Abstract
We have studied the concentration and temperature dependent influence of cholesterol, stigmasterol, and sitosterol on the global structure and the bending fluctuations of fluid dimyristoyl phosphatidylcholine and palmitoyl oleoyl phosphatidylcholine bilayers applying small-angle x-ray scattering, as well as dilatometry and ultrasound velocimetry. Independent of the lipid matrix, cholesterol was found to be most efficient in modulating bilayer thickness and elasticity, followed by sitosterol and stigmasterol. This can be attributed to the additional ethyl groups and double bond at the C17 alkyl side-chain of the two plant sterols. Hence, it seems that some flexibility of the sterol hydrocarbon chain is needed to accommodate within the lipid bilayer. In addition, we did not observe two populations of membranes within the putative liquid-ordered/liquid-disordered phase coexistence regime of binary sterol/lipid mixtures. Instead, the diffraction patterns could be interpreted in terms of a uniform phase. This lends further support to the idea of compositional fluctuations of unstable sterol rich domains recently brought up by fluorescence microscopy experiments, which contrasts the formation of stable domains within the miscibility gap of binary lipid/sterol mixtures.
INTRODUCTION
Mammalian cells contain cholesterol localized in the plasma membrane, where it participates in the regulation of membrane fluidity, as well as in the activity and metabolism of many membrane bound proteins (1–5). It is also believed that cholesterol is responsible for organizing membrane lipids laterally into submicrometer domains, commonly referred to as rafts (6–8). Although direct evidence for their existence seems still to be lacking, lipid rafts are conceived as an important part of several cellular functions, such as intracellular trafficking of lipids and lipid-anchored proteins, endocytosis, and signal transduction.
Plant sterols are structurally related to cholesterol and are found mainly in plasma membranes of higher plant cells, where they take over the role of cholesterol (9). Sitosterol [(24R)-ethylcholest-5-en-3β-ol] and stigmasterol [(24S)-ethylcholesta-5E,22-dien-3β-ol] are the predominant sterols in plant membranes (9,10). Traces of plant sterols (including sitosterol and stigmasterol) are, however, also found in mammalian tissues, where they represent a large proportion of dietary sterols (11). Nevertheless, plant sterols are of huge biological and medical relevance. For example, β-sitosterol as a single substance and also when combined with other plant sterols, such as stigmasterol reduces cholesterol levels within the blood (12,13) most likely by blocking its absorption (14). Further, plant sterols seem to be of importance for an efficient functioning of the immune system (15) and may cut the risk for prostate cancer by reducing the levels of testosterone and its more active forms (16). Additionally some plant sterols, which are structurally similar to β-sitosterol were reported to inhibit growth of cancer cells (17).
Biophysical studies on artificial membranes have shown that cholesterol positions itself into lipid bilayers such that the hydroxyl group interacts with the lipid headgroup, and the side chain at C17 aligns to the lipid's fatty acid chain (9) This induces disorder in the lamellar gel phase of lipid membranes and order in the fluid phase (18). Moreover, several studies on binary lipid/cholesterol mixtures have indicated a fluid-fluid phase separation into liquid-disordered (Ld) and liquid-ordered (Lo), domains (19–21). These phase diagrams have been, however, put in doubt recently (22–26) and there is growing experimental evidence that cholesterol instead leads to a progressive transformation of the Ld to the Lo phase in absence of a miscibility gap. In turn, Ld and Lo phase coexistence is well established in ternary lipid/cholesterol mixtures (24).
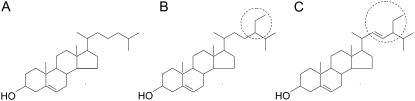
Plant sterols can be used as biomolecular probes to identify specific structural features of cholesterol such as its abilities to modulate acyl chain order (27–30), elasticity (30,31) and lateral organization (32,33). Previous reports comparing cholesterol to plant sterols show differential effects on the bilayer permeability (34–37), chain melting temperature (38), hydrocarbon chain order (39), condensation efficacy (40) and on the membrane's elastic behavior (41). It has been also reported that sterols reduce in general the thermal area expansion coefficient of the lipid bilayer (42,43). However, because of additional ethyl or methyl groups and double bonds, cholesterol derivatives are usually less effective in this respect. An exception seems to be lanosterol, which condenses lipid bilayer more than cholesterol (43). Of particular interest to this study are reports comparing the effects of cholesterol, sitosterol and stigmasterol (35,38–40). Sitosterol and stigmasterol differ structurally from cholesterol only with respect to the C17 side chain by an additional ethyl group at C23 (sitosterol) and a double bond at C22 in addition to this ethyl group (stigmasterol) (Fig. 1).
FIGURE 1.
Chemical structures of cholesterol (A), sitosterol (B), and stigmasterol (C). The encircled regions highlight the structural differences.
Schuler et al. (35) found that sitosterol was more efficient in reducing the water permeability in egg phosphatidylcholine (PC) and soy bean PC compared to cholesterol. Bernsdorff and Winter (39), in turn observed, using steady-state fluorescence anisotropy measurements on dipalmitoyl phosphatidylcholine (DPPC)/sterol mixtures, that sitosterol was less effective than cholesterol with respect to hydrocarbon chain ordering. Further, stigmasterol, was reported to have slightly higher ordering capabilities than sitosterol. This latter finding was contrasted by the groups of Slotte and Yu, who concluded based on differential scanning calorimetric (DSC), resonance energy transfer and detergent solubilization (38), as well as monolayer studies (40), that stigmasterol had smaller condensing effects than sitosterol. Both studies agreed, however, with Bernsdorff and Winter (39) in that cholesterol lead to the most pronounced increase in hydrocarbon chain order.
In view of the lack of a structural study these controversies prompted us to compare the effects of cholesterol on the structural and elastic properties of model membranes composed of the disaturated and monounsaturated lipids dimyristoyl phosphatidylcholine (DMPC) and palmitoyl oleoyl phosphatidylcholine (POPC) to those induced by sitosterol and stigmasterol using a different experimental approach, namely a combination of small-angle x-ray scattering, dilatometry and ultrasound velocimetry. Generally, we found a bilayer condensation for all three sterols with increasing concentration independent of its lipid composition. However, cholesterol was most effective in ordering the hydrocarbon chains followed by sitosterol and stigmasterol. Although this is in agreement with previous studies (38,40), it is remarkable, because stigmasterol differs from sitosterol only by a single additional double bond (Fig. 1). We also found that the observed differential effects are much less pronounced in POPC than in DMPC alluding to the role of hydrocarbon chain composition, which might explain the results of Schuler et al. (35). An additional outcome of this study of general interest is the absence of a fluid-fluid phase separation for all three binary sterol/lipid mixtures yielding further support to the recent discussion on the presence of a uniform phase above the chain melting transition.
MATERIALS AND METHODS
Sample preparation
DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) and POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids (Birmingham, AL), cholesterol, β-sitosterol and stigmasterol from Sigma-Aldrich (St. Louis, MO) and used without further purification. Lipid and sterol stock solutions were prepared by dissolving weighted amounts of dry lipid or sterol powder in chloroform. Sterol concentrations of 1, 5, 7, 10, 20, 30, and 40 mol % within the lipid bilayer were obtained by mixing appropriate amounts of the stock-solutions. The organic lipid/sterol solutions were evaporated at room temperature under a gentle stream of nitrogen and then placed under vacuum for 12 h to form a thin lipid film on the bottom of glass vials. The dry lipid films were subsequently resuspended in 18 MΩ/cm water (UHQ PS, USF Elga, Wycombe, UK) and incubated for 4 h at 45°C with vigorous intermittent vortex mixing. The final lipid concentrations were 50 mg/ml for x-ray and 3 mg/ml for dilatometry and ultrasound velocimetry.
Small and wide-angle x-ray scattering
Small and wide-angle x-ray scattering (SWAXS) experiments were carried out at the Austrian SAXS beamline (44) (Sincrotrone, Trieste, Italy). Two linear one-dimensional gas detectors were used covering the q ranges (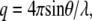 where θ is the scattering angle and λ the x-ray wavelength) between 0.01 and 0.6 Å−1 for SAXS and 0.67–1.95 Å−1 for WAXS, respectively. Eight keV photons were selected. The angular dependence of the scattered intensity was calibrated using silver behenate (d = 58.38 Å) for the SAXS regime and para-bromo benzoic acid (45) for the WAXS regime. The instrumental resolution was determined to have a full width at half maximum of
where θ is the scattering angle and λ the x-ray wavelength) between 0.01 and 0.6 Å−1 for SAXS and 0.67–1.95 Å−1 for WAXS, respectively. Eight keV photons were selected. The angular dependence of the scattered intensity was calibrated using silver behenate (d = 58.38 Å) for the SAXS regime and para-bromo benzoic acid (45) for the WAXS regime. The instrumental resolution was determined to have a full width at half maximum of 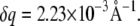 The lipid dispersions were measured in quartz glass capillaries (diameter 1 mm) and tempered in a home-made sample holder block of brass, which was connected to a circulating water bath (Unistat CC, Huber, Offenburg, Germany). For any given temperature the sample was equilibrated before exposure for a period of 10 min. Exposure times were 2–3 min.
The lipid dispersions were measured in quartz glass capillaries (diameter 1 mm) and tempered in a home-made sample holder block of brass, which was connected to a circulating water bath (Unistat CC, Huber, Offenburg, Germany). For any given temperature the sample was equilibrated before exposure for a period of 10 min. Exposure times were 2–3 min.
Background corrected SAXS patterns were analyzed in the full q-range using the program GAP (Global Analysis Program). The technique has been described previously in detail (46,47) (see Pabst (48) for recent review). From the fits to the scattered intensities I = S(q) |F(q)|2/q2 (S(q)…structure factor; F(q)…form factor) we directly obtained the lamellar repeat distance d and further the bilayer thickness dB applying (49)
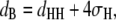 |
(1) |
where dHH is the headgroup-to-headgroup thickness and σH the width of the Gaussian peak applied to model electron density profile of the headgroup region (46). The bending fluctuation or Caillé parameter (50,51),
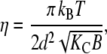 |
(2) |
was directly obtained from the fits and depends on membrane bending rigidity Kc and the bulk modulus of interactions B (52). We further obtained the lateral area per lipid by (49)
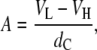 |
(3) |
where 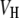 is the volume of the lipid headgroup (= 319 Å3 (53)) and
is the volume of the lipid headgroup (= 319 Å3 (53)) and 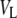 is the partial lipid volume, which is obtained from dilatometric measurements (see below). The hydrocarbon chain length dC is given by
is the partial lipid volume, which is obtained from dilatometric measurements (see below). The hydrocarbon chain length dC is given by
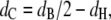 |
(4) |
where 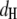 is the headgroup thickness that is set to 10 Å (54).
is the headgroup thickness that is set to 10 Å (54).
Dilatometry and ultrasound velocimetry
The density 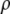 and ultrasound velocity
and ultrasound velocity 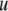 of the liposomal dispersions have been determined simultaneously using the DSA 5000 density and sound analyzer (Anton Paar, Graz, Austria). The density is measured according to the vibrating tube principle (55,56) and the ultrasound velocity is obtained from the propagation time of short 3 MHz acoustic pulses over a fixed distance (57). Temperature control was given by a built in Peltier circuit to within 10−3°C and the claimed accuracy of density and ultrasound velocity are 10−6 g/cm3 and 0.1 m/s, respectively. Sedimentation or flotation effects were found to be negligible by monitoring changes in density and sound velocity values over a period of 30 min. Further, we have repeatedly measured different samples yielding a good overall data reproducibility.
of the liposomal dispersions have been determined simultaneously using the DSA 5000 density and sound analyzer (Anton Paar, Graz, Austria). The density is measured according to the vibrating tube principle (55,56) and the ultrasound velocity is obtained from the propagation time of short 3 MHz acoustic pulses over a fixed distance (57). Temperature control was given by a built in Peltier circuit to within 10−3°C and the claimed accuracy of density and ultrasound velocity are 10−6 g/cm3 and 0.1 m/s, respectively. Sedimentation or flotation effects were found to be negligible by monitoring changes in density and sound velocity values over a period of 30 min. Further, we have repeatedly measured different samples yielding a good overall data reproducibility.
From the density data we calculated apparent specific partial lipid volumes 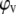 using the relation (56)
using the relation (56)
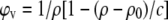 |
(5) |
where ρ0 is the density of the solvent, ρ the density of solution, and c the solute concentration. The partial molecular volume of sterol (VS) and lipid (VL) have been derived according to Greenwood et al. (58) by first calculating the volume per molecule:
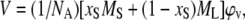 |
(6) |
where NA is Avogadro's number, xS = NS/(NS + NL) the sterol fraction (NS is the number of sterols and NL the number of lipid molecules), and MS and ML the molecular weights of sterol and lipid, respectively. The partial molecular volumes were then given by
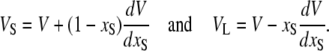 |
(7) |
The sound velocity number was obtained from the sound velocities of water u0 and the lipid/water dispersion u according to (59)
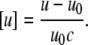 |
(8) |
This allowed us to estimate volume compressibility of the bilayers relative to the solvent using (60),
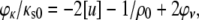 |
(9) |
where 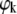 is the specific partial adiabatic compressibility and
is the specific partial adiabatic compressibility and 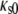 the adiabatic compressibility coefficient of the solvent given by the Laplace equation
the adiabatic compressibility coefficient of the solvent given by the Laplace equation
 |
(10) |
RESULTS
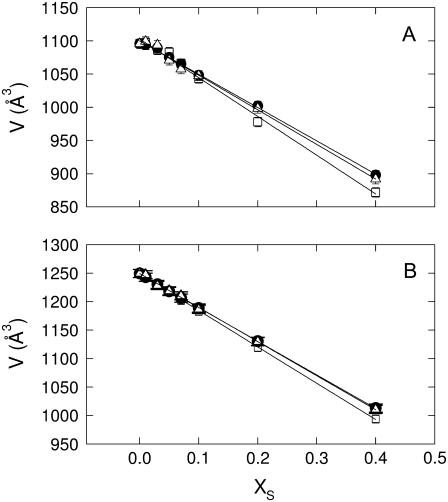
Fig. 2 shows the behavior of the molecular volumes of the studied lipid/sterol mixtures as a function of sterol content. Temperatures of 35°C were chosen for DMPC (Fig. 2 A) and 25°C for POPC (Fig. 2 B), respectively, to be well above the chain melting transition and thus also distant from any pretransitional effects (49,61). A linear decrease of V is observed for all lipid-sterol mixtures, indicating lipid condensation as previously reported for cholesterol/lipid mixtures (58). The same authors, however, observed in the case of DMPC not a single, but also a second linear decrease of V at very high cholesterol content with a slightly smaller slope, which we did not find. The reasons for this disagreement may be manifold. One obvious argument relates to the different experimental approach applied. However, the 5°C higher temperature applied in this study may also lead to this difference. To address this point adequately further systematic work would be necessary. This is, however, not the scope of this study. Nevertheless, we note that our derived partial molecular volumes for DMPC, POPC and cholesterol (Table 1) agree reasonably well with those reported by Greenwood et al. (58). Table 1 further shows the results for the partial molecular volumes, VS, of sitosterol and stigmasterol in the presence of DMPC and POPC, which are both larger than that of cholesterol. As discussed previously (58), the volume condensation effect of sterols is included in their apparent partial molecular volumes and not in the partial lipid volume, VL. Consequently, our results indicate that DMPC bilayers can be more condensed than POPC bilayers for all studied sterols, which can be attributed to its monounsaturated acyl chain in agreement with several previous studies (58,62,63). Addressing the differential condensation capabilities of the three studied sterols is more subtle, because VS also contains the bare or intrinsic volume of the sterols. Greenwood et al. (58) have suggested an intrinsic volume of 630 Å3 for cholesterol from similar measurements on a series of model membranes, which is close to our value of 611 ± 6 Å3 in POPC bilayers. Indeed the same authors have suggested that POPC undergoes no or insignificant volume condensation, at least in the studied cholesterol concentration range. Hence, we may propose that the partial molecular volumes for sitosterol and stigmasterol obtained in POPC membranes will be close to their intrinsic volumes. In fact, their values are within experimental error equal and larger than VS of cholesterol as expected from their chemical structure (Fig. 1). Using thus, VS in POPC as approximate reference for the intrinsic volumes we calculate the relative volume changes in DMPC as ∼−1.4% for cholesterol, ∼−0.9% for sitosterol, and ∼−1.1% for stigmasterol. The relative volume change in the presence of cholesterol compares well with a recent value derived from pressure perturbation calorimetry in POPC at 2°C (23). The results further indicate that cholesterol leads to a stronger volume condensation than the two plant sterols. However, given the involved uncertainties this conclusion may seem ambiguous. Moreover, the condensation capabilities of sterols are usually much more expressed in the lateral area per lipid or membrane thickness (23).
FIGURE 2.
The partial molecular volume values of various lipid /sterol mixtures as a function of sterol content. (A) Results for DMPC at 35°C in presence of cholesterol (□), sitosterol (•), and stigmasterol (Δ). (B) POPC at 25°C in presence of cholesterol (□), sitosterol (•), and stigmasterol (Δ).
TABLE 1.
Partial molecular volumes of lipid/sterol mixtures
| Lipid | T (°C) | VL (Å3) | VCH (Å3) | VSI (Å3) | VST (Å3) |
|---|---|---|---|---|---|
| POPC | 25 | 1250.0 ± 2 | 611.0 ± 6 | 658.7 ± 5 | 651.0 ± 8 |
| DMPC | 35 | 1103.0 ± 2 | 520.0 ± 18 | 598.5 ± 8 | 580.0 ± 16 |
VL is the partial molecular volume of the lipid. VCH, VSI, and VST are partial volumes of the cholesterol, sitosterol, and stigmasterol, respectively.
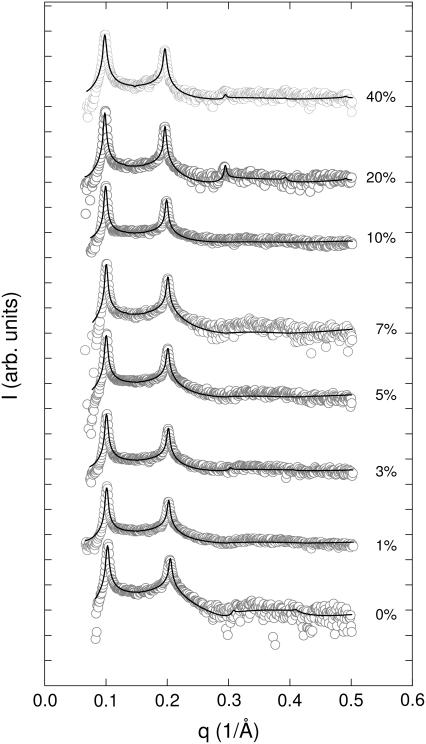
We have, therefore, carried out SAXS experiments under equivalent conditions. As an example for the various diffraction data recorded Fig. 3 shows the SAXS patterns of DMPC at 35°C in the presence of various concentrations of stigmasterol. Fig. 3 also presents the global fits (46–48) to the scattering data, which show a good overall agreement. The good agreement of the fit with the experimental data is a general result and observed for all other SAXS data (not shown). This is remarkable because of two aspects. First of all because the applied electron density model does not include additional electron density contributions close to the headgroup, previously observed in diffraction studies on PC/cholesterol mixtures (26,64). Hence, their contribution to the scattered intensity seems to be small, which justifies not taking it into account explicitly in the applied electron density model. The second aspect is somewhat more important. We have applied only a single bilayer model and were able to obtain good fits for all data. This means that the various lipid/sterol mixtures studied appear to be in a uniform phase. We will address this issue in the next section in more detail.
FIGURE 3.
X-ray diffraction patterns of fully hydrated DMPC/stigmasterol MLVs at 35°C. Numbers to the right of the SAXS patterns indicate stigmasterol concentration in mol %. The solid lines show the best fit to the data applying a global analysis technique (see Materials and Methods).
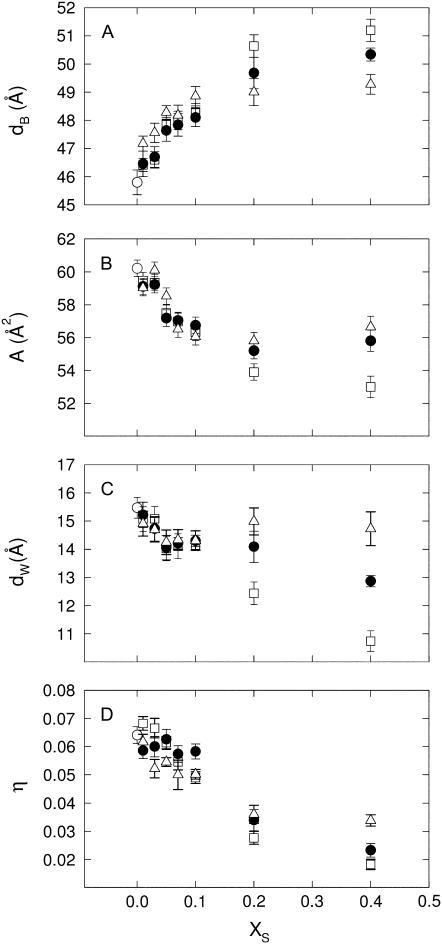
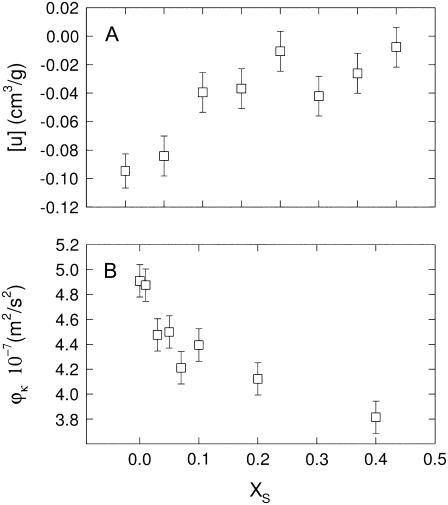
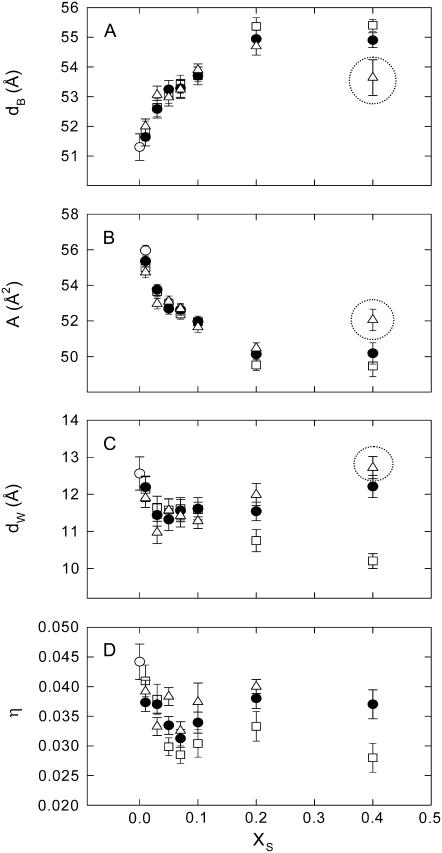
Returning to Fig. 3, we note that the Bragg peaks shifted to lower q-values with sterol concentration concurring with the appearance of higher order peaks. This indicates an increase of the lamellar repeat spacing coupled to an increase order within the lipid bilayer. These general trends are reflected in Fig. 4, which presents the structural parameters dB, dW, A, and η retrieved from the global analysis of all DMPC/sterol mixtures. All shown binary systems exhibited a nonlinear increase of the membrane thickness (Fig. 4 A), observed previously in several diffraction studies on fluid bilayers in the presence of cholesterol (62,64–66). The increase of dB can be attributed to an ordering of the lipid hydrocarbon chains mainly caused by van der Waals forces between sterols and lipids (67). Besides this universal membrane thickening induced by the studied sterols, the overall extent of dB increase is different for the various sterols and mainly visible at higher sterol concentrations. The total amount of the bilayer thickening is ∼5 Å for cholesterol, but only ∼4 Å and ∼3 Å for sito- and stigmasterol, respectively. Similarly, we found that the bilayer condensation as evidenced by the area per lipid was more pronounced in the presence of cholesterol than in the presence of the two plant sterols (Fig. 4 B). Again the effect appears to be less pronounced for stigmasterol than for sitosterol. Due the condensation of the lipid bilayer we expected an increase of the bending rigidity (31) that would lead to a decrease of the bilayer separation and the bending fluctuations. Indeed, we observed both effects (Fig. 4, C and D) again following the order cholesterol > sitosterol > stigmasterol with respect to their effectiveness. Apparently, there seems to be a slight difference in the behavior of dW and η on the sterol concentration and the fluctuations are found to be much more reduced than the bilayer separation. Previously, we made the experience that dW is much more sensitive to bilayer interactions than η (49). With respect to the quantitative dependence on xS, dW should be therefore more reliable than η. We stress, however, that qualitatively both parameters decrease and show the same qualitative differences between the various sterols, which can be understood as an effect of reduced bilayer flexibility. Rigidification of the bilayer due to the condensation effect of sterols can be also observed in terms of the sound velocity number and the specific partial adiabatic compressibility (68,69). We found an increase of [u] and a decrease of 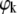 as a function of sterol content for all three sterols (Fig. 5). However, no differential effects could be observed for the three sterols, most likely due to instrumental limitations.
as a function of sterol content for all three sterols (Fig. 5). However, no differential effects could be observed for the three sterols, most likely due to instrumental limitations.
FIGURE 4.
Global structural parameters of DMPC at 35°C in presence of cholesterol (□), sitosterol (•), and stigmasterol (Δ). (A) Concentration dependence of the membrane thickness (dB). (B) Lateral area per lipid (A). (C) Interstitial water layer (dW). (D) Caillé parameter (η). (○) correspond to values obtained for the pure lipid.
FIGURE 5.
Sound velocity number (A) and specific partial adiabatic compressibility (B) for binary DMPC/cholesterol mixtures at 35°C.
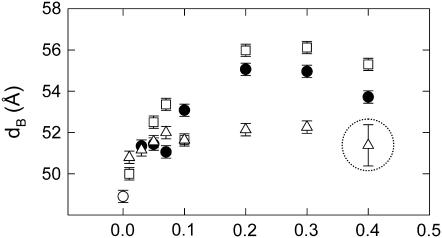
As a next step, we compared our observations for DMPC to monounsaturated bilayers. Fig. 6 presents our results for the structural parameters in POPC at 7°C. This particular temperature was chosen to have a comparable temperature difference to the chain melting temperature Tm as in the case of DMPC (Tm = −3.5°C for POPC (70)). Analogously to our results for DMPC we found a nonlinear increase of dB, as well as a nonlinear decrease of A (Fig. 6, A and B). In agreement with our dilatometry results (Table 1) and several previous reports (58,62,71) the overall condensation effect for all sterols is, however, not as much pronounced as in the case of DMPC. The total amount of bilayer thickening is ∼4 Å for cholesterol, ∼3.5 Å for sitosterol and ∼2.5 Å for stigmasterol. The latter value is of lower certainty due to the formation of stigmasterol crystallites (Fig. 7). The formation of stigmasterol precipitates was verified by repeated sample preparations. Cholesterol is known to form precipitates in lipid bilayers by exceeding its solubility limit (72), or by lipid peroxidation (73). These reports suggest that, depending on the nature of the phospholipid and the history of the sample, the cholesterol crystallites can be in the form of cholesterol monohydrate, anhydrous cholesterol or a mixture of both forms. Analogously, we expect a similar scenario for stigmasterol, because it can not fully dissolve at 40 mol % within POPC. This is remarkable, because POPC/stigmasterol mixtures were prepared as all other samples using only chloroform as organic solvent (see Materials and Methods). Stigmasterol precipitation seems, therefore, to be related to a packing incompatibility of stigmasterol with the asymmetric and monounsaturated fatty acid chains of POPC due to the double bond at C22.
FIGURE 6.
Global structural parameters of POPC at 7°C in presence of cholesterol (□), sitosterol (•), and stigmasterol (Δ). (○) correspond to values obtained for the pure lipid. Encircled data points highlight the structural parameters obtained in the presence of stigmasterol crystallites.
FIGURE 7.
SAXS pattern of POPC in the presence of 40 mol % of stigmasterol at 7°C. Arrows indicate the first and second order reflection of stigmasterol monohydrate (d = 34 Å). The inset shows the corresponding WAXS pattern.
Furthermore, an increase of temperature to 25°C led to an increase of the differential condensation effects as evidenced in Fig. 8, although stigmasterol was still not fully able to dissolve within the POPC bilayers. Nevertheless, the order of cholesterol > sitosterol > stigmasterol was preserved with respect to the sterol capacity to cause ordering of the hydrocarbon chains. Thus, it appears that a temperature increase enhances the differences of the sterol activities, but does not change the above given order in their efficiency.
FIGURE 8.
Bilayer thickness of POPC 25°C as a function of cholesterol (□), sitosterol (•), and stigmasterol (Δ) concentration. (○) corresponds to the value obtained for the pure lipid. The circle marks the membrane thickness obtained in the presence of stigmasterol crystallites.
DISCUSSION
We have provided experimental evidence that cholesterol, sitosterol and stigmasterol lead to different responses of disaturated and monounsaturated lipid bilayers in the fluid phase with respect to structural parameters and bending fluctuations. All studied sterols lead to an increase of order within these bilayers, well known from several previous studies, which have mainly focused on cholesterol (43,64,66). However, there is a subtle difference between the studied sterols and we found the condensing effect to be less expressed for plant sterols compared to cholesterol following the order stigmasterol < sitosterol < cholesterol. The differential effects of the various studied sterols can be understood qualitatively by the additional ethyl group on the side chain of sitosterol, as well as the double bond and ethyl group of stigmasterol (Fig. 1), which render the plant sterols less flexible and as a consequence also less soluble in the bilayer compared to cholesterol. Nevertheless, it is highly remarkable that even the additional double bond of stigmasterol at C22 leads to different condensation properties compared to sitosterol.
A previous DSC study on the impact of bulky ethyl or methyl groups and the length of the C17 sterol side chain on the thermotropic phase behavior concluded that the differential effects were mainly due to the sterol side chain length, whereas the side chain structure had only minor influence (74). In turn, using DSC, resonance energy transfer and detergent-induced solubilization, Halling and Slotte (38) more recently found that the composition of the C17 side chain is also of significance for the sterols effects on lipid bilayer. In this particular study the importance of double bonds in the structure of sterol side chain has been shown and cholesterol was found to induce tightest packing in POPC bilayers, followed by sitosterol and stigmasterol. Most recently, Su et al. (40) reported similar results using dipalmitoyl PC monolayers. Our present findings are in broad agreement with these studies. Our results, however, disagree with Bernsdorff and Winter (39), who reported that stigmasterol affects dipalmitoyl PC bilayers more strongly than sitosterol. This might be due to the fluorescence probe used, which detected only the “interaction between the rigid system of the sterols with the upper acyl chain region of the phospholipids” (39). As this region is intrinsically relatively rigid their measurements may not be very sensitive to small modifications of the alkyl side chain of the sterols, which are located deeper toward the center of the bilayer. Bernsdorff and Winter (39) explained their results by smaller volume fluctuations of stigmasterol, as compared to sitosterol, due to the rigidity of the double bond at C22. A double bond exhibits, however, in addition π-orbitals, which align normal with respect to the σ-orbitals and, therefore, extend effectively the electron cloud along the bond. Moreover, a rigid C=C segment can be easily envisioned to increase the steric repulsion amplitude of the whole C17 alkyl side chain by decreasing its overall packing flexibility. Both effects yield toward an additional repulsion of neighboring hydrocarbon chains and thus to a less effective condensation property of stigmasterol, as observed in our experiments.
Further, and again in agreement with previous studies (58,62,63) we found that the condensing effect of cholesterol is less expressed in unsaturated lipids than in saturated lipids. In general, the less favorable cross-relaxation rates between cholesterol and unsaturated chains (63) are a plausible explanation for this effect. The same holds true for the two studied plant sterols as judged from our results. Additionally, the above given ranking is not changed and sitosterol is always less effective than cholesterol unlike the observation made in polyunsaturated lipid mixtures of egg PC or soy bean PC (35). Nevertheless, the differences regarding the effects on the membrane structure and elasticity between the three studied sterols become less pronounced (Fig. 6) and we cannot exclude sitosterol inducing more order in polyunsaturated membranes than cholesterol.
Our study further shows clearly that double-bond interactions have significant consequences for the solubility of stigmasterol in POPC, and both unsaturated moieties on either the lipid acyl chain or sterol alkyl side chain seem to be responsible for this effect. We further note that the solubility limit of stigmasterol in soy bean PC bilayers was reported to be 16 mol % (35), which indicates that this effect is even more pronounced for polyunsaturated lipids. POPC features a cis-double bond at the C9 position of the unsaturated acyl chain, which is inducing an inflexible “kink” of the chain. On the other hand, stigmasterol has an additional double bond at the C22 position of its alkyl side chain. Hence, the reduced solubility may be explained by the mismatch between the trans-double bond of the sterol molecule (length ∼17 Å) and the cis-double bond of the lipid hydrocarbon chain as a kind of stereo-chemical packing incompatibility.
Additionally we found that temperature has an important influence on the condensation of the membrane by the three studied sterols. In essence, differences in their abilities in inducing order within the bilayer are much more enhanced at higher temperature (Fig. 8). This can be understood by considering the interplay between entropy and van der Waals interactions between the hydrocarbon chains. With temperature entropy within the bilayer increases, leading to more pronounced mutual steric repulsion between the cis double-bond of the unsaturated POPC hydrocarbon chain and the trans double-bond on the stigmasterol side chain.
Finally, this study adds also to the discussion of binary lipid/sterol phase diagrams. We were able to fit all SAXS data with a single phase model. Hence, we were not able to observe a coexistence of 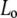 and
and 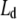 phases in a certain range of sterol concentrations proposed originally by Ipsen et al. (20) and found in several spectroscopic studies (see, for example, refs. (19,21)). Instead, the binary mixtures appear to be in a uniform phase with a gradual change from Ld to Lo with sterol concentration in agreement with recent fluorescence microscopy measurements (24,25). At first sight our findings might not be a convincing argument in favor of a uniform phase at all sterol concentrations, because x-ray diffraction experiments in general report on structural properties of the sample average and small details will get blurred, especially if one concentrates only on the scattered intensities found in the Bragg peaks. The global analysis technique, however, models the full q-range, hence, taking also into account the diffuse intensities between the peaks (48). Therefore, it is in principle sensitive to coexisting domains with different structural properties as shown recently for phosphatidylglycerols (75). The prerequisites are, however, that the domains are large, stable on the time-scale of the diffraction experiment, differ significantly in membrane thickness and display a sufficiently large volume fraction. In this case the maximum difference of membrane thickness between pure bilayers and bilayers containing 40 mol % sterol is ∼5 Å (Fig. 4 A). Assuming that this corresponds to the difference in thickness between the Ld and Lo phases we should be able to detect the coexisting domains at this data quality given an approximate 1:1 volume fraction of the two phases. This latter condition should be fulfilled at some point in the studied sterol concentration range. In support of this assumption, a similar difference of dB values between Ld and Lo phases was recently reported for a ternary dioleoyl PC/DPPC/cholesterol mixture (26). In that case, however, a clear phase separation in form of two populations of lamellar lattices has been observed. This means that the two coexisting domains were large and stable enough to come into registry. Obviously this is not the case for our binary lipid/cholesterol mixtures, nor are we aware of any x-ray diffraction study reporting coexisting lamellar lattices in such systems. Consequently, even if such domains exit in our samples they are too small and unstable to be detected. On thermodynamic grounds this means, however, that these domains can only approximately be considered as phases. The correct way to view fluid lipid/sterol mixtures would consequently be in terms of nonrandom mixtures with compositional fluctuations of sterol/lipid complexes, i.e., a dynamic formation and dissipation of nanoscopic Lo domains (23,76). Considering the different experimental time scales, the disagreement between the spectroscopic techniques and fluorescence microscopy or diffraction studies can be reconciled. Spectroscopic techniques may detect the compositional fluctuations, which get averaged out on the larger time scales involved in diffraction experiments or fluorescence microscopy.
phases in a certain range of sterol concentrations proposed originally by Ipsen et al. (20) and found in several spectroscopic studies (see, for example, refs. (19,21)). Instead, the binary mixtures appear to be in a uniform phase with a gradual change from Ld to Lo with sterol concentration in agreement with recent fluorescence microscopy measurements (24,25). At first sight our findings might not be a convincing argument in favor of a uniform phase at all sterol concentrations, because x-ray diffraction experiments in general report on structural properties of the sample average and small details will get blurred, especially if one concentrates only on the scattered intensities found in the Bragg peaks. The global analysis technique, however, models the full q-range, hence, taking also into account the diffuse intensities between the peaks (48). Therefore, it is in principle sensitive to coexisting domains with different structural properties as shown recently for phosphatidylglycerols (75). The prerequisites are, however, that the domains are large, stable on the time-scale of the diffraction experiment, differ significantly in membrane thickness and display a sufficiently large volume fraction. In this case the maximum difference of membrane thickness between pure bilayers and bilayers containing 40 mol % sterol is ∼5 Å (Fig. 4 A). Assuming that this corresponds to the difference in thickness between the Ld and Lo phases we should be able to detect the coexisting domains at this data quality given an approximate 1:1 volume fraction of the two phases. This latter condition should be fulfilled at some point in the studied sterol concentration range. In support of this assumption, a similar difference of dB values between Ld and Lo phases was recently reported for a ternary dioleoyl PC/DPPC/cholesterol mixture (26). In that case, however, a clear phase separation in form of two populations of lamellar lattices has been observed. This means that the two coexisting domains were large and stable enough to come into registry. Obviously this is not the case for our binary lipid/cholesterol mixtures, nor are we aware of any x-ray diffraction study reporting coexisting lamellar lattices in such systems. Consequently, even if such domains exit in our samples they are too small and unstable to be detected. On thermodynamic grounds this means, however, that these domains can only approximately be considered as phases. The correct way to view fluid lipid/sterol mixtures would consequently be in terms of nonrandom mixtures with compositional fluctuations of sterol/lipid complexes, i.e., a dynamic formation and dissipation of nanoscopic Lo domains (23,76). Considering the different experimental time scales, the disagreement between the spectroscopic techniques and fluorescence microscopy or diffraction studies can be reconciled. Spectroscopic techniques may detect the compositional fluctuations, which get averaged out on the larger time scales involved in diffraction experiments or fluorescence microscopy.
It has not escaped our notice that in particular the area per lipid (Fig. 4 B) shows sudden changes at ∼20 mol % sterol content. Although more experimental data in this concentrations regime would be needed to prove the significance of this finding this observation compares at least for DMPC surprisingly well with the break points observed in the partial molecular volumes by Greenwood et al. (58). For POPC no such break point was observed. The most appropriate explanation appears to be that this marks the sterol concentration up to which the condensation takes place. Because the volume condensation is much smaller than the area condensation (23), it might be difficult to be detected in dilatometric measurements on mono- or di-unsaturated lipid/sterol mixtures, which are known to interact less preferentially with sterols (58,62,63). Further, Heerklotz and Tsapatsis (23) have shown that the volume condensation decreases significantly with temperature. We therefore expect that dilatometric experiments are able to resolve a volume condensation effect in POPC at sufficiently low temperature. It would be further interesting to discuss these findings in terms of the models described in Edholm and Nagle (77), however, this is not the intention of this study.
In conclusion, this study shows clearly that small differences in sterol structure can give rise to significant differences in membrane properties. These effects also depend on the lipid composition (degree of hydrocarbon chain saturation) and on temperature conditions. The ability of sterols to modify membrane properties in different ways points to the mutual interplay between sterols and membrane mechanical properties, and its potential relevance for evolution (78). Their different modulation of membrane properties suggests that the small differences in their molecular architecture are associated with their different roles in biological systems.
Acknowledgments
We thank Bernadette Zanner and Barbara Sartori for assistance in sample preparation.
This work was supported by the Austrian Science Fund (P17112-B03 to G.P.).
Editor: Mark Girvin.
References
- 1.Yeagle, P. L. 1985. Cholesterol in the cell membranes. Biochim. Biophys. Acta. 822:267–287. [DOI] [PubMed] [Google Scholar]
- 2.Yeagle, P. L. 1991. Modulation of membrane function by cholesterol. Biochimie. 73:1303–1310. [DOI] [PubMed] [Google Scholar]
- 3.Finegold, L., editor. 1993. Cholesterol in Membrane Models. CRC Press, Boca Raton, FL.
- 4.Addona, G. H., H. Sandermann, Jr., M. A. Kloczewiak, and K. W. Miller. 2003. Low chemical specificity of the nicotinic acetylcholine receptor sterol activation site. Biochim. Biophys. Acta. 1609:177–182. [DOI] [PubMed] [Google Scholar]
- 5.Lagane, B., G. Gaibelet, E. Meilhoc, J. M. Masson, L. Cezanne, and A. Lopez. 2000. Role of sterols in modulating the human mu-opioid receptor function in Saccharomyces cerevisiae. J. Biol. Chem. 275:33197–33200. [DOI] [PubMed] [Google Scholar]
- 6.Edidin, M. 2003. The state of lipid rafts: From model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 32:257–283. [DOI] [PubMed] [Google Scholar]
- 7.Simons, K., and W. L. C. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269–295. [DOI] [PubMed] [Google Scholar]
- 8.Brown, D. A. 2006. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda). 21:430–439. [DOI] [PubMed] [Google Scholar]
- 9.Demel, R. A., and B. D. Kruyff. 1976. The functions of sterols in membranes. Biochim. Biophys. Acta. 457:109–132. [DOI] [PubMed] [Google Scholar]
- 10.Laggner, P., M. Filek, M. Szechynska-Hebda, and M. Kriechbaum. 2003. X-ray structure investigations of winter wheat membrane systems. II. Effect of phytohormones on structural properties of mixed phospholipid-sterols membranes. Plant Sci. 165:271–275. [Google Scholar]
- 11.Kuksis, A., L. Marai, J. Myher, and K. Geher. 1976. Identification of plant sterols in plasma and red blood cells of man and experimental animals. Lipids. 11:581–586. [DOI] [PubMed] [Google Scholar]
- 12.Lees, A. M., H. Y. Mok, R. S. Lees, M. A. McCluskey, and S. M. Grundy. 1977. Plant sterols as cholesterol-lowering agents: clinical trials in patients with hypercholesterolemia and studies of sterol balance. Atherosclerosis. 28:325–338. [DOI] [PubMed] [Google Scholar]
- 13.Jones, P. J. 1999. Cholesterol-lowering action of plant sterols. Curr. Atheroscler. Rep. 1:230–235. [DOI] [PubMed] [Google Scholar]
- 14.Grundy, S. M., E. H. Ahrens, Jr., and J. Davignon. 1969. The interaction of cholesterol absorption and cholesterol synthesis in man. J. Lipid Res. 10:304–315. [PubMed] [Google Scholar]
- 15.Bouic, P. J. D. 1996. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 18:693–700. [DOI] [PubMed] [Google Scholar]
- 16.Awad, A. B., C. S. Fink, H. Williams, and U. Kim. 2001. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur. J. Cancer Prev. 10:507–513. [DOI] [PubMed] [Google Scholar]
- 17.Bradford, P. G., and A. B. Awad. 2007. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 51:161–170. [DOI] [PubMed] [Google Scholar]
- 18.Mouritsen, O. G., and K. Jorgensen. 1994. Dynamical order and disorder in lipid bilayers. Chem. Phys. Lipids. 73:3–25. [DOI] [PubMed] [Google Scholar]
- 19.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 20.Ipsen, J. H., G. Karlstrom, O. G. Mouritsen, H. Wennerstrom, and M. J. Zuckermann. 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 905:162–172. [DOI] [PubMed] [Google Scholar]
- 21.Loura, L. M. S., A. Fedorov, and M. Prieto. 2001. Fluid-fluid membrane microheterogeneity: A fluorescence resonance energy transfer study. Biophys. J. 80:776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMullen, T. P., and R. N. McElhaney. 1995. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim. Biophys. Acta. 1234:90–98. [DOI] [PubMed] [Google Scholar]
- 23.Heerklotz, H., and A. Tsamaloukas. 2006. Gradual change or phase transition: characterizing fluid lipid-cholesterol membranes on the basis of thermal volume changes. Biophys. J. 91:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veatch, S. L., and S. L. Keller. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. [DOI] [PubMed] [Google Scholar]
- 25.Veatch, S. L., K. Gawrisch, and S. L. Keller. 2006. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys. J. 90:4428–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmakar, S., B. R. Sarangi, and V. A. Raghunathan. 2006. Phase behaviour of lipid-cholesterol membranes. Solid State Commun. 139:630–634. [Google Scholar]
- 27.Korstanje, L. J., G. Ginkel, and Y. Levine. 1990. Effects of steroid molecules on the dynamical structure of dioleoylphosphatidylcholine and digalactosyldiacylclycerol bilayers. Biochim. Biophys. Acta. 1022:155–162. [DOI] [PubMed] [Google Scholar]
- 28.Urbina, J. A., S. Pekerar, H.-B. Le, J. Petterson, B. Montetz, and E. Oldfield. 1995. Molecular order and dynamics of phosphatidylcholine bilayer membranes in the presence of cholesterol, ergosterol and lanosterol: a comparative study using 2H-, 13C- and 31P-NMR spectroscopy. Biochim. Biophys. Acta. 1238:163–176. [DOI] [PubMed] [Google Scholar]
- 29.Yeagle, P. L. 1985. Lanosterol and cholesterol have different effects on phospholipid acyl chain ordering. Biochim. Biophys. Acta. 815:33–36. [DOI] [PubMed] [Google Scholar]
- 30.Endress, E., S. Bayerl, K. Prechtel, C. Maier, R. Merkel, and T. M. Bayerl. 2002. The effect of cholesterol, lanosterol, and ergosterol on lecithin bilayer mechanical properties at molecular and microscopic dimensions: A solid-state NMR and micropipet study. Langmuir. 18:3293–3299. [Google Scholar]
- 31.Henriksen, J., A. C. Rowat, and J. H. Ipsen. 2004. Vesicle fluctuation analysis of the effects of sterols on membrane bending rigidity. Eur. Biophys. J. 33:732–741. [DOI] [PubMed] [Google Scholar]
- 32.Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- 33.Wenz, J. J., and F. J. Barrantes. 2003. Steroid structural requirements for stabilizing or disrupting lipid domains. Biochemistry. 42:14267–14276. [DOI] [PubMed] [Google Scholar]
- 34.Benz, R., and D. Cros. 1978. Influence of sterols on ion transport trough lipid bilayer membrane. Biochim. Biophys. Acta. 506:256–280. [DOI] [PubMed] [Google Scholar]
- 35.Schuler, I., A. Milon, Y. Nakatani, G. Ourisson, A. M. Albrecht, P. Benveniste, and M. A. Hartman. 1991. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 88:6926–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demel, R. A., K. R. Bruckdorfer, and L. L. van Deenen. 1972. The effect of sterol structure on the permeability of lipomes to glucose, glycerol and Rb+. Biochim. Biophys. Acta. 255:321–330. [DOI] [PubMed] [Google Scholar]
- 37.Demel, R. A., K. R. Bruckdorfer, and L. L. van Deenen. 1972. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim. Biophys. Acta. 255:311–320. [DOI] [PubMed] [Google Scholar]
- 38.Halling, K. K., and J. P. Slotte. 2004. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim. Biophys. Acta. 1664:161–171. [DOI] [PubMed] [Google Scholar]
- 39.Bernsdorff, C., and R. Winter. 2003. Differential properties of the sterols cholesterol, ergosterol, beta-sitosterol, trans-7-dehydrocholesterol, stigmasterol and lanosterol on DPPC bilayer order. J. Phys. Chem. B. 107:10658–10664. [Google Scholar]
- 40.Su, Y., Q. Li, L. Chen, and Z. Yu. 2007. Condensation effect of cholesterol, stigmasterol, and sitosterol on dipalmitoylphosphatidylcholine in molecular monolayers. Colloids Surf. A. Physicochem. Eng. Asp. 293:123–129. [Google Scholar]
- 41.Hildenbrand, M. F., and T. M. Bayerl. 2005. Differences in the modulation of collective membrane motions by ergosterol, lanosterol, and cholesterol: a dynamic light scattering study. Biophys. J. 88:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Needham, D., T. McIntosh, and E. Evans. 1988. Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers. Biochemistry. 27:4668–4673. [DOI] [PubMed] [Google Scholar]
- 43.Pencer, J., M. P. Nieh, T. A. Harroun, S. Krueger, C. Adams, and J. Katsaras. 2005. Bilayer thickness and thermal response of dimyristoylphosphatidylcholine unilamellar vesicles containing cholesterol, ergosterol and lanosterol: a small-angle neutron scattering study. Biochim. Biophys. Acta. 1720:84–91. [DOI] [PubMed] [Google Scholar]
- 44.Amenitsch, H., M. Rappolt, M. Kriechbaum, H. Mio, P. Laggner, and S. Bernstorff. 1998. First performance assessment of the small-angle X-ray scattering beamline at ELETTRA. J. Synchrotron Radiat. 5:506–508. [DOI] [PubMed] [Google Scholar]
- 45.Ohura, K., S. Kashino, and M. Haisa. 1972. The crystal and molecular structure of p-bromobenzoic acid. Bull. Chem. Soc. Jpn. 45:2651–2652. [Google Scholar]
- 46.Pabst, G., M. Rappolt, H. Amenitsch, and P. Laggner. 2000. Structural information from multilamellar liposomes at full hydration: full q-range fitting with high quality X-ray data. Phys. Rev. E. 62:4000–4009. [DOI] [PubMed] [Google Scholar]
- 47.Pabst, G., R. Koschuch, B. Pozo-Navas, M. Rappolt, K. Lohner, and P. Laggner. 2003. Structural analysis of weakly ordered membrane stacks. J. Appl. Cryst. 63:1378–1388. [Google Scholar]
- 48.Pabst, G. 2006. Global properties of biomimetic membranes: perspectives on molecular features. Biophys. Rev. Lett. 1:57–84. [Google Scholar]
- 49.Pabst, G., J. Katsaras, V. A. Raghunathan, and M. Rappolt. 2003. Structure and interactions in the anomalous swelling regime of phospholipid bilayers. Langmuir. 19:1716–1722. [Google Scholar]
- 50.Caillé, A. 1972. Remarques sur la diffusion des rayons X dans les smectiques A. C. R. Acad. Sc. Paris B. 274:891–893. [Google Scholar]
- 51.Zhang, R., R. M. Suter, and J. F. Nagle. 1994. Theory of the structure factor of lipid bilayers. Phys. Rev. E. 50:5047–5060. [DOI] [PubMed] [Google Scholar]
- 52.De Gennes, P. G., and J. Prost. 1993. The Physics of Liquid Crystals. Oxford University Press, Oxford.
- 53.Sun, W. J., R. M. Suter, M. A. Knewtson, C. R. Worthington, S. Tristram-Nagle, R. Zhang, and J. F. Nagle. 1994. Order and disorder in fully hydrated unoriented bilayers of gel phase dipalmitoylphosphatidylcholine. Phys. Rev. E. 49:4665–4676. [DOI] [PubMed] [Google Scholar]
- 54.McIntosh, T. J., and S. A. Simon. 1986. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 25:4948–4952. [DOI] [PubMed] [Google Scholar]
- 55.Kratky, O., H. Leopold, and H. Stabinger. 1973. The determination of the partial specific volume of proteins by the mechanical oscillator technique. Methods Enzymol. 27:98–110. [DOI] [PubMed] [Google Scholar]
- 56.Laggner, P., and H. Stabinger. 1976. The partial specific volume changes involved in the thermotropic phase transitions of pure and mixed lecithins. In Colloid and Interface Science. M. Kerker, editor. Academic Press, New York. 91–96.
- 57.Schneditz, D., T. Kenner, H. Heimel, and H. Stabinger. 1989. A sound-speed sensor for the measurement of total protein concentration in disposable, blood-perfused tubes. J. Acoust. Soc. Am. 86:2073–2080. [Google Scholar]
- 58.Greenwood, A. I., S. Tristram-Nagle, and J. F. Nagle. 2006. Partial molecular volumes of lipids and cholesterol. Chem. Phys. Lipids. 143:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarvazyan, A. 1982. Development of methods of precise ultrasonic measurements in small volumes of liquids. Ultrasonics. 20:151–154. [Google Scholar]
- 60.Sarvazyan, A. P. 1991. Ultrasonic velocimetry of biological compounds. Annu. Rev. Biophys. Biophys. Chem. 20:321–342. [DOI] [PubMed] [Google Scholar]
- 61.Pabst, G., H. Amenitsch, D. P. Kharakoz, P. Laggner, and M. Rappolt. 2004. Structure and fluctuations of phosphtidylcholines in the vicinity of the main phase transition. Phys. Rev. E. 70:021908. [DOI] [PubMed] [Google Scholar]
- 62.Rappolt, M., M. F. Vidal, M. Kriechbaum, M. Steinhart, H. Amenitsch, S. Bernstorff, and P. Laggner. 2003. Structural, dynamic and mechanical properties of POPC at low cholesterol concentration studied in pressure/temperature space. Eur. Biophys. J. 31:575–585. [DOI] [PubMed] [Google Scholar]
- 63.Huster, D., and K. Gawrisch. 2000. New insights into biomembrane structure from two-dimensional nuclear Overhauser enhancement spectroscopy. In Lipid Bilayers. Structure and Interactions. J. Katsaras and T. Gutberlet, editors. Springer, Berlin, Heidelberg, New York. 109–125.
- 64.Hung, W. C., M. T. Lee, F. Y. Chen, and H. W. Huang. 2007. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 92:3960–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallova, J., D. Uhrikova, J. Hanulova, J. Teixeria, and P. Balgavy. 2004. Bilayer thickness in unilamellar extruded 1,2-dimyristoleoyl and 1,2-dierucoyl phosphatidylcholine vesicles: SANS contrast variation study of cholesterol effect. Colloids Surf. B. Biointerfaces. 38:11–14. [DOI] [PubMed] [Google Scholar]
- 66.McIntosh, T. J. 1978. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 513:43–58. [DOI] [PubMed] [Google Scholar]
- 67.Tabas, I. 2002. Cholesterol in health and disease. J. Clin. Invest. 110:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halstenberg, S., T. Heimburg, T. Hianik, U. Kaatze, and R. Krivanek. 1998. Cholesterol-induced variations in the volume and enthalpy fluctuations of lipid bilayers. Biophys. J. 75:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hianik, T., M. Haburcák, K. Lohner, E. Prenner, F. Paltauf, and A. Hermetter. 1998. Compressibility and density of lipid bilayers composed of polyunsaturated phospholipids and cholesterol. Colloids Surf. A. Physicochem Eng. Asp. 139:189–197. [Google Scholar]
- 70.Pabst, G., A. Hodzic, J. Strancar, S. Danner, M. Rappolt, and P. Laggner. 2007. Rigidification of neutral lipid bilayers in the presence of salts. Biophys. J. 93:2688–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rukmini, R., S. S. Rawat, S. C. Biswas, and A. Chattopadhyay. 2001. Cholesterol organization in membranes at low concentrations: effects of curvature stress and membrane thickness. Biophys. J. 81:2122–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epand, R. M., R. F. Epand, D. W. Hughes, B. G. Sayer, N. Borochov, D. Bach, and E. Wachtel. 2005. Phosphatidylcholine structure determines cholesterol solubility and lipid polymorphism. Chem. Phys. Lipids. 135:39–53. [DOI] [PubMed] [Google Scholar]
- 73.Jacob, R. F., and J. T. Mason. 2005. Lipid peroxidation induces cholesterol domain formation in model membranes. J. Biol. Chem. 280:39380–39386. [DOI] [PubMed] [Google Scholar]
- 74.McMullen, T. P., C. Vilcheze, R. N. McElhaney, and R. Bittman. 1995. Differential scanning calorimetric study of the effect of sterol side chain length and structure on dipalmitoylphosphatidylcholine thermotropic phase behavior. Biophys. J. 69:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pabst, G., S. Danner, S. Karmakar, G. Deutsch, and V. A. Raghunathan. 2007. On the propensity of phosphatidylglycerols to form interdigitated phases. Biophys. J. 93:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feigenson, G. W. 2006. Phase behavior of lipid mixtures. Nat. Chem. Biol. 2:560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edholm, O., and J. F. Nagle. 2005. Areas of molecules in membranes consisting of mixtures. Biophys. J. 89:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bloch, K. E. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14:47–92. [DOI] [PubMed] [Google Scholar]