Abstract
Purpose
Achieving simultaneous single and clear visual experience during postnatal development depends on the temporal relationship between accommodation and vergence, in addition to their accuracies. This study was designed to examine one component of the dynamic relationship, the latencies of the responses.
Methods
Infants and adults were tested in three conditions i) Binocular viewing of a target moving in depth at 5cm/s (closed loop) ii) monocular viewing of the same target (vergence open loop) iii) binocular viewing of a low spatial frequency Difference of Gaussian target during a prism induced step change in retinal disparity (accommodation open loop).
Results
There was a significant correlation between accommodation and vergence latencies in binocular conditions for infants from 7 to 23 weeks of age. Some of the infants, as young as 7 or 8 weeks, generated adult-like latencies of less than 0.5 s. Latencies in the vergence open loop and accommodation open loop conditions tended to be shorter for the stimulated system than the open loop system in both cases, and all latencies were typically less than 2 seconds across the infant age range.
Conclusions
Many infants between 7 and 23 weeks of age were able to generate accommodation and vergence responses with latencies of less than a second in full binocular closed loop conditions. The correlation between the latencies in the two systems suggests that they are limited by related factors from the earliest ages tested.
Keywords: infant, accommodation, vergence, latency, cue
INTRODUCTION
Single and clear visual experience in a dynamic environment requires concurrent adjustment of both the alignment and focus of the eyes. A binocular single image is achieved using vergence eye movements to align the eyes with the object of interest and then by neurally combining the retinal images into a single representation. A focused retinal image is achieved when ocular accommodation overcomes the difference between an eye’s refractive error and the dioptric distance of the object being viewed.
Accommodation responses and vergence eye movements, in reality, do not act in isolation. They are coupled in human adults and typically occur concurrently even when only one system is directly stimulated (Maddox, 1887; Alpern & Ellen, 1956; Fincham & Walton, 1957). Under monocular viewing conditions an accommodation response is correlated with a change in alignment of the eyes even though the retinal disparity cue is absent (this response is termed accommodative vergence) and when there is no change in blur stimulus a vergence response is still correlated with a change in focus of the eyes (this response is termed vergence accommodation). The sensorimotor neural pathways for vergence and accommodation are linked at the levels of both the cortex and mid-brain (Judge & Cumming, 1986; Zhang, Mays & Gamlin, 1992) and models of the control of these coupled responses have been developed (Eadie & Carlin, 1995).
Misalignment and defocus of the eyes can both influence the postnatal development of the visual system during the critical or sensitive period. Studies of humans with clinical abnormalities (e.g. Banks, Aslin & Letson, 1975; Birch & Stager, 1985) have suggested, and studies of animal models (e.g. Kiorpes & Boothe, 1980; Harwerth, Smith, Boltz, Crawford & von Noorden, 1983; Kiorpes, Kiper, O’Keefe, Cavanaugh & Movshon, 1998) have demonstrated, the disruptive effects of abnormal visual experience on the development of binocularity and the contrast sensitivity function, among other aspects of visual performance.
With regard to typical postnatal development, low gain accommodation and vergence responses are present soon after birth (e.g. Banks, 1980; Aslin, 1977) and a number of studies have suggested that the interdependent coupling of accommodation and vergence is present in early infancy: Aslin and Jackson (1979) were the first to demonstrate the presence of a convergence response under monocular viewing conditions in infants as young as 2 months of age, Bobier, Guinta, Kurtz and Howland (2000) found that retinal disparity drove accommodation by 4 months of age, and Turner, Horwood, Houston & Riddell (2002) demonstrated the presence of accommodation and vergence responses in both binocular and monocular conditions after two months with mixed evidence at younger ages.
Infants must therefore achieve a balance in their use of the independent and coupled components of accommodation and vergence responses in a dynamic environment if they are to achieve single and clear vision simultaneously. Although the collection of data from infants is complicated by their short attention span and reduced response repertoire, the goals of this study were to provide a qualitative understanding of the interactions between accommodation and vergence responses in dynamic conditions, and to provide the first quantitative description of their different response latencies. The approach taken was to measure the latencies of open loop and closed loop accommodation and vergence responses of human infants.
In adults the latencies of accommodation and vergence and their coupled responses are well matched down to the scale of milliseconds, although the absolute values vary across experimental conditions in different studies. The general relationship is shown in the data of Heron, Charman & Schor (2001) (Table 1), while the following discussion provides data from other studies. With all cues present, typical human adult accommodative latencies are on the order of 300 to 400 ms (e.g. Campbell & Westheimer, 1960; Phillips, Shirachi & Stark, 1972) while vergence latencies are between 100 and 200 ms (Rashbass & Westheimer, 1961; Krishnan, Farazian & Stark, 1973). In studies of the coupled responses, Wilson (1973) found that the accommodative vergence latency was longer than the disparity driven vergence latency, by about 200 to 300 ms. The accommodative vergence latency was still shorter than the accommodative latency however (which Heron & Winn (1989) found to change little between monocular and binocular conditions). Schor, Lott, Pope & Graham (1999) found a similar result, the accommodative vergence latency was around 175 ms while that of accommodation was around 300 ms. Suryakumar, Meyers, Irving & Bobier (2007) have recently reported data for vergence accommodation. They found latencies of approximately 190 ms for disparity-driven vergence, 240 ms for blur-driven accommodation and 290 ms for vergence accommodation. This is in good agreement with Krishnan, Shirachi & Stark (1977) who found an average vergence accommodation latency of 260 ms. Thus, in adults, all of these components of the responses are capable of contributing to performance within half a second of stimulus onset. Our goal was to determine whether a similar relationship exists for infants; vergence being faster than accommodation and the coupled response typically being slower than the direct response for each system.
Table 1.
Mean latencies for adult accommodation and vergence responses in open loop conditions, from Heron, Charman & Schor (2001). Average latencies for 13 subjects from 16 to 48 years of age.
| Heron, Charman & Schor (2001) Response | Mean Latency (ms) | |
|---|---|---|
| Far to Near | Near to Far | |
| Accommodation | 317 ± 142 | 301 ± 126 |
| Accommodative Convergence | 148 ± 95 | 168 ± 92 |
| Convergence | 132 ± 74 | 116 ± 39 |
| Convergence Accommodation | 362 ± 197 | 272 ± 176 |
A comparison of infants’ latencies in reduced-cue conditions with those in fully naturalistic conditions would also provide evidence about the potential importance of different cues in naturalistic conditions. For example, a significant delay in the reduced cue responses relative to the full cue responses would indicate a small role for the remaining reduced cue components in the initial period of a full cue response.
To date, only accommodation latencies in binocular viewing have been measured during infancy (Tondel & Candy, 2007) – they were typically less than a second after 8 weeks of age when infants tracked a smoothly moving target. The latencies of the other components have not been measured and so infants’ ability to maintain synchronized single and clear vision in a highly dynamic environment is largely unknown. Tondel & Candy (2007) and Aslin (1977) have demonstrated that infants have the capability to track moving targets with accommodation and vergence respectively, at least after approximately three months of age, but the temporal relationship between the systems is not understood.
METHODS
Three experimental conditions were presented using two sets of apparatus. In one set, the subjects viewed a moving target in binocular and monocular conditions. Binocular viewing provided all of the naturalistic accommodative and vergence cues and feedback, and therefore was a full ‘closed loop’ condition (CL). The monocular viewing was considered reduced-cue or ‘open loop’ for the vergence system (VOL) because the direct cue for vergence, retinal disparity, was absent (e.g. Maddox, 1887; Alpern & Ellen, 1956; Fincham & Walton, 1957). The fact that the target moved in real space meant that there were also naturalistic proximity cues in this apparatus. In the second apparatus, the subject viewed a stationary low spatial frequency target binocularly while a prism was introduced before one of their eyes to manipulate retinal disparity. The subject could experience a change in accommodation without detecting any change in blur of this stimulus. As blur is the direct cue for accommodation, and its feedback information was removed, this condition was considered ‘open loop’ for the accommodative system (AOL) (Kotulak & Schor, 1987; Suryakumar et al., 2007). The target in this apparatus remained stationary and therefore only presented a stationary proximity cue – in competition with the step retinal disparity stimulus.
Subjects
The subjects were recruited from the local community. A total of 67 infants between 6 and 23 weeks of age were tested in the CL and VOL conditions. They were all reported to be full term by their parents. Eighteen of them came for between two and four visits (at two to four week intervals), giving a total of 90 sessions. Five pre-presbyopic adults were tested for comparison. A total of 42 infants between 7 and 21 weeks of age were tested in the AOL condition. Four of them were tested twice (after a two to four week interval), giving a total of 46 sessions. Four pre-presbyopic adults were tested for comparison. Twenty-six of the infants and one adult were tested in all three conditions in their visit.
The infants’ parents and the adult subjects all gave informed consent before taking part in the data collection. The study followed the tenets of the Declaration of Helsinki and was approved by the Indiana University Bloomington Campus Committee for the Protection of Human Subjects.
Procedure
Accommodation and vergence responses were recorded simultaneously using a commercially available video-based eccentric photorefractor, the PowerRefractor (Multi Channel Systems). The data were gathered at 25Hz from a distance of 1m, which enabled infants to be placed in a relatively naturalistic setting (Choi, Weiss, Schaeffel, Seidemann, Howland, Wilhelm & Wilhelm, 2000; Blade & Candy, 2006). The instrument’s defocus and gaze-position measurements were not calibrated for each individual subject as the only absolute quantitative analyses were completed in the time domain. Blade and Candy (2006) have demonstrated that the default defocus calibrations in the software are sufficiently accurate for these time-based latency analyses for both infants and adults (the slopes of the relative calibration slopes are close to one). With regard to measures of vergence, the instrument’s handbook states that the gaze position data are accurate to within two degrees and the current stimuli were all greater than 5.5 degrees (see figures 3 & 4).
Figure 3.
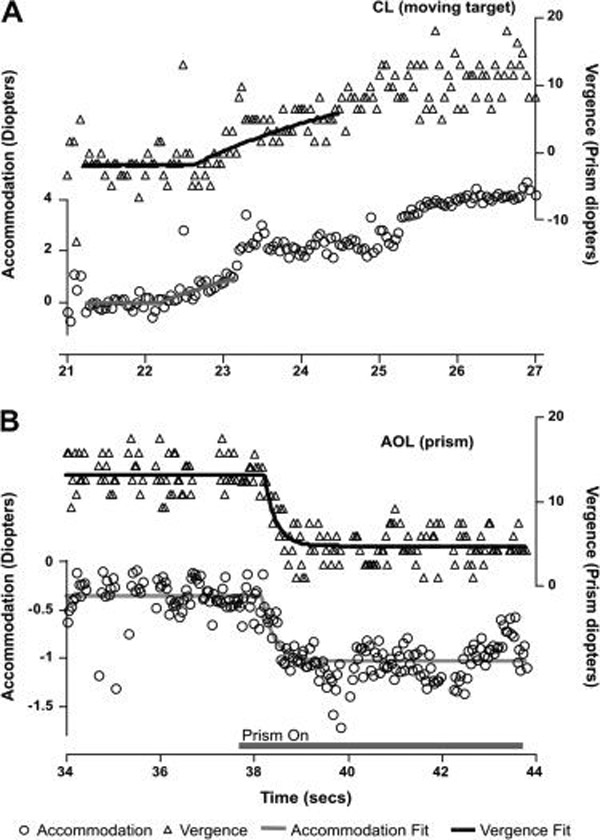
Examples of the functions fit to the accommodation and vergence data collected in the CL (panel A, an 11 week-old) and AOL (panel B, an adult) conditions. The R2 values for the CL fits were 0.46 for the accommodation data (48 data points) and 0.49 for the vergence data (82 data points). This individual’s CL data are presented as examples of fits that were restricted in length to reduce the impact of mismatches between the stimulus and response function shapes on the latency estimate.
Figure 4.
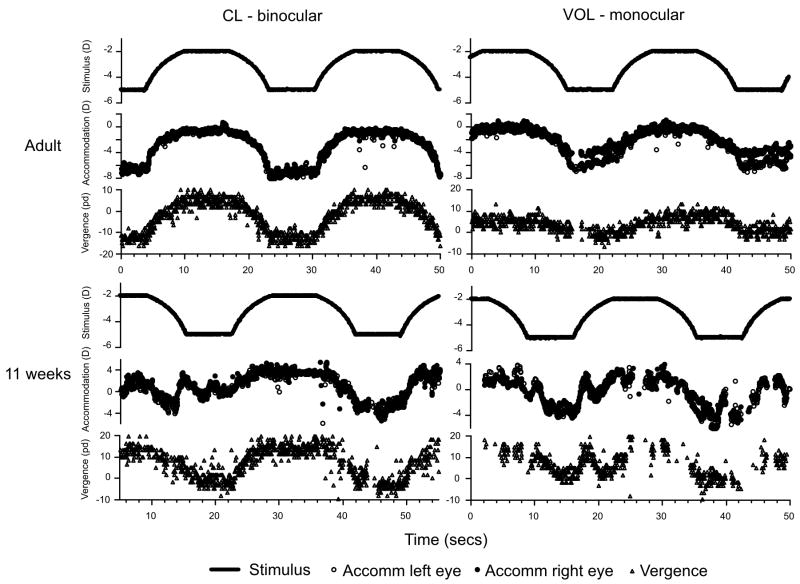
Examples of raw data collected from an adult and an infant in the CL and VOL conditions. The infant’s accommodation data in the VOL, monocular, condition demonstrate that the accommodation is consensual at this age. The accommodation and vergence data are plotted relative to infinity, although the instrument was not calibrated for individual subjects (in terms of dioptric calibration, angle lambda or the Hirschberg ratio). The temporal structure of the response is therefore accurate, but the dioptric or angular response accuracy was not tested.
The infants wore no optical correction and were placed in a car seat or on their parent’s lap, with their head gently supported and stabilized. The adults, who were either emmetropic or wearing their habitual soft contact lens correction, were seated on a chair with no chinrest. The axis of the photorefractor camera was aligned with the bridge of the subject’s nose and the target was positioned centrally between the subject’s eyes in the real-time image from the photorefractor. The room was kept in dim illumination to attract the subjects’ attention to the task.
The ‘closed loop’ (CL) and vergence ‘open loop’ (VOL) apparatus
A high contrast colored picture of a clown was used as the target. The image was 3 cm by 2 cm in size and had a broad spatial frequency amplitude spectrum. It was mounted on a small internally illuminated box. The luminance of the target was 30 cd/m2 unless the subject’s pupils were small, in which case it was reduced to cause pupil dilation above the required 3mm minimum for the photorefractor to function. As the infants could not be instructed to maintain fixation on the stimulus, the adults were merely told to look at the clown with no further instruction (Horwood, Turner, Houston & Riddell, 2001).
A stepper motor was used to move the target along a track between viewing distances of 20 and 50 cm (see Figure 1, panel A). The target was immediately below the camera axis, at angles of 3 deg for the 50 cm viewing distance and 7 deg for the 20 cm viewing distance. The stimulus velocity was 5 cm/s, which approximated a 0.5 D/s movement, although it was really exponential in shape on a dioptric scale. The movement duration was six seconds. The target was automatically paused for eight seconds between movements, but it could also be paused manually as necessary during the data collection. The stimulus position as a function of time was recorded using a linear potentiometer sampling at 5 KHz. These position data were synchronized with the photorefractor recordings using a trigger pulse at the start of each recording. The movement of the stimulus was correlated with audible noises from the motor (located adjacent to the camera at the 1m distance), which helped attract the infants’ attention. Occasionally toys were also used to attract an infant’s attention, but only when the stimulus was stopped between movements and only at the current distance of the target. Thus these additional cues were not informative about the target position during its motion.
Figure 1.
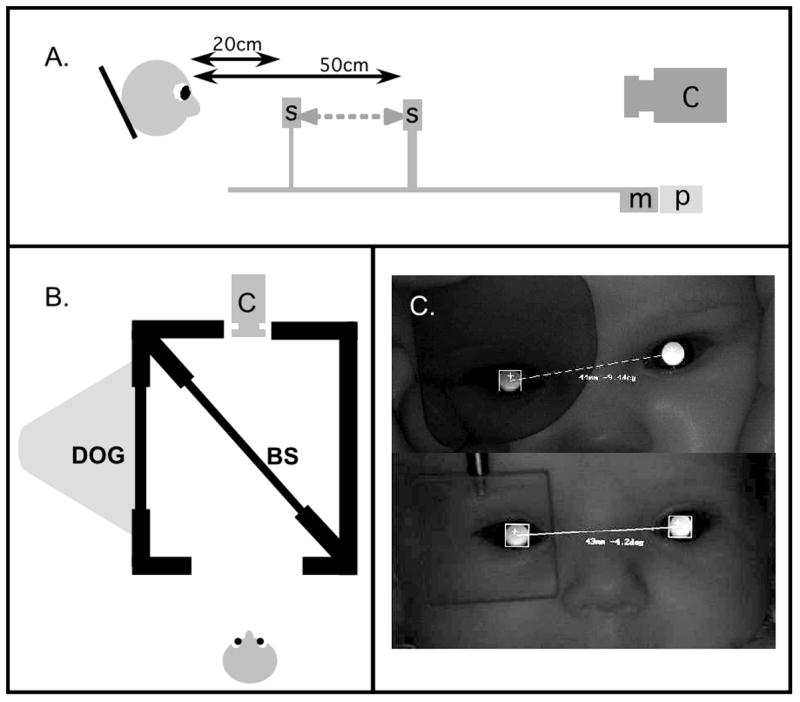
The experimental apparatus. Panel A: For the CL and VOL ramp protocols, the stimulus, S, was moved by a motor, m, along a track in front of the stabilized subject. The stimulus position was recorded using a linear potentiometer, p, and the responses were recorded using a photorefractor, c. Panel B: In the AOL protocol, the subject viewed a DOG target via a beamsplitter, while responses were recorded with the photorefractor. Panel C: An IR filter was placed before one of the subject’s eyes in the VOL condition. The photorefractor could collect data binocularly, as shown in the top image, although the subject could not see through the filter. A prism was placed before the eye for the AOL condition, as shown in the bottom image.
The monocular, VOL, viewing conditions were generated by placing a highpass glass filter with a cutoff at 850 nm (Edmunds Optics) over one eye (see Figure 1, panel C). The subject could not detect the target when viewing through this filter and so they were rendered monocular even though the photorefractor could collect data through the filter and hence record binocularly.
At least six stimuli were presented (three in each direction) in both the binocular and monocular conditions. While the stimuli were designed to elicit detectable responses from both adults and infants based on data in the literature, the repetitions were performed to monitor data quality and allow for inattention and intermittent cooperation. The binocular CL data were always collected before the monocular, VOL, data to increase the likelihood of cooperation as young infants have been noted to resist occlusion of an eye (Currie & Manny, 1997; Turner et al, 2002).
The accommodation ‘open loop’ (AOL) apparatus
In the AOL viewing condition, the subjects were positioned in front of a large black box containing a 58 cm diameter beamsplitter rotated about a vertical axis (see Figure 1, panel B). Three walls of the box contained 30 cm diameter apertures. The subject viewed through one aperture, the photorefractor was aligned with their eyes through a second, and the stimulus target was presented through the third. Thus the target and photorefractor camera were on the same optical axis.
The target was designed to minimize blur cues and feedback to produce accommodation ‘open loop’ conditions. A vertically oriented ‘difference of Gaussians’ (DOG) was used, that only contained low spatial frequencies and yet provided effective vergence information when viewed binocularly (Kotulak & Schor, 1987). The luminance profile of the two-dimensional target was initially defined using the following equation:
where x is spatial position in the horizontal dimension and σ is the space constant, both in degrees. The space constant was set to 1.6 degrees. This function was extended uniformly along the vertical dimension.
The profile was then multiplied by another, two-dimensional, luminance Gaussian function with the following equation:
where p is the radial distance from the center of the image. This Gaussian was used to minimize the luminance at the edge of the target and therefore minimize the spatial contrast at the edge of the aperture (the target is shown in figure 2). A Fourier transform of the final combination demonstrated that the amplitude spectrum (contrast as a function of spatial frequency) fell to below 0.15% by 0.5cpd. This spatial frequency content has been shown to provide a poor accommodative stimulus for adults (Charman & Tucker, 1978; Kotulak & Schor, 1987) and pre-school children (Suryakumar & Bobier, 2004).
Figure 2.
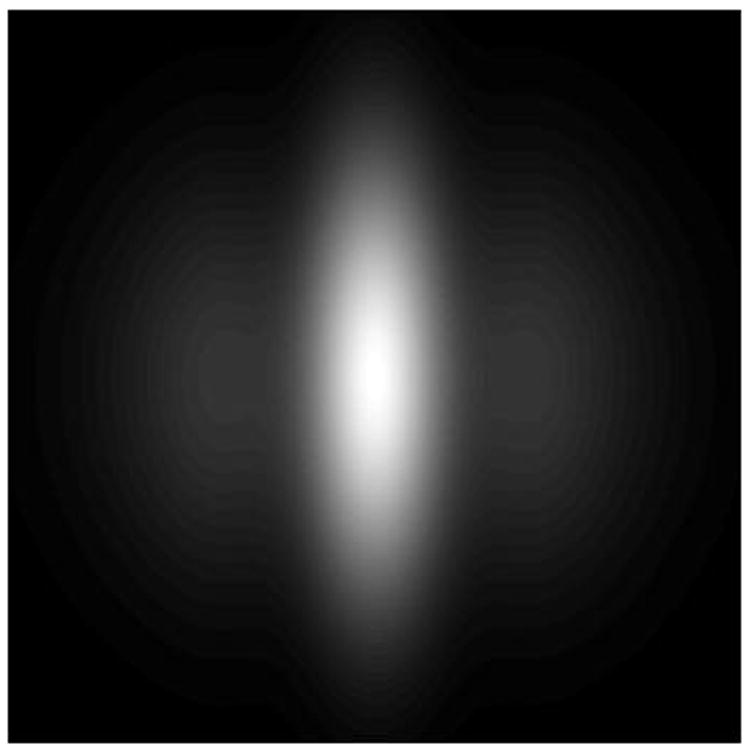
The difference of Gaussian (DOG) target used to provide accommodation open-loop conditions.
The target was printed on an overhead transparency using the full luminance look-up table range and mounted in the appropriate aperture in the box. It subtended 15.6 by 15.6 degrees. Diffuser material was mounted over the transparency to further reduce the contrast at high spatial frequencies, and then the target was back-illuminated uniformly with an incandescent bulb. The maximum luminance at the center of the stimulus was 38.5 cd/m2 and the minimum at the periphery was 0.1 cd/m2.
The dynamic stimulus to the oculomotor system in this apparatus was provided by placing a stick prism (Bernell Corp) before one eye. The goal was to provide a change in retinal disparity with no detectable change in blur. Two prism powers were used (one and ten prism diopters) to test the validity of the responses. Both prisms might provide a distraction while held close to the face, but the one prism diopter prism would provide less change in retinal disparity than the ten prism diopter version, and therefore should stimulate less vergence and accommodation. Each prism, in base out orientation, was placed over one eye and then removed at least three times during a recording session. The timing of the introduction and removal of the prism was confirmed using the recorded photorefraction video images (sampling every 40ms).
Data Analysis
After each infant visit, and before the data were examined, an experimenter noted a subjective assessment of the session on a scale from zero to five based on the infant’s behavior and cooperation. A score of zero implied that the infant was sleepy or fussy, and a score of five indicated sustained calm attention. All of the infant sessions given a subjective score of zero by the experimenter were excluded from the data analysis. Other photorefractor images were excluded if the subject’s pupils fell below the photorefractor’s minimum acceptable size of 3mm, if the eye position was greater than 15 degrees eccentricity from the pupillary axis (to avoid apparent changes in accommodation resulting from peripheral changes in refraction (Navarro, Artal & Williams, 1993; Seidemann, Schaeffel, Guirao, Lopez-Gil & Artal, 2002), or if the refraction estimate was outside the +4 to −6D working range of the instrument (Choi et al., 2000).
Further analysis was only performed on responses that were clearly stimulus-driven. A response was considered stimulus-driven, or scorable, if it started after the beginning of the stimulus, the final position was in the expected direction of change, there was a stable position before and after the response, there were no missing data due to blinks that made the latency estimation ambiguous, and data from both eyes were present. While this set of criteria risks excluding some repeatable and interesting form of immature behavior that infants might exhibit, most of the data were excluded under the missing data or unstable starting or ending position criteria and visual inspection revealed no consistent tendency for other strategies, such as responses occurring in the wrong direction for example. These criteria provided a fair representation of the qualitative aspects of the data. Each subject’s first response that met the inclusion criteria was included in the quantitative analysis. Any further responses were included in an analysis of repeatability. For the binocular viewing condition, the eye included in the analysis was selected using a criterion of minimizing the number of missing data points (due to any effect of blinks for example).
The ‘closed loop’ (CL) and vergence ‘open loop’ (VOL) conditions
The latencies of the accommodation and vergence responses were estimated by fitting the initial section of the appropriate stimulus function to the beginning of the response. The approach is described in and was used to fit the ramp data in Tondel & Candy (2007). It is described briefly here. We wished to minimize the assumptions made about the shape of the infants’ responses to the exponential stimulus and to avoid having any assumptions about response shape influence the latency estimate. Therefore only the beginning of the stimulus function and response were used.
The stimulus position data were fit with a function first. The fit extended from at least one second before to at least one second after the completion of the movement. The following equation was used for each disaccommodation or divergence stimulus:
| 1 |
where: B = average stimulus position before the movement
Tb = time at the beginning of the stimulus movement
Te= time at the end of the stimulus movement
A = amplitude of the stimulus movement
Ta = time constant
B, Tb, Te, A and Ta were all free parameters in the fit. The function was reversed for the fit to the accommodation/convergence stimuli, which moved in the opposite direction. All of these fits had an R2 greater than 0.985.
The initial section of this stimulus function was then fit to the beginning of the response data to provide a visually acceptable fit (Figure 3, panel A, and see Figure 3 in Tondel & Candy, 2007). The fit extended from approximately one second before the response to at least one second after the beginning of the response. The free parameters in the fit to the response, R(t), were Tbr and Br.
| 2 |
where: Br = average accommodation/vergence before the beginning of the response
Tbr = time at the beginning of the response
The latency of the response could then be calculated in seconds from Tbr−Tb.
Again, this function was reversed for the fit to the accommodation/convergence responses in the opposite direction.
The analysis of the vergence data required an additional step to calculate the stimulus function. The vergence stimulus needed to be converted from meter angles (1/viewing distance) to the angular unit of prism diopters for comparison with the photorefractor response output. The photorefractor provides a vergence measurement in prism diopters (based on the eyes’ horizontal gaze position in degrees and a population average of the Hirschberg ratio). The conversion from meter angles to prism diopters requires the subject’s interpupillary distance (IPD) for distant viewing. Each adult’s IPD was measured and used to make their individual conversion. For the infants, the IPD was determined using a function derived from measurements of 18 infants viewing in the distance (ages 6 to 20 weeks). A linear regression was used to calculate IPD in millimeters as a function of age in weeks over this range (IPD = 0.49* age + 34.8 with an R2 of 0.43). (These data were collected over a narrower age range and sampled more frequently than the function described by MacLachlan and Howland (2002), who averaged their data collected during the first year. Hence a direct comparison of the two studies cannot be made).
The accommodation ‘open loop’ (AOL) condition
The latencies of the accommodation and vergence responses were determined after introduction and removal of the prism in the AOL condition (Figure 3). The motion of the prism effectively had a step temporal profile, and so the exponential function in equation 1 was fit directly to the response data, as has been done in the past for responses to step stimuli (Beers & Van Der Heijde, 1994; Yamada & Ukai, 1997; Tondel & Candy, 2007; but see also e.g. Semmlow & Yuan (2002) and Bharadwaj and Schor (2006) for other approaches to fitting the data).
Before the fitting procedure was undertaken, the vergence data recorded by the photorefractor were also corrected for the optical effect of the prism in this AOL condition. The photorefractor records gaze position by tracking the position of the first Purkinje image relative to the pupil center. Introducing a prism before a stationary eye shifts the Purkinje image by an amount equivalent to the prism power. This shift would be recorded as an eye movement by the photorefractor. An eye that rotates to compensate for the prism will realign the Purkinje image with the pupil center mimicking the conditions present before the prism was introduced, and suggesting no change in eye position after introduction of the prism. A correction for the presence of the prism was therefore subtracted from the vergence data whenever the prism was present in the recorded video images.
The latency was finally determined by calculating the difference between the time at which the prism was first aligned in the video and the start of the fitted accommodation or vergence response (Tb). The response function was fit over a range from at least three seconds before the prism alignment to three seconds after that time.
The R2 values for the fits to the CL, VOL and AOL accommodation data had a mean and standard deviation of 0.53 ± 0.21 in the infants and 0.66 ± 0.24 in the adults. The corresponding values for the fits to the vergence data were 0.39 ± 0.21 in the infants and 0.48 ± 0.31 in the adults. These fits were typically performed on approximately 100 data points, and were also confirmed to be visually acceptable.
All of the fits were performed using Matlab, and the statistical analyses were completed using SPSS.
RESULTS
Forty infants, aged 6 to 23 weeks, provided scorable CL data (48 sessions) and eleven infants, aged 12 to 22 weeks, provided scorable VOL data. The VOL subjects were all in the group that provided CL data. Thirteen infants, aged 7 to 17 weeks, provided scorable AOL data. Seven of these infants were in the group that provided CL data (see Table 2).
Table 2.
Number of subjects who participated in each condition and the number who provided usable data.
| Condition | Closed Loop | Vergence Open Loop | Accommodation Open Loop | All 3 |
|---|---|---|---|---|
| Infants Attempted | 67 (6–23 weeks of age) | 42 | 26 | |
| Sessions Run | 90 | 46 | 26 | |
| Infants providing usable data | 40 (6–23 weeks) | 11 (12–22 weeks) | 13 (7 to 17 weeks) | 3 |
Raw data
Figure 4 shows examples of raw data recorded from an adult and an 11 week-old infant. Their accommodation and vergence responses are shown for the CL and VOL viewing conditions. A negative refraction indicates that the subject was focused myopically and a positive value indicates that the subject was focused hyperopically (the accommodation data from the two eyes overlie each other). These data have been shifted by 1D (the camera’s viewing distance) to make them approximately relative to infinity. Equally, a negative vergence indicates convergence and a positive vergence indicates divergence. The data have not been corrected for each individual’s angle lambda, and therefore the recorded vergence position is likely to be more divergent than the true position of the visual axes. This does not affect the outcome of the latency analysis as it is a constant offset and the data are analyzed in terms of their dynamics only. These data demonstrate that the adult and infant were able to respond to the stimulus in the appropriate direction and with response durations related to those of the stimuli. They were able to do this under binocular and monocular conditions indicating that, consistent with previous literature, this 11 week-old (and adult) generated both accommodation and vergence responses when one eye was occluded -- in the absence of retinal disparity cues (Aslin & Jackson, 1979; Turner et al., 2002). This is indicative of an accommodative vergence response.
Figure 5 shows examples of responses to the prism in the AOL condition. Panels A and B show adult and seven week-old infant data after correction for the optical effect of the 10 pd prism. The data demonstrate convergence and an increase in accommodation when the base out prism is introduced and divergence and disaccommodation when the prism is removed. Nine infants, from 7 to 17 weeks of age, provided responses to both the 10pd and 1pd prisms (seven of them in both directions). The mean vergence response was 7.4pd to the 10pd prism and 0.8pd to the 1pd prism (t = −8.40, df = 15, p < 0.001). The mean accommodation response was 2.08 D to the 10pd prism and 0.39 D to the 1pd prism (t = 5.64, df = 15, p < 0.001). This result is consistent with the findings of Bobier et al (2000) who also noted that infants’ responses depended on prism power, and demonstrates that the 10pd responses were due to the change in retinal disparity rather than any distraction resulting from the presence of the prism. These data from the nine infants are also in good agreement with the infant stimulus CA/C ratio of 0.17 D/pd found by Bobier et al, in that the mean infant CA/C ratios calculated for the 10pd data are 0.21 D/pd for the stimulus ratio and 0.28 D/pd for the response ratio.
Figure 5.
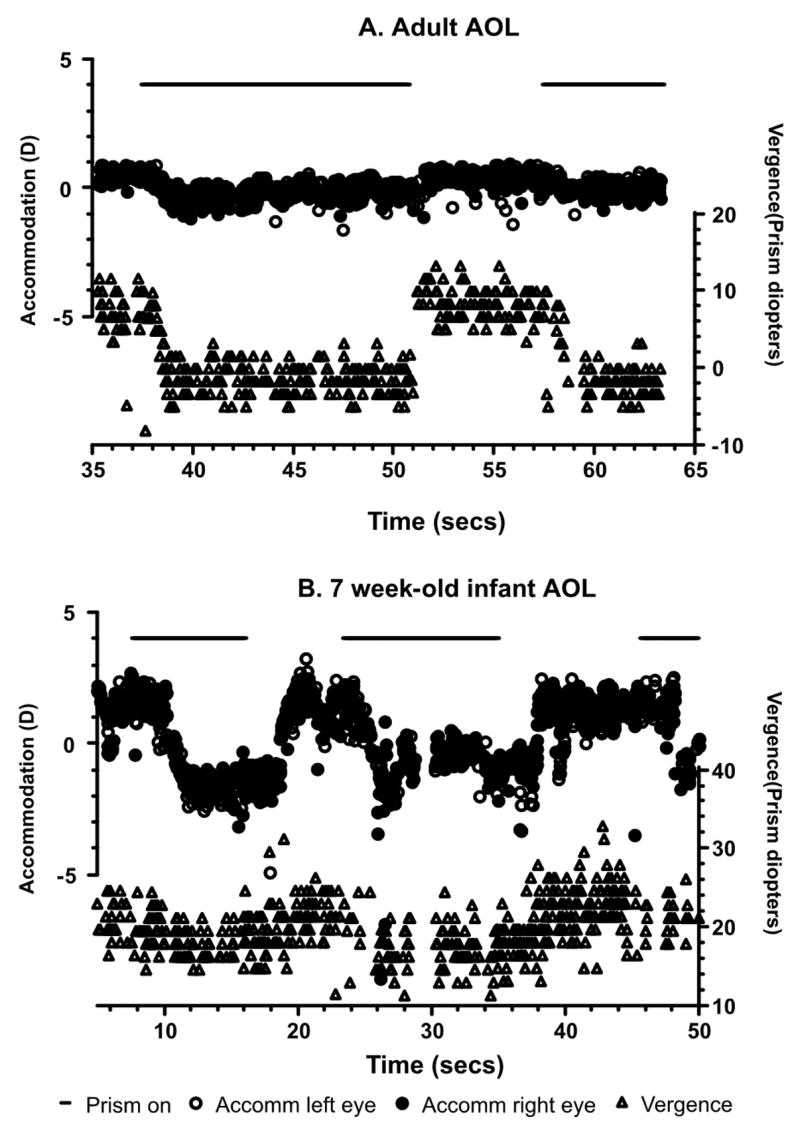
Examples of raw data collected from an adult and an infant in the AOL conditions. The infant data suggest a larger accommodative response than the adult, which would be consistent with the results of Bobier et al (2000), who found infants to have a larger CA/C ratio than adults.
Latency data
Latencies derived from the fits to the data are shown in Table 3 and Figures 6–8. Figure 6, Panel A shows accommodative and vergence latencies for the far to near (FN) stimulus in binocular CL viewing conditions. Each subject’s first response that met the inclusion criteria is presented. The absolute latencies of the responses depend on a combination of the each subject’s capability and their motivation. The data suggest that at least a number of infants were capable of responding in an adult-like timeframe. Considering the infant group only, the accommodation and vergence latencies were correlated with each other (n = 36, Pearson correlation = 0.88, p < 0.001), but neither the accommodation nor vergence latencies were correlated with age (Accommodation latencies: Pearson correlation = 0.05, p = 0.75, Vergence latencies: Pearson correlation = 0.005, p = 0.98). With regard to the difference in latencies between the accommodation and vergence responses, vergence was on average faster than accommodation in adults by 14 ms (range 20 ms slower to 60 ms faster across the three subjects). In the infants the mean difference was −138 ms (SD +/− 267 ms across 36 infants), implying that the accommodation response was faster than the vergence response in these infants (the mean difference was significantly different from zero; t = −3.101, df = 35, p = 0.004 in a two-tailed t test). The difference in latencies in the infants was also not correlated with age or the average of the individual’s accommodation and vergence latencies (age Pearson correlation = 0.10, p = 0.56; average latency Pearson correlation = −0.003, p = 0.99).
Table 3.
Mean response latency (and standard deviation) for the different viewing conditions.
| Condition | Accommodation (ms) | Vergence (ms) | Difference (ms) | ||||
|---|---|---|---|---|---|---|---|
| NF | FN | NF | FN | NF | FN | ||
| CL | Infant | 617 (±533) n = 33 | 692 (±528) n = 36 | 951 (±680) n = 33 | 830 (±545) n = 36 | −335 (±354) n = 33 | −138 (±267) n = 36 |
| Adult | 517 (±230) n = 4 | 221 (±136) n = 3 | 487 (±160) n = 4 | 208 (±94) n = 3 | 30 (±201) n = 4 | 14(±42) n = 3 | |
| VOL | Infant | 779(±349) n = 8 | 844 (±624) n = 3 | 1215 (±355) n = 8 | 1157 (±710) n = 3 | − 435 (±386) n = 8 | − 313 (±135) n = 3 |
| Adult | 412 (±319) n = 5 | 163 (±113) n = 2 | 746 (±200) n = 5 | 417 (±166) n = 2 | −334 (±444) n = 5 | −254 (±53) n = 2 | |
| AOL | Infant | 1302 (±732) n = 12 | 1185 (±686) n = 6 | 927 (±611) n = 12 | 1192 (±647) n = 6 | 375 (±402) n = 12 | 6 (±395) n = 6 |
| Adult | 544 (±163) n = 3 | 556 (±256) n = 4 | 185 (±85) n = 3 | 334 (±249) n = 4 | 359 (±182) n = 3 | 222 (±59) n = 4 | |
Figure 6.
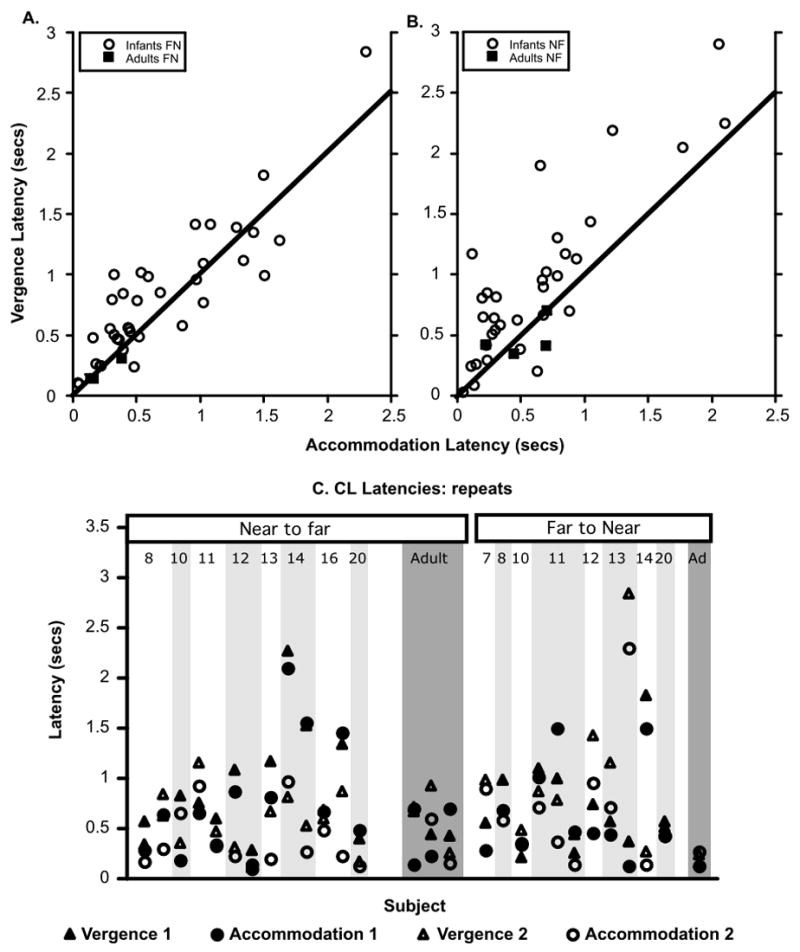
Accommodative and vergence latencies of infants and adults for the CL, (binocular) conditions. Panel A: Relationship between the accommodation and vergence latencies of all the subjects in the far to near direction. Panel B: Relationship between the accommodation and vergence latencies of all the subjects in the near to far direction. Panel C: The data from the subjects who provided more than one set of latencies, in either the NF or FN condition. The ages of the infants in weeks are shown at the top of the graph.
Figure 8.
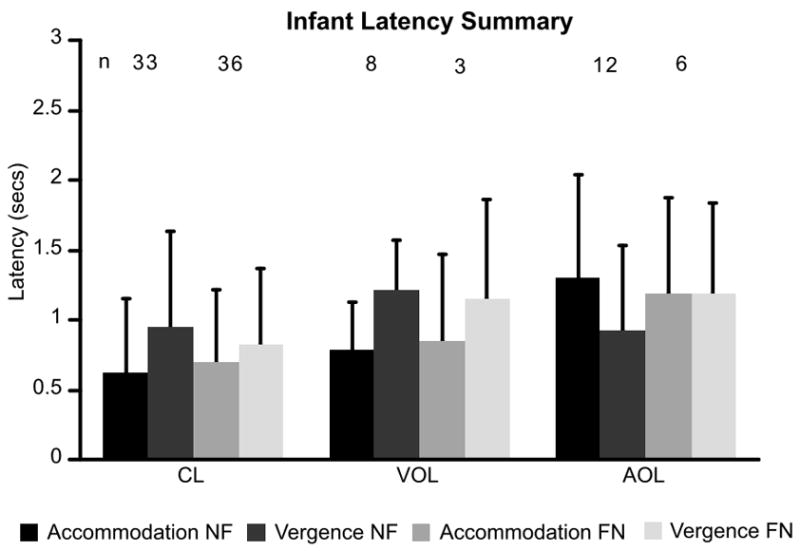
A summary of the infant accommodation and vergence latency data across the different stimulus directions (NF or FN) and different viewing conditions. The numbers of subjects in each group are included at the top of the graph.
The latencies of the responses in the opposite direction (NF) demonstrated the same effects, as shown in Figure 6, panel B. The accommodation and vergence latencies were correlated in infants (n = 33, Pearson correlation = 0.86, p < 0.001) and neither the accommodation nor vergence latencies, nor their difference, were correlated with infant age over this range (Accommodation latencies: Pearson correlation = 0.27, p = 0.13, Vergence latencies: Pearson correlation = 0.15, p = 0.42 & difference in latency: Pearson correlation = 0.12, p = 0.50). The mean latency difference for the responses in this direction was −335 ms for the infants (SD +/− 354 ms across the 33 responses) and 30 ms for the adults (range −210 to 270 across 4 responses). The mean difference in the infants was again significantly different from zero (t = −5.417, df = 32, p < 0.001 in a two-tailed t test) suggesting that accommodation was faster than vergence.
Twenty-one infants provided scorable responses in both the near to far and far to near CL directions. A repeated measures ANOVA was performed to determine the effect of direction. The data were collapsed across age, as age was not significantly related to latency difference in the full datasets. The within-subject factors were response (accommodation or vergence), and direction (NF or FN). The response main effect and the interaction between direction and response both had a significance of <0.05 (response, F(1, 20) = 17.9, p < 0.001; interaction, F(1, 20) = 5.09, p = 0.035). Consistent with the full datasets, accommodation was initiated more quickly than vergence and the difference was greater for NF than FN in these within-subject data. The main effect of direction was not significant (F(1, 20) = 0.21, p = 0.65).
The latency data from subjects who provided repeated responses for the same stimulus direction are shown in Figure 6, panel C. The data are labeled according to the subject’s age, and demonstrate that some of even the youngest infants were capable of responding with adult-like latencies. The change in mean latency across repetitions is presumably indicative of attentional or motivational factors, although each response will also contain random variation. One would expect the difference between the latencies of the two systems, however, to remain more stable (assuming that this difference results from stable independent delay in the two systems occurring only after the influence of common factors such as attention). The mean absolute change in difference between the accommodation and vergence latencies in infants was 293 ms (SD +/− 259 ms, n = 24) and in adults was 331 ms (range 114 to 513 across the 3 subjects). These data seem to suggest considerable variability relative to typical latency values in the studies of adult subjects described above, which is likely to reflect the precision of the measurement technique (Figures 3 & 4).
These same analyses were performed for the VOL and AOL latencies. The data are shown in Figure 7. The VOL data are shown on the left side and the AOL data on the right side of each panel. The data are in the same format as Figure 6. Panel A shows the data from individuals for the FN direction, panel B shows the data from the NF direction, and panel C shows the data from subjects who completed repeated responses. The numbers of subjects providing usable data in these conditions were smaller and therefore the analyses had less statistical power. Correlations were not calculated for sample sizes of ten or less and only the significant correlations are reported.
Figure 7.
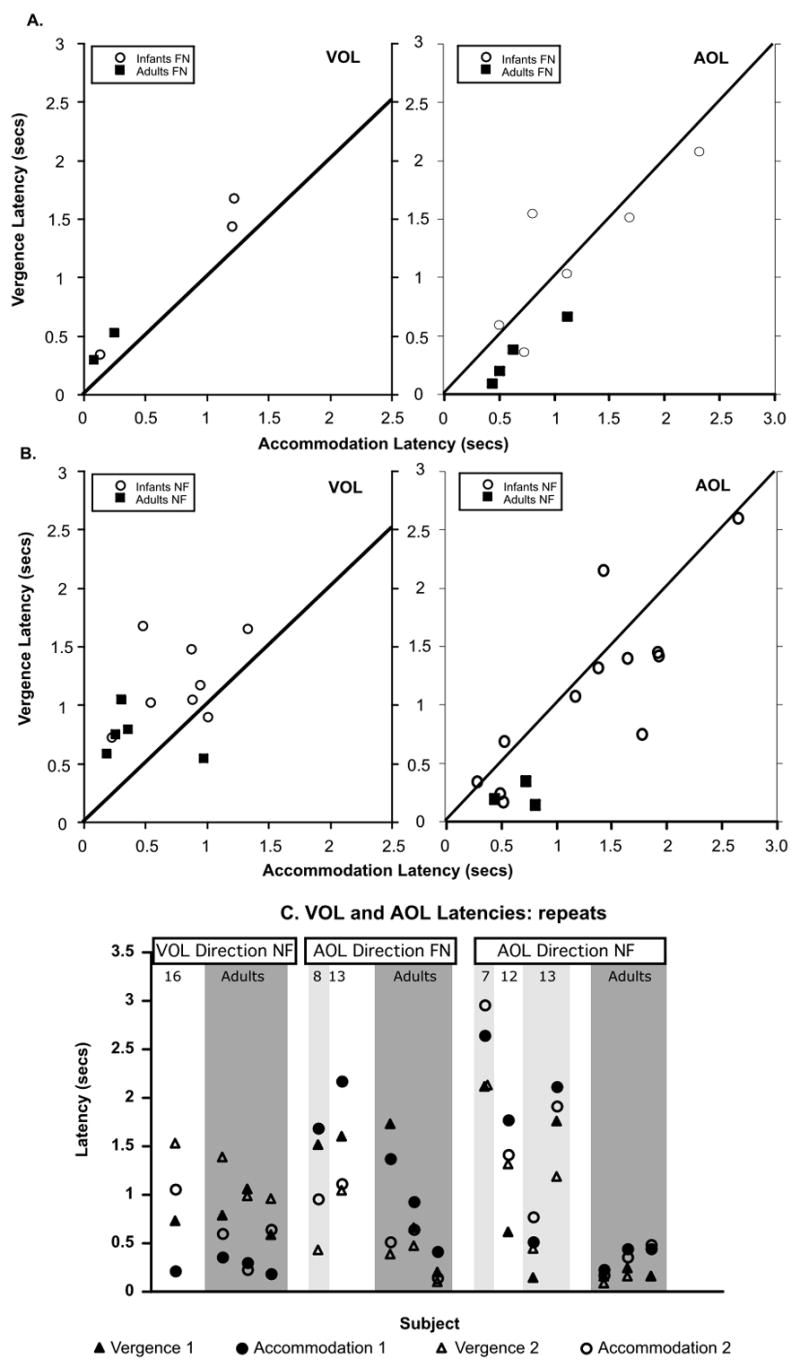
Relationship between the accommodation and vergence latencies of all the subjects in the VOL (monocular) and the AOL (DOG) conditions.
Considering panel A, both the adult and the infant accommodation responses are faster than the vergence responses in the, monocular, VOL condition (the mean infant difference was −313 ms, range −224 to −469 across the three infants, and the two adult values were −292 ms and −217 ms). Thus the infant accommodation system again appears to initiate a response more rapidly than the vergence system, while there is also a trend towards this in the adults. This relationship moved towards the reverse in the AOL conditions. The infant AOL mean latency difference was 6 ms (SD +/− 395), and the adult AOL mean difference was 222 ms (SD +/− 59).
With regard to performance in the opposite direction, in panel B, the infant VOL mean difference was −435 ms (SD +/− 386), which was significantly different from zero (t = −3.19, df = 7, p = 0.015 in a two tailed test). The adult VOL mean latency difference was −334 ms (SD +/− 444), which was not significantly different from zero (t = −3.684, df = 4, p = 0.168 in a two tailed test). The infant AOL accommodation and vergence latencies were correlated (n = 12, Pearson correlation = 0.84, p = 0.001), and the mean difference between them was 375ms (SD +/− 402), which was significantly different from zero (t = 3.227, df = 11, p = 0.008). The adult mean difference was 359ms (range 200ms to 558ms across the three subjects). Thus the relationship between the accommodative and vergence latencies appeared consistent across stimulus direction.
The data from individual subjects who provided repeated responses for the same stimulus direction are shown in Panel C. The only infant to provide repeated responses in the VOL condition had a change in difference of 35ms. The three adults had a mean absolute change in difference of 151 ms (range of 7 ms to 349 ms across the three). The mean absolute change in difference between the accommodation and vergence latencies in infants in the AOL condition was 443 ms (+/−338). In adults the mean absolute change was 146 ms (+/−173).
A univariate ANOVA was performed on the entire set of infant data (only one pair of latencies from each infant was included in each condition, which resulted in 98 sets of accommodation and vergence latencies). Accommodation and vergence latencies (response) were treated as repeated measures, while viewing condition (CL, VOL or AOL) and direction (NF or FN) were treated as between subject factors. The data are shown in a bar chart in Figure 8. The response and viewing condition main effects both had a significance of <0.05 (response: F(1, 92) = 8.58, p = 0.004 & viewing condition: F(2, 92) = 3.321, p < 0.04). Overall, the mean vergence latency was 946ms and the mean accommodation latency was 783ms. With regard to viewing condition, in Figure 8 it can be seen that the AOL latencies were slower than those for the CL condition (post-hoc testing with Bonferroni correction: CL vs AOL, p = 0.043, was the only test with p < 0.05). With regard to interactions, viewing condition by response had a significance of <0.001 (F(2, 92) = 12.07). In Figure 8 it can be seen that the accommodation and vergence latencies tend to reverse their relative order in the AOL viewing condition. The response by direction by viewing condition interaction also had a significance of p < 0.01 (F(2, 92) = 4.924). The latencies of subjects who provided scorable data in more than one viewing condition were consistent with the results of this ANOVA performed on the full set of data.
DISCUSSION
Closed loop viewing conditions
The mean adult accommodation and vergence latencies in closed loop viewing conditions were in reasonable agreement with the literature discussed above, although somewhat more variable. Previous studies of adult subjects have typically sampled the responses at significantly higher sampling rates (e.g. Heron et al (2001) at 102.4Hz and Schor et al (1999) at 200Hz), and the 40ms sampling interval of the photorefraction technique undoubtedly added noise to the latency estimates (suggested in panel C of Figures 6 & 7).
The infants providing usable data demonstrated longer mean latencies than the adults, with no significant difference between the stimulus directions. Even though the mean was longer, some of the infants demonstrated adult like latencies as early as seven or eight weeks of age. The other longer latencies may be the result of poor attention during some of the trials or variation in true optimal performance across these infants. These longer latencies are still relatively short in the context of the duration of events in an infant’s typical visual environment however.
The correlation between accommodation and vergence latencies, while not indicating causation, does imply that the two systems are not completely independent in infancy even in binocular viewing conditions where blur and retinal disparity have the potential to act as independent cues for accommodation and vergence respectively. The common variation in the two systems may result from a common, potentially immature, processing delay (e.g. the developmental progression of myelin in the two systems, or the role of coupling between accommodation and vergence in the responses) or the influence of an additional external factor, such as voluntary or attentional effects, that is common to both systems.
The accommodation response was initiated more rapidly than the vergence response in binocular conditions in infants, while there was a trend towards the reverse in the adults. The adult trend is consistent with the previous literature, which has found vergence to have a shorter latency than accommodation, although the difference is smaller in the current data (e.g. Campbell & Westheimer, 1960; Phillips et al., 1972; Rashbass & Westheimer, 1961; Krishnan et al., 1973). A reversal in the relationship in infancy could reflect some combination of immaturity of the sensory or motor visual system as binocular function matures, the relatively greater biomechanical compliance of the young lens during accommodation, or any bias towards rapid accommodation derived from the presence of the proximity cue in addition to blur and retinal disparity in the ramp stimulus.
The previous studies of infants’ binocular performance, in which accommodation and vergence were recorded simultaneously, collected single samples of steady-state responses at a number of viewing distances. Aslin and Dobson (1983), Hainline, Riddell, Grose-Fifer& Abramov (1992) and Turner et al (2002) all noted instances in which accommodation and vergence levels were not correlated. Hainline et al and Turner et al both summarized their data with statements that some infants made accurate responses with one system and not the other in binocular conditions, implying that the relationship between accommodation and vergence was immature. They found some infants with an immature relationship at least until 6 months of age. The results of the current study of dynamics suggest that these mismatches in responses were not the result of long latency differences between the two systems. The accommodation and vergence responses typically appear mismatched on the order of milliseconds with regard to latency.
Vergence open-loop and accommodation open-loop viewing conditions
The data collected from the two eyes in the monocular, vergence open loop, condition demonstrated that accommodation was consensual in the infants (e.g. Figure 4). This was true from the earliest ages that data were collected. The combination of accommodation and vergence data collected in the AOL and VOL conditions also suggests that the coupled responses are present at least from the end of the second month after birth. This is consistent with the results of Aslin and Jackson (1979) and Turner et al (2002) in that a vergence response was recorded in monocular viewing conditions, and with the results of Bobier et al (2000) in that an accommodation response was recorded after a change in retinal disparity in the accommodation open-loop condition.
The apparent accommodative vergence and vergence accommodation responses were also initiated after relatively short latencies (Figure 8), and in both cases the infants’ indirect coupled response tended to a longer latency than the direct response. The adult data also demonstrated this relative latency effect, which is not consistent with the data of Wilson (1973) and Schor et al (1999) who both found that vergence had a shorter latency than accommodation in monocular, VOL, viewing conditions in adult humans. Cumming and Judge (1986) found the monocular vergence latency to also be shorter than the accommodative latency in monkeys, by around 50ms. This difference in results is not easily explained in terms of our methodology, even though the sampling interval was relatively long and the data are noisy, as the relationship qualitatively reversed when the same instrument was used to collect the data in the AOL conditions. The fact that a ramp stimulus including proximal cues was used for the VOL condition rather than a step stimulus with no proximal cues could have been a factor (Rosenfield, Ciuffreda & Hung, 1991; Schor, Alexander, Cormack & Stevenson, 1992).
With regard to the potential role for the different response components in naturalistic binocular performance, the removal of retinal disparity information in the VOL conditions resulted in an increase of 100–200 ms in the vergence latency relative to the accommodation latency in infants. In the AOL conditions, the removal of blur information resulted in a slowing of the accommodation response by approximately 500 to 650 ms relative to the vergence latency. Thus these data suggest that the blur-driven accommodation and disparity-driven vergence responses play an important role in the early period of the infant response, but that the indirect coupled components of the response are capable of contributing at least within half a second of response onset. In adults the absence of retinal disparity in the VOL conditions delayed the vergence response by 260 to 360 ms relative to the accommodation response, and the absence of the blur cue delayed the accommodation latency by 160 to 360 ms relative to the vergence latency in the AOL condition. These values are somewhat higher than those found in the previous literature described above, by approximately a factor of two, but they are in the appropriate direction.
The overall success rates in collecting scorable responses in the VOL and AOL conditions were relatively low. Our experience in the monocular, vergence open loop, condition was similar to that of Currie and Manny (1997) and Turner et al (2002) who both found that infants did not respond well to a stimulus when one eye was occluded. The proximity information in the VOL condition used here was consistent with the change in blur information and could therefore have been used to boost the response (Rosenfield et al., 1991; Schor et al., 1992). Even so, the success rate was still low. There was no change in proximity (size) of the stimulus with change in retinal disparity in the AOL condition and so the proximal cue was in conflict with the disparity cue. It is unclear whether this discrepancy prevented some infants from generating a detectable response in that condition. It is important to remember that the quantitative results above are derived from a limited proportion of the sample tested.
Conclusion
This study suggests that blur-driven accommodation responses, retinal disparity driven vergence responses and the coupled responses all have latencies on the order of milliseconds in young infants, and therefore that cortical synapses undergoing activity-dependent refinement are typically experiencing correlations between focusing and alignment on this timescale. The durations of the accommodation and vergence responses were also noted to be well-matched in these data (see figure 4). In the clinical context, the consequences of the coupled responses must therefore also be considered even prior to three months of age when sensory binocular function appears to undergo a rapid maturation.
Acknowledgments
This research was supported by an NEI grant, R01 EY014460, awarded to TRC.
We would like to thank Bill Monette for building the experimental equipment, Diane Goss for recruiting the infant subjects, the infants and parents for their participation, and Jingyun Wang and Diane Goss for help with the data collection. We would also like to thank Shrikant Bharadwaj for helpful discussion. This research was supported by an NEI grant, R01 EY014460, awarded to TRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpern M, Ellen P. A quantitative analysis of the horizontal movements of the eyes in the experiment of Johannes Mueller. I. Method and results. American Journal of Ophthalmology. 1956;42(4 Part 2):289–296. doi: 10.1016/0002-9394(56)90380-4. [DOI] [PubMed] [Google Scholar]
- Aslin RN. Development of binocular fixation in human infants. Journal of Experimental Child Psychology. 1977;23(1):133–150. doi: 10.1016/0022-0965(77)90080-7. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Dobson V. Dark vergence and dark accommodation in human infants. Vision Research. 1983;23(12):1671–1678. doi: 10.1016/0042-6989(83)90182-7. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Jackson RW. Accommodative-convergence in young infants: development of a synergistic sensory-motor system. Can J Psychol. 1979;33(4):222–231. doi: 10.1037/h0081722. [DOI] [PubMed] [Google Scholar]
- Banks MS. The development of visual accommodation during early infancy. Child Development. 1980;51(3):646–666. [PubMed] [Google Scholar]
- Banks MS, Aslin RN, Letson RD. Sensitive period for the development of human binocular vision. Science. 1975;190(4215):675–677. doi: 10.1126/science.1188363. [DOI] [PubMed] [Google Scholar]
- Beers AP, Van Der Heijde GL. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vision Research. 1994;34(21):2897–2905. doi: 10.1016/0042-6989(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Bharadwaj SR, Schor CM. Dynamic control of ocular disaccommodation: first and second-order dynamics. Vision Research. 2006;46(6–7):1019–1037. doi: 10.1016/j.visres.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR. Monocular acuity and stereopsis in infantile esotropia. Investigative Ophthalmology & Visual Science. 1985;26(11):1624–1630. [PubMed] [Google Scholar]
- Blade PJ, Candy TR. Validation of the PowerRefractor for measuring human infant refraction. Optometry & Vision Science. 2006;83(6):346–353. doi: 10.1097/01.opx.0000221402.35099.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobier WR, Guinta A, Kurtz S, Howland HC. Prism induced accommodation in infants 3 to 6 months of age. Vision Research. 2000;40(5):529–537. doi: 10.1016/s0042-6989(99)00196-0. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Westheimer G. Dynamics of accommodation responses of the human eye. Journal of Physiology. 1960;151:285–295. doi: 10.1113/jphysiol.1960.sp006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman WN, Tucker J. Accommodation as a function of object form. Am J Optom Physiol Opt. 1978;55(2):84–92. doi: 10.1097/00006324-197802000-00004. [DOI] [PubMed] [Google Scholar]
- Choi M, Weiss S, Schaeffel F, Seidemann A, Howland HC, Wilhelm B, Wilhelm H. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor) Optometry & Vision Science. 2000;77(10):537–548. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Cumming BG, Judge SJ. Disparity-induced and blur-induced convergence eye movement and accommodation in the monkey. J Neurophysiol. 1986;55(5):896–914. doi: 10.1152/jn.1986.55.5.896. [DOI] [PubMed] [Google Scholar]
- Currie DC, Manny RE. The development of accommodation. Vision Res. 1997;37(11):1525–1533. doi: 10.1016/s0042-6989(97)85022-5. [DOI] [PubMed] [Google Scholar]
- Eadie AS, Carlin PJ. Evolution of control system models of ocular accommodation, vergence and their interaction. Medical & Biological Engineering & Computing. 1995;33(4):517–524. doi: 10.1007/BF02522508. [DOI] [PubMed] [Google Scholar]
- Fincham EF, Walton J. The reciprocal actions of accommodation and convergence. Journal of Physiology. 1957;137(3):488–508. doi: 10.1113/jphysiol.1957.sp005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainline L, Riddell P, Grose-Fifer J, Abramov I. Development of accommodation and convergence in infancy. Behavioural Brain Research. 1992;49(1):33–50. doi: 10.1016/s0166-4328(05)80192-5. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Boltz RL, Crawford ML, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: spatial modulation sensitivity. Vision Research. 1983;23(12):1501–1510. doi: 10.1016/0042-6989(83)90162-1. [DOI] [PubMed] [Google Scholar]
- Heron G, Charman WN, Schor CM. Age changes in the interactions between the accommodation and vergence systems. Optometry & Vision Science. 2001;78(10):754–762. doi: 10.1097/00006324-200110000-00015. [DOI] [PubMed] [Google Scholar]
- Heron G, Winn B. Binocular accommodation reaction and response times for normal observers. Ophthalmic Physiol Opt. 1989;9(2):176–183. doi: 10.1111/j.1475-1313.1989.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Horwood AM, Turner JE, Houston SM, Riddell PM. Variations in accommodation and convergence responses in a minimally controlled photorefractive setting. Optometry & Vision Science. 2001;78(11):791–804. doi: 10.1097/00006324-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. Journal of Neurophysiology. 1986;55(5):915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Boothe RG. The time course for the development of strabismic amblyopia in infant monkeys (Macaca nemestrina) Investigative Ophthalmology & Visual Science. 1980;19(7):841–845. [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience. 1998;18(16):6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotulak JC, Schor CM. The effects of optical vergence, contrast, and luminance on the accommodative response to spatially bandpass filtered targets.[erratum appears in Vision Res 1988;27(10):361] Vision Research. 1987;27(10):1797–1806. doi: 10.1016/0042-6989(87)90108-8. [DOI] [PubMed] [Google Scholar]
- Krishnan VV, Farazian F, Stark L. An analysis of latencies and prediction in the fusional vergence system. American Journal of Optometry & Archives of American Academy of Optometry. 1973;50(12):933–939. doi: 10.1097/00006324-197312000-00001. [DOI] [PubMed] [Google Scholar]
- Krishnan VV, Shirachi D, Stark L. Dynamic measures of vergence accommodation. American Journal of Optometry & Physiological Optics. 1977;54(7):470–473. doi: 10.1097/00006324-197707000-00007. [DOI] [PubMed] [Google Scholar]
- MacLachlan C, Howland HC. Normal values and standard deviations for pupil diameter and interpupillary distance in subjects aged 1 month to 19 years. Ophthalmic & Physiological Optics. 2002;22(3):175–182. doi: 10.1046/j.1475-1313.2002.00023.x. [DOI] [PubMed] [Google Scholar]
- Maddox EE. Investigations in the relation between convergence and accommodation of the eyes. Journal of anatomy and physiology. 1887;21:21–42. [PMC free article] [PubMed] [Google Scholar]
- Navarro R, Artal P, Williams DR. Modulation transfer of the human eye as a function of retinal eccentricity. J Opt Soc Am A. 1993;10(2):201–212. doi: 10.1364/josaa.10.000201. [DOI] [PubMed] [Google Scholar]
- Phillips S, Shirachi D, Stark L. Analysis of accommodative response times using histogram information. American Journal of Optometry & Archives of American Academy of Optometry. 1972;49(5):389–400. doi: 10.1097/00006324-197205000-00001. [DOI] [PubMed] [Google Scholar]
- Rashbass C, Westheimer G. Disjunctive eye movements. Journal of Physiology. 1961;159:339–360. doi: 10.1113/jphysiol.1961.sp006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield M, Ciuffreda KJ, Hung GK. The linearity of proximally induced accommodation and vergence. Invest Ophthalmol Vis Sci. 1991;32(11):2985–2991. [PubMed] [Google Scholar]
- Schor CM, Alexander J, Cormack L, Stevenson S. Negative feedback control model of proximal convergence and accommodation. Ophthalmic & Physiological Optics. 1992;12(3):307–318. [PubMed] [Google Scholar]
- Schor CM, Lott LA, Pope D, Graham AD. Saccades reduce latency and increase velocity of ocular accommodation. Vision Res. 1999;39(22):3769–3795. doi: 10.1016/s0042-6989(99)00094-2. [DOI] [PubMed] [Google Scholar]
- Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A Opt Image Sci Vis. 2002;19(12):2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- Semmlow JL, Yuan W. Adaptive modification of disparity vergence components: an independent component analysis study. Investigative Ophthalmology & Visual Science. 2002;43(7):2189–2195. [PubMed] [Google Scholar]
- Suryakumar R, Bobier WR. Gain and movement time of convergence-accommodation in preschool children. Optom Vis Sci. 2004;81(11):835–843. doi: 10.1097/01.opx.0000145026.42124.fc. [DOI] [PubMed] [Google Scholar]
- Suryakumar R, Meyers JP, Irving EL, Bobier WR. Vergence accommodation and monocular closed loop blur accommodation have similar dynamic characteristics. Vision Research. 2007;47(3):327–337. doi: 10.1016/j.visres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Tondel GM, Candy TR. Human infants’ accommodation responses to dynamic stimuli. Investigative Ophthalmology & Visual Science. 2007;48(2):949–956. doi: 10.1167/iovs.06-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Horwood AM, Houston SM, Riddell PM. Development of the response AC/A ratio over the first year of life. Vision Research. 2002;42(22):2521–2532. doi: 10.1016/s0042-6989(02)00268-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. A centre for accommodative vergence motor control. Vision Res. 1973;13(12):2491–2503. doi: 10.1016/0042-6989(73)90246-0. [DOI] [PubMed] [Google Scholar]
- Yamada T, Ukai K. Amount of defocus is not used as an error signal in the control system of accommodation dynamics. Ophthalmic & Physiological Optics. 1997;17(1):55–60. [PubMed] [Google Scholar]
- Zhang Y, Mays LE, Gamlin PD. Characteristics of near response cells projecting to the oculomotor nucleus. Journal of Neurophysiology. 1992;67(4):944–960. doi: 10.1152/jn.1992.67.4.944. [DOI] [PubMed] [Google Scholar]