Abstract
This article discusses three largely unrecognized aspects related to fluid movement in ocular tissues; namely, a) the dynamic changes in water permeability observed in corneal and conjunctival epithelia under anisotonic conditions; b) the indications that the fluid transport rate exhibited by the ciliary epithelium is insufficient to explain aqueous humor production; and c) the evidence for fluid movement into and out of the lens during accommodation. We have studied each of these subjects in recent years and present an evaluation of our data within the context of the results of others who have also worked on electrolyte and fluid transport in ocular tissues. We propose that 1) the corneal and conjunctival epithelia, with apical aspects naturally exposed to variable tonicities, are capable of regulating their water permeabilities as part of the cell-volume regulatory process, 2) fluid may directly enter the anterior chamber of the eye across the anterior surface of the iris, thereby representing an additional entry pathway for aqueous humor production, and 3) changes in lens volume occur during accommodation, and such changes are best explained by a net influx and efflux of fluid.
Keywords: unidirectional water fluxes, net water fluxes, electrolyte transport, corneal epithelium, conjunctival epithelium, ciliary body epithelium, crystalline lens
Introduction
Most ocular tissues transport fluid. This property is widely recognized in the cases of the corneal endothelium and ciliary body epithelium, which are largely responsible for corneal deturgescence and aqueous humor formation respectively. When isolated under in vitro conditions, the epithelia of the cornea, conjunctiva and lens have also exhibited fluid transport activity. We recently provided an overview of the established transporters and channels likely to underpin such fluid transport in these latter tissues, and suggested possible functional roles for active fluid movement by these epithelia (Candia, 2004; Candia and Alvarez, 2005). More recently, Levin and Verkman (2006) presented a fundamentally similar overview, but emphasized the central roles of CFTR and aquaporins (AQPs) in the fluid transport effected by most ocular epithelia. As such, these authors implicitly espoused the commonly held view that the underlying linkage between fluid and electrolyte transport is via a transcellular local osmotic mechanism, a concept rigorously defended by Mathias and Wang (2005).
In contrast, Fischbarg and his associates have recently provided data on the corneal endothelium that are inconsistent with the transcellular local osmotic hypothesis, whereby AQPs are required to facilitate fluid transport, and instead they promote the idea that net fluid movement across this tissue occurs paracellularly driven by an electro-osmotic coupling to re-circulating Na+ currents at the level of the intercellular tight-junctions (Fischbarg, 2003, 2006; Fischbarg et al., 2006). Succinctly, their model rests upon various observations including the findings that fluid transport persists in the face of inhibited transcellular HCO3- and Cl- secretion (Diecke et al., 2007), and that in vitro measurements of net fluid transport across endothelial cells isolated from AQP1 null mice exhibited fluid transport rates largely similar to those obtained with cells isolated from control animals (Fischbarg et al., 2006). In addition, Hill’s group have provided reviews on the evidence for paracellular fluid transport (Shachar-Hill and Hill, 2002) and on work with AQP knockout mice (Hill et al., 2004), while Larsen et al. (2007) have also analyzed the Na+ recirculation theory as it pertains to ion-coupled water transport in both low- and high resistance epithelia. Conversely, although Mathias and Wang (2005) do not question the possible validity of fluid transport models employing electro-osmosis, they deduced that local osmosis will also be present and sufficient to generate the nearly isotonic fluid that is transported. Their conclusions were based on theoretical models containing high transcellular water permeability provided by AQPs in both apical and basolateral membranes.
Fischbarg (2003, 2006) does not dispute an important role for AQPs in transcellular water movement across tissues normally exposed to large transepithelial osmotic gradients (e.g., kidney collecting ducts), but questions their necessity as water conduits for the secretory or absorptive functions of “low-volume fluid transporting” epithelia, such as those in the eye. Instead, he suggests that AQPs may be involved in cell volume regulation given his group’s recent findings of impaired regulatory volume decrease (RVD) in corneal endothelial cells from AQP knockout mice. They posit that the cytoplasmic aspects of the water channels may interact with other regulatory proteins (Fischbarg et al., 2006), a hypothetical notion that has recently been buttressed by recent work on AQP5, which was demonstrated to co-localize with TRPV4 (the transient receptor potential vanalloid 4), a molecule thought to function as an osmosensor (Liedtke 2005a, 2005b; Liu et al., 2006). It was shown that AQP5 and TRPV4 concertedly controlled regulatory volume decrease in salivary gland cells (Liu et al., 2006). Hill et al. (2004, 2006) have also suggested a role for AQPs in osmosensing.
Interestingly, histological observations by Levin and Verkman (2006) of corneas from AQP-deficient mice have provided somewhat curious findings that perhaps may be related to changes in cell volume regulatory processes. For example, they report increased corneal stromal and epithelial thickness in mice lacking AQP5, the aquaporin expressed at the epithelial apical surface, but reduced corneal thickness in mice deficient in AQP1, the only AQP found in the endothelium. Presumably, AQP5 was needed to prevent in vivo swelling. This suggests a possible role of the epithelium in in vivo corneal detergescence as reported by Klyce (1975, 1977). On the other hand, AQP1 deficiency did not lead to swelling in vivo. However, AQP1 was required in order to restore corneal thickness in corneas pre-exposed to hypotonic solutions, implying a role for AQP1 in the deturgescence of pre-swollen corneas (Levin and Verkman, 2006).
Moreover, it is also important to note that the in vivo corneal endothelium does not transport a net flow of fluid. This occurs because either 1) there is a recirculation - a flow in one direction in one pathway and the same flow in the opposite direction in another pathway (i.e., a level flow), or 2) the negative, imbibition pressure of the stroma is balanced by a force produced by the transport mechanisms, and there is no net flow across either pathway (i.e., a static head). This question has not yet been resolved. Furthermore, net flows are always determined in swollen corneas, which is not the in vivo situation; or with isolated, or cultured, corneal endothelial cells which are isolated from the negative pressure of the stroma. Thus, one must question if Fischbarg’s circulating Na+ currents cease, or do not exist in vivo, when there is no net flow across the corneal endothelium, or if the paracellular fluid transport proposed by Fischbarg’s model is balanced by an equal and opposite transcellular movement of fluid towards the stroma.
Overall, the disparate observations noted above, as well as the conflicting views on the epithelial mechanisms for solute-solvent coupling in fluid transport, require further analysis and experimentation, and cannot be easily resolved at the present time. As such, the purpose of this article is to highlight other unsettled phenomena in ocular fluid transport physiology that have remained unrecognized and warrant further thought. We will focus on the effects of anisotonic conditions on the epithelial water permeability of the cornea and conjunctiva, the meager fluid transport activity of the isolated ciliary body epithelium, and the apparent fluid transport that accompanies shape changes of the lens under conditions mimicking the accommodative process.
Measurements of Water Fluxes and Water Permeability
We have applied two commonly used methods to measure water fluxes across various ocular epithelia: a) unidirectional/diffusional flow with tritiated water (3H2O); and b) net water flow by volumetric/gravitational procedures.
With method “a”, the diffusion permeability coefficient, Pdw, is expressed in cm/s and given by:
where, A is the area of the membrane (cm2), Vw is the partial volume of water (cm3/mol), Cw is the concentration of water (mol/cm3), and Jdw is the measured flux in cm3/s.
In this case, a 2-compartment chamber is used. The tissue is mounted between compartments; 3H2O is added to one side and samples are taken periodically from both sides to determine the diffusion of 3H2O, which is proportional to Jdw. Details of the method and evaluations have been published (Candia and Zamudio, 1995; Candia et al., 1997, 1998a, 1998b).
With method “b”, the osmotic permeability coefficient, Pf, is expressed in cm/s and can be calculated from the following expression:
where, Jv is in cm3/s and ΔCs is the difference in solute concentration (mol/cm3). For this approach, a two-compartment arrangement could also be used, along with a graduated capillary tube, or appropriate detection system, in order to directly measure Jv as a function of time. Examples of this approach were given by Maurice (1972) and by Fischbarg and his associates (Narula et al., 1992; Yang et al., 2000). This method is analogous to the gravimetric methods used earlier to quantify fluid movement across the urinary bladder (Parisi and Bourguet, 1983; Finkelstein, 1987), and we have recently presented our application of this approach on the ciliary epithelium (Candia et al., 2005).
Unidirectional fluxes of water determined with 3H2O (method “a”) are usually large and similar in both directions. Thus, a small difference (the net volumetric flow, which is detected directly with method “b”) is difficult to detect by method “a” and is usually not calculated as a difference between two unidirectional fluxes. For example, in the case of the conjunctival epithelium, we demonstrated that such unidirectional water fluxes (Jdw) across the tissue were statistically identical in either direction (Candia et al., 2006), and have a magnitude ≈ 60-fold larger than the reported values for the net flux (Jv) of fluid secreted to the tear side by the isolated conjunctiva (≈ 4 - 6 μl · h-1 · cm-2, data from Shiue et al., 2000 and Li et al., 2001 using their applications of method “b” (a volumetric approach). Because of this discrepancy in magnitude, it is unfeasible (if not impossible) to calculate Jv as the difference between the two, relatively large, unidirectional fluxes in the opposite directions. However, method “a” (a diffusional approach) is useful for determining the effects of agents or various experimental conditions on water permeability (Pdw); because although labeled water will cross cell membranes via all available pathways - lipid bilayer, AQPs, and other channels, we have demonstrated that measurements of Jdw, which reflect Pdw, change equally in both directions when an experimental maneuver changes the water permeability of the epithelium (Candia et al., 1997; 1998a; 1998b; 2006).
The discovery of the aquaporins (Preston and Agree, 1991; Preston et al., 1992; King and Agre, 1996) led us to re-examine some of the phenomenological data of water fluxes that had remained unexplained, such as the apparent “rectification” phenomenon that existed in measurements of transepithelial water movement across the amphibian bladder (Candia, Mia and Yorio, 1997). Briefly, arginine vasopressin (AVP) elicits as much as a 40-fold increase in net water fluxes across the bladder when the mucosal-side osmolarity is diluted 10-fold. But an AVP induction of a net water flux is not obtained when the osmotic gradient is in the opposite direction (serosal-side hypotonic), implying a rectification phenomenon. However, this condition (i.e., serosal-side hypotony) elicits a reduction of unidirectional water fluxes in both directions indicating no rectification, but rather a simple decrease in permeability by the unilateral hypotonic conditions from the basal side. It is possible that the basolateral water permeability could have been down regulated, when these cells are swollen from their basolateral sides, due to either aquaporin closure or the removal of constitutive water channels from the membrane. Such reduction in water permeability, which was also observed in corneal and conjunctival epithelia (Candia et al., 1998a, 1998b), may be part of a regulatory mechanism to help maintain cell volume. Consistent with this possibility, it was suggested that water permeability mediated by AQP4 might be regulated by a gating mechanism (Zelenina et al., 2002).
Another observation that has received varied interpretations is the conflicting values obtained for Pf and Pdw in an individual tissue. Furthermore, whereas AVP increases Pf by 40 times, it only increases Pdw no more than 6 fold (Hays and Franki, 1970; Parisi and Bourguet, 1983). The accepted interpretation is that in bulk flow (where the radius of the pore is much larger than that of a water molecule, and laminar flow occurs) a difference between Pf and Pdw is predicted so that Pf/Pdw ≫1 for pores of large radius. This explanation also predicts that when a pore is only slightly larger than a water molecule, so that it restricts water translocation to a single file movement, Pf = Pdw (Finkelstein, 1987). Determinations of the most constricted pore region of the aquaporin structure indicate a diameter of 4 Å (Ren et al., 2001), thereby sufficient to only allow for single-file water movement. Thus, an alternative interpretation must be found for the discrepancy in Pf and Pdw values. As noted above, we have suggested that the imposed osmotic gradient necessary for Pf measurements induces, in itself, cell volume changes that in turn modify membrane water permeability via an effect on aquaporins, or other water-transporting channels, i.e., possibly either gating or retrieval of the channels into submembrane endosomes. This phenomenon would not be elicited when measuring Pdw in the absence of an osmotic gradient, but detected as a change in Pdw in the presence of such gradient. Because it is now clear that the large-pore radius theory cannot be applied to the aquaporins, this reasoning may explain published discrepancies between Pf and Pdw.
It is also important to note that although the units of Pf and Pdw are identical (cm/sec), these paramaeters are actually different measurements. Jdw is directly proportional to the activity (concentration) of water, whereas Jv is proportional to the solute concentration, which decreases the activity of water as solute levels are increased. For a detailed analysis of these phenomena, see Katchalsky and Curran (1967), and Finkelstein (1987).
Water Permeability Studies on Isolated Corneal and Conjunctival Epithelia
Our studies examined the water permeability characteristics of the epithelia of the frog cornea and rabbit conjunctiva isolated in Ussing-type chambers under short-circuited conditions (Candia et al. 1998a, 1998b). While the frog corneal epithelium is similar to the toad bladder in that its apical aspect had relatively lower water permeability than its basolateral surface (Candia et al., 1998a), such sidedness is not a characteristic of the conjunctiva in that neither surface is rate limiting to transepithelial water movement (Candia et al., 1998b). More importantly, it was found that a reduction in Jdw in response to basolateral hypotony was a trait common to both of these ocular tissues, as we initially observed with the amphibian bladder (Candia et al., 1997). Furthermore, unidirectional water fluxes across both the frog corneal epithelium and the rabbit conjunctiva are also inhibited by unilateral hypertonic conditions (Candia et al., 1998a; Candia et al., 2006).
Typically, the unilateral introduction of anisotonic media produced ≈20-30% reductions in Jdw in both tissues, which were reversible upon restoring the control physiological osmolality. The corneas were more responsive to changes in bath tonicity from the stromal, or basolateral side of the preparations, than from the tear side. With the conjunctiva, the introductions of either hypo- or hypertonic solutions against the tear- or stromal-sides of the preparations were equally effective in reducing Jdw, with the exception that in order to obtain such reduction with increased tonicity from the stromal side (medium osmolality increased by adding sucrose), conditions inhibiting regulatory volume increase (RVI) mechanisms (e.g., pretreatment with amiloride and bumetanide, or Na+-free Tyrode’s solution) were also required. Putatively, when present in the stromal-side bath, sucrose may diffuse slowly through the relatively thick conjunctival stroma so that its concentration in the lateral spaces may rise in a manner sufficiently limited that intrinsic RVI mechanisms (e.g., Na+/H+ exchange, NKCC activity) could come into play and maintain cell volume. As such, the apparent down-regulation of membrane Pdw would not occur under conditions with an adequate RVI response, but would do so under conditions with which RVI mechanisms were inhibited. Thus, these experiments suggested that the reductions in epithelial membrane permeability occurred secondarily to changes in cell volume (Candia et al., 2006); presumably the epithelial cell volume had to be perturbed markedly in order to elicit the decline in Pdw.
In both corneal and conjunctival epithelia, the transepithelial water fluxes (Jdw) seem to be predominately transcellular because data were obtained that indicate independence between unidirectional water fluxes and the integrity of the paracellular pathway - 1) there is no correlation between control levels of Jdw and control transepithelial electrical resistance (Rt) values, which inversely reflect paracellular conductances; and 2) mannitol fluxes, which solely traverse paracellular pathways, are increased by both hypertonic and hypotonic conditions that reduce Jdw comparably (Candia et al., 1998b; 2006). Apparently, the unilateral anisotonic environment compromised the integrity of the tight junctions leading to the mannitol flux increase, thereby indicating that the concomitant reduction of Jdw occurred via an inhibition of a transcellular pathway.
Further evidence for a predominately transcellular route for Jdw was obtained using Arrhenius plots, which indicated that the tonicity-elicited water permeability reduction occurred at the level of “water transporting” channels (Candia et al., 1998a; 2006). Moreover, in experiments with the conjunctiva, transepithelial fluxes of n-butanol, a highly lipophilic compound, were measured under anisotonic conditions to test whether or not water movement across the lipid bilayer was affected due to any putative alteration of tissue geometry in vitro (Candia et al., 2006). The unilateral anisotonic conditions did not affect the transconjunctival n-butanol fluxes (suggesting that the integrity of the lipid bilayer was not affected), but did increase the energy of activation (Ea), implying that the tonicity shifts produced less water movement through water channels (Candia et al., 1998a; 2006).
Data from the latter approach on the conjunctiva suggested that most of the transepithelial water diffusion (Jdw) occurs via water-transporting channels presumably at both surfaces and perhaps, as well, through the communicating junctions within the multilayered epithelium. This is based upon the finding that the control Ea for water diffusion of 4.93 kcal/mole that was calculated with the conjunctiva, resides between the value of 2.7 kcal/mole reported for isolated endosomes containing the water channels of the anuran urinary bladder (Verkman, 1992), and a value of 10.2 kcal/mole obtained with native Xenopus oocytes (Zhang and Verkman, 1991), which naturally exhibit relatively low intrinsic water permeability. An approximate 30-40% increase in Ea was calculated with conjunctivae under anisotonic conditions (Candia et al., 2006), implying a partial reduction in water movement via channel-mediated pathways; similar increases in Ea were obtained with the frog corneal epithelium under hypotonic conditions (Candia et al. 1998a).
However, we have not yet identified the specific channels that are “down-regulated” by non-physiological osmolalities. The possibility that AQPs could be involved in the observed reduction in Jdw evoked by the anisotonic conditions remains an open question. Both the corneal and conjunctival epithelia exhibit an identical AQP distribution with AQP5 residing in the apical domain and AQP3 in the basolateral membranes (Verkman, 2003; Oen et al., 2006). Both of these AQPs have reported sensitivities to mercurial agents (Kuwahara et al., 1997), but the use of compounds such as HgCl2 elicits a rapid loss of the transepithelial short-circuit current (a measure of net electrolyte transport across the tissue) and an elimination of Rt (to that of the solution resistance) suggesting that Hg2+ is injurious to the epithelium (Candia et al., 1998a, see Fig. 1; Candia et al., 2006). As such, the fact that subsequent tonicity changes do not evoke further reductions in Jdw in HgCl2-pretreated conjunctivae (Candia et al., 2006) does not in itself demonstrate a role for AQPs in the apparent down regulation in water permeability, given that the epithelium may no longer be biochemically viable, because Hg2+ (at levels 1-2 orders of magnitude lower than that uses to block AQPs) reacts with numerous biological ligands and produces cytotoxic effects (Issa et al., 2003; Kim and Sharma, 2004; Shih et al., 2005). The putative future development of non-toxic AQP blockers would be important for determining a possible role for AQPs in the observed phenomena. Alternatively, should tonicity changes reduce the Jdw, and hence the calculated Pdw, of murine tissues, such reductions could be examined with aquaporin-deficient mice.
Figure 1.
Immediate effects of changes in osmolarity of the apical-side solution. See text for explanation.
It is also possible that changes in the activity of ionic channels that are known to exhibit relatively high water permeabilities could underlie the reductions in water fluxes evoked by osmolality perturbations. CFTR might be a candidate in this regard given that its reported water permeability is in the order of that seen with AQP3 (Pohl, 2004). In addition, some classes of K+ channels also appear to have equally high water permeabilities as the aquaporins (Pohl, 2004, see Fig. 4). Ionic channels (possibly carrying water) typically close under hypertonic conditions and contribute towards RVI mechanisms; thus, such closure might explain the reduction in Jdw with elevated osmolality. However, the opposite would be expected with hypotonic solutions, with which ionic channels open as part of the RVD response. Hence, the reduction in Jdw under anisotonic conditions may not necessarily involve the same channels when tissues are exposed to hypo- and hypertonic media. However, it should also be pointed out that the expression levels of AQPs in membranes appear to be in the order of 1,000 AQP monomers per square micrometer, which is generally greater than the density of ion channels, or ≈ 1 channel/μm2 (Verkman and Mitra, 2000), thereby suggesting that AQPs may make a larger contribution than ion channels to the membrane water permeability phenomena described herein.
Figure 4.
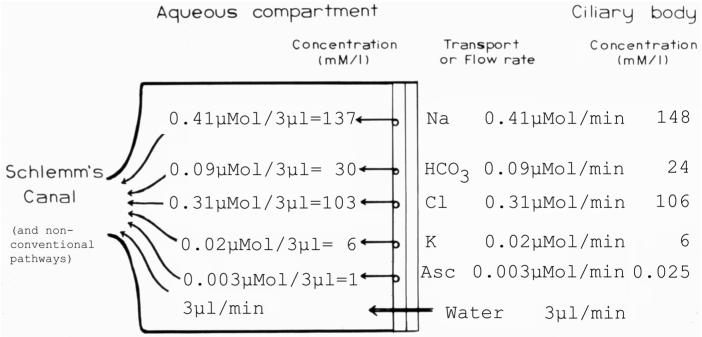
Model of the transport rates that would have to occur across the in vivo rabbit bilayered ciliary epithelium to maintain steady-state electrolyte concentrations of the aqueous compartment, given an aqueous humor flow rate of 3 μl/min. In the case of sodium, for example, the continuous loss of 3 μl of aqueous through the outflow pathways each minute requires 0.41 μmole of Na to cross the ciliary epithelium per minute to maintain aqueous [Na] at 137 mM. In the rabbit, only bicarbonate and ascorbate (Asc) are actively transported “uphill” into the aqueous compartment from lower plasma levels, which equal the levels within the ciliary body stroma (shown on the right-side column of the figure). Na, Cl and K values are from Gerometta et al. (2005), bicarbonate and ascorbate values are from Kinsey (1953), and the 3 μl/min rate of aqueous humor flow in rabbits is from Murray and Bartels (1993).
In our experiments, the tonicity changes were introduced unilaterally on either the tear or stromal sides of the preparations in separate experiments. We initially adopted this protocol with the amphibian bladder to re-examine the “rectification” phenomenon discussed above, and kept it with the ocular tissues to determine the relative responsiveness of the corneal and conjunctival epithelia to unilateral changes in bath tonicity. With these latter tissues, one would expect the osmolality of the stromal-side milieu to remain fairly constant in vivo. However, the apical aspects of these epithelia are naturally exposed to varied osmolalities including virtually pure water, or strongly hyposmotic solutions, as well as elevated tonicities in the cases of individuals with lacrimal gland deficiencies (Farris et al., 1986). As such, the demonstrated reduction of water permeability upon the imposition of anisotonic conditions in the tear-side bath seems relevant to the physiology of the epithelia lining the ocular surface; and as noted above, the conjunctiva is more sensitive to tear-side tonicity changes than is the corneal epithelium.
A second observation consistent with the finding that the corneal apical surface is rate limiting to transepithelial water movement was obtained by the use of the polyene antibiotic, amphotericin B, which has also been used as an artificial water channel in lipid bilayers (Holz and Finkelstein, 1970). Its addition to the tear-side bath of the frog cornea increased Jdw by 20% (Candia et al., 1998a), but such addition to the rabbit conjunctiva does not elicit marked effects (Candia et al., 1998a), indicating that the conjunctival water permeability of the apical surface cannot be increased above that of the control levels, likely due to the presence of AQP5, which we have recently identified in this domain (Oen et al., 2006).
Upon initially finding that the imposition of stromal-side hypotonicity rapidly reduced Jdw across the epithelia of the bladder, cornea and conjunctiva, we proposed that the water permeability of the basolateral membrane could have been down regulated when these cells began to swell from their serosal sides, due to either AQP closure or the removal of constitutive water channels from the membrane (Candia et al., 1997, 1998a, and 1998b). As such, the reduction of basolateral water permeability would have the beneficial effect of slowing the inflow of water, thereby abetting solute-based RVD mechanisms, and contribute towards cell-volume maintenance. This idea has to be reconciled with more recent reports suggesting possible roles for AQPs in both RVD and osmosensing (Fischbarg et al., 2006; Hill et al. 2004, 2006; Liu et al., 2006). Clearly, a unifying explanation for these phenomena is a contemporary question.
In the case of tear-side hypertonic conditions with the conjunctiva, the decline in Jdw was gradual with the largest changes measured between 45 and 60 min after the application of the tonicity increase (Candia et al., 2006). Unequivocally, water must enter the epithelium for RVI mechanisms to restore cell volume and a reduction in water permeability would not be advantageous. It is tempting to speculate that in this case water-transporting channels closed after RVI mechanisms had reverted cell volume closer to that of the control state. If so, it is not immediately apparent as to why the epithelium would putatively express lower water permeability after cell volume had recovered, presumably to, or close to, its control value. However, it should be noted that the new steady-state was attained with the cellular compartment hypertonic and that by itself may be a factor affecting water permeability.
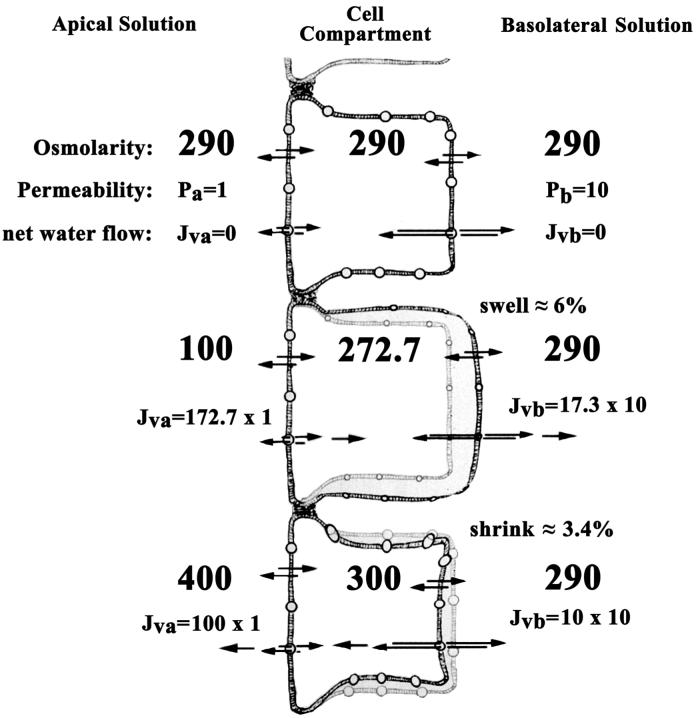
Nevertheless it is clear from our data that the water permeability of epithelia is dynamic and mutable under anisotonic conditions. To illustrate a possible functional role for this property we provide the following examples to show how we would expect an epithelial cell layer to react to tonicity changes from either side bathing solution. Our models use the hypothetical situation that the water permeability of the basolateral membrane is ten times larger than that of the apical aspect, a feature that might exist in the corneal epithelium. Permeability (P) and net water flow (Jv) are given in arbitrary units for illustrative purposes.
Figure 1, top cell, shows the system at equilibrium. Assuming no ionic transport mechanisms and minimal pressure gradients, there is no net water flow (Jv) across either the apical or basolateral membrane because the osmolarity is the same in the three compartments. Notice, however, that the permeability of the basolateral membrane is ten times larger than that of the apical. This includes the intrinsic permeability of the basolateral membrane and a possible larger surface area. If the diffusional water flow (Jdw) could be measured separately across each membrane, it is expected that it would be much larger across the basolateral membrane.
The center cell shows what happens before volume regulatory mechanisms are activated (or if they are inexistent) when the apical-side bath osmolality is reduced to 100. The cell will swell; the cell compartment osmolality will be reduced to a value intermediary between the osmolalities of the two bathing solutions until the gradient across each membrane times its permeability gives equal fluxes (172.7 × 1 = 17.3 × 10). Such equality of fluxes is required for a steady state of the transepithelial flux and cellular osmolyte concentration. The cell needs to increase its volume about 6% to reduce its concentration from 290 to 272.7. If regulatory volume decrease mechanisms exist, solute extrusion would occur with concomitant volume reduction maintaining the cellular osmolality at 272.7. It is clear that steady-state osmolality of the cell, 272.7, is proportionally closer to the bath in contact with the membrane of the larger permeability. It is calculated by:
By a similar reasoning, it is easy to calculate the steady state cell osmolality (300) and net water flow towards the apical solution (100) when the apical side is made hypertonic (400). Initially the cell reaches this osmolality by shrinking about 3.4% (Figure 1, bottom cell). Such putative changes in volume would be minimal and would not severely affect cellular mechanisms. Furthermore, this explanation illustrates the tolerance that epithelia, such as that of the cornea, exhibit against extreme osmolality changes on the apical side.
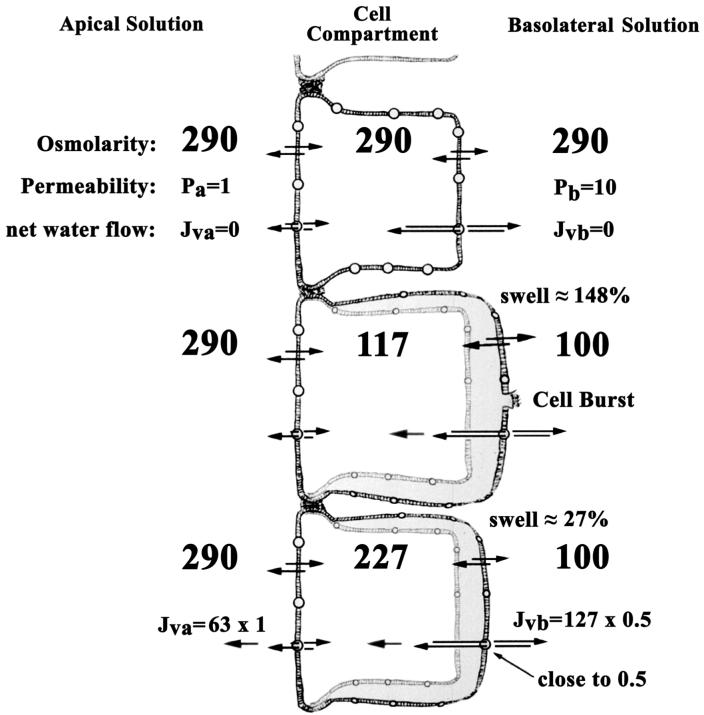
Figure 2, shows the consequences of reducing the osmolality of the basolateral side to 100 mOsM. The cellular compartment would only reach its predicted steady-state osmolality upon swelling 148%, or about 2.5 times its original volume. Red cells burst under these conditions, but intact epithelial layers do not. The rapidity of the swelling precludes prevention by regulatory volume decrease mechanisms that are likely present. However, as indicated by our results, epithelial cells do not burst in response to osmolalities approaching this example. We can explain this phenomenon by introducing a regulatory mechanism that, as the cell reaches a larger volume set point, the permeability of the basolateral side is reduced, let’s say to 0.5 from 10 (Figure 2, bottom cell). In this situation, only a 27% increase in volume would be necessary to reach a steady state before volume regulatory decrease mechanisms are activated to reduce cell volume in the presence of the osmotic gradient.
Figure 2.
Immediate effects of hypo-osmolarity in the basolateral-side solution. See text for explanation.
In short, we posit that changes in water permeability in response to osmotic challenge may represent an unrecognized epithelial property that contributes towards cell-volume regulation, but the identities of the channels involved, and the signaling transduction pathways, remain to be elucidated.
Fluid and Electrolyte Transport Studies on Isolated Ciliary Body Epithelia
Few studies have attempted to measure net fluid fluxes (Jv) across isolated ciliary body preparations applying method “b” (a volumetric approach) described above. Cole (1966) and Burstein et al. (1984) described elaborate mounting protocols for such measurements, but neither reported fluid transport rates comparable to the generally accepted rate of in vivo aqueous formation (i.e., ≈3-4 μl/min in rabbits; Murray and Bartels, 1993). We also designed our own approach to quantify Jv across ciliary epithelia (CE) from rabbit, bovine and porcine preparations and obtained rates of fluid transport ≈60-fold less than the rate of aqueous formation (Candia et al., 2005, 2007).
However, it is important to point out that it is difficult to accurately compare in vitro fluid transport by isolated CE preparations and in vivo rates of aqueous humor formation. Firstly, not all of the ciliary processes and pars plana are exposed to the bathing medium in vitro, and the actual surface area of the processes in the divided chambers that are used is an estimate (Candia et al., 2005, 2007). Secondly, it may reasonably be supposed that the transport activity of the isolated CE in vitro is unlikely to be identical with that in the intact animal due to the absence of blood supply, a condition under which a partial collapse of the processes may occur, and/or hormones or other paracrine agents that stimulate active electrolyte transport may be lacking. Nevertheless, the detected fluid movement in the blood-to-aqueous direction that we and Burstein et al. (1984) observed solely reflects the secretory activity of the isolated CE (albeit possibly compromised) since these in vitro arrangements precluded contributions from ultrafiltration, as well as externally applied osmotic or pressure gradients.
Although the transport activity of the CE in vitro may not be as great as in the intact animal, it is clear that the tissue is not physically damaged by its mounting within divided chambers (Candia et al., 2005). Transepithelial osmotic gradients produced by unilaterally adding 90 mM and 200 mM sucrose to the isolated bovine and rabbit preparations, respectively, were applied in order to calculate the osmotic permeability (Pf) of these epithelia. Both specimens exhibited Pf values of ≈10-3 cm/sec (Candia et al., 2005), a reasonable value for an epithelium, and one that is similar to that of the unstimulated toad bladder, which expresses an increased Pf upon treatment with anti-diuretic hormone (Hayes and Leaf, 1962). We do not yet know whether or not the imposition of the transepithelial osmotic gradient with sucrose also affected Pdw as seen with the cornea and conjunctiva as described in the previous section of this article. Yet if there had there been structural damage to the CE cells during the isolation and mounting of the ciliary body in the divided chambers, the imposition of the transepithelial osmotic gradient could have resulted in a larger fluid displacement from one hemichamber to the other, and a non-physiological osmotic permeability (Pf) would have been calculated. Of course, extensive damage to the tissue would not necessarily result in large transmural fluid displacements if the refection coefficient of the cells to sucrose had decreased and the solute permeated the tissue. With an intact epithelium, the imposed sucrose gradient necessary to calculate Pf can only dissipate slowly via the paracellular pathway, the integrity of which was corroborated by the measurements of mannitol fluxes (Candia et al., 2005).
More importantly, approaches for measuring Jv in vitro could prove relevant for future work controlling of the secretory activity of the CE; the possibility of reduced transport activity in vitro is not withstanding in this regard. A putative lack of an effect by an agent capable of reducing aqueous humor formation in vivo on the in vitro fluid transport rate would be an indication that in vivo there are other important components involved in the production of aqueous humor.
It is well established that the driving forces for fluid production across the ciliary epithelium are the osmotic gradient (created by ionic transport mechanisms) and a possible hydrostatic pressure difference. The relative contribution of these forces to the production of aqueous humor is still an open question, and a contribution from both appears likely (Beneyto et al., 1995; Bill,1973; Macri,1982; Macri and Cevario, 1978). The fact that a pseudo-facility can be measured indicates that a change in intraocular pressure not only influences the efflux, but also the influx of aqueous humor. During tonometry, when an external pressure is applied to the eye, an increased outflow, as well as a decreased inflow, is observed. Thus, there is a pressure-dependent component in the aqueous humor inflow.
The importance of the osmotic force developed by active ionic transport became apparent from the initial work of Cole (1969, 1977). Following this, several investigators were able to isolate the ciliary body epithelia of toad, shark, rabbit, and bovine with which active transport mechanisms for Cl-, Na+ and HCO3- were described (To et al., 2002). Complementing this work, other investigators have used the isolated tissue to look for transporters with intracellular electrodes (Chu and Green, 1990; Wiederholt and Zadunaisky, 1986), fluorescent probes (Butler et al., 1994; Wolosin et al., 1991), electron probe (Bowler et al., 1996; Macknight et al., 2000), and patch clamp techniques (Chen and Sears, 1997; Chen et al., 1998; Edelman et al.,1995; Wang et al., 2000), or have used cultured CE cells to look for channels using microelectrodes (Helbig et al., 1989c) or patch-clamping (Chen et al., 1994; CoCa-Prados et al., 1995, 1996; Yantorno et al., 1992), and for transporters using radioisotopes (Helbig et al. 1988, 1989a, 1989b), or fluorescent techniques (Tao et al., 1998; Hou et al., 1998, 2000). As a result of this wealth of information, several models have been proposed to explain aqueous humor production by the ciliary epithelium (Civan, 1997; Civan and Macknight, 2004; Jacob and Civan, 1996). Although the potential ions responsible for creating the necessary osmotic gradient have been identified, a quantitative accounting of the rate of ionic transport necessary for a fluid production of about 2-4 μl/min is still elusive. Indeed, it was calculated (Chu and Candia, 1987; Krupin et al., 1984) that the measured rate of ionic transport in the isolated rabbit ciliary epithelium could only account for about 15% of the observed fluid production in vivo. Moreover, there are no publications reporting a measured rate of active transport that can justify the in vivo rate of fluid transport. But as noted above, the in vitro transport rates may not represent the rates in vivo. Yet, it still appears likely that aqueous humor production is a concerted contribution by both active and passive processes, with the strongest indication for the latter coming from the pressure-dependent component of aqueous humor inflow as evidenced by the existence of pseudo-facility.
Despite the intensive effort noted above, a satisfying electrolyte transport model consistent with all the available experimental data does not exist. This situation may be due in part to the complexity of the CE and the variations in transport elements among the species studied. In the rabbit tissue, for example, the negative polarity of the aqueous side of the epithelium may be due to a blood-to-aqueous net transport of HCO3-, since removal of HCO3- from both bathing solutions reverses the electrical potential difference (PD) (Krupin et al., 1984; Pesin and Candia, 1982; Sears et al., 1991), whereas in the bovine tissue, a Cl- transport from blood-to-aqueous seems to be responsible for the same PD orientation (To et al., 1998a, 1998b; Do and To, 2000). Moreover, with the isolated rabbit ciliary epithelium, Kishida et al. (1982) and Crook et al. (2000) also described a net Cl- transport as a component of the short-circuit current (ISC) across the tissue, although in the latter study there was a large discrepancy between the net Cl- flux and the ISC.
It is widely known that the composition of the aqueous humor of different species varies, particularly with regard to the Cl- and HCO3- concentrations. In humans, for example, Cl- concentration is higher and HCO3- concentration is lower in the posterior chamber than their concentrations in the plasma, whereas in the rabbit the reverse is true (Cole, 1970, 1984; Davson et al., 1952; Kinsey, 1953; Gerometta et al., 2005). Most of the transport work with the isolated rabbit ciliary epithelium has yielded results consistent with the in vivo higher-than-plasma concentration of HCO3- in the aqueous (Krupin et al., 1984; Sears et al., 1991; Carre et al., 1992; McLaughlin et al., 1998). Even in the work of Crook et al. (2000), bumetanide inhibited only 43% of the ISC, the remainder of which may be a net HCO3- flux. Evidence indicative of a net HCO3- flux across the rabbit CE was provided by Wolosin and coworkers, who demonstrated an asymmetry in the expression of HCO3- transporters in the non-pigmented and pigmented epithelia (Wolosin et al., 1991, 1993; Butler et al. 1994). The latter exhibited the alkali-loading Na+-dependent Cl-/HCO3- exchanger, while the dominant bicarbonate transporters of the former resulted in HCO3- efflux. This expression of bicarbonate transporters would favor a net bicarbonate transport from blood-to-aqueous. In the isolated bovine ciliary body, in contrast, previous findings show a net Cl- transport towards the aqueous (To et al., 1998, 2001; Do and To, 2000), which is consistent with a higher-than-plasma concentration of Cl- in the aqueous humor, as found in humans.
In collaboration with Chi-ho To, whom we lent our closed system for measuring transepithelial radiolabeled CO2 and bicarbonate fluxes (Candia, 1996), we published an explanation of the effects of the carbonic anhydrase inhibitor, acetazolamide, on the bovine ciliary epithelium (To et al., 2001). In this study, we empirically ruled out the existence of a net bicarbonate transport across the bovine CE and instead showed that the inhibitory effects of acetazolamide on the ISC across the CE resulted from an inhibition of a net Cl- flux in the stromal-to-aqueous direction that was HCO3- dependent. We developed a vectorial model to explain the fact that the net Cl- flux was 2-3 fold larger than the ISC due to the secretion of Na+ and K+ into the aqueous that partially subtracts from the Cl- flux. Our results (To et al., 2001) suggested how carbonic anhydrase inhibitors could reduce intraocular pressure in the absence of net HCO3- transport as a driving force for fluid secretion; i.e., by reducing cellular H+ and HCO3- (generated from metabolic CO2 production), which are exchanged with Na+ and Cl- via Na+/H+ and Cl-/HCO3- exchangers, thereby reducing the net Cl- transport.
The net fluid flow (Jv) is bicarbonate dependent in the rabbit CE and Cl- dependent in the bovine CE (Candia, et al., 2005), consistent with the ionic transport mechanisms summarized above. Moreover, we would expect Jv across the bovine preparation to also exhibit a bicarbonate dependency, given the HCO3- -dependent Cl- fluxes that we described with this tissue (To et al., 2001). However, this HCO3- condition was not tested on the bovine CE Jv measurements, but was done with porcine preparations, with which bumetanide inhibited Jv by ≈40%, while the removal of CO2/HCO3- reduced Jv by ≈50% (Candia et al., 2007), seemingly consistent with a bicarbonate-dependent Cl- flux mechanism.
Yet, more importantly, the relative contributions of secretion by the CE and ultrafiltration to aqueous humor formation have still not been determined. As mentioned above, the large discrepancy between the in vitro measures of Jv and the rates of aqueous humor formation does not by necessity demonstrate a large role for ultrafiltration in the in vivo production of aqueous humor; nor do the volumetric approaches with the CE provide any estimate of the relative proportions between secretion by the CE and ultrafiltration. An alternative, interesting arrangement for characterizing the mechanisms underlying aqueous humor formation involves the use of an in vitro perfused bovine eye (Shahidullah et al., 2003). With this methodology, a perfusion pressure is applied, and 40% of aqueous humor formation was ouabain insensitive (Shahidullah et al., 2003), suggesting that this may represent a proportion of the formation due to passive ultrafiltration. However, Shahidullah et al. (2003) point out that their resultant estimation of 40% ultrafiltration in aqueous humor formation may be an overestimation, because the inhibition of active transport processes with ouabain should have led to a reduction in the intraocular pressure, which would have favored more passive ultrafiltration of aqueous humor. Such passive ultrafiltration is commonly envisaged to occur via the paracellular pathways between the cells of the CE.
But there is still another possible pathway that would allow for the ouabain-insensitive fluid flow. Freddo (2001) has detailed an impressive overview of extensive studies done by his group and colleagues showing that the entry pathway for protein into the aqueous is limited to the anterior surface of the iris, i.e., proteins directly enter the anterior chamber near the angle and are not found in the posterior chamber. This direct entry pathway to the anterior chamber strongly implies that fluid can also passively enter via this route.
Current interpretations of aqueous humor dynamics indicate that all fluid entering the anterior chamber comes from the posterior chamber via the pupil. Thus, the solute composition of fluid in the anterior chamber should be equal to that in the posterior chamber. This equality will not hold for any solute with a higher concentration in the posterior chamber than plasma (due to active transport by the CE) if there is a dilution in the anterior chamber by plasma, or if such solutes are transported back to the posterior chamber across the iris epithelium.
One could also posit a priori that the solute content of fluid directly entering the anterior chamber across the anterior aspect of the iris would resemble that of the plasma. As such, compounds such as ascorbate that are actively transported by the CE into the aqueous, so that the aqueous levels are about ≈25 to 50-fold higher than plasma (Kinsey, 1953; To et al., 2002; Table 1), would result in an ascorbate concentration gradient from the posterior chamber to the front of the iris, where the ascorbate levels would be diluted with ascorbate-deficient fluid directly entering the anterior chamber. Consistent with this possibility, measurements by Kinsey (1953) of the respective ascorbate concentrations in posterior and anterior aqueous samples from rabbits showed levels of 1.30 and 0.96 mM; i.e., a 35% higher concentration in the posterior chamber. We recently confirmed these findings by Kinsey in rabbit and our unpublished results are shown in Table 1.
Table 1.
Ascorbate concentrations (mM) of aqueous humor in anterior and posterior chambers of rabbit eye
| Rabbit sample | Anterior Chamber | Posterior Chamber |
|---|---|---|
| 1R | 1.05 | 1.32 |
| 1L | 0.97 | 1.53 |
| 2R | 1.30 | 1.32 |
| 2L | 1.24 | 1.82 |
| 3R | 1.10 | 1.45 |
| 3L | 1.07 | 1.37 |
| 4R | 1.01 | 1.32 |
| 4L | 0.98 | 1.41 |
| 5L | 0.94 | 1.04 |
| 6L | 0.99 | 1.17 |
| 7R | 0.91 | 1.56 |
| 7L | 0.85 | 1.30 |
| 8R | 1.13 | 1.77 |
| 8L | 1.13 | 1.56 |
| Mean: | 1.05 | 1.42* |
| SEM: | 0.03 | 0.06 |
| n: | 14 | 14 |
| Posterior : Anterior ratio | 1.36 |
Significantly larger than the anterior chamber value as paired data, P<0.001. Rabbits were pretreated with bilateral, topical applications of 0.5% proparacaine HCl. After 15 min, 30 μl and 50 μl of aqueous were drawn from the posterior and anterior chambers respectively as described by Kinsey (1953). Twenty microliter aliquots of these samples were used in the ascorbate assay of Liu et al. (1982). In two cases the aqueous samples were not used because the iris was punctured; injured rabbits were immediately sacrificed by placement under general anesthesia and CO2 asphyxiation.
A caveat can be taken that ascorbate consumption by the lens and/or other ocular tissues, such as the cornea, could account for the difference in the concentrations. However, Kinsey (1953) also found a higher concentration of HCO3- in the posterior aqueous (34.1 mM) than in the anterior (27.7 mM), a measurement consistent with the net HCO3- transport expected to exist across the CE of the rabbit, which has plasma HCO3- levels of 24 mM. Although Kinsey (1953) recognized that a gain of water across the blood-aqueous barrier of the anterior chamber could account for the lower concentrations of ascorbate and bicarbonate in the anterior aqueous, he felt it more likely that these anions left the anterior chamber by diffusion via iris vessels given the favorable gradient of the aqueous-to-plasma concentrations. We prefer the former possibility because of the demonstration of Freddo (2001) for the direct entry of plasma proteins into the anterior chamber across the anterior aspect of the iris.
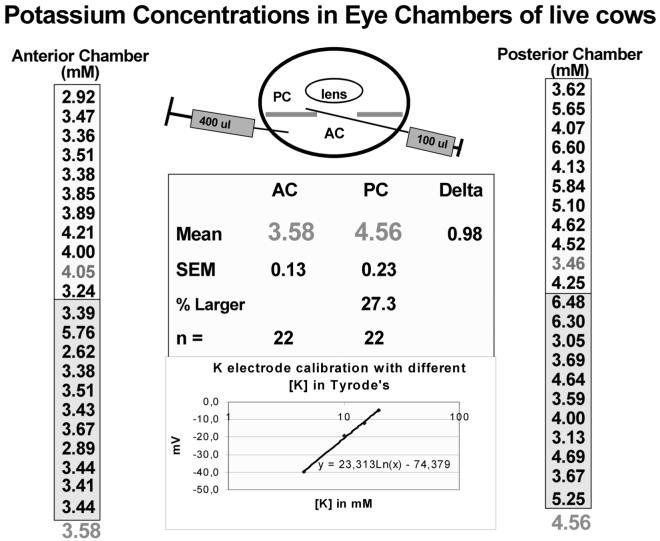
A higher K+ concentration in the posterior chamber than the anterior chamber might be predicted given our models for a putative net K+ flux across the bovine CE (To et al., 2001). In fact, Kinsey (1953) had also provided preliminary data showing higher K+ levels in the posterior chamber than the anterior chamber of rabbit. His potassium measurements were relative, and exact concentrations were not obtained. We recently examined this question and measured a 27% higher concentration of K+ in the bovine posterior aqueous than in the anterior chamber using K+-selective electrodes (Figure 3). Thus in general, the composition of the posterior aqueous differs from that of the anterior in those electrolytes actively transported by the CE from the plasma (so that the posterior aqueous levels are higher than plasma), with the lower levels of the anterior chamber possibly reflecting a dilution from fluid leaving the iris. As such, another undefined component of “aqueous humor production” might exist.
Figure 3.
Aqueous humor potassium concentrations of the anterior and posterior chambers of the in vivo bovine eye. Cows at an Argentinean corral were immobilized within a narrow passage ending in a yoke that loosely closed around the neck separating the body from the head and allowing manipulations of head and neck without discomfort to the animal. These animals were destined to imminent slaughter under the supervision of a local veterinarian according to approved procedures. Two drops of topical proparacaine 0.5% (Alcon, Argentina) were instilled on the eyes as an anesthetic. Following this, an ophthalmologist (R. Gerometta of the Departamento de Oftalmología, Facultad de Medicina, Universidad Nacional Del Nordeste (UNNE), Corrientes, Argentina) performed paracentesis drawing about 0.1 ml from the posterior chamber and 0.4 ml from the anterior chamber. These samples were immediately stored on ice in capped vials for analysis with a K+-sensitive electrode at UNNE, which was done upon returning to the lab. Paired posterior and anterior samples from individual eyes were collected over the course of 2 days (distinct shaded backgrounds for each date of collection) and one mean for the posterior and anterior chambers was tabulated. The potassium concentration of the posterior chamber was higher than that of the anterior in 21 of the 22 eyes assayed.
One should also note that the methods used to measure the rate of so called “aqueous humor production” do not actually measure the production of aqueous humor into the posterior chamber, but instead measure the exit of aqueous humor from the anterior chamber (Lee and Brubaker, 1982; Topper et al., 1984; Vogh et al., 1989), which may include a contributing flow of fluid from the iris into the anterior chamber.
Finally, it should be noted that an aqueous humor outflow of 3 μl/min (as found in rabbit) carries 0.41 μmole of Na+, 0.31 μmole of Cl-, 0.02 μmole of K+, 0.09 μmole of HCO3-, and 0.003 μmole of ascorbate (Figure 4) that must be replenished by transport into the posterior chamber if all aqueous humor is produced into that compartment. However, as alluded to above, there are no publications reporting a measured rate of active transport that can account for these levels of electrolytes to cross the CE each min. We have extensive experience measuring Na+ and Cl- fluxes across isolated CE preparations from rabbit and never obtained a net Na+ or Cl- flux across this tissue (Pesin and Candia, 1982; Chu and Candia, 1987). Thus, we are suspicious of the net Cl- flux data of Kishida et al. (1982) and Crook et al. (2000) in rabbit, and find the data in the latter erroneous, given that the reported net Cl- flux was orders of magnitude greater than the simultaneously measured short-circuit current. Alternatively, if one considers the rate of active transport of Cl- reported for the cat (Holland and Gipson, 1970), the net Cl- flux of 2.89 μmole/cm2/hr represents 0.289 μmole/min across the CE if one assumes a total surface area in cat of 6 cm2 as reported for rabbit (Silverman et al. 2001). Thus, this Cl- transport rate seems about right. However, no evidence of a net Na+ flux of 0.41 μmole/min has been reported across the CE in any species. Moreover, because in live rabbits the aqueous levels of Cl- in the aqueous are not higher than plasma (Gerometta et al., 2005), and we found no evidence for active transport of Cl- across the rabbit CE, we suggest that a major role of the rabbit ciliary epithelium appears to be the active transport of bicarbonate and ascorbate into the posterior chamber (Kinsey, 1953; and Figure 4). As such, we also propose that a direct flow of fluid from the iris to the anterior chamber is a real possibility.
Fluid Transport by the Crystalline Lens during Accommodation
The lens is an asymmetrical organ system with localized transport properties. Early work on lens asymmetry emphasized the influence of the lens epithelium in conferring to the lens its asymmetrical electrophysiological properties (Candia et al., 1970; Candia, Bentley and Mills, 1971; Candia, 1973; Duncan, Juett and Croghan, 1977). When isolated in a double chamber that separated the anterior from the posterior lens surfaces, an anteriorly directed, positive electrical potential developed, as a result of an electrogenic Na+-K+ pump located in the surface, basolateral membrane of the epithelium (Duncan et al., 1980), along with parallel basolateral K+ conductance(s) (Alvarez, Wolosin and Candia, 1991). Logically, it was assumed that the activity of this monolayer was relatively uniform, and that a positive current emanated from the anterior pole to the equator. However, Patterson and coworkers (Robinson and Patterson, 1982; Patterson, 1988; Wind, Walsh and Patterson, 1988a, 1988b) using the vibrating probe, showed in frog and rat lenses a non-homogenous distribution of currents around the lens surface.
Initially, the findings of Patterson’s group were not widely received, and no other laboratory attempted to reproduce their results. Nevertheless, based on Patterson’s model, in which ionic currents exited around the equator and re-entered the lens at the poles, Mathias, Rae and Baldo (1997) developed a theoretical model for electrolyte and fluid circulation inside the lens. This latter model also included information obtained from impedance analysis and structural studies.
Subsequently, Candia and Zamudio (2002) designed a novel Ussing-type chamber that enabled the short-circuiting approach to be used to characterize the regional distribution of Na+ and K+ currents around the surface of the rabbit lens. A non-uniform current distribution was found in the epithelium from the anterior pole to the equator due to the absence of the Na+-K+ pump-generated current at the anterior polar region, where passive inflow of Na+ occurred, and the dominance of K+ conductances plus the pump current at the equatorial surface, as well as, an area just anterior to it. As such, this work represented the first independent confirmation of the findings of Patterson and coworkers, as well as provided the identification of an additional asymmetrical aspect in the lens, namely the absence of Na+-K+ pump-generated current at the anterior-most surface. Consistent with this characteristic, Gao et al. (2000) estimated that the pump-current density at the frog lens equator was 20-fold larger than that at the anterior pole.
Our interest in electrolyte and fluid transport in ocular epithelial led us to apply method “b” (a volumetric approach described above) to measure Jv across distinct regions of the isolated bovine lens (Candia, 2004) in order to determine if data consistent with the model of Mathias et al. (1997) for an internal fluid circulation within the lens could be acquired. Measurements largely in accord with the Mathias model were indeed obtained under control steady-state conditions with inward fluid movement detected across the anterior face and fluid efflux across the equatorial and posterior surfaces (Candia, 2004). The posterior fluid efflux was not predicted, and suggests that the electrolyte transport mechanisms at the posterior aspect of the bovine lens may differ from that of other more widely studied lens species (e.g., frog, rat and rabbit).
Yet the high water permeability exhibited by the bovine lens suggested to us that fluid might readily enter and exit the lens when its shape is changed. This had also been suggested by earlier studies (Cotlier et al., 1968) demonstrating that lenses swell or shrink when exposed to anisotonic solutions, an indication of high capsular and fibril water permeability. We thus decided to examine the possibility that fluid movement, to and from the lens, accompanies the shape changes that occur during the accommodation process. However, for these studies, we have not applied either a volumetric or diffusional approach to directly measure water movement, but instead calculated changes in lens volume under conditions mimicking accommodation.
Accommodation is the ability of the normal eye to focus on the retina the image of objects situated within a range of distances. This change in refractive power is accomplished in lower vertebrates by an anterior-posterior movement of the crystalline lens (Gillum, 1976), and in higher primates, including man, by changes in lens curvature with minimal displacement within the eye (Garner and Yap, 1997; Glasser and Kaufman, 1999). Both the mechanisms of accommodation and its decrease with age (presbyopia) have been the subject of numerous studies (Atchison, 1995; Croft et al., 2001; Glasser et al., 2001). The lens is suspended inside the eye by the attachment to the capsule (around the equatorial region) of the zonulae, which are in turn attached on their opposite end to the ciliary body. The accepted theory, although there are those who dispute it (Schachar et al., 1996), is that when the ciliary muscle is relaxed and flattened against the sclera, the zonulae are under tension and thus pulling eccentrically on the lens equator. This action forces the lens to adopt a more flattened shape with a larger equatorial diameter and a shorter anterior-to-posterior length. Such change in shape of the human lens has been actually recorded in vivo with high resolution MRI (Strenk et al., 1999, 2004). When the subject is focusing on infinite, the ciliary muscle is relaxed, but the zonulae and the lens capsule are under tension deforming the pliable lens content. When the subject focuses on a short distance, the ciliary muscle contracts, its distance to the lens decreases, the zonulae relax and the lens as a whole adopts a more rounded shape, which is its normal tendency. The failures of any of these components (ciliary muscle, less pliable lens content, shortened distance from the ciliary muscle to the equator) have been accepted as contributing factors for presbyopia (Atchison, 1995; Croft et al., 2001; Garner and Yap, 1997; Glasser and Kaufman, 1999; Glasser et al., 2001).
However, it was not the purpose of our work to study the mechanisms of accommodation and this introduction does not attempt to cover this subject exhaustively. We merely propose the possible existence of a fluid flow between the lens and the intraocular fluids during accommodation. This possibility has not been considered (except by Reiff (1995)) in the many publications on accommodation. The fundament of this assertion is based on physical laws governing the relation between surface and volume of a body changing shape. To introduce this concept, two extreme situations are described below.
First, let’s assume a sphere with a given volume and a surface area which cannot change. It can be deformed or folded, but it cannot be stretched or retracted. The sphere is a body that contains a given volume with minimum surface. An example could be a soccer ball with a leather (un-stretchable) surface. When inflated it assumes a spherical shape, and this shape does not change despite being kicked around for 90 minutes. The only way to flatten it, is to let some air out (thus, reducing volume at constant surface area).
The other extreme will be an impermeable rubber balloon. We can deform its shape by pressing (or other means) without changing its volume because its surface stretches. What is the situation with the lens capsule and its contents? The capsule is rather stiff and more than 100 times stiffer than the lens content due to its collagen composition (Krag and Andreassen, 2003, see Table 2). Nevertheless, it is possible that the lens capsule stretches when pulled by the zonulae to adopt its flattened shape for far distance focusing. There is no study that has conclusively determined what changes in the lens during accommodation: surface, volume or both. Strenk et al. (2004) in commenting on their MRI study of in vivo human lenses conclude: “a quite unexpected finding is that the lens CSA (cross sectional area) increases with accommodation. Because the CSA of the lens is larger in the accommodated state when external tension is minimal, the reduction in lens CSA must be produced by the increased zonular tension required to flatten the lens. These accommodative changes in lens CSA reflect accommodative changes in lens volume and suggest that the lens material is slightly compressed when accommodation is relaxed and the external forces exerted on the lens are greatest. These results challenge a long-held belief that the lens is incompressible, based on the fact that it contains a large amount of water which is incompressible.” Despite the fact that the lens compressibility (i.e., Poisson’s ratio) has never been measured, they suggest the possibility that the lens material may be compressed and uncompressed with elastic recovery during the two extremes of accommodation. Thus, they have demonstrated changes in volume during accommodation, but they do not consider the possibility that fluid may be moving in and out of the lens (our hypothesis) rather than being compressed. Their demonstration is based in a two-dimensional approach: the CSA changes during accommodation. Assuming symmetry around the anterior-posterior axis, the result can be extrapolated to the 3-dimensional lens.
As indicated before, the other report suggesting possible fluid movement during accommodation is that of Reiff (1995). Although his concern is the change in intraocular pressure during accommodation, he states: “Another factor in the lens’ ability to regulate intraocular pressure during accommodation would be the effect of the change in shape of the lens during accommodation on the transport of water into and out of the lens. If, as described (Koretz & Handelman, 1988), the lens capsule were relatively constant in total surface area over a short period of time, a change in shape from approximately an oblate spheroid to one approaching a globular spheroid, as occurs during accommodation, would allow the lens to imbibe more water because the maximum volume for a given surface area is the sphere.”
Contrary to this, Koopmans et al. (2003) reported that they were able to change the accommodative power of isolated, cortex-emptied and polymer-refilled human lens capsules, where volume was constant. However, it is possible that the postmortem capsule could be substantially stretched, which is the only way that the shape and curvature can be changed at constant volume. Given this contemporary interest to replace the content of the capsular bag of cataractous lenses with a gel-like material that could be deformed by the action of the ciliary muscle, thereby re-establishing accommodation after cataract surgery, it should be made clear that if the surface area of the capsule does not change enough, then the change in shape of the “refilled lens capsule” will be limited because the internal gel will not get out of, or into, the lens. The natural lens has two important physical properties difficult to reproduce - its high protein content for a refraction index larger than that of the aqueous humor, and a fluid content that can permeate its capsule.
We recently described in detail our approach to calculate volume from lens CSA (Gerometta et al., 2007). In this work we characterized 1) an in vitro model: the isolated iris-ciliary body-zonulae-lens complex (CB-Z-L) dissected from bovine, and 2) a theoretical model: the graphical construction of human lenses from structural parameters acquired from the literature. The bovine lens was chosen because it could be freshly procured, its large size allowed for calculation of small volume changes, and it could be abundantly obtained at a low cost. We are aware that the bovine lens has a limited accommodative amplitude given the structure of its internal fibers (Kuszak et al., 2006), but it served our purpose.
Because of the complex geometric shape of the lens, an accurate measurement of its volume is not easy to determine with certainty. Hence, we developed a method to compute the volume of the lens from lateral photographs (Gerometta et al., 2007). These photographs enabled the CSA to be computed with software. We calculated the volume of isolated bovine lenses in conditions simulating accommodation by forcing shape changes with a custom-built stretching device in which the CB-Z-L was placed. Two measurements were taken (CSA and center of mass) to calculate volume. Mechanically stretching the CB-Z-L increased the equatorial length, and decreased the anterior-to-posterior length, the CSA, and the lens volume. The control parameters were restored when the lenses were stretched and relaxed in an aqueous physiological solution, but not when submerged in oil, a condition with which fluid leaves the lens and does not re-enter. This suggests that changes in lens CSA previously observed in human could have resulted from fluid movement out of the lens. If the change in volume was due to compression of its content, as suggested by Strenk et al. (2004), the lens should have regained its rounded shape when tension was relaxed even when immersed in oil. In the bovine, our measurements indicated a reversible 6-8% change in the volume of the lens under the conditions simulating accommodation.
Although the lens can swell or shrink under the influence of osmotic forces, there are no studies showing that a few microliters can be expelled across the anterior face in less than a second, which is the time required in accommodation. We don’t know the force developed inside the lens when stretched, but it should be sufficient to move fluid at the rate required. For technical reasons related to the design of our “stretching apparatus” (rotating a wheel), it took several seconds to stretch the lens, but we felt no particular resistance (Gerometta et al., 2007). With a more advanced technology, we were able to determine that the bovine lens regains its volume in less than 200 milliseconds after the release of the stretching tension (unpublished data). Although we demonstrated a change in volume of the lens, it turned out from our analysis that a change in the surface area of the lens during accommodation is also required (Gerometta et al., 2007). We are presently in the processes of developing an approach to quantify the extent to which the lens surface area might also change.
In short, accommodation could involve changes in capsular surface, but it also clearly involves changes in lens volume. During accommodation, the shape and arrangement of the lens fibers should change (Kuszak et al., 2006) and the fibers maybe compressed somewhat. This could result in fluid movement from the more internal fibers towards the periphery, each layer contributing a minute amount of the volume that finally emerges at the lens surface. As such, this requires good communication between fibers laterally, as described by Zampighi et al. (2002) for the extensive interconnection of fibers with aquaporins. Presumably, the rich expression of aquaporins within the lens may have a role during accommodation and their possible impairment could be one more factor contributing towards presbyopia.
General Summary
Overall, we have presented in this article unresolved issues related to fluid transport in distinct ocular tissues that we have studied over the past decade. Perhaps others might find the phenomena discussed herein sufficiently intriguing to merit complementary study and comment. Clearly an important future advance would be the development of non-toxic aquaporin inhibitors, the use of which could provide insight on each of the three subject areas that we have highlighted.
Acknowledgements
This work was supported by National Institutes of Health grants EY00160, EY01867, EY13749 and EY15857, and by an unrestricted grant from Research to Prevent Blindness, Inc., NY, USA. We are also most grateful to Rosana Gerometta of the Departamento de Oftalmología, Facultad de Medicina, Universidad Nacional Del Nordeste (UNNE), Corrientes, Argentina, and to Chi-Wing Kong and Aldo Zamudio of the Department of Ophthalmology, Mount Sinai School of Medicine, New York, for their expert assistance in the various protocols described herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez LJ, Wolosin JM, Candia OA. Contribution from a pH- and tonicity-sensitive K+ conductance to toad translens short-circuit current. Exp. Eye Res. 1991;52:283–292. doi: 10.1016/0014-4835(91)90092-s. [DOI] [PubMed] [Google Scholar]
- Atchison DA. Accommodation and presbyopia. Ophthalmic Physiol. Opt. 1995;15:255–272. [PubMed] [Google Scholar]
- Beneyto Martin P, Fernandez-Vila PC, Perez TM. Determination of the pseudofacility by fluorophotometry in the human eye. Int. Ophthalmol. 1995;19:219–223. doi: 10.1007/BF00132690. [DOI] [PubMed] [Google Scholar]
- Bill A. The role of ciliary blood flow and ultrafiltration in aqueous humor formation. Exp. Eye Res. 1973;16:287–298. doi: 10.1016/0014-4835(73)90094-8. [DOI] [PubMed] [Google Scholar]
- Bowler JM, Peart D, Purves RD, Carre DA, Macknight AD, Civan MM. Electron probe X-ray microanalysis of rabbit ciliary epithelium. Exp. Eye Res. 1996;62:131–139. doi: 10.1006/exer.1996.0017. [DOI] [PubMed] [Google Scholar]
- Burstein NL, Fischbarg J, Liebovitch L, Cole DF. Electrical potential, resistance, and fluid secretion across isolated ciliary body. Exp. Eye Res. 1984;39:771–779. doi: 10.1016/0014-4835(84)90076-9. [DOI] [PubMed] [Google Scholar]
- Butler GA, Chen M, Stegman Z, Wolosin JM. Na(+-) Cl(-)- and HCO3(-)- dependent base uptake in the ciliary body pigment pigment epithelium. Exp. Eye Res. 1994;59:343–349. doi: 10.1006/exer.1994.1116. [DOI] [PubMed] [Google Scholar]
- Candia OA. Microelectrode and short-circuiting techniques for the study of ion transport in the lens. Exp. Eye Res. 1973;15:219–223. doi: 10.1016/0014-4835(73)90122-x. [DOI] [PubMed] [Google Scholar]
- Candia OA. A novel system to measure labelled CO2 and HCO3- fluxes across epithelia: corneal epithelium as model tissue. Exp. Eye Res. 1996;63:137–149. doi: 10.1006/exer.1996.0102. [DOI] [PubMed] [Google Scholar]
- Candia OA. Electrolyte and fluid transport across corneal, conjunctival and lens epithelia. Exp. Eye Res. 2004;78:527–535. doi: 10.1016/j.exer.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Candia OA, Alvarez LJ. Water and ion transport in ocular tissues. Physiol Minirev. 2006;1:48–57. [Google Scholar]
- Candia OA, Alvarez LJ, Zamudio AC. Regulation of water permeability in rabbit conjunctival epithelium by anisotonic conditions. Am. J. Physiol. Cell. Physiol. 2006;290:C1168–78. doi: 10.1152/ajpcell.00254.2005. [DOI] [PubMed] [Google Scholar]
- Candia OA, Bentley PJ, Mills CD. Short-circuit current and active Na transport across isolated lens of the toad. Am. J. Physiol. 1971;220:558–564. doi: 10.1152/ajplegacy.1971.220.2.558. [DOI] [PubMed] [Google Scholar]
- Candia OA, Bentley PJ, Mills CD, Toyofuku H. Asymmetrical distribution of the potential difference in the toad lens. Nature. 1970;227:852–853. doi: 10.1038/227852a0. [DOI] [PubMed] [Google Scholar]
- Candia OA, Mia A, Yorio T. Evidence of basolateral water permeability regulation in amphibian urinary bladder. Biol. Cell. 1997;89:331–339. [PubMed] [Google Scholar]
- Candia OA, Patarca R, Alvarez LJ. Reduction of water permeability by anisotonic solutions in frog corneal epithelium. Invest. Ophthalmol. Vis. Sci. 1998;39:378–384. [PubMed] [Google Scholar]
- Candia OA, Shi XP, Alvarez LJ. Reduction in water permeability of the rabbit conjunctival epithelium by hypotonicity. Exp. Eye Res. 1998;66:615–624. doi: 10.1006/exer.1998.0462. [DOI] [PubMed] [Google Scholar]
- Candia OA, To CH, Gerometta RM, Zamudio AC. Spontaneous fluid transport across isolated rabbit and bovine ciliary body preparations. Invest. Ophthalmol. Vis. Sci. 2005;46:939–947. doi: 10.1167/iovs.04-0950. [DOI] [PubMed] [Google Scholar]
- Candia OA, To CH, Law CS. Fluid transport across the isolated porcine ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 2007;48:321–327. doi: 10.1167/iovs.06-0432. [DOI] [PubMed] [Google Scholar]
- Candia OA, Zamudio AC. Regional distribution of the Na(+) and K(+) currents around the crystalline lens of rabbit. Am. J. Physiol. Cell. Physiol. 2002;282:C252–62. doi: 10.1152/ajpcell.00360.2001. [DOI] [PubMed] [Google Scholar]
- Candia OA, Zamudio AC. Chloride-activated water permeability in the frog corneal epithelium. J. Membr. Biol. 1995;143:259–266. doi: 10.1007/BF00233454. [DOI] [PubMed] [Google Scholar]
- Carre DA, Tang CS, Krupin T, Civan MM. Effect of bicarbonate on intracellular potential of rabbit ciliary epithelium. Curr. Eye Res. 1992;11:609–624. doi: 10.3109/02713689209000734. [DOI] [PubMed] [Google Scholar]
- Chen S, Inoue R, Inomata H, Ito Y. Role of cyclic AMP-induced Cl conductance in aqueous humour formation by the dog ciliary epithelium. Br. J. Pharmacol. 1994;112:1137–1145. doi: 10.1111/j.1476-5381.1994.tb13202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sears M. A low conductance chloride channel in the basolateral membranes of the non-pigmented ciliary epithelium of the rabbit eye. Curr. Eye Res. 1997;16:710–718. doi: 10.1076/ceyr.16.7.710.5064. [DOI] [PubMed] [Google Scholar]
- Chen S, Wan XL, Sears M. pICln can regulate swelling-induced Cl- currents in either layer of rabbit ciliary epithelium. Biochem. Biophys. Res. Commun. 1998;246:59–63. doi: 10.1006/bbrc.1998.8571. [DOI] [PubMed] [Google Scholar]
- Chu TC, Candia OA. Electrically silent Na+ and Cl- fluxes across the rabbit ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1987;28:445–450. [PubMed] [Google Scholar]
- Chu TC, Green K. Bicarbonate and DIDS effects on intracellular potential difference in rabbit ciliary epithelium. Curr. Eye Res. 1990;9:233–239. doi: 10.3109/02713689009044518. [DOI] [PubMed] [Google Scholar]
- Civan MM. Transport by the ciliary epithelium of the eye. News Physiol Sci. 1997;12:158–162. [Google Scholar]
- Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp. Eye Res. 2004;78:625–631. doi: 10.1016/j.exer.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Anguita J, Chalfant ML, Civan MM. PKC-sensitive Cl- channels associated with ciliary epithelial homologue of pICln. Am. J. Physiol. 1995;268:C572–9. doi: 10.1152/ajpcell.1995.268.3.C572. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M, Sanchez-Torres J, Peterson-Yantorno K, Civan MM. Association of ClC-3 channel with Cl- transport by human nonpigmented ciliary epithelial cells. J. Membr. Biol. 1996;150:197–208. doi: 10.1007/s002329900044. [DOI] [PubMed] [Google Scholar]
- Cole DF. Aqueous humor formation. Doc. Ophthalmol. 1966;21:116–238. [Google Scholar]
- Cole DF. Evidence for active transport of chloride in ciliary epithelium of the rabbit. Exp. Eye Res. 1969;8:5–15. doi: 10.1016/s0014-4835(69)80074-6. [DOI] [PubMed] [Google Scholar]
- Cole DF. Aqueous and Ciliary Body. In: Graymore CN, editor. Biochemistry of the eye. Academic Press; New York: 1970. pp. 115–125. [Google Scholar]
- Cole DF. Secretion of the aqueous humour. Exp. Eye Res. 1977;25(Suppl):161–176. doi: 10.1016/s0014-4835(77)80015-8. [DOI] [PubMed] [Google Scholar]
- Cole DF. Ocular Fluids. In: Davson H, editor. The Eye. Academic Press; New York: 1984. pp. 269–390. [Google Scholar]
- Cotlier E, Kwan B, Beaty C. The lens as an osmometer and the effects of medium osmolarity on water transport, 86Rb efflux and 86Rb transport by the lens. Biochim. Biophys. Acta. 1968;150:705–722. doi: 10.1016/0005-2736(68)90060-6. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Kaufman PL. Accommodation and presbyopia. Int. Ophthalmol. Clin. 2001;41:33–46. doi: 10.1097/00004397-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Crook RB, Takahashi K, Mead A, Dunn JJ, Sears ML. The role of NaKCl cotransport in blood-to-aqueous chloride fluxes across rabbit ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 2000;41:2574–2583. [PubMed] [Google Scholar]
- Davson H, Matchett PA, Roberts JR. Comparative studies of the distribution of chloride between plasma and aqueous humour. J. Physiol. 1952;116:47P–48P. [PubMed] [Google Scholar]
- Diecke FP, Ma L, Iserovich P, Fischbarg J. Corneal endothelium transports fluid in the absence of net solute transport. Biochim. Biophys. Acta. 2007 doi: 10.1016/j.bbamem.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do CW, To CH. Chloride secretion by bovine ciliary epithelium: a model of aqueous humor formation. Invest. Ophthalmol. Vis. Sci. 2000;41:1853–1860. [PubMed] [Google Scholar]
- Ducan G, Delamere NA, Paterson CA, Neville MC. Contribution of an electrogenic pump to the electrical characteristics of frog lens membranes. Exp Eye Res. 1980;30:105–107. doi: 10.1016/0014-4835(80)90128-1. [DOI] [PubMed] [Google Scholar]
- Duncan G, Juett JR, Croghan PC. A simple chamber for measuring lens asymmetry potentials. Exp. Eye Res. 1977;25:391–398. doi: 10.1016/0014-4835(77)90106-3. [DOI] [PubMed] [Google Scholar]
- Edelman JL, Loo DD, Sachs G. Characterization of potassium and chloride channels in the basolateral membrane of bovine nonpigmented ciliary epithelial cells. Invest. Ophthalmol. Vis. Sci. 1995;36:2706–2716. [PubMed] [Google Scholar]
- Farris RL, Stuchell RN, Mandel ID. Tear osmolarity variation in the dry eye. Trans. Am. Ophthalmol. Soc. 1986;84:250–268. [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A. Distinguished lecture series of the Society of General Physiologists. Vol. 4. John Wiley & Sons; New York: 1987. Water Movement through Lipid Bilayers, Pores and Plasma Membranes. Theory and Reality. [Google Scholar]
- Fischbarg J. Aquaporins and fluid transport: an evolving relationship. Cell. Mol. Biol. (Noisy-Le-Grand) 2006;52:28–33. [PubMed] [Google Scholar]
- Fischbarg J. On the mechanism of fluid transport across corneal endothelium and epithelia in general. J. Exp. Zoolog A. Comp. Exp. Biol. 2003;300:30–40. doi: 10.1002/jez.a.10306. [DOI] [PubMed] [Google Scholar]
- Fischbarg J, Diecke FP, Iserovich P, Rubashkin A. The Role of the Tight Junction in Paracellular Fluid Transport across Corneal Endothelium. Electro-osmosis as a Driving Force. J. Membr. Biol. 2006;210:117–130. doi: 10.1007/s00232-005-0850-8. [DOI] [PubMed] [Google Scholar]
- Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp. Eye Res. 2001;73:581–592. doi: 10.1006/exer.2001.1056. [DOI] [PubMed] [Google Scholar]
- Gao J, Sun X, Yatsula V, Wymore RS, Mathias RT. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. J. Membr. Biol. 2000;178:89–101. doi: 10.1007/s002320010017. [DOI] [PubMed] [Google Scholar]
- Garner LF, Yap MK. Changes in ocular dimensions and refraction with accommodation. Ophthalmic Physiol. Opt. 1997;17:12–17. [PubMed] [Google Scholar]
- Gerometta RM, Malgor LA, Vilalta E, Leiva J, Candia OA. Cl-concentrations of bovine, porcine and ovine aqueous humor are higher than in plasma. Exp. Eye Res. 2005;80:307–312. doi: 10.1016/j.exer.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Gerometta R, Zamudio AC, Escobar DP, Candia OA. Volume change of the ocular lens during accommodation. Am. J. Physiol. Cell. Physiol. 2007;293:C797–804. doi: 10.1152/ajpcell.00094.2007. [DOI] [PubMed] [Google Scholar]
- Gillum W. Mechanisms of accommodation in vertebrates. Ophthalmic Semin. 1976;1:253–286. [PubMed] [Google Scholar]
- Glasser A, Croft MA, Kaufman PL. Aging of the human crystalline lens and presbyopia. Int. Ophthalmol. Clin. 2001;41:1–15. doi: 10.1097/00004397-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- Hays RM, Leaf A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J. Gen. Physiol. 1962;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig H, Korbmacher C, Erb C, Nawrath M, Knuuttila KG, Wistrand P, Wiederholt M. Coupling of 22Na and 36Cl uptake in cultured pigmented ciliary epithelial cells: a proposed role for the isoenzymes of carbonic anhydrase. Curr. Eye Res. 1989a;8:1111–1119. doi: 10.3109/02713688909000036. [DOI] [PubMed] [Google Scholar]
- Helbig H, Korbmacher C, Kuhner D, Berweck S, Wiederholt M. Characterization of Cl-/HCO3- exchange in cultured bovine pigmented ciliary epithelium. Exp. Eye Res. 1988;47:515–523. doi: 10.1016/0014-4835(88)90091-7. [DOI] [PubMed] [Google Scholar]
- Helbig H, Korbmacher C, Nawrath M, Erb C, Wiederholt M. Sodium bicarbonate cotransport in cultured pigmented ciliary epithelial cells. Curr. Eye Res. 1989b;8:595–598. doi: 10.3109/02713688908995759. [DOI] [PubMed] [Google Scholar]
- Helbig H, Korbmacher C, Wohlfarth J, Coca-Prados M, Wiederholt M. Electrical membrane properties of a cell clone derived from human nonpigmented ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1989c;30:882–889. [PubMed] [Google Scholar]
- Hill AE, Shachar-Hill B. A new approach to epithelial isotonic fluid transport: an osmosensor feedback model. J. Membr. Biol. 2006;210:77–90. doi: 10.1007/s00232-005-0847-3. [DOI] [PubMed] [Google Scholar]
- Hill AE, Shachar-Hill B, Shachar-Hill Y. What are aquaporins for? J. Membr. Biol. 2004;197:1–32. doi: 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]
- Holland MG, Gipson CC. Chloride ion transport in the isolated ciliary body. Invest. Ophthalmol. 1970;9:20–29. [PubMed] [Google Scholar]
- Holz R, Finkelstein A. The water and nonelectrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J. Gen. Physiol. 1970;56:125–145. doi: 10.1085/jgp.56.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Delamere NA. Studies on H(+)-ATPase in cultured rabbit nonpigmented ciliary epithelium. J. Membr. Biol. 2000;173:67–72. doi: 10.1007/s002320001008. [DOI] [PubMed] [Google Scholar]
- Hou Y, Pierce WM, Jr, Delamere NA. The influence of ascorbic acid on active sodium transport in cultured rabbit nonpigmented ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 1998;39:143–150. [PubMed] [Google Scholar]
- Issa Y, Watts DC, Duxbury AJ, Brunton PA, Watson MB, Waters CM. Mercuric chloride: toxicity and apoptosis in a human oligodendroglial cell line MO3.13. Biomaterials. 2003;24:981–987. doi: 10.1016/s0142-9612(02)00436-2. [DOI] [PubMed] [Google Scholar]
- Jacob TJ, Civan MM. Role of ion channels in aqueous humor formation. Am. J. Physiol. 1996;271:C703–20. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- Katchalsky A, Curran PF. Nonequilibrium Thermodynamics in Biophysics. Harvard University Press; Cambridge, MA: 1967. [Google Scholar]
- Kim SH, Sharma RP. Mercury-induced apoptosis and necrosis in murine macrophages: role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol. Appl. Pharmacol. 2004;196:47–57. doi: 10.1016/j.taap.2003.11.020. [DOI] [PubMed] [Google Scholar]
- King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu. Rev. Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Kinsey VE. Comparative chemistry of aqueous humor in posterior and anterior chambers of rabbit eye, its physiologic significance. AMA Arch. Ophthalmol. 1953;50:401–417. doi: 10.1001/archopht.1953.00920030409001. [DOI] [PubMed] [Google Scholar]
- Kishida K, Sasabe T, Iizuka S, Manabe R, Otori T. Sodium and chloride transport across the isolated rabbit ciliary body. Curr. Eye Res. 1982;2:149–152. doi: 10.3109/02713688208997688. [DOI] [PubMed] [Google Scholar]
- Klyce SD. Transport of Na, Cl, and water by the rabbit corneal epithelium at resting potential. Am J Physiol. 1975;228:1446–1452. doi: 10.1152/ajplegacy.1975.228.5.1446. [DOI] [PubMed] [Google Scholar]
- Klyce SD. Enhancing fluid secretion by the corneal epithelium. Invest Ophthalmol Vis Sci. 1977;16:968–973. [PubMed] [Google Scholar]
- Koopmans SA, Terwee T, Barkhof J, Haitjema HJ, Kooijman AC. Polymer refilling of presbyopic human lenses in vitro restores the ability to undergo accommodative changes. Invest. Ophthalmol. Vis. Sci. 2003;44:250–257. doi: 10.1167/iovs.02-0256. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Handelman GH. How the human eye focuses. Sci. Am. 1988;259:92–99. doi: 10.1038/scientificamerican0788-92. [DOI] [PubMed] [Google Scholar]
- Krag S, Andreassen TT. Mechanical properties of the human lens capsule. Prog. Retin. Eye Res. 2003;22:749–767. doi: 10.1016/s1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- Krupin T, Reinach PS, Candia OA, Podos SM. Transepithelial electrical measurements on the isolated rabbit iris-ciliary body. Exp. Eye Res. 1984;38:115–123. doi: 10.1016/0014-4835(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Kuszak JR, Mazurkiewicz M, Jison L, Madurski A, Ngando A, Zoltoski RK. Quantitative analysis of animal model lens anatomy: accommodative range is related to fiber structure and organization. Vet. Ophthalmol. 2006;9:266–280. doi: 10.1111/j.1463-5224.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Gu Y, Ishibashi K, Marumo F, Sasaki S. Mercury-sensitive residues and pore site in AQP3 water channel. Biochemistry. 1997;36:13973–13978. doi: 10.1021/bi9711442. [DOI] [PubMed] [Google Scholar]
- Larsen EH, Mobjerg N, Nielsen R. Application of the Na(+) recirculation theory to ion coupled water transport in low- and high resistance osmoregulatory epithelia. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2007;148:101–116. doi: 10.1016/j.cbpa.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Lee DA, Brubaker RF. Effect of phenylephrine on aqueous humor flow. Curr. Eye Res. 1982;2:89–92. doi: 10.3109/02713688208997681. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporins and CFTR in ocular epithelial fluid transport. J. Membr. Biol. 2006;210:105–115. doi: 10.1007/s00232-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Kuang K, Yerxa B, Wen Q, Rosskothen H, Fischbarg J. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl(-) and fluid secretion. Am. J. Physiol. Cell. Physiol. 2001;281:C595–602. doi: 10.1152/ajpcell.2001.281.2.C595. [DOI] [PubMed] [Google Scholar]
- Liedtke W. TRPV4 as osmosensor: a transgenic approach. Pflugers Arch. 2005a;451:176–180. doi: 10.1007/s00424-005-1449-8. [DOI] [PubMed] [Google Scholar]
- Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J. Physiol. 2005b;567:53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TZ, Chin N, Kiser MD, Bigler WN. Specific spectrophotometry of ascorbic acid in serum or plasma by use of ascorbate oxidase. Clin. Chem. 1982;28:2225–2228. [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay B, Nakamoto T, Singh B, Liedtke W, Melvin JE, Ambudkar I. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 2006;281:15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- Macknight AD, McLaughlin CW, Peart D, Purves RD, Carre DA, Civan MM. Formation of the aqueous humor. Clin. Exp. Pharmacol. Physiol. 2000;27:100–106. doi: 10.1046/j.1440-1681.2000.03208.x. [DOI] [PubMed] [Google Scholar]
- Macri FJ. The pressure dependency of aqueous humor formation in the anesthetized cat. Ophthalmic Res. 1982;14:279–283. doi: 10.1159/000265203. [DOI] [PubMed] [Google Scholar]
- Macri FJ, Cevario SJ. The formation and inhibition of aqueous humor production. A proposed mechanism of action. Arch. Ophthalmol. 1978;96:1664–1667. doi: 10.1001/archopht.1978.03910060290023. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol. Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- Mathias RT, Wang H. Local osmosis and isotonic transport. J. Membr. Biol. 2005;208:39–53. doi: 10.1007/s00232-005-0817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DM. The location of the fluid pump in the cornea. J. Physiol. 1972;221:43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin CW, Peart D, Purves RD, Carre DA, Macknight AD, Civan MM. Effects of HCO3- on cell composition of rabbit ciliary epithelium: a new model for aqueous humor secretion. Invest. Ophthalmol. Vis. Sci. 1998;39:1631–1641. [PubMed] [Google Scholar]
- Murray DL, Bartels SP. The relationship between aqueous humor flow and anterior chamber protein concentration in rabbits. Invest. Ophthalmol. Vis. Sci. 1993;34:370–376. [PubMed] [Google Scholar]
- Narula P, Xu M, Kuang KY, Akiyama R, Fischbarg J. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am. J. Physiol. 1992;262:C98–103. doi: 10.1152/ajpcell.1992.262.1.C98. [DOI] [PubMed] [Google Scholar]
- Oen H, Cheng P, Turner HC, Alvarez LJ, Candia OA. Identification and localization of aquaporin 5 in the mammalian conjunctival epithelium. Exp. Eye Res. 2006;83:995–998. doi: 10.1016/j.exer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Parisi M, Bourguet J. The single file hypothesis and the water channels induced by antidiuretic hormone. J. Membr. Biol. 1983;71:189–193. doi: 10.1007/BF01875460. [DOI] [PubMed] [Google Scholar]
- Patterson JW. Characterization of the equatorial current of the lens. Ophthalmic Res. 1988;20:139–142. doi: 10.1159/000266570. [DOI] [PubMed] [Google Scholar]
- Pesin SR, Candia OA. Na+ and Cl- fluxes, and effects of pharmacological agents on the short-circuit current of the isolated rabbit iris-ciliary body. Curr. Eye Res. 1982;2:815–827. doi: 10.3109/02713688209020017. [DOI] [PubMed] [Google Scholar]
- Pohl P. Combined transport of water and ions through membrane channels. Biol. Chem. 2004;385:921–926. doi: 10.1515/BC.2004.120. [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc. Natl. Acad. Sci. U. S. A. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Reiff TR. The role of the ocular lens in the regulation of intraocular pressure. J. Theor. Biol. 1995;175:267–269. doi: 10.1006/jtbi.1995.0140. [DOI] [PubMed] [Google Scholar]
- Ren G, Reddy VS, Cheng A, Melnyk P, Mitra AK. Visualization of a water- selective pore by electron crystallography in vitreous ice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1398–1403. doi: 10.1073/pnas.041489198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR, Patterson JW. Localization of steady currents in the lens. Curr. Eye Res. 1982;2:843–847. doi: 10.3109/02713688209020020. [DOI] [PubMed] [Google Scholar]
- Schachar RA, Tello C, Cudmore DP, Liebmann JM, Black TD, Ritch R. In vivo increase of the human lens equatorial diameter during accommodation. Am. J. Physiol. 1996;271:R670–6. doi: 10.1152/ajpregu.1996.271.3.R670. [DOI] [PubMed] [Google Scholar]
- Sears ML, Yamada E, Cummins D, Mori N, Mead A, Murakami M. The isolated ciliary bilayer is useful for studies of aqueous humor formation. Trans. Am. Ophthalmol. Soc. 1991;89:131–52. discussion 152-4. [PMC free article] [PubMed] [Google Scholar]
- Shachar-Hill B, Hill AE. Paracellular fluid transport by epithelia. Int. Rev. Cytol. 2002;215:319–350. doi: 10.1016/s0074-7696(02)15014-5. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Wilson WS, Yap M, To CH. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Invest. Ophthalmol. Vis. Sci. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- Shih CM, Ko WC, Yang LY, Lin CJ, Wu JS, Lo TY, Wang SH, Chen CT. Detection of Apoptosis and Necrosis in Normal Human Lung Cells Using 1H NMR Spectroscopy. Ann. N. Y. Acad. Sci. 2005;1042:488–496. doi: 10.1196/annals.1338.042. [DOI] [PubMed] [Google Scholar]
- Shiue MH, Kulkarni AA, Gukasyan HJ, Swisher JB, Kim KJ, Lee VH. Pharmacological modulation of fluid secretion in the pigmented rabbit conjunctiva. Life Sci. 2000;66:PL105–11. doi: 10.1016/s0024-3205(99)00638-4. [DOI] [PubMed] [Google Scholar]
- Silverman RH, Lizzi FL, Ursea BG, Rondeau MJ, Eldeen NB, Kaliscz A, Lloyd HO, Coleman DJ. High-resolution ultrasonic imaging and characterization of the ciliary body. Invest. Ophthalmol. Vis. Sci. 2001;42:885–894. [PubMed] [Google Scholar]
- Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest. Ophthalmol. Vis. Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- Strenk SA, Strenk LM, Semmlow JL, DeMarco JK. Magnetic resonance imaging study of the effects of age and accommodation on the human lens cross-sectional area. Invest. Ophthalmol. Vis. Sci. 2004;45:539–545. doi: 10.1167/iovs.03-0092. [DOI] [PubMed] [Google Scholar]
- Tao W, Prasanna G, Dimitrijevich S, Yorio T. Endothelin receptor A is expressed and mediates the [Ca2+]i mobilization of cells in human ciliary smooth muscle, ciliary nonpigmented epithelium, and trabecular meshwork. Curr. Eye Res. 1998;17:31–38. doi: 10.1076/ceyr.17.1.31.5256. [DOI] [PubMed] [Google Scholar]
- To CH, Do CW, Zamudio AC, Candia OA. Model of ionic transport for bovine ciliary epithelium: effects of acetazolamide and HCO. Am. J. Physiol. Cell. Physiol. 2001;280:C1521–30. doi: 10.1152/ajpcell.2001.280.6.C1521. [DOI] [PubMed] [Google Scholar]
- To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin. Exp. Optom. 2002;85:335–349. [PubMed] [Google Scholar]
- To CH, Mok KH, Do CW, Lee KL, Millodot M. Chloride and sodium transport across bovine ciliary body/epithelium (CBE) Curr. Eye Res. 1998;17:896–902. doi: 10.1076/ceyr.17.9.896.5138. [DOI] [PubMed] [Google Scholar]
- To CH, Mok KH, Tse SK, Siu WT, Millodot M, Lee KL, Hodson S. In vitro bovine ciliary body/epithelium in a small continuously perfused Ussing type chamber. Cell Struct. Funct. 1998;23:247–254. doi: 10.1247/csf.23.247. [DOI] [PubMed] [Google Scholar]
- Topper JE, McLaren J, Brubaker RF. Measurement of aqueous humor flow with scanning ocular fluorophotometers. Curr. Eye Res. 1984;3:1391–1395. doi: 10.3109/02713688409000834. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Water channels in cell membranes. Annu. Rev. Physiol. 1992;54:97–108. doi: 10.1146/annurev.ph.54.030192.000525. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Role of aquaporin water channels in eye function. Exp. Eye Res. 2003;76:137–143. doi: 10.1016/s0014-4835(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am. J. Physiol. Renal Physiol. 2000;278:F13–28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- Vogh BP, Godman DR, Maren TH. Measurement of aqueous humor flow following scleral injection of sulfacetamide as marker: effect of methazolamide, timolol, and pilocarpine. J. Ocul. Pharmacol. 1989;5:293–302. doi: 10.1089/jop.1989.5.293. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen L, Jacob TJ. The role of ClC-3 in volume-activated chloride currents and volume regulation in bovine epithelial cells demonstrated by antisense inhibition. J. Physiol. 2000;524(Pt 1):63–75. doi: 10.1111/j.1469-7793.2000.t01-1-00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederholt M, Zadunaisky JA. Membrane potentials and intracellular chloride activity in the ciliary body of the shark. Pflugers Arch. 1986;407(Suppl 2):S112–5. doi: 10.1007/BF00584939. [DOI] [PubMed] [Google Scholar]
- Wind BE, Walsh S, Patterson JW. Effect of ouabain on lens equatorial currents. Invest. Ophthalmol. Vis. Sci. 1988;29:1753–1755. [PubMed] [Google Scholar]
- Wind BE, Walsh S, Patterson JW. Equatorial potassium currents in lenses. Exp. Eye Res. 1988;46:117–130. doi: 10.1016/s0014-4835(88)80070-8. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Bonanno JA, Hanzel D, Machen TE. Bicarbonate transport mechanisms in rabbit ciliary body epithelium. Exp. Eye Res. 1991;52:397–407. doi: 10.1016/0014-4835(91)90035-d. [DOI] [PubMed] [Google Scholar]
- Wolosin JM, Chen M, Gordon RE, Stegman Z, Butler GA. Separation of the rabbit ciliary body epithelial layers in viable form: identification of differences in bicarbonate transport. Exp. Eye Res. 1993;56:401–409. doi: 10.1006/exer.1993.1054. [DOI] [PubMed] [Google Scholar]
- Yang H, Reinach PS, Koniarek JP, Wang Z, Iserovich P, Fischbarg J. Fluid transport by cultured corneal epithelial cell layers. Br. J. Ophthalmol. 2000;84:199–204. doi: 10.1136/bjo.84.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantorno RE, Carre DA, Coca-Prados M, Krupin T, Civan MM. Whole cell patch clamping of ciliary epithelial cells during anisosmotic swelling. Am. J. Physiol. 1992;262:C501–9. doi: 10.1152/ajpcell.1992.262.2.C501. [DOI] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Micro-domains of AQP0 in lens equatorial fibers. Exp Eye Res. 2002;75:505–519. doi: 10.1006/exer.2002.2041. [DOI] [PubMed] [Google Scholar]
- Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am. J. Physiol. Renal Physiol. 2002;283:F309–18. doi: 10.1152/ajprenal.00260.2001. [DOI] [PubMed] [Google Scholar]
- Zhang RB, Verkman AS. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am. J. Physiol. 1991;260:C26–34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]