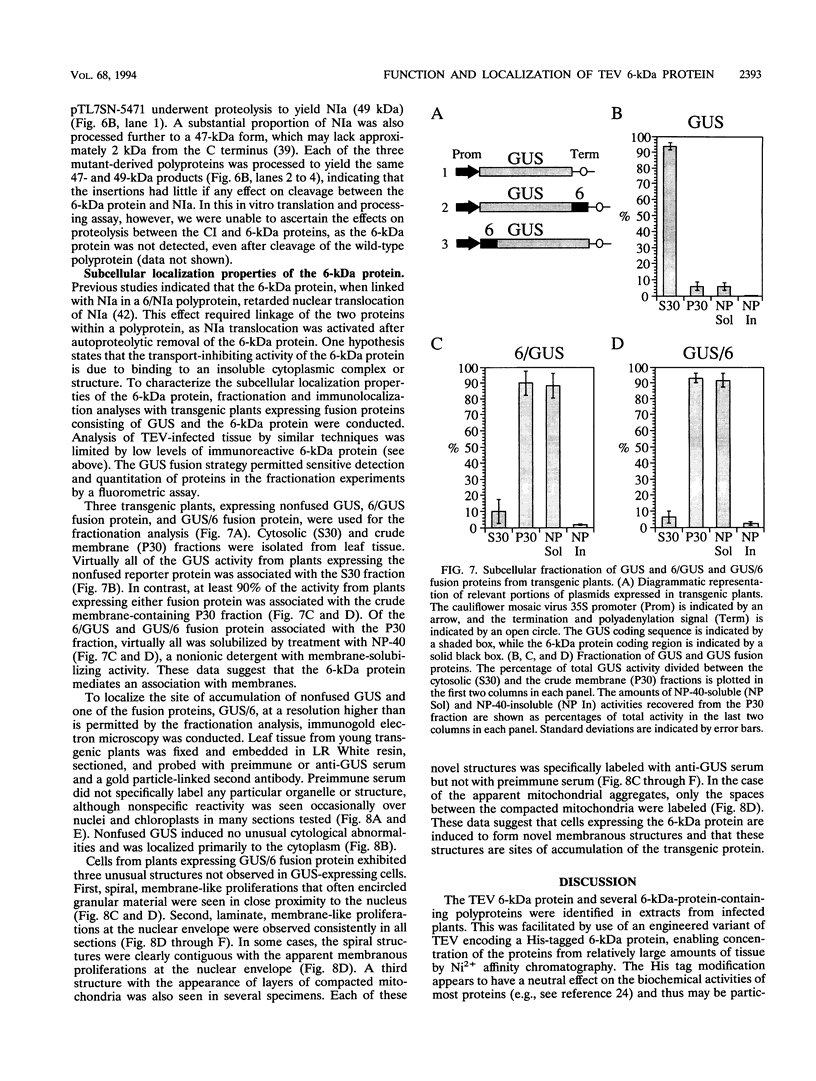
Abstract
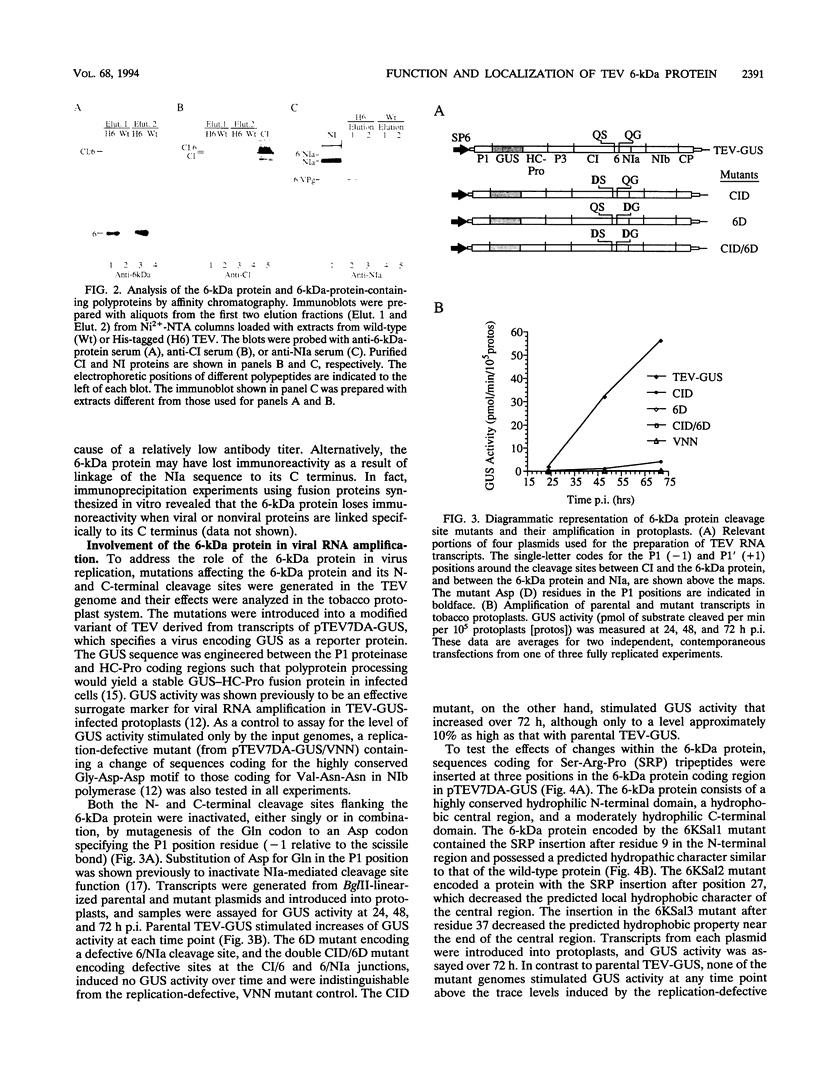
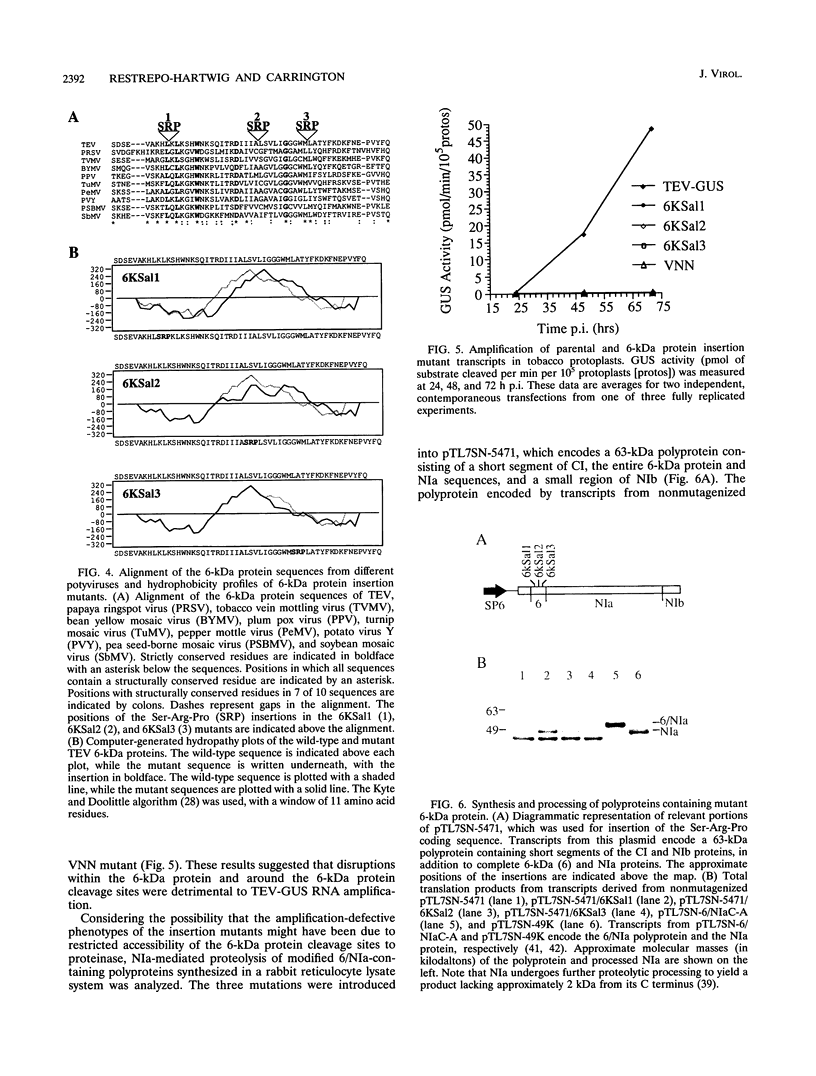
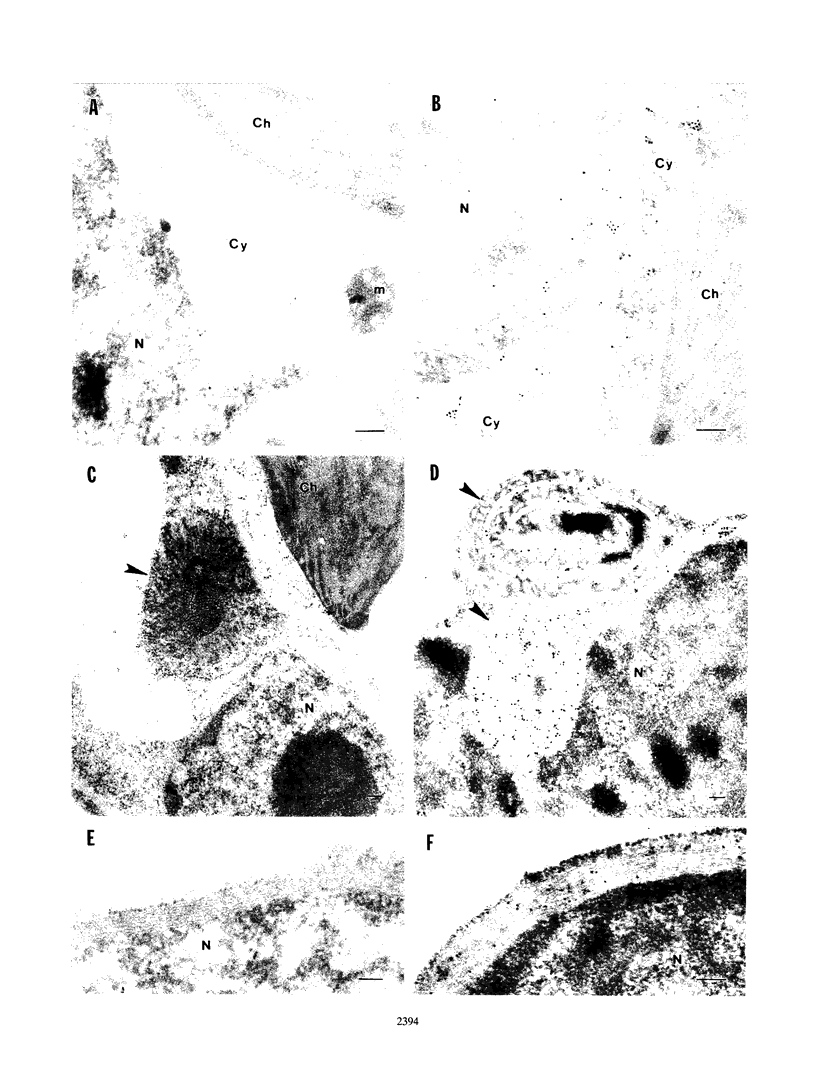
The tobacco etch potyvirus (TEV) genome encodes a polyprotein that is processed by three virus-encoded proteinases. Although replication of TEV likely occurs in the cytoplasm, two replication-associated proteins, VPg-proteinase (nuclear inclusion protein a) (NIa) and RNA-dependent RNA polymerase (nuclear inclusion protein b) (NIb), accumulate in the nucleus of infected cells. The 6-kDa protein is located adjacent to the N terminus of NIa in the TEV polyprotein, and, in the context of a 6-kDa protein/NIa (6/NIa) polyprotein, impedes nuclear translocation of NIa (M. A. Restrepo-Hartwig and J. C. Carrington, J. Virol. 66:5662-5666, 1992). The 6-kDa protein and three polyproteins containing the 6-kDa protein were identified by affinity chromatography of extracts from infected plants. Two of the polyproteins contained NIa or the N-terminal VPg domain of NIa linked to the 6-kDa protein. To investigate the role of the 6-kDa protein in vivo, insertion and substitution mutagenesis was targeted to sequences coding for the 6-kDa protein and its N- and C-terminal cleavage sites. These mutations were introduced into a TEV genome engineered to express the reporter protein beta-glucuronidase (GUS), allowing quantitation of virus amplification by a fluorometric assay. Three-amino-acid insertions at each of three positions in the 6-kDa protein resulted in viruses that were nonviable in tobacco protoplasts. Disruption of the N-terminal cleavage site resulted in a virus that was approximately 10% as active as the parent, while disruption of the C-terminal processing site eliminated virus viability. The subcellular localization properties of the 6-kDa protein were investigated by fractionation and immunolocalization of 6-kDa protein/GUS (6/GUS) fusion proteins in transgenic plants. Nonfused GUS was associated with the cytosolic fraction (30,000 x g centrifugation supernatant), while 6/GUS and GUS/6 fusion proteins sedimented with the crude membrane fraction (30,000 x g centrifugation pellet). The GUS/6 fusion protein was localized to apparent membranous proliferations associated with the periphery of the nucleus. These data suggest that the 6-kDa protein is membrane associated and is necessary for virus replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunoch D. A., Das P., Browning M. E., Hari V. A temporal study of the expression of the capsid, cytoplasmic inclusion and nuclear inclusion proteins of tobacco etch potyvirus in infected plants. J Gen Virol. 1991 Mar;72(Pt 3):487–492. doi: 10.1099/0022-1317-72-3-487. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987 Sep;160(1):220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Dougherty W. G. Mutational analysis of tobacco etch virus polyprotein processing: cis and trans proteolytic activities of polyproteins containing the 49-kilodalton proteinase. J Virol. 1988 Jul;62(7):2313–2320. doi: 10.1128/jvi.62.7.2313-2320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Parks T. D., Dougherty W. G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989 Feb;8(2):365–370. doi: 10.1002/j.1460-2075.1989.tb03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Dougherty W. G. Small nuclear inclusion protein encoded by a plant potyvirus genome is a protease. J Virol. 1987 Aug;61(8):2540–2548. doi: 10.1128/jvi.61.8.2540-2548.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D. Cap-independent enhancement of translation by a plant potyvirus 5' nontranslated region. J Virol. 1990 Apr;64(4):1590–1597. doi: 10.1128/jvi.64.4.1590-1597.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Leinicke A. J. Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell. 1991 Sep;3(9):953–962. doi: 10.1105/tpc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Haldeman R., Dolja V. V., Restrepo-Hartwig M. A. Internal cleavage and trans-proteolytic activities of the VPg-proteinase (NIa) of tobacco etch potyvirus in vivo. J Virol. 1993 Dec;67(12):6995–7000. doi: 10.1128/jvi.67.12.6995-7000.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja V. V., McBride H. J., Carrington J. C. Tagging of plant potyvirus replication and movement by insertion of beta-glucuronidase into the viral polyprotein. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10208–10212. doi: 10.1073/pnas.89.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty W. G., Carrington J. C., Cary S. M., Parks T. D. Biochemical and mutational analysis of a plant virus polyprotein cleavage site. EMBO J. 1988 May;7(5):1281–1287. doi: 10.1002/j.1460-2075.1988.tb02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D. Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology. 1991 Aug;183(2):449–456. doi: 10.1016/0042-6822(91)90974-g. [DOI] [PubMed] [Google Scholar]
- Giachetti C., Hwang S. S., Semler B. L. cis-acting lesions targeted to the hydrophobic domain of a poliovirus membrane protein involved in RNA replication. J Virol. 1992 Oct;66(10):6045–6057. doi: 10.1128/jvi.66.10.6045-6057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachetti C., Semler B. L. Role of a viral membrane polypeptide in strand-specific initiation of poliovirus RNA synthesis. J Virol. 1991 May;65(5):2647–2654. doi: 10.1128/jvi.65.5.2647-2654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., de Martynoff G., Lou J., Hipskind R. A., Nordheim A., Stunnenberg H. G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawson R. H., Hearon S. S. The association of pinwheel inclusions with plasmodesmata. Virology. 1971 May;44(2):454–456. doi: 10.1016/0042-6822(71)90277-7. [DOI] [PubMed] [Google Scholar]
- Laín S., Martín M. T., Riechmann J. L., García J. A. Novel catalytic activity associated with positive-strand RNA virus infection: nucleic acid-stimulated ATPase activity of the plum pox potyvirus helicaselike protein. J Virol. 1991 Jan;65(1):1–6. doi: 10.1128/jvi.65.1.1-6.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laín S., Riechmann J. L., García J. A. RNA helicase: a novel activity associated with a protein encoded by a positive strand RNA virus. Nucleic Acids Res. 1990 Dec 11;18(23):7003–7006. doi: 10.1093/nar/18.23.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M. T., García J. A. Plum pox potyvirus RNA replication in a crude membrane fraction from infected Nicotiana clevelandii leaves. J Gen Virol. 1991 Apr;72(Pt 4):785–790. doi: 10.1099/0022-1317-72-4-785. [DOI] [PubMed] [Google Scholar]
- Maynell L. A., Kirkegaard K., Klymkowsky M. W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992 Apr;66(4):1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. F., Rhoads R. E., Hunt A. G., Shaw J. G. The VPg of tobacco etch virus RNA is the 49-kDa proteinase or the N-terminal 24-kDa part of the proteinase. Virology. 1990 Sep;178(1):285–288. doi: 10.1016/0042-6822(90)90405-g. [DOI] [PubMed] [Google Scholar]
- Murphy J. F., Rychlik W., Rhoads R. E., Hunt A. G., Shaw J. G. A tyrosine residue in the small nuclear inclusion protein of tobacco vein mottling virus links the VPg to the viral RNA. J Virol. 1991 Jan;65(1):511–513. doi: 10.1128/jvi.65.1.511-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt R., Jaspars E. M. Purification and characterization of brome mosaic virus RNA-dependent RNA polymerase. Virology. 1990 Sep;178(1):189–194. doi: 10.1016/0042-6822(90)90393-6. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Hartwig M. A., Carrington J. C. Regulation of nuclear transport of a plant potyvirus protein by autoproteolysis. J Virol. 1992 Sep;66(9):5662–5666. doi: 10.1128/jvi.66.9.5662-5666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo M. A., Freed D. D., Carrington J. C. Nuclear transport of plant potyviral proteins. Plant Cell. 1990 Oct;2(10):987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann J. L., Laín S., García J. A. Highlights and prospects of potyvirus molecular biology. J Gen Virol. 1992 Jan;73(Pt 1):1–16. doi: 10.1099/0022-1317-73-1-1. [DOI] [PubMed] [Google Scholar]
- Riechmann J. L., Laín S., García J. A. The genome-linked protein and 5' end RNA sequence of plum pox potyvirus. J Gen Virol. 1989 Oct;70(Pt 10):2785–2789. doi: 10.1099/0022-1317-70-10-2785. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Hanecak R., Dorner L. F., Wimmer E. A membrane-associated precursor to poliovirus VPg identified by immunoprecipitation with antibodies directed against a synthetic heptapeptide. Cell. 1982 Feb;28(2):405–412. doi: 10.1016/0092-8674(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M., Shaw J. G., Rhoads R. E. Mapping of the tobacco vein mottling virus VPg cistron. Virology. 1988 Apr;163(2):635–637. doi: 10.1016/0042-6822(88)90307-8. [DOI] [PubMed] [Google Scholar]
- Takegami T., Kuhn R. J., Anderson C. W., Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Verchot J., Koonin E. V., Carrington J. C. The 35-kDa protein from the N-terminus of the potyviral polyprotein functions as a third virus-encoded proteinase. Virology. 1991 Dec;185(2):527–535. doi: 10.1016/0042-6822(91)90522-d. [DOI] [PubMed] [Google Scholar]
- Wright R., Basson M., D'Ari L., Rine J. Increased amounts of HMG-CoA reductase induce "karmellae": a proliferation of stacked membrane pairs surrounding the yeast nucleus. J Cell Biol. 1988 Jul;107(1):101–114. doi: 10.1083/jcb.107.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]