3223(91)90108-x. [DOI] [PubMed] [Google Scholar]
- Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–7. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Davey RT, Jr, Ostrowski M, Shawn Justement J, Engel D, Mullins JI, Fauci AS. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nature Med. 2000;6:757–61. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Wtimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Donchin E, Karis D, Bashore T, Coles MGH, Gratton G. Cognitive psychophysiology and human information processing. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology: systems, processing, and applications. New York: Gilford Press; 1986. pp. 244–67. [Google Scholar]
- Duncan-Johnson CC. P300 latency: a new metric of information processing. Psychophysiology. 1981;18:207–15. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Evers S, Grotemeyer KH, Riechelt D, Luttmann S, Husstedt IW. Impact of antiretroviral treatment on AIDS dementia: a longitudinal prospective event-related potential study. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:143–8. doi: 10.1097/00042560-199802010-00007. [DOI] [PubMed] [Google Scholar]
- Ferrando SJ, Rabkin JG, van Gorp W, Lin SH, McElhiney M. Longitudinal improvement in psychomotor processing speed is associated with potent combination antiretroviral therapy in HIV-1 infection. J Neuropsychiatry Clin Neurosci. 2003;15:208–14. doi: 10.1176/jnp.15.2.208. [DOI] [PubMed] [Google Scholar]
- Gangadharar BN, Ancy J, Janakiramaiah N, Umapathy C. P300 amplitude in non-bipolar, melancholic depression. J Affect Disord. 1993;28:57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242:535–60. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Grant I, Akinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107:828–86. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli: 1 Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol. 1995a;94:191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal J, Birabin A. Intracerebral potentials to rare target and distractor stimuli: 2 Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol. 1995b;94:229–50. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hasenne M, Pitchot W, Gonzalez-Moreno A, Urcelay Zaldua I, Ansseau M. Suicidal behavior in depressive disorder: an event-related potential study. Biol Psychiatry. 1996;40:116–22. doi: 10.1016/0006-3223(95)00372-x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ. The HNRC 500—neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–51. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Ackerman PT, Dykman RA. Auditory event-related potentials in attention and reading disabled boys. Int J Psychophysiol. 1986;3:263–73. doi: 10.1016/0167-8760(86)90035-8. [DOI] [PubMed] [Google Scholar]
- Husstedt IW, Frohne L, Bockenholt S, Frese A, Rahmann A, Heese C, Riechelt D, Evers S. Impact of highly active antiretroviral therapy on cognitive progressing in HIV infection: cross-sectional and longitudinal studies of event-related potentials. AIDS Res Hum Retroviruses. 2002;18:485–90. doi: 10.1089/088922202317406628. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin RA, McCutchan JA, Chandler J, Grant I. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC group. Arch Neurol. 1993;50:250–5. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Johnson R. The amplitude of the P300 component of the event-related potentials: review and synthesis. In: Ackles P, Jennings JR, Coles MGH, editors. Advances in psychophysiology: a research annual. Greenwich, CT: JAI Press; 1988. pp. 69–137. [Google Scholar]
- Johnson JR. Developmental evidence for modality dependent P300 generators: a normative study. Psychophysiology. 1989;26:651–67. doi: 10.1111/j.1469-8986.1989.tb03167.x. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35:23–33. [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–9. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contribution of the temporal–parietal junction to the auditory P3. Brain Res. 1989;502:109–16. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Komiti A, Judd F, Grech P, Mijch A, Hoy J, Williams B, Streen A, Lloyd JH. Depression in people living with HIV/AIDS attending primary care and outpatient clinics. Aust N Z J Psychiatry. 2003;37:70–7. doi: 10.1046/j.1440-1614.2003.01118.x. [DOI] [PubMed] [Google Scholar]
- Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol (Berl) 1990;80:393–400. doi: 10.1007/BF00307693. [DOI] [PubMed] [Google Scholar]
- Levy JK, Fernandez F, Holmes VF, Gagen M, Pirozzolo FJ. Verbal-memory disturbances associated with HIV infection. J Clin Exp Neuropsychol. 1987;9:45. [Google Scholar]
- Matelli M, Luppino G, Fogassi L, Rizzolatti G. Thalamic input to inferior area 6 and area 4 in the macaque monkey. J Comp Neurol. 1989;311:445–62. doi: 10.1002/cne.902800311. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC, Williamson PD, Spencer DD. Task-dependent field potentials in human hippocampal formation. J Neurosci. 1989;9:4253–60. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, Cohen BA, Sheridan K, Machado AM, Van Gorp WG, Visscher B. Neuropsychological performance in HIV-1-infected homosexual men: the Multi-center AIDS Cohort Study (MACS) Neurology. 1990;40:197–203. doi: 10.1212/wnl.40.2.197. [DOI] [PubMed] [Google Scholar]
- Navia BA, Price RW. Dementia complicating AIDS. Psychiatry Ann. 1986;16:158–66. [Google Scholar]
- Ollo C, Johnson R, Grafman J. Signs of cognitive change in HIV disease: an event-related brain potential study. Neurology. 1991;41:209–15. doi: 10.1212/wnl.41.2_part_1.209. [DOI] [PubMed] [Google Scholar]
- Onofrj M, Fulgente T, Malatesta G, Bazzono S, Colamartino P, Gami D. P3 recordings in patients with bilateral temporal lobe lesions. Neurology. 1992;42:1762–7. doi: 10.1212/wnl.42.9.1762. [DOI] [PubMed] [Google Scholar]
- Patel SH, Kolson DL, Glosser G, Matozzo I, Ge Y, Babb JS, Mannon LJ, Grossman RI. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. Am J Neuroradiol. 2002;23:543–9. [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Christensen C, Ford J, Kopell B. Apparent response incompatibiity effects on P3 latency depend on the task. Electroencephalogr Clin Neurophysiol. 1986;64:424–37. doi: 10.1016/0013-4694(86)90076-3. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 clinical utility and control of variability. J Clin Neurophysiol. 1998;15:14–33. doi: 10.1097/00004691-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Polich J, Basho S. P3a and P3b auditory ERPs in HIV patients receiving anit-viral medication. Clin Electroencephalogr. 2002;33:97–101. doi: 10.1177/155005940203300305. [DOI] [PubMed] [Google Scholar]
- Polich J, Ilan A, Poceta JS, Mitler MM, Darko DF. Neuroelectric assessment of HIV: EEG, ERP, and viral load. Int J Psychophysiol. 2000;38:97–108. doi: 10.1016/s0167-8760(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood–brain barrier. Ann Neurol. 1993;34:339–50. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, Dhawan V, Ginos JZ, Price RW. The metabolic pathology of the AIDS dementia complex. Ann Neurol. 1987;22:700–6. doi: 10.1002/ana.410220605. [DOI] [PubMed] [Google Scholar]
- Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areass. J Neurosci. 1984;4:539–60. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager LK, D’Souza MP. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. J Am Med Assoc. 1998;280:67–71. doi: 10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- Schroeder MM, Handelsman L, Torres L, Dorfman D, Rinaldi P, Jacobson J, Wiener J, Ritter W. Early and late cognitive event-related potentials mark stages of HIV-1 infection in the drug-user risk group. Biol Psychiatry. 1994;35:54–69. doi: 10.1016/0006-3223(94)91168-1. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E, Sokolik ME, Baudina P, Musolino E, Liegeois-Chauvel C, Chauvel P. The intra-cranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalogr Clin Neurophysiol. 1990;76:235–48. doi: 10.1016/0013-4694(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Urretavizcaya M, Moreno I, Benlloch L, Cardoner N, Serrallonga J, Menchon JM, Vallejo J. Auditory event-related potentials in 50 melancholic patients: increased N100, N200, and P300 latencies and diminished P300 amplitude. J Affect Disord. 2003;74:293–7. doi: 10.1016/s0165-0327(02)00016-2. [DOI] [PubMed] [Google Scholar]
- Valente SM. Depression and HIV disease. J Assoc Nurses AIDS Care. 2003;14:41–51. doi: 10.1177/1055329002250993. [DOI] [PubMed] [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34:131–56. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Verleger R, Heide W, Butt C, Kompf D. Reduction of P3b potentials in patients with temporo-parietal lesions. Cogn Brain Res. 1994;2:103–16. doi: 10.1016/0926-6410(94)90007-8. [DOI] [PubMed] [Google Scholar]
- White DA, Heaton RK, Monsch AU. Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals. The HNRC Group. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:304–15. doi: 10.1017/s1355617700000308. [DOI] [PubMed] [Google Scholar]
- Wilkie F, Eisdorfer C, Morgan R, Loewenstein D, Szapocnik J. Cognition in early human immunodeficiency virus infection. Arch Neurol. 1990;7:433–40. doi: 10.1001/archneur.1990.00530040085022. [DOI] [PubMed] [Google Scholar]