Abstract
Background
Most children with Tourette syndrome (TS) experience a marked decline in the severity of tic symptoms during adolescence. Currently no clinical measures can predict whose tic symptoms will persist into adulthood. Previous cross-sectional imaging studies have identified reduced caudate nucleus volumes in subjects with TS.
Objective
To evaluate whether caudate nucleus volumes in childhood can predict the severity of tic or obsessive–compulsive symptoms at follow-up in early adulthood.
Methods
In a prospective longitudinal study, clinical status and basal ganglia volumes of 43 children with TS were measured on high-resolution magnetic resonance images before age 14 years. Follow-up clinical assessments were conducted after age 16 years, an average of 7.5 years later. Linear regression and Tobit regression analyses were used to assess the association of basal ganglia volumes measured in childhood with the severity of tic and obsessive–compulsive disorder (OCD) symptoms at the time of childhood MRI and at follow-up in early adulthood.
Results
Volumes of the caudate nucleus correlated significantly and inversely with the severity of tic and OCD symptoms in early adulthood. Caudate volumes did not correlate with the severity of symptoms at the time of the MRI scan.
Conclusions
Caudate volumes in children with Tourette syndrome predict the severity of tic and obsessive–compulsive symptoms in early adulthood. This study provides compelling evidence that morphologic disturbances of the caudate nucleus within cortico-striatal-thalamo-cortical circuits are central to the persistence of both tics and obsessive–compulsive symptoms into adulthood.
Tourette syndrome (TS) is a childhood-onset neuropsychiatric disorder that is characterized by the presence of motor and vocal tics, together with a range of semicompulsory behaviors. Obsessive–compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD) frequently co-occur with TS in clinically identified samples.1 Childhood-onset OCD also co-occurs with TS within families and in community samples more often than chance2 and is therefore thought to represent a variable manifestation of a common underlying set of vulnerability genes.3 Tics usually begin during the first decade of life and typically reach their peak severity early in the second decade.4 In one-half to two-thirds of all children who have TS, the severity of tic symptoms attenuate dramatically during adolescence, often remitting completely by early adulthood.4,5 When tic symptoms persist into adulthood, the clinical presentation can be severe, and can include self-injurious motor tics or socially stigmatizing utterances and gestures.6 Currently, no clinical features or biologic measures are known to predict which children will have tics that persist into adulthood.
Pathologic processes based within the basal ganglia have long been hypothesized to be of central importance in the etiology of TS.7-10 One of the most consistently reported findings in brain imaging studies of the disorder is reduced volume of the caudate nucleus in children and adults with TS compared with those of healthy control subjects.7-9 This prospective, longitudinal study seeks to test the hypothesis that volumes of the caudate nucleus measured in childhood predict persistence of tic and OCD symptoms into adulthood.
Methods
Subjects
Children with TS were recruited from the Tic and Obsessive-Compulsive Disorders Specialty Clinic at the Yale Child Study Center. Written informed consent was obtained for all participants. The 43 children recruited into this prospective longitudinal study had volumetric MRI scans performed before age 14 years and were older than 16 years at the time of follow-up. All subjects were included in published findings from previous imaging studies.9-12 Eligibility for follow-up evaluation required that a subject both participate in a volumetric MRI scan and receive a diagnosis of TS before age 14 years. Exclusion criteria for original MRI image obtainment included previous seizure, history of head trauma with loss of consciousness, ongoing or past substance abuse, lifetime history of psychotic illness, or an IQ less than 80. Subjects were paid for their participation.
Sixty-one subjects were eligible to participate in this study based on previous imaging data. We were unable to locate several of those eligible to participate (n = 4), and others refused to participate (n = 14), leaving 43 participating subjects. Those declining participation did not differ significantly from those who did participate in the follow-up on age, sex, medication use, IQ, worst-ever or current severity of tic or OCD symptoms, or comorbid diagnoses at the time of MRI (table). Participating subjects did have a slightly although not significantly higher rate of comorbid OCD and a lower rate of ADHD compared with nonparticipating subjects at the time of MRI.
Table. Demographic comparison at the time of MRI between participating and nonparticipating subjects eligible for follow-up interview.
| Participants | Nonparticipants | p Value | |
|---|---|---|---|
| n | 43 | 18 | |
| Age, y | 11.4 ± 1.6 | 10.9 ± 1.0 | 0.27 |
| Sex, M/F | 34/9 | 13/5 | 0.55 |
| Handedness, R/L | 36/7 | 16/2 | 0.58 |
| IQ | 112 ± 15.2 | 111 ± 11.3 | 0.80 |
| OCD | 15 (35%) | 3 (17%) | 0.16 |
| ADHD | 8 (19%) | 6 (33%) | 0.20 |
| Any tic medication | 21 (49%) | 5 (29%) | 0.13 |
| Dopamine antagonists | 10 (23%) | 4 (22%) | 0.95 |
| SSRI use | 4 (9%) | 0 | 0.18 |
| YGTSS score | 19.9 ± 8.7 | 21.5 ± 6.5 | 0.55 |
| YGTSS score—worst ever | 28.7 ± 6.2 | 25.9 ± 6.3 | 0.11 |
| CY-BOCS score | 1.9 ± 3.1 | 2.6 ± 5.3 | 0.52 |
| CY-BOCS score—worst ever | 3.6 ± 6.6 | 4.8 ± 9.5 | 0.57 |
Data are presented as mean ± SD. Eligibility required being older than 16 years at follow-up interview and having a volumetric MRI scan before age 14 years. Exclusionary criteria for initial MRI scan included previous seizure, history of head trauma with loss of consciousness, ongoing or past substance abuse, or an IQ less than 80. There were no significant differences between any of the variables examined between participating and nonparticipating subjects. Participating subjects had a notably higher rate of comorbid obsessive–compulsive disorder (OCD) and lower rate of attention deficit hyperactivity disorder (ADHD) than nonparticipating subjects. Although nonparticipating subjects had a much lower rate of comorbid OCD, they had a higher average CY-BOCS score than participating subjects. This finding reflects the phenomenon that the nonparticipating subjects with comorbid OCD had greater OCD symptom severity at Time 1.
SSRI = selective serotonin reuptake inhibitor; YGTSS =Yale Global Tic Severity Scale; CY-BOCS = Children's Yale-Brown Obsessive-Compulsive Scale.
Clinical measures at the time of MRI
Clinical measures at the time of MRI included the Yale Global Tic Severity Scale (YGTSS)13 and the Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS),14 rated at the time of scan for worst-ever symptom severity. Other comorbid psychiatric illnesses were surveyed using the Schedule for Tourette and Other Behavioral Syndromes.15-17 IQ was estimated with the Kaufman Brief Intelligence Test.18 Neuropsychiatric diagnoses were established through a best-estimate consensus procedure performed by two child psychiatrists (B.S.P. and J.F.L.) after reviewing all available materials.
MRI scan and morphometric procedures
A full description of the MRI pulse sequence and morphometric procedures has been previously described.10 Briefly, MRI scans were obtained using a single GE Signa 1.5-T scanner (GE Signa, Milwaukee, WI). Basal ganglia volumes were determined by investigators blinded to subject characteristics and hemisphere on UNIX workstations using ANALYZE 7.5 software. Hand tracing was used to define the basal ganglia after the images were enlarged eightfold to minimize mechanical tracing error. Whole brain volume included gray and white matter tissue and ventricular, cisternal, and sulcal CSF. Interclass correlation coefficients were greater than 0.95 for the caudate and putamen, greater than 0.90 for the globus pallidus, and greater than 0.99 for whole brain volume.
Follow-up assessments
Forty-three subjects and their parents were interviewed either in-person (n = 14) or via telephone by experienced clinician assessors (n = 29). All follow-up assessments were conducted blind to the morphological measurements and clinical features obtained at the time of the MRI scan in childhood. Assessment of symptoms and functioning at the time of follow-up included current and worst-ever YGTSS and CY-BOCS ratings, a Global Assessment Scale rating,19 medication history, detailed inquiry about ADHD symptoms, and screening for comorbid psychiatric conditions with Structured Clinical Interview for DSM-IV Axis I Disorders. Eight clinical evaluations relied solely on information provided by a parent who recently lived in the same home as the subject after consent was obtained from both parties. CY-BOCS ratings were not completed for three subjects whose parents felt unqualified to answer questions regarding their child's obsessions.
Statistical analyses
Statistical analyses were performed with SAS version 9.1 (SAS Institute Inc., Cary, NC). Because of the skewing toward zero of YGTSS and CY-BOCS ratings at follow-up (15 subjects had no tic symptoms and 25 had no OCD symptoms), a left-censored regression model, Tobit regression, was used for these analyses. Current YGTSS and CY-BOCS ratings at follow-up were entered as the dependent variable in a model, the basal ganglia volumes (in cm3) were entered as the independent variable, and whole brain volume (scaling effects), sex, and age at the time of MRI scan were entered as covariates.
Although YGTSS and CY-BOCS ratings were significantly intercorrelated (r = 0.59, p < 0.001), we nevertheless elected to examine tic and OCD severity as two separate outcome measures because they represent ratings of two distinct psychiatric conditions that are have different natural histories and treatments. That is, although these severity measures are highly intercorrelated and not statistically independent, we deemed independent assessment of these outcome measures to be relevant clinically. Furthermore, the intercorrelation of these measures was not perfect, and differing strengths of correlation between volumes and severity measures may be revealing in terms of the pathophysiology of these different, albeit related, conditions.
For analysis of data at the time of MRI, a simple linear regression model was used because these outcome data were normally distributed. Unadjusted YGTSS and CY-BOCS ratings at the time of MRI were entered separately as the dependent variables, and caudate volume, whole brain volume, sex, and age at the time of MRI scan were entered as covariates in this model.
Separate Tobit regression models were used to test our a priori hypothesis that total caudate volume would correlate with the severity of tic or OCD symptoms at follow-up in early adulthood. Strict Bonferroni correction was invoked for the two a priori hypotheses, and thus the threshold for statistical significance was reduced to p < 0.025. Based on findings of previous cross-sectional studies, we anticipated that we would not detect a significant correlation of basal ganglia volumes with symptom severity at the time of MRI.10
In exploratory analyses, the effects on outcome measures of left-sided, right-sided, or total (left + right) volumes for other basal ganglia regions were examined individually in separate left-censored regression models for analyzing follow-up symptom severity. These included putamen, globus pallidus, striatum (caudate + putamen), lenticular nucleus (putamen + globus pallidus), and total basal ganglia (caudate + putamen + globus pallidus). The threshold of significance for exploratory analyses was set at p < 0.05 to maximize hypothesis generation.
All significant findings were assessed carefully for the possible confounding influences of medication use, comorbid ADHD, sex effects, and time interval between MRI acquisition and follow-up assessment. Medication classes that were considered included use of α2 agonists, dopamine receptor antagonists (atypical and traditional neuroleptics), and selective serotonin reuptake inhibitors (SSRIs) at the times of MRI scan. Each medication class or comorbid diagnosis was added separately as a covariate to the simple or left-censored linear regression model. Confounding effects were reported as significant if the model that included confounding covariates induced a significant change in the parameter estimate of the basal ganglia measurement of interest. A significant effect on the basal ganglia covariate was defined as a change in the parameter estimate greater than 1 standard error for simple linear (or Tobit) regression analysis.
Results
Subjects
Average age at the time of MRI was 11.4 (range 8.5 to 13.9, SD 1.6) and at follow-up was 18.7 years (range 16 to 23 years, SD 1.7). The average interval between MRI and follow-up was 7.5 years (range 3.8 to 12.8 years, SD 1.9). Participants were on average experiencing far fewer tic symptoms at the time of follow-up than at the time of the MRI in childhood. Only 8 subjects (19%) were experiencing tics of moderate or greater severity (YGTSS score >20) at follow-up, whereas 22 subjects (51%) had tics of that severity at the time of MRI. All 43 subjects reported experiencing tics of that severity at some time in their lives. Average YGTSS score at follow-up was 9.8 (range 0 to 39 [possible range 0 to 50], SD 10.6), compared with 19.9 (range 5 to 46 [possible range 0 to 50], SD 8.7) at the time of MRI. In the 19 subjects (44%) who reported experiencing OCD symptoms of moderate or greater severity (CY-BOCS score ≥10) at some time in their life, average CY-BOCS score at follow-up was 11.6 (range 0 to 34 [possible range 0 to 40], SD 9.3), compared with 3.8 (range 0 to 12 [possible range 0 to 40], SD 4.2) at the time of MRI. A more detailed analysis of the longitudinal course of tic and OCD symptoms in this study sample has been described previously.5
Tic severity at the time of follow-up in early adulthood
Volumes of the total (left + right) caudate nucleus measured in childhood correlated inversely with the severity of tics at the time of follow-up in early adulthood (figure 1A). Furthermore, in exploratory analyses, left- and right-sided caudate volumes, as well as all basal ganglia structures that included the caudate nucleus as a component (i.e., the striatum and total basal ganglia), correlated significantly and inversely with follow-up tic severity (figure 2A). Smaller right putamen volume also correlated with increased tic severity at follow-up. None of the other basal ganglia measurements correlated significantly with tic severity at follow-up.
Figure 1.
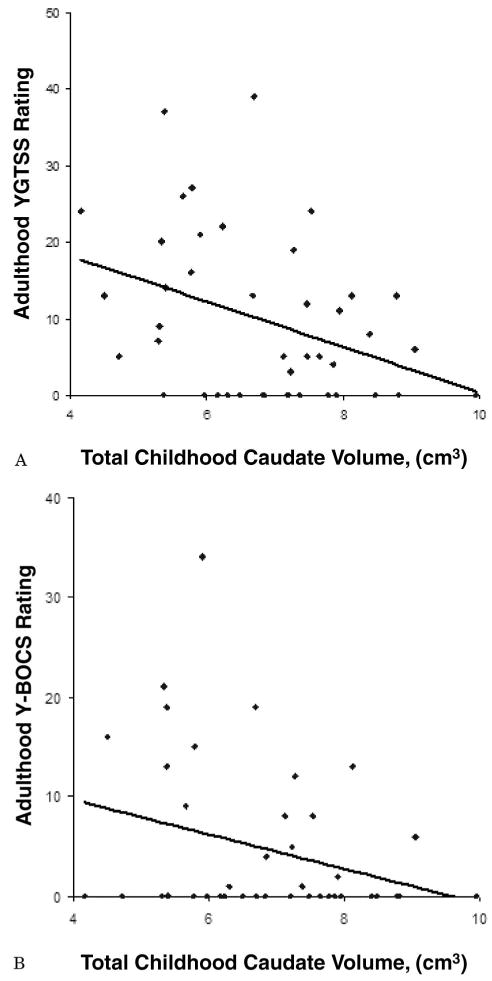
Trend lines represent the significant inverse correlation for total caudate nucleus volume in childhood and Yale Global Tic Severity Scale (YGTSS; A) and Yale-Brown Obsessive-Compulsive Scale (Y-BOCS; B) symptoms in late adolescence. Caudate volumes in childhood were associated inversely with tic and obsessive–compulsive symptom severity at follow-up in early adulthood.
Figure 2.
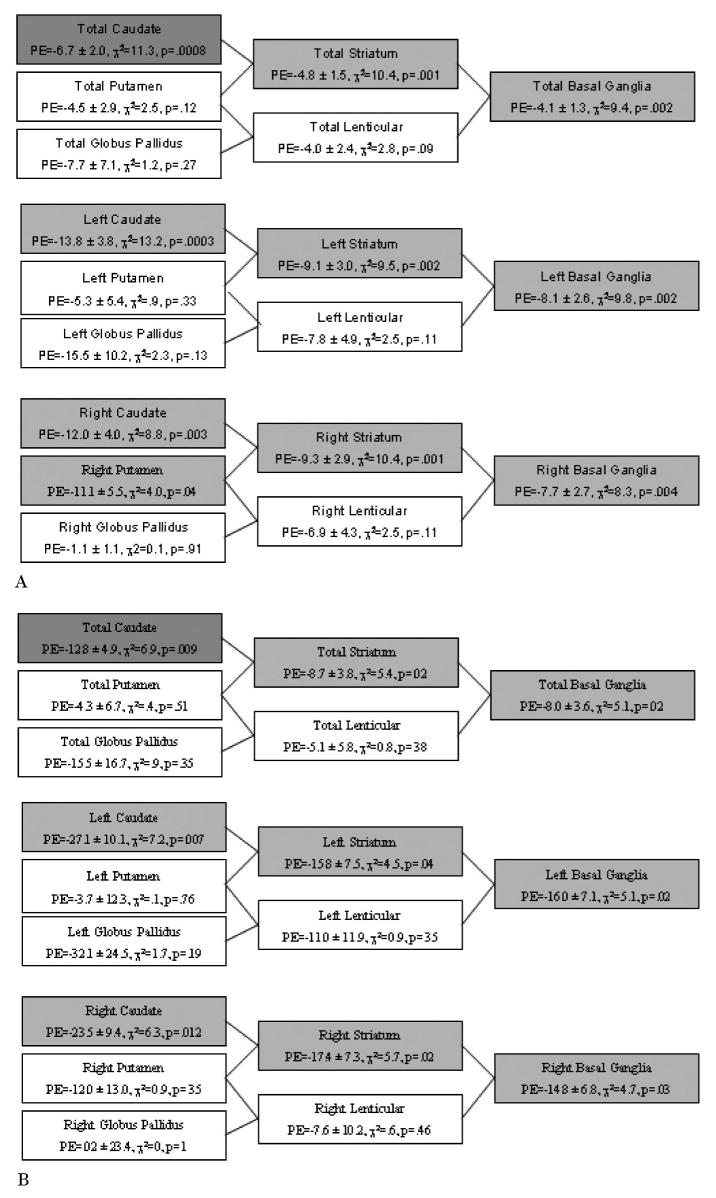
Summary of the associations of the volumes of various anatomic groupings of basal ganglia nuclei with the severity of tic (A) and obsessive-compulsive disorder (OCD; B) symptoms at follow-up in early adulthood. Basal ganglia regions in which significant associations were detected in exploratory analyses (p values < 0.05) are shaded in light gray, and significant findings for a priori analyses (p values < 0.025) are shaded in dark gray. Parameter estimates (PE) are presented as mean ± standard error for each cm3 change in basal ganglia volume.
OCD severity at the time of follow-up in early adulthood
Total caudate volume also correlated significantly and inversely with the severity of OCD symptoms at follow-up (figure 1B). In exploratory analyses, both right- and left-sided caudate volumes individually also correlated significantly and inversely with the severity of OCD symptoms at follow-up. Additionally, all basal ganglia measurements that included the caudate nucleus as a component correlated significantly and inversely with CY-BOCS score at follow-up (figure 2B). None of the correlations for other basal ganglia regions reached significance.
Tic and OCD severity at the time of childhood MRI
We detected no association between caudate volume and tic severity (β = −2.0, t = −1.8, p = 0.08) or OCD severity (β = 0.2, t = 0.6, p = 0.59) at the time of MRI scan.
Confounding effects
No significant medication effects were observed for analyses of SSRI, dopamine receptor antagonists, or α2 agonist use at the times of MRI or at follow-up. Additionally, no significant effects of sex, comorbid ADHD, or follow-up time interval were observed in our analyses.
Discussion
Our study demonstrated that reduced volumes of the caudate nucleus in childhood were associated with more severe tic and OCD symptoms in late adolescence and early adulthood. This finding is consistent with the long-standing hypothesis that the primary disturbances in cortico-striato-thalamo-cortical circuits, thought to be central to the etiology of TS, are centered on neural projections into or out of the caudate nucleus.9-11,20 Previous neuroimaging studies have demonstrated that reduced caudate volumes are a defining morphologic feature in children and adults with TS.9,10 Caudate volumes were reduced in the more severely affected monozygotic co-twins with TS, and D2 dopamine receptor binding increased in the head of the caudate in direct proportion to the severity of tics.8,21 In fMRI studies, activation of the caudate nucleus, together with activation of prefrontal and parietal cortices, has been shown to play an important role in the willful suppression of tics in persons with TS.11 Volumes of the caudate nucleus in this and previous neuroimaging studies, however, have not correlated significantly with the severity of tics in children with TS at the time of MRI scanning,10 nor have they been associated significantly with the diagnosis of OCD in pediatric OCD patients with or without comorbid tics.22 Therefore, our findings suggest that volumes of the caudate nucleus measured in childhood correlate more strongly with the severity of tics and OCD symptoms in adulthood than in childhood, after the usual developmental perturbations observed as the waxing and waning of symptoms in childhood and adolescence have settled down to more stable adult levels. Success in predicting this longer term, more stable outcome of the TS phenotype and the absence of significant correlations in temporal cross-section with the fluctuating severity of symptoms at any point in childhood are in fact consistent with the status of reduced caudate volumes as a putative marker for a trait vulnerability within the CNS of persons with TS.10
Although genetic determinants likely contribute to the presence of smaller caudate volumes in persons with TS, epigenetic effects and the interaction of epigenetic effects with genetic determinants must also be considered in the etiology of reduced caudate volumes in children who have TS. In genetically identical, monozygotic twins who have TS, for example, smaller caudate volumes have been demonstrated in the twin with more severe tics, emphasizing the likely contributions of epigenetic influences on caudate volume and the severity of illness in persons with TS.8 Further research is necessary to identify these environmental and potentially preventable developmental antecedents of TS. Postmortem and further complementary in vivo imaging studies are also needed to assess the structural and functional integrity of subregions of the caudate nucleus and the specific cellular correlates of reduced caudate volumes.
Our study has several limitations. First, the study population represents a clinically referred population of TS patients, and thus the study sample likely had a higher rate of comorbid illness and more severe tic symptoms than does the general population of individuals with TS.23 Second, 70% of the eligible study sample participated in follow-up interviews. Although clinical characteristics of nonparticipating subjects did not differ significantly at the time of MRI, these measures may have differed at the time of follow-up. Third, one-half of the study sample was taking psychotropic medications at the time of MRI scanning. Efforts were made to discern any effects of the medication that could have compromised the analyses or confounded our findings, and no such effects were found. Nevertheless, the possibility of such effects cannot be discounted entirely.
The findings of this study highlight the importance of prospective, longitudinal neuroimaging studies in the research of individuals who have TS or other complex neuropsychiatric disorders. In our cross-sectional data at the time of MRI acquisition in childhood, we detected no significant associations of caudate volume with the severity of tics. However, these same caudate volumes measured in childhood predicted quite robustly the severity of tic and OCD symptoms in adulthood in the longitudinal component of the study 7.5 years later. This seeming ability of caudate volumes to predict adult outcome underscores the likely centrality of the caudate nucleus in the pathophysiology of TS and tic-related OCD, and it further argues powerfully that reduced caudate volumes in childhood are not simply an epiphenomenal or compensatory effect of a more pathophysiologically central disturbance elsewhere in the brain. Therefore, the findings of this study emphasize the importance of prospective, longitudinal imaging studies of childhood-onset illnesses in discerning whether anatomic and functional abnormalities detected in temporal cross-section represent the causes or consequences of an illness.24
Caudate volumes alone likely do not represent a clinically useful predictor of adult outcome in children with TS. However, the possibility that MRI-based measures of brain structure and function could eventually be useful clinically in predicting long-term outcome in children with TS warrants further study. Further study is especially warranted given that caudate volumes were more reliable predictors of adult outcome than were any clinical characteristics in this study or in other large samples.5 Predictors of long-term outcome in children with TS could eventually influence the decision of whether and when to initiate medications, one of the most difficult decisions in the care of children who have this troubling illness.
Acknowledgments
The authors thank Drs. Lawrence Scahill, Heping Zhang, Robert King, Paul Lombroso, and Lawrence Staib and Ms. Lilya Katsovich at Yale University School of Medicine; Ronald Whiteman, Georgette Quackenbush, Victoria Stein, Laura Martin, Kathleen Durkin, and Rebecca Straus at Columbia College of Physicians and Surgeons; and John Gore at Vanderbilt University for their technical assistance.
Supported in part by grants MH74677 (B.S.P.), MH59139 (B.S.P.), MH068318 (B.S.P.), MHK02-74677 (B.S.P.), MH49351 (J.F.L.), MH30929, and RR00125 from the National Institutes of Health, Rockville, MD; a grant from the Tourette Syndrome Association, New York, NY (B.S.P.); the Suzanne Crosby Murphy Endowment at Columbia University; and support from the Smart Family Foundation, Jay and Jean Kaiser, Mr. Eric Brooks, and the Chasanoff Family at the Yale University Child Study Center.
Footnotes
Disclosure: The authors report no conflicts of interest.
Contributor Information
Michael H. Bloch, Yale Child Study Center, Yale University School of Medicine, New Haven, CT.
James F. Leckman, Yale Child Study Center, Yale University School of Medicine, New Haven, CT.
Hongtu Zhu, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons, New York.
Bradley S. Peterson, Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons, New York.
References
- 1.Walkup JT, Khan S, Schuerhulz L, Paik YS, Leckman JF, Schultz RT. Attention deficit hyperactivity disorder and learning disabilities. In: Leckman JF, Cohen DJ, editors. Tourette's syndrome: Tics, obsessions, compulsions—developmental psychopathology and clinical care. New York: John Wiley and Sons; 1998. [Google Scholar]
- 2.Peterson BS, Pine DS, Cohen P, Brook JS. Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders in an epidemiological sample. Am Acad Child Psychiatr. 2001;40:685–695. doi: 10.1097/00004583-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Leckman JF, Pauls DL, Zhang H, Rosario-Campos MC, Katsovich L, Kidd KK. Tourette Syndrome Association International Consortium for Genetics. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am J Med Genet. 2003;116B:60–68. doi: 10.1002/ajmg.b.10001. [DOI] [PubMed] [Google Scholar]
- 4.Leckman JF, Zhang H, Vitale A, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 5.Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Leckman JF. Clinical predictors of future tic and OCD severity in children with Tourette's syndrome. Arch Pediatr Adolesc Med. doi: 10.1001/archpedi.160.1.65. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain. 2000;123:425–462. doi: 10.1093/brain/123.3.425. [DOI] [PubMed] [Google Scholar]
- 7.Peterson BS, Leckman JF, Arnsten A, et al. Neuroanatomical circuitry. In: Leckman JF, Cohen DJ, editors. Tourette's syndrome: Tics, obsessions, compulsions—developmental psychopathology and clinical care. New York: John Wiley and Sons; 1998. pp. 230–260. [Google Scholar]
- 8.Hyde TM, Aaronson BA, Randolph C, Weinberger DR. Cerebral morphometric abnormalities in Tourette's syndrome: a quantitative MRI study of monozygotic twins. Neurology. 1995;45:1176–1182. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- 9.Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Leckman JF. Reduced basal ganglia volumes in Tourette's syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43:941–949. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- 10.Peterson BS, Thomas P, Kane MJ, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 11.Peterson BS, Skudlarski P, Anderson AW, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 12.Peterson BS, Staib L, Scahill L, et al. Regional brain and ventricular volumes in Tourette's syndrome. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 13.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Severity Scale: initial testing of a clinician-rated scale of tic-severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosini P, Metz C, Prabucki K, Lee JC. Videotape reliability of the third revised edition of the K-SADS. J Am Acad Child Adolesc Psychiatry. 1989;28:723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Pauls DL, Hurst CR. Schedule for Tourette and other behavioral syndromes. New Haven: Yale University Child Study Center; 1996. [Google Scholar]
- 18.Naugle RI, Chelune GJ, Tucker GD. Validity of the Kaufman Brief Intelligence Test. Psychol Assess. 1993;5:182–186. [Google Scholar]
- 19.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 20.Mink JW. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr Neurol. 2001;25:190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- 21.Wolff SS, Jones DW, Knable MB, et al. Tourette's syndrome: prediction of phenotypic variation in monozygotic twins by caudate nucleus D2 receptor binding. Science. 1996;273:1225–1227. doi: 10.1126/science.273.5279.1225. [DOI] [PubMed] [Google Scholar]
- 22.Szeszko PR, MacMillan S, McMeniman M, et al. Brain structural abnormalities in psychotropic drug-naïve pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161:1049–1061. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- 23.Peterson BS, Leckman JF. The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry. 1998;44:1337–1348. doi: 10.1016/s0006-3223(98)00176-0. [DOI] [PubMed] [Google Scholar]
- 24.Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Dev Psychopathol. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]


