Abstract
Background
Tourette syndrome (TS) and Attention-Deficit/Hyperactivity Disorder (ADHD) are common and debilitating neuropsychiatric illnesses that typically onset in the pre-school years. Recently, both conditions have been subject to neuroimaging studies, with the aim of understanding their underlying neurobiological correlates.
Objective
The relation of TS and ADHD is discussed against the background of findings from previous Magnetic Resonance Imaging (MRI) studies.
Methods
We review the designs and major findings of previous studies that have examined TS with comorbid ADHD, and we briefly contrast these findings with those in ADHD without comorbid tic disorders.
Results
The frequent comorbidity of TS and ADHD may reflect a common underlying neurobiological substrate, and studies confirm the hypothesized involvement of fronto-striatal circuits in both TS and ADHD. However, poor inhibitory control and volumetric reductions in fronto-striatal circuits appear to be core features of ADHD, whereas reduced volumes of the caudate nucleus, together with activation and hypertrophy of prefrontal regions that likely help to suppress tics, seem to be core features of TS.
Conclusion
The etiological relationship between TS and ADHD must be clarified further with cross-sectional and, if possible, longitudinal imaging studies that examine samples of substantial size, including subgroups with pure TS and ADHD, as well as with comorbid conditions.
Keywords: Tourette syndrome, Attention-Deficit/Hyperactivity Disorder, neuroimaging
Introduction
TS and ADHD are common neuropsychiatric disorders found in children, and they frequently co-occur in the same individual [60, 61]. Tic disorders are characterized by bouts of brief, involuntary or semi-voluntary movements and sounds. Tic disorders are classified as either transient (present for less than one year), chronic (motor or vocal tics lasting longer than one year), or as Tourette syndrome (TS), in which the presence of chronic motor and vocal tics endure beyond one year [3, 74]. ADHD is characterized by hyperactivity, inattention, and impulsivity. The DSM-IV classification of ADHD [3] distinguishes three subtypes: predominantly hyperactive, predominantly inattentive, and combined type, in which both hyperactive and inattentive symptoms are present. To date, neuroimaging studies involving tic disorders have focused exclusively on patients with TS and not on persons with either transient or chronic tics. Our review will thus comprise only neuroimaging studies with a focus on TS and comorbid ADHD. We will consistently use the term “comorbid” to mean “co-occurring” or “co-existing,” because this is the term used in the reviewed literature. We use “comorbid” as a descriptive term to indicate that both illnesses are present in the same individual.
Whereas the neurobiologic relationship between TS and ADHD is not yet clear, at least three etiological links between ADHD and TS have been proposed:
-
(1)
TS and ADHD are variant expressions of a common set of vulnerability genes [17], with one subtype of ADHD being a variant expression of the underlying vulnerability genes for TS [47]. Indeed, some investigators have suggested that both conditions evolve from disturbances in a shared neural substrate, such as dysfunction of the basal ganglia [18, 47].
-
(2)
Two differing genotypes underlie TS and ADHD. The frequent co-occurrence of TS and ADHD is likely attributable to a clinical referral bias in which the referral of children with hyperactivity or other behavioral problems to TS clinics is based on a misattribution of their problematic behaviors to tics [70].
-
(3)
ADHD may be independent of TS in some individuals, whereas in other persons the ADHD may be secondary to TS [47].
Gaining a comprehensive overview of the findings from neuroimaging studies of TS with co-morbid ADHD may therefore help to clarify further which of these mutually exclusive hypotheses concerning the neurobiologic relationship of these two disorders is correct.
Brain morphology has been a central interest in neuroimaging studies because it can serve as a measurable, mediating link between genotype and behavioral phenotype [44]. If a common genetic vulnerability does indeed underlie some subtypes of TS and ADHD, we can therefore expect to find a number of morphometric similarities in patients with either of the two conditions. The generalizability of the findings, however, maybe limited because of the possibility that some TS subgroups may share a genetic vulnerability with ADHD, whereas others do not. On the other hand, if the high comorbidity rate of these disorders stems from a clinical referral bias, findings derived from measures in brain morphology should differ in patients with either TS or ADHD or both. Reviewing the discriminative anatomy may possibly help in defining endophenotypes or subtypes for each of these conditions [26]. For example, abnormalities in brain morphology, as detected in a common set of morphometric measures (within basal ganglia-based circuits, for example), may distinguish individuals who have TS alone from those who have either TS with comorbid ADHD, or TS with comorbid OCD [50]. We will therefore focus on studies that have reported on similarities and differences in brain morphometry between TS and ADHD.
Issues involved in imaging children with TS and comorbid ADHD
MRI has proven useful and appropriate in investigating pediatric populations affected by neuropsychiatric disorders, in that it operates without using the radioactive tracers required by Positron Emission Tomography (PET) and Single-Photon Emission Computerized Tomography (SPECT). Moreover, the safety of MRI has led to its successful application in longitudinal studies requiring the repeated examination of children [23, 24, 65]. The vast majority of published imaging studies in childhood neuropsychiatric illnesses, however, have used a cross-sectional study design with cases compared to controls. Conclusions concerning developmental trajectories must therefore be drawn with caution, and findings from these studies should be confirmed with longitudinal investigations [37, 49]. Although studies are able to detect differences in brain structure on a group level, to date, a single MRI examination cannot confirm or discount in a single patient a diagnosis of TS, ADHD, or other neuropsychiatric disorders (despite the frequent inquiries of clinicians regarding the potential uses of MRI as a tool for improving and supporting individual diagnoses), due to the subtlety of the deviations. In the future, however, such tools combined with other biological and clinical measures may be applicable to the subtyping of different neuropsychiatric disorders. Most likely this subtyping will involve several brain imaging measures (possibly even involving different MR modalities), as neuropsychiatric disorders are characterized by an impairment in brain circuits rather than by a single brain lesion.
All studies that aim to detect biological correlates of TS in brain morphometry must control in their statistical analyses for the high rates of comorbidity of both ADHD and OCD in individuals with TS [61]. In imaging studies of the neural basis of TS, individuals with co-existing conditions typically have not been excluded, whereas individuals with tics have often been excluded from studies that focus on ADHD [11, 14, 56]. A few reasons may explain the differences in inclusion and exclusion practices, including:
-
(1)
Individuals with ADHD and comorbid TS, or even with simple tic symptoms, may represent a subtype of ADHD that is distinct from that of the majority of individuals with ADHD [16].
-
(2)
Comorbidity with either ADHD or OCD is the rule rather than the exception in TS [61], and a representative sample of individuals with TS explicitly should also include individuals who have comorbid conditions.
-
(3)
The lower prevalence of pure TS (i.e., without either ADHD or OCD) makes it less reasonable to exclude all patients with comorbid conditions in order to build pure TS samples, because studies would loose statistical power or stall recruitment.
Thus, three diagnostic groups typically have been identified in studies exploring the neurobiologic basis of TS: TS only, TS with comorbid ADHD, and TS with comorbid OCD. Most studies use statistical models that include all patients, while covarying for co-existing conditions (like ADHD or OCD). The statistical findings, however, should be confirmed in additional analyses by restricting inclusion to the primary diagnostic group of interest (e.g., TS only) and the control group [54, 55, 58]. Despite a loss of statistical power, this approach can help to exclude the possibility that findings attributed to the main diagnostic entity (e.g., TS) in fact reflect a co-existing condition (e.g., ADHD or OCD) [10]. Others have suggested that a corresponding contrast group (i.e., individuals with ADHD only) ideally should be included in neuroimaging studies of brain structure in persons with TS and comorbid ADHD [10]. Only a few studies, however, have included a contrast group with ADHD only, and the sample sizes of these studies were comparatively small [7, 10, 21, 34]. We will therefore review the main findings from imaging studies on individuals with TS and comorbid ADHD, as well as those from relevant studies of “pure” TS.
Findings from neuroimaging studies of TS with comorbid ADHD
Basal ganglia
The current model for tic generation and modulation involves various portions of cortico-striato-thalamo-cortical (CSTC) circuits [2, 38, 39], which comprise segregated, largely parallel loops between the cortex and subcortex [2]. The cortex projects to striatal portions of these circuits; the striatum in turn sends projections to the globus pallidus (the substantia nigra and the thalamus), whose various nuclei send projections back to the cortex. Although the etiology of tics is unknown, they are thought by some to occur when a cluster of hypofunctional striatal neurons becomes active. This neuronal activity likely causes a non-voluntary inhibition of the efferent neurons of the basal ganglia, which in turn may lead to the disinhibition of a motor pattern generator in the cortex, ultimately releasing an involuntary movement [1].
Early imaging studies reported conflicting findings concerning the volumes of the basal ganglia (Table 1). The first volumetric imaging study of the basal ganglia in TS [52], which included 14 adult patients and 14 healthy controls, found decreased volumes of the left lenticular nucleus (putamen and globus pallidus combined) in the TS group compared with the control group. This TS sample, however, did not include individuals with comorbid ADHD.
Table 1.
Overview of anatomical studies dealing with volumes of basal ganglia, the midsagittal area of the Corpus Callosum and volumes of cortical regions involving samples of children and adults with TS and with comorbid conditions
| Study | Area of Interest | Regional volumes corrected for brain size | Diagnosis (n, age/years, gender, comorbidities) | Controls (n, age/years, gender) | Main Findings |
|---|---|---|---|---|---|
| Peterson et al. [52] | Basal Ganglia | Brain size index | 14 TS 31.8 (SD 8.5) 11m; 3 f | 14 HC 32.4 (SD 8.8) 11m; 3f | Volume of the left lenticular nucleus was reduced (P < .025) in TS |
| Singer et al. [66] | Basal Ganglia | Area of the 5 largest intracranial intracranial slices × slice thickness | 19 TS 11.8 (7–16) 14m; 5f 18 TS+ADHD 11.1 (9–13) 15m;3f | 18 HC 9.8 (6–15) 14m; 4f | Lenticular nucleus symmetry was either reduced or reversed; boys with TS showed trend toward a smaller left putamen (P < .08) |
| Hyde et al. [31] | Basal Ganglia | Total brain volume | 10 monozygotic twin pairs with TS 16.3 (9–31) 16m; 4f | No healthy controls; all participants had either TS or chronic tic disorder | Caudate nucleus volumes were smaller in the more severely affected twin (6%; P < .01) |
| Castellanos et al. [10] | Basal Ganglia, Anterior frontal region | Total brain volume | 14 TS+ADHD 10.4 (SD 1.9) boys only 26 ADHD 10.7 (SD 1.9) boys only | 31 HC 10.9 (SD 1.9) | Rightward asymmetry of putamen reversed in the ADHD and TS+ADHD groups (P < .009) |
| Zimmerman et al. [76] | Basal Ganglia, Ventricles | Area of the 5 largest intracranial slices intracranial slices × slice thickness | 19 TS 11 (7–15) girls only 8 girls with comorbid ADHD | 21 HC 10.7 (8–15) | No robust differences between girls with TS and controls |
| Peterson et al. [55] | Basal Ganglia | Whole brain volume | 154 TS 18.7 (6–63) 115m; 39f Comorbidities: ADHD: 41 (26.6%) OCD: 51 (33.1%) | 130 HC 21.0 (6–63) 71m; 59f | Caudate nucleus fl in the TS group, independent of age (P = .01). Smaller lenticular nucleus in TS adults (P < .02) |
|
| |||||
| Peterson et al. [51] | Corpus Callosum | Midsagittal head area | 14 TS 18–49 11m; 3f | 14 HC 18–49 11m; 3f | Corpus callosum reduced by 18% in the TS group (P < .006) |
| Baumgardner et al. [7] | Corpus Callosum | Intracranial area | 16 TS 12.6 (SD 2.2) 13m; 3f 21 TS+ADHD 11.2 (SD 1.6) 19m; 2f 13 ADHD 11.3 (SD 1.4) | 27 HC 10.8 (SD 2.6) 21m; 6f | Compared with HCs, the rostral body of the callosum was 17% larger in the TS group (P < .007). TS+ADHD: intermediate CC size. Pure ADHD: smaller CC (P < .004). |
|
| |||||
| Moriarty et al. [45] | Corpus Callosum, Basal Ganglia | Brain size index | 17 TS 35 (17–62) 11m; 6f | 8 HC 33 (20–45) 4m; 4f | TS group had an enlarged CC and loss of asymmetry in caudate nucleus. |
| Mostofsky et al. [46] | Corpus Callosum | Intracranial area | 10 TS 10.9 (7.6–14.6) girls only 9 TS+ADHD 10.4 (8.4–13.7) girls only | 22 HC 11.1 (8–14.5) girls only | No group-specific differences |
| Plessen et al. [58] | Corpus Callosum | Whole brain volume | 158 TS 18.5 (SD 13.3) 117m; 41f Comorbidities: ADHD: 42 (27 %) OCD: 49 (31 %) | 121 HC 19.7 (SD 12.6) 67m; 54f | CC smaller in the TS group (P < .005). Prominent interaction with age, with TS children having smaller CCs and TS adults larger CCs. |
|
| |||||
| Peterson et al. [54] | Cortical Regions, Ventricles | Whole brain volume | 155 TS 18.7 (SD 13.4) 114m; 41f Comorbidities: ADHD:36 (23%) OCD: 62 (40%) | 131 HC 20.8 (SD 13.4) 72m; 59f | TS subjects dorsal prefrontal volumes (P < .0004) and parieto-occipital regions (P < .002), but inferior occipital volumes ↓ (P < .03). |
| Fredericksen et al. [21] | Frontal Volume, White/Gray Matter Composition | Total frontal volume | 11 TS 10.7 (SD 2.2) boys only 14 TS+ADHD 11.7 (SD 2.4) boys only 12 ADHD 10.6 (SD 1.7) boys only | 26 HC 10.6 (SD 2.7) boys only | TS had larger proportion of white matter in the right rontal lobe (P < .01), no differences of total frontal volume for TS, but ADHD reduced frontal volume (P < .05). |
| Kates et al. [34] | Four frontal regions, deep white matter | Whole brain volume | 13 TS 9.9 (SD 1.1) boys only 13 ADHD 9.4 (SD 1.2) boys only | 13 HC 10.0 (SD 1.5) | Decrease of deep white matter in the left frontal lobe in TS (P = .02). In ADHD volume reductions of the prefrontal cortex (left P = .002; right P = .03) |
The findings of reduced volumes of the left lenticular nucleus were confirmed in an MR volumetric study in children with TS [66], in which 18 children with TS and comorbid ADHD and 19 children with TS only were compared to 18 control children. Children with TS and comorbid ADHD showed a trend toward a smaller left globus pallidus in both males and females. Moreover, normal lenticular asymmetry was either reduced or reversed (right-larger-than-left lenticular nucleus) in the group of subjects with TS and in the group with TS and comorbid ADHD.
In another study [30], basal ganglia volumes were measured in 10 pairs of monozygotic twins (aged 9–31) who were discordant across co-twins for the severity of their tics. Volumes of the lenticular nucleus did not differ between co-twins, but the caudate nuclei were smaller (on average, by 6%) in the twins who had the more severe tic symptoms. The absence of a control group in this study design, however, prevented a determination of whether both twins presented a genetically determined reduction in volume of the caudate. Moreover, the analyses in this study did not control for comorbid disorders [10].
The most recent anatomical MRI study of the basal ganglia examined the volumes of the caudate nucleus, globus pallidum, and putamen in 154 children and adults with TS (26.6% with comorbid ADHD and 33.1% with OCD) and 130 healthy control subjects [55]. This study, the largest of its kind, represented considerable advances in neuroimaging methodology, particularly in its increased image resolution and contrast, thus allowing for more precise measurement of basal ganglia volumes. Results from this study indicated that volumetric abnormalities in the basal ganglia of both children and adults with TS were specific to the caudate nucleus. Smaller volumes of the putamen and globus pallidus, on the other hand, were found in adults but not in children with TS. Comorbid ADHD was not significantly associated with alterations of basal ganglia volumes beyond the alterations that were accounted for by the presence of TS (OCD, by contrast, was associated with a smaller putamen). The smaller caudate volumes in the children with TS are consistent with findings of a smaller caudate nucleus in children with ADHD below the age of 16 [14], yet around the age of 16, caudate volumes appeared to normalize in teenagers with “pure” ADHD [14].
In the first prospective study in TS of imaging measures as predictors of clinical course, children from this sample were examined as young adults to measure tic severity and OCD symptoms [8]. This analysis showed that the volume of the caudate nucleus in childhood significantly predicted tic severity and OCD symptom severity in adulthood. Again, comorbid ADHD had no significant statistical influence on outcome.
The results of these anatomical imaging studies have several implications for understanding the role of the basal ganglia in the pathophysiology of TS and comorbid ADHD. First, the presence of significantly smaller caudate nuclei in both children and adults suggests that hypoplasia of the caudate nucleus may represent a trait morphological abnormality in persons with TS. Second, decreased caudate nucleus volumes that persist into adulthood imply that the caudate nucleus is not a prime target for plastic changes in response to the presence of tics, nor is it a likely candidate for the cause of the normal attenuation of tic symptoms during adolescence. Third, the co-occurrence of ADHD did not statistically alter the findings on basal ganglia volumes in subjects with TS.
Cortical regions
Whereas anatomical studies suggest that reduced volumes of the caudate nuclei may be the locus of trait abnormalities in children and adults with TS, cortical portions of CSTC circuits are thought to be involved in the modulation and suppression of tic symptoms in individuals with tics [38, 39, 53, 67]. The modulation of tics through prefrontal regions is thought to occur via fronto-striatal circuits. These circuits mediate self-regulatory control in various domains that are known to mature late in the time course of brain development [22, 43], during late childhood and adolescence [65, 68]. In a sample of 155 children and adults with TS and 131 normal control subjects, a cortical parcellation schema based on callosal landmarks was used to parcellate the cerebrum into eight cortical regions that included both white and gray matter. The prefrontal region was segmented into left and right dorsal prefrontal and orbitofrontal regions. Larger dorsal prefrontal volumes were detected in children with TS, whereas smaller dorsal prefrontal volumes were observed in adults with TS [54]. Larger cortical volumes in both the orbitofrontal and parieto-occipital regions were significantly associated with fewer tic symptoms in adults with TS. In a separate analysis of the children from this sample (age <18 years), 42 pure TS subjects (no lifetime diagnosis of OCD or ADHD) who had no prior exposure to typical or atypical neuroleptics were compared with 67 healthy controls. Results were similar to those obtained for the comparison of the entire sample of TS and control children, suggesting that neither comorbid ADHD/OCD nor prior neuroleptic exposure influenced the findings significantly. Diagnoses of ADHD or OCD in the TS subjects, however, were associated as covariate main effects with larger cerebral volumes at trend levels of significance (P = .09 and P = .06 respectively), and a diagnosis of ADHD in TS subjects was associated with smaller ventricular volumes at a trend level of significance (P = .06).
These inverse correlations of prefrontal volumes with age suggest that the larger prefrontal volumes found in children with TS could represent compensatory or adaptive processes in the brains of these children that help to attenuate the severity of tics. This developmentally based interpretation should be regarded with caution because of the cross-sectional design of the study. Nevertheless, it is supported by findings from an fMRI study of effortful tic suppression in adults with TS, which showed that prefrontal cortices activated robustly during the suppression of tics [53]. The frequent need to suppress tics continuously in diverse social settings would activate these prefrontal regions regularly and frequently. This repeated activation could then induce activity-dependent, plastic hypertrophy of prefrontal cortices in children and adolescents with TS, probably through a reduced rate of pruning of the prefrontal regions during brain maturation [65, 68].
Activity-dependent plasticity and hypertrophy within prefrontal regions in turn would help to attenuate tic severity by increasing inhibitory reserve and the capacity for self-regulatory control. This interpretation of the anatomical and fMRI findings in prefrontal cortices is consistent with the known role of the dorsal prefrontal region in subserving self-regulatory control [22, 43, 71]. In addition, a higher proportion of white matter has been detected in the right frontal lobe of 11 boys with TS, but not 14 boys with comorbid TS-ADHD and 12 with ADHD-only, compared to 26 HC subjects [21]. The total volume of the frontal lobe (i.e., combined cortical gray and white matter) in the TS group, however, did not differ from that in the control group in this study. In contrast, both individuals with TS and comorbid ADHD and individuals with pure ADHD had smaller overall frontal lobes. A higher proportion of frontal white matter in the TS group supports the hypothesis that fronto-striatal tracts are involved in the pathophysiology of TS. Statistical analyses were performed while using TS and ADHD as variables in the statistical models. This approach, however, did not allow investigators to distinguish morphometric differences of the brain that were specific to either TS or ADHD from the morphometric differences specific to comorbid TS and ADHD.
The hypertrophic prefrontal cortical regions in children with TS contrast with findings in children with ADHD alone, who have repeatedly exhibited reduced prefrontal volumes [11, 13, 20, 69], consistent with their problems in controlling impulsive behavior [15]. Findings of smaller prefrontal regions in adults with TS, however, agree with the few neuroanatomical studies of adults with ADHD, which have reported reduced orbitofrontal volumes [28] and reduced overall volumes of cortical gray matter, prefrontal regions, and ACC [63].
Corpus callosum
The corpus callosum (CC) has been a central focus in studying the biological correlates of TS since the early reports suggesting the presence of abnormal brain lateralization in the disorder. The unilateral character of the initial findings from the basal ganglia—i.e., decreased volumes of the left lenticular nucleus [52] and a loss of the physiological left-greater-than-right asymmetry of the basal ganglia [66]—raised the question of whether individuals with TS would exhibit loss of the normal asymmetric organization of the CNS [75]. In this respect, the CC is a structure of central interest because of the various roles it plays in lateralization of the brain [62]. First, the CC is thought to mirror brain asymmetry and hemispheric specialization [5]. Second, anatomical changes of the CC may represent indirect evidence for alterations in cortical tissue, especially for alterations of the frontal and prefrontal cortical areas, which send widespread, intertwined fibers through the genu and the body of the CC [29].
The first study of the CC in individuals with TS, which included 14 young adults with TS and 14 control subjects, revealed a reduced size of all subregions of the CC in the TS group compared with controls [51]. This study, however, did not include individuals with comorbid ADHD. A second study compared the size of the CC in 16 children with TS, in 21 children with TS-ADHD, in 13 children with ADHD only, and in 21 control children [7]. The group with TS alone had a larger CC on average, whereas the group with comorbid TS-ADHD had an intermediate CC size, and the group with ADHD alone had a reduced CC size. To evaluate the effect of gender on CC size in TS, another study included 10 girls with TS, 9 girls with a comorbid TS-ADHD, and 22 control girls [46]. The absence of differences in CC size across diagnostic groups was interpreted as indicating that differences in the size of the CC in the TS group were restricted to boys. These studies shared the disadvantage of not having realigned midsagittal MR images prior to measuring the CC [59]. The failure to realign midsagittal slices when defining the CC may increase inter-individual differences in measurement of its size, simply because of differences in midline placement of the heads of subjects in the scanner.
The most recent study of CC size [58] examined 158 subjects with TS and 121 controls (5 to 65 years of age) in a cross-sectional design using CCs realigned to true midline. Subjects with TS had smaller overall CCs compared with controls. These reductions in size were found to depend significantly on the age of the subject, with smaller CCs present primarily in children with TS compared with healthy control children and larger CCs primarily in adults with TS compared with healthy adults. The sizes of the CC and its subregions correlated with the severity of motor tics, indicating that a smaller CC may have a protective or compensatory function in subjects with TS. Having a smaller CC could therefore represent a compensatory, plastic response to the presence of tics, similar to the postulated role of the larger prefrontal cortex found in children with TS. Furthermore, CC size correlated inversely with volumes of the prefrontal cortex in both the TS and control groups, although the magnitudes of these inverse correlations were significantly greater in the TS group. This exaggeration presumably represented an adaptive or compensatory process in the brains of individuals with TS, given that smaller CCs and larger prefrontal cortices were both associated with less severe tic symptoms. The putative plastic process that produced a smaller CC may have reduced excitatory input from the CC to the inhibitory GABAergic interneurons in the prefrontal cortex [9, 36]. This postulated reduction in numbers of inhibitory GABAergic interneurons would produce enhanced prefrontal output (because the prefrontal cortex receives less transcallosal inhibition), which in turn may increase the suppression of tics (which are themselves mediated through fronto-striatal connections). Over time, the prefrontal cortex may become hypertrophic because of frequent use, thereby enhancing the prefrontal self-regulatory control in persons with TS. As an alternative explanation, a smaller CC in children with TS may signal a less severe form of TS and produce an outcome with fewer tic symptoms, and hence it could have a protective function in the disorder. A larger CC and smaller prefrontal regions, in contrast, would signal the presence of a more severe form of TS. The positive associations of CC size with tic severity in this study do not support this latter alternative; however, its cross-sectional design prevents definitive support for one or the other of the two hypotheses.
Findings of smaller CCs in subjects with TS have recently been extended to the fiber tracts that compose the CC as measured by Diffusion Tensor Imaging (DTI) in 20 boys with TS (5 boys with a comorbid ADHD) and 20 control boys (aged 10–18 years) [57]. DTI measurements [6] showed that boys with TS had lower Fractional Anisotropy values in all regions of the CC. The degree of anisotropy depends both on the number of fibers and on the thickness of the myelin sheaths that surround nerve fibers [48]. Recent studies document the correlation of the degree of myelination with changes of anisotropy, as measured by DTI during brain maturation in infants and toddlers [27]. Thus, these DTI findings again point to the presence of reduced interhemispheric connectivity in children with TS. A comorbid diagnosis of ADHD did not influence the findings, as shown by excluding all individuals with ADHD (and with OCD) from statistical analyses.
The impetus for examining the CC in TS stemmed from previous reports of reduced structural brain asymmetry of the basal ganglia in patients with TS. The hypothesis of abnormal functional brain lateralization, however, has not been confirmed in subsequent studies or in other reports studying anatomical and functional brain lateralization in TS [41, 54, 55]. Reported findings of morphometric and functional deviations of callosal measures [41, 57, 58] are, however, consistent with findings that implicate prefrontal brain regions in tic suppression in TS [53, 54].
Conclusion
Evidence from neuroimaging studies that included both individuals with TS alone, and individuals with TS and comorbid ADHD, suggest that comorbid ADHD does not significantly alter the primary findings in samples of individuals with TS, thus providing a degree of support for the hypothesis of a shared genetic vulnerability as expressed in deviations of brain morphology in these comorbid conditions. Moreover, these studies support the hypothesized involvement of the striatum in the genesis of tics, while at the same time suggesting strongly that reduced volumes of the caudate nucleus represent a manifestation of a genetic vulnerability to TS [8, 55]. The non-significant influence of comorbid ADHD on basal ganglia volumes in individuals with TS may suggest that a reduced volume of the caudate nucleus represents a shared, genetically mediated etiology for TS and ADHD. This possibility is further strengthened by neuroimaging findings from children and adolescents with ADHD alone, which show a reduced size of the right [11-13] and left caudate nucleus [11, 20, 64]. The functional significance of a smaller caudate nucleus may, however, differ across diagnostic groups. Striatal dysfunction in ADHD has been implicated in impaired inhibition: children with ADHD show a reduced activation of striatal regions during a “go-no-go” task compared with healthy control children [19, 72]. Children and adults with TS, on the other hand, show impaired probabilistic learning, which is mediated by striatal regions [35, 42]. These findings suggest that a smaller caudate may have differing functional implications in TS and ADHD.
When examined in isolation, TS and ADHD differ in their influences on regions of the brain outside of the basal ganglia. For example, children and adolescents with ADHD have reduced prefrontal volumes [11, 13, 20], especially in the inferior aspects of prefrontal cortices [69], whereas children with TS have larger prefrontal cortices, especially its dorsal portions [54]. Moreover, a recent study confirmed that adults with ADHD have reduced prefrontal volumes compared with normal controls [63]. Findings from studies of individuals with pure ADHD therefore initially may appear to contradict the idea that comorbid ADHD in individuals with TS does not alter findings of increased prefrontal volume in children with TS [54]. One explanation may be that the ADHD observed in children with TS represents a subtype distinct from that of ADHD alone [50]. Alternatively, the continuous need in children with TS to control tics, thereby leading to increased prefrontal volumes, could outweigh the influence of comorbid ADHD on development of the frontal cortex. However, this overriding influence of TS on prefrontal development would arguably attenuate symptoms of hyperactivity in these patients, a phenomenon that has not yet been observed. On the contrary, tics themselves may cause distractibility or even a secondary attention deficit in some individuals [47, 70]. Indeed, the use of relatively small sample sizes in studies of children with co-existing TS and ADHD may have generated underpowered mapping of cerebral measures.
Whereas a reduction in the size of the corpus callosum has been reported both in children and adolescents with pure ADHD [7, 25, 32, 33, 40, 64] (for an overview, see [62]) and in those with TS [58], these reductions may be associated with a different mechanism for each condition. In ADHD, one could speculate that a smaller CC (especially the most anterior part of the CC) may represent a decrease in the number of CC fibers stemming from hypoplasticity of the prefrontal cortex. To our knowledge, this hypothesis has not yet been tested formally in any study. In persons with TS, however, a smaller CC has been shown to correlate with an increase in prefrontal volumes [58]. The reduced size of the CC in TS has thus been understood as a compensatory mechanism that likely enhances prefrontal functioning, thereby facilitating self-regulatory control of tic behavior; less callosal “trafficking” may reduce inhibitory influences on prefrontal functioning (thought to be mediated via GABAergic interneurons) (Fig. 1).
Fig. 1.
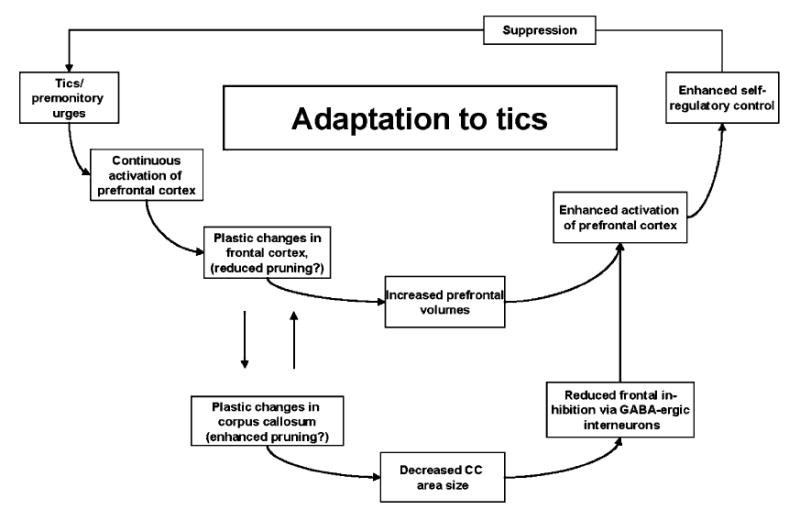
Suggested model for the interplay of prefrontal and callosal changes that enhance tic suppression. Tic suppression is most likely mediated through prefrontal cortical fibers that ultimately control motor activity through CSTC circuits. Reduced interhemispheric connectivity may lead to reduced inhibition of prefrontal neurons and thus may facilitate tic suppression. We suggest that mechanisms of experience-dependent axonal and synaptic pruning are more pronounced in the CC of those individuals in the TS population who have been successful in developing the ability to suppress tics, whereas the pruning in the cortical regions is slowed as a result of continuous activation due to tic suppression
Based on findings of reduced volumes of the caudate nuclei in children with TS and in children with ADHD alone, as well as in children with TS and comorbid ADHD, we conclude that findings from neuroimaging studies so far do not exclude the possibility of a shared genetic vulnerability in TS and ADHD for the basal ganglia. The effects of TS on morphometric features of other brain regions (e.g., prefrontal cortical regions) differ from those of ADHD, however. The hypertrophic dorsal prefrontal cortex in children with TS appears to evolve as an activity-dependent, neuroplastic response to the need to suppress tics, whereas prefrontal hypoplasia in children with ADHD alone has been hypothesized to be a core feature of the condition that therefore is likely genetically mediated [73]. Thus to date, neuroimaging studies cannot conclude unambiguously whether TS and ADHD share an underlying genetic vulnerability that is expressed in coincident features of brain structures, or whether the high rates of comorbidity are mainly attributable to a clinical referral bias. The etiological relationship between TS and ADHD must be clarified further with cross-sectional and, if possible, longitudinal neuroimaging studies that use samples of substantial size, and that include groups with pure TS, pure ADHD [4], and individuals with comorbid conditions (i.e., using a 2 × 2 factorial design; Banaschewski et al., this issue).
Acknowledgments
This work was supported by the Center for Child and Adolescent Mental Health, University of Bergen, Norway, and in part by a grant from the Tourette Syndrome Association, NIMH grants MH01232, MH59139, MH068318, and K02-74677, the Suzanne Crosby Murphy Endowment at Columbia University College of Physicians and Surgeons, and the Thomas D. Klingenstein & Nancy D. Perlman Family Fund.
Contributor Information
Kerstin J. Plessen, Center for Child and Adolescent Mental Health, University of Bergen, P.O. Box 7800, 5020 Bergen, Norway, Tel.: +47-55/588670, Fax: +47-55/588379, E-Mail: kerstin.plessen@rbup.uib.no, Columbia College of Physicians & Surgeons and the New York State Psychiatric Institute New York (NY), USA, Division of Psychiatry, Haukeland University Hospital, Bergen, Norway
Jason M. Royal, Columbia College of Physicians & Surgeons and the New York State Psychiatric Institute, New York (NY), USA
Bradley S. Peterson, Columbia College of Physicians & Surgeons and the New York State Psychiatric Institute, New York (NY), USA
References
- 1.Albin R, Mink J. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, DeLong MR, Strick PL. Parallel organization fo functionally segregated circuits linking basal ganglia and cortex. Annual Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 4.Banaschewski T, Neale BM, Rothenberger A, Roessner V. Comorbidity of tic disorders and ADHD. Conceptual and methodological considerations. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):I/5–I/14. doi: 10.1007/s00787-007-1002-8. [DOI] [PubMed] [Google Scholar]
- 5.Banich M. Interacting hemispheres: a means of modulating attention. In: Zaidel E, Iacoboni M, editors. The parallel brain. MIT Press; Cambridge, Massachusetts: 2003. pp. 267–270. [Google Scholar]
- 6.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Baumgardner TL, Singer HS, Denckla MB, Rubin MA, Abrams MT, Colli MJ, Reiss AL. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47:477–482. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- 8.Bloch M, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr DB, Sesack SR. Callosal terminals in the rat prefrontal cortex: synaptic targets and association with GABA-immunoreactive structures. Synapse. 1998;29:193–205. doi: 10.1002/(SICI)1098-2396(199807)29:3<193::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Castellanos F, Giedd J, Hamburger S, Marsh W, Rapoport J. Brain morphometry in Tourette’s syndrome: the influence of comorbid attention-deficit/hyperactivity disorder. Neurology. 1996;47:1581–1583. doi: 10.1212/wnl.47.6.1581. [DOI] [PubMed] [Google Scholar]
- 11.Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Vaituzis AC, Blumenthal JD, Nelson J, Bastain TM, Zijdenbos A, Evans AC, Rapoport JL. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 12.Castellanos FX, Giedd JN, Eckburg P, Marsh WL, Vaituzis AC, Kaysen D, Hamburger SD, Rapoport JL. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:1791–1796. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 14.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport J L. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos FX, Tannock R. Neuroscience of attention-deficit/ hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 16.Comings DE. Clinical and molecular genetics of ADHD and Tourette syndrome. Two related polygenic disorders. Ann N Y Acad Sci. 2001;931:50–83. doi: 10.1111/j.1749-6632.2001.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 17.Comings DE, Comings BG. A controlled family history study of Tourette’s syndrome, I: Attention-deficit hyperactivity disorder and learning disorders. J Clin Psychiatry. 1990;51:275–280. [PubMed] [Google Scholar]
- 18.Comings DE, Comings BG. Tourette’s syndrome and attention deficit disorder with hyperactivity: are they genetically related? J Am Acad Child Psychiatry. 1984;23:138–146. doi: 10.1097/00004583-198403000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 20.Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 21.Fredericksen K, Cutting L, Kates W, Mostofsky S, Singer H, Cooper K, Lanham D, Denckla M, Kaufmann W. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58:85–89. doi: 10.1212/wnl.58.1.85. [DOI] [PubMed] [Google Scholar]
- 22.Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- 23.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 24.Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 25.Giedd JN, Castellanos FX, Casey B, et al. Quantitative morphology of the Corpus Callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 26.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 27.Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S. Pediatric diffusion tensor imaging: Normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Hesslinger B, Tebartz van Elst L, Thiel T, Haegele K, Hennig J, Ebert D. Frontoorbital volume reductions in adult patients with attention deficit hyperactivity disorder. Neurosci Lett. 2002;328:319–321. doi: 10.1016/s0304-3940(02)00554-2. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application to the midsagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Hyde T, Aaronson B, Randolph C, Rickler K, Weinberger D. Relationship of birth weight to the phenotypic expression of Gilles de la Tourette’s syndrome in monozygotic twins. Neurology. 1992;42:652. doi: 10.1212/wnl.42.3.652. [DOI] [PubMed] [Google Scholar]
- 31.Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette’s syndrome: a quantitative MRI study of monozygotic twins. Neurology. 1995;45:1176–1182. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- 32.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Arch Neurol. 1990;47:919–926. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- 33.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: morphometric analysis of MRI. J Learn Disabil. 1991;24:141–146. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- 34.Kates WR, Frederikse M, Mostofsky SH, Folley BS, Cooper K, Mazur-Hopkins P, Kofman O, Singer HS, Denckla MB, Pearlson GD, Kaufmann W E. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Res. 2002;116:63–81. doi: 10.1016/s0925-4927(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 35.Keri S, Szlobodnyik C, Benedek G, Janka Z, Gadoros J. Probabilistic classification learning in Tourette syndrome. Neuropsychologia. 2002;40:1356–1362. doi: 10.1016/s0028-3932(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 36.Kimura F, Baughman RW. GABAergic transcallosal neurons in developing rat neocortex. Eur J Neurosci. 1997;9:1137–1143. doi: 10.1111/j.1460-9568.1997.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 37.Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- 38.Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 39.Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: a relentless drumbeat–driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47:537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 40.Lyoo IK, Noam GG, Lee CK, Lee HK, Kennedy BP, Renshaw PF. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biol Psychiatry. 1996;40:1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- 41.Margolis A, Donkervoort M, Kinsbourne M, Peterson B S. Interhemispheric connectivity and executive functioning in adults with Tourette syndrome. Neuropsychology. 2006;20:66–76. doi: 10.1037/0894-4105.20.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh R, Alexander GM, Packard MG, Zhu H, Wingard JC, Quackenbush G, Peterson BS. Habit learning in Tourette syndrome: a translational neuroscience approach to a developmental psychopathology. Arch Gen Psychiatry. 2004;61:1259–1268. doi: 10.1001/archpsyc.61.12.1259. [DOI] [PubMed] [Google Scholar]
- 43.Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson B S. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 45.Moriarty J, Varma A, Stevens J, Fish M, Trimble M, Robertson M. A volumetric MRI study of Gilles de la Tourette’s syndrome. Neurology. 1997;49:410–415. doi: 10.1212/wnl.49.2.410. [DOI] [PubMed] [Google Scholar]
- 46.Mostofsky S, Wendlandt J, Cutting L, Denckla M, Singer H. Corpus callosum measurements in girls with Tourette syndrome. Neurology. 1999;53:1345–1347. doi: 10.1212/wnl.53.6.1345. [DOI] [PubMed] [Google Scholar]
- 47.Pauls DL, Leckman JF, Cohen DJ. Familial relationship between Gilles de la Tourette’s syndrome, attention deficit disorder, learning disabilities, speech disorders, and stuttering. J Am Acad Child Adolesc Psychiatry. 1993;32:1044–1050. doi: 10.1097/00004583-199309000-00025. [DOI] [PubMed] [Google Scholar]
- 48.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 49.Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Dev Psychopathol. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- 50.Peterson BS, Klein J. Neuroimaging of Tourette’s syndrome neurobiologic substrate. In: Peterson BS, editor. Child psychiatry clinics of North America: neuroimaging. WB Saunders Co; Philadelphia, PA: 1997. pp. 343–364. [Google Scholar]
- 51.Peterson BS, Leckman J, Duncan J, Wetzles R, Riddle M, Hardin M, Cohen D. Corpus callosum morphology from magnetic resonance images in Tourette’s syndrome. Psychiatry Res. 1994;55:85–99. doi: 10.1016/0925-4927(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 52.Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, Leckman JF. Reduced basal ganglia volumes in Tourette’s syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43:941–949. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- 53.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 54.Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 55.Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal Ganglia volumes in patients with gilles de la tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 56.Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plessen KJ, Grüner R, Lundervold A, Xu D, Hirsch J, Hammar A, Lundervold AJ, Wentzel-Larsen T, Lie SA, Bansal R, Gass A, Peterson BS, Hugdahl K. Reduced white matter connectivity in the Corpus Callosum of children with Tourette syndrome. J Child Psychol Psychiatry. 2006;47(10):1013–1022. doi: 10.1111/j.1469-7610.2006.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, Leckman JF, Bansal R, Peterson BS. Altered interhemispheric connectivity in individuals with Tourette’s disorder. Am J Psychiatry. 2004;161:2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- 59.Rauch R, Jinkins J. Variability of corpus callosal area measurements from midsagittal MR images: effect of subject placement within the scanner. AJNR Am J Neuroradiol. 1996;17:27–28. [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson MM. Attention deficit hyperactivity disorder, tics and Tourette’s syndrome: the relationship and treatment implications. A commentary. Eur Child Adolesc Psychiatry. 2006;15:1–11. doi: 10.1007/s00787-006-0505-z. [DOI] [PubMed] [Google Scholar]
- 61.Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123(Pt 3):425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- 62.Roessner V, Banaschewski T, Uebel H, Becker A, Rothenberger A. Neuronal network models of ADHD – lateralization with respect to interhemispheric connectivity reconsidered. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I71–79. doi: 10.1007/s00787-004-1007-5. [DOI] [PubMed] [Google Scholar]
- 63.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 64.Semrud-Clikeman M, Filipek PA, Biederman J, Steingard R, Kennedy D, Renshaw P, Bekken K. Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J Am Acad Child Adolesc Psychiatry. 1994;33:875–881. doi: 10.1097/00004583-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 66.Singer H, Reiss A, Brown J, Aylward E, Shih B, Chee E, Harris E, Reader M, Chase G, Bryan R. Volumetric MRI changes in basal ganglia of children with Tourette’s syndrome. Neurology. 1993;43:950–956. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- 67.Singer HS. Tourette’s syndrome: from behaviour to biology. Lancet Neurol. 2005;4:149–159. doi: 10.1016/S1474-4422(05)01012-4. [DOI] [PubMed] [Google Scholar]
- 68.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 69.Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 70.Spessot AL, Peterson BS. Tourette syndrome: a multifactorial, developmental pychopathology. In: Cicchetti D, Cohen DJ, editors. Manual of developmental psychopathology. John Wiley; New York: 2006. pp. 436–469. [Google Scholar]
- 71.Spessot AL, Plessen KJ, Peterson BS. Neuroimaging of developmental psychopathologies: the importance of self-regulatory and neuroplastic processes in adolescence. Ann N Y Acad Sci. 2004;1021:86–104. doi: 10.1196/annals.1308.010. [DOI] [PubMed] [Google Scholar]
- 72.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev. 2006;26(4):396–432. doi: 10.1016/j.cpr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 74.WHO. ICD-10: The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostical Guideline. World Health Organization; Geneva: 1992. [Google Scholar]
- 75.Witelson SF. Clinical neurology as data for basic neuroscience: Tourette’s syndrome and the human motor system. Neurology. 1993;43:859–861. doi: 10.1212/wnl.43.5.859. [DOI] [PubMed] [Google Scholar]
- 76.Zimmerman AM, Abrams MT, Giuliano JD, Denckla MB, Singer HS. Subcortical volumes in girls with tourette syndrome: support for a gender effect. Neurology. 2000;54:2224–2229. doi: 10.1212/wnl.54.12.2224. [DOI] [PubMed] [Google Scholar]


