Abstract
We present a study of the relationship between blood flow and skin temperature under different dynamics of skin-temperature-change: locally induced thermal shock and well controlled, gradual change. First, we demonstrate memory phenomena for blood flow and skin temperature under both conditions. Secondly, we point out that the “hysteresis” loops obtained are dependent on initial conditions, indicating physiological response times of more than twenty minutes. We also show that under thermal shock the level of blood flow is preserved up to some characteristic temperature limit, independently of subject.
Thermal homeostasis in humans is mainly achieved by regulation of the level of blood flow in the skin. Accordingly, blood perfusion through the vessels in the skin surface constantly adjusts to the skin temperature (1), and the skin temperature and heat loss rate changes as a result. In this way, fluctuations in skin blood flow are subject to thermoregulation (2). This enables us to examine the dynamics of thermoregulation from the point of view of the associated timescales of fluctuation. Experiments have demonstrated entrainment of blood flow by suitable periodic thermal perturbation (2,3) and, correspondingly, phase coherence between simultaneously measured skin blood flow and skin temperature (4). Nonlinear interplay characterized by negative feedback control has been proposed to model the complex relationship between these two physiological measures (5). However, whereas most of the above studies relate either mean values or fluctuations of skin blood flow and temperature (6–8), here we seek the relationship between these two variables when skin temperature is locally: i), reduced by thermal shock and ii), gradually changed. We expect that the relationship obtained will support the hypothesis of feedback control with embedded nonlinearity, providing insight into the underlying physiology and into the dynamical behavior and characteristic response times of biochemical mechanisms, such as the promotion of vasoconstriction by noradrenalin and nitric oxide synthase (9). To the best of our knowledge, no similar results on the time dependence of the relationship between skin blood flow and temperature have been reported.
The data used to study the level of blood flow under thermal shock are the same as reported in an earlier study by Bandrivskyy et al. (4). Simultaneous, continuous control recordings of basal skin blood flow and skin temperature, with the sensors placed together on the volar aspect of the forearm, were performed for 30 min; then under conditions of skin cooling for 20 min, then for 30 min thereafter, during the recovery period. Skin cooling was induced by application of an ice pack to the measurement area and its immediate vicinity, through a layer of fabric to lessen discomfort. Measurements were made on 10 healthy subjects. A temperature sensor (Thermilinear, YSI, Yellow Springs, OH) connected to a signal conditioning unit (Cardiosignals, Jožef Stefan Institute, Ljubljana Slovenia) was used to record skin temperature. The resolution of temperature was 0.0003°C within the measurement range of between 20 and 40°C, using 16-bit A/D conversion scaled to ±5 V. A Laser-Doppler probe (DP1T-V2), connected to a DRT4 unit (Moor Instruments, Axminster, UK) was used to record skin blood flow. This technique allows noninvasive assessment of microvascular blood perfusion, expressed here in arbitrary perfusion units (AU). The recorded signal indicates the integrated blood perfusion within the sampling volume, i.e., perfusion in the capillaries, arterioles, venules, and dermal vascular plexa (10). The sampling rate for both signals was 400 Hz. For analysis, the signals were resampled to 10 Hz.
The mean basal values of skin temperature and blood flow were 33.4 ± 0.6°C and 7 ± 4 AU, respectively. During cooling with the ice pack, no distinctive plateau in skin temperature was reached within 20 min. The lowest skin temperature recorded for any subject was 25.1°C, and the maximum mean temperature difference achieved for any subject was 6.8°C. Conversely, it took ∼8 min for every subject to reach a plateau temperature during the recovery period. This temperature was 32 ± 1°C and this is not statistically different from the basal temperature. The mean blood flow was 6 ± 2 AU, also not significantly different from the basal level.
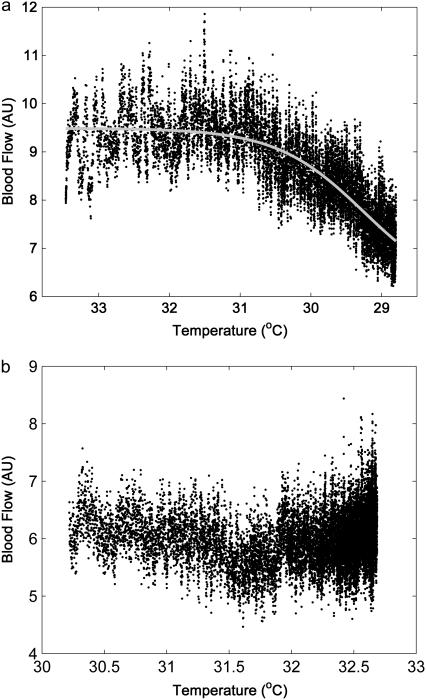
The relationship between blood flow and skin temperature during cooling is shown in Fig. 1 a. The values are medians of blood flow samples across 10 recordings plotted against corresponding median values of the skin temperature samples. The x axis is reversed to correspond to the initial and final states in real time. The curve obtained is steeply sigmoid (r2 = 0.67, p < 0.001). It can be seen that as long as skin temperature is above 31°C, the level of skin blood flow is maintained at a constant level. It responds rapidly to a further decrease in skin temperature, indicating a temperature threshold at which vascular constriction begins. Furthermore, when the same graph was plotted for the recovery period (Fig. 1 b), a spontaneous increase in skin temperature was followed by a slow increase in blood flow. Again, there is no immediate response but rather a slow increase, becoming more rapid after a temperature of ∼31°C is reached. The values of blood flow recorded for a given skin temperature differed between the induced cooling and recovery warming period (independent of the subject considered), indicating a hysteresis-like response. In this case, the rate of skin temperature change was allowed to depend on the homeostatic response to the cooling and recovery stimuli, and so may be asymmetric. Therefore, we performed another set of measurements during which the rate of change in temperature was well controlled.
FIGURE 1.
Skin blood flow versus skin temperature during local cooling with an ice pack (a) and recovery period (b), obtained in 10 healthy subjects. Median values of each sample of blood flow across 10 recordings are plotted against corresponding medians of the skin temperature. Sampling rate is 10 Hz.
For local skin cooling and heating, we used a copper plate (∼10 cm2 in area) with a small aperture (∼8 mm2) in the center to accommodate the Laser-Doppler probe. Peltier elements linked to the plate and to the temperature controller (Temperature Controller, MTTC-1410, Melcor, Trenton, NJ) controlled the temperature of the plate to within ± 0.1°C. The temperature module was built in the Lancaster University Department of Physics' Workshop to meet the required specifications. Skin temperature under the copper plate was measured with the same sensor as used in the earlier cooling study. This temperature sensor was placed on the volar aspect of the lower arm, and the temperature control module, with incorporated blood flow probe, was placed over it. The distance between the flow and temperature sensors was minimized. The signal sampling rate was 1200 Hz, re-sampled to 10 Hz before further analysis. Subjects lay supine on a bed in a ventilated room at 20°C.
Three healthy subjects participated in the study, two males and one female (mean age 30.3 years). After 15 min of rest, simultaneous measurements of skin temperature and blood flow were made as follows. After it was set to 24°C, and skin temperature was allowed to reach a steady state, the temperature of the module was gradually increased to 42°C, and then decreased to 24°C. The temperature of the module was changed in constant steps at a rate of 1°C/min, as monitored on the temperature controller. At this rate it took 37 min to round the loop between limiting values. Afterward, the subjects were allowed 30 min to recover, and a similar procedure was repeated; starting from 42°C, the temperature was lowered to 24°C and increased back to 42°C. The selected temperature limits have been found to cause a maximal level of localized vasoconstriction and vasodilation (6).
The stepwise module temperature change led to an approximately linear response of the skin temperature. However, the skin temperature recorded under the copper plate generally differed from the temperature of the copper plate itself. The skin temperature and plate temperature matched exactly only between 31 and 32°C when heating from 24°C, and between 36 and 37°C when cooling from 42°C. These particular values correspond to the lower and upper limits of the “neutral zone” of skin temperature, when a skin area of ∼15 cm2 is studied (11), so the recorded skin temperature lagged slightly behind the changing plate temperature in the neutral zone, for both heating and cooling. The decrease in blood flow shown in Fig. 1 a, when the temperature falls below 31–32°C, suggests a homeostatic response to the temperature falling below the lower limit value of the neutral zone. The higher limit value (36–37°C) also corresponds to the regime of temperature-related structural changes in hemoglobin (12), and this may play a role in the increased level of blood flow during warming.
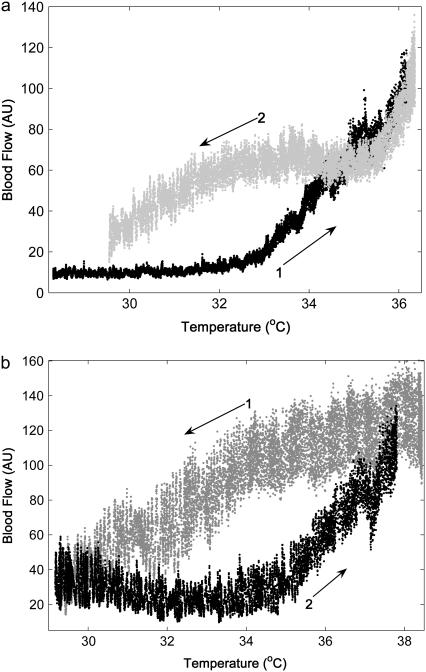
When each sample of blood flow is plotted against the corresponding skin temperature sample, plots like those in Fig. 2, a and b, are obtained. Fig. 2 shows a representative result for one subject. It can be seen that the level of blood flow depends on skin temperature with memory. In addition, two different paths are obtained depending on the initial state.
FIGURE 2.
Relationship between blood flow and skin temperature when skin temperature is changed in steps of 1°C/min within a cycle between 24 and 42°C; starting from 24°C (a) and from 42°C (b). Each sample value of blood flow is plotted against the corresponding sample of skin temperature. Sampling rate is 10 Hz.
In conclusion, our results demonstrate that the response of skin blood flow to a change in skin temperature may exhibit a path contingent upon rate-dependent memory. The memory phenomenon is evident when skin is subject to a cold shock as well as during gradual, well-controlled skin-temperature change. We have shown that a steady level of skin blood flow must be significantly perturbed by a thermal shock to cause vasodilation/constriction. Thus, the differing temperature flow curves obtained during heating and cooling may be due to true (path-dependent) hysteresis, indicating bistable or multistable blood flow levels for a given temperature stimulus. In addition, sensory integration of stimuli over time may result in a delayed temperature response (a rate-dependent memory effect). Slowing the rate of controlled temperature change would reduce rate-dependent effects, reducing the differences in blood flow patterns during heating and cooling and narrowing the hysteresis curves. However, a (slower) rate of 2°C temperature change every 5 min was found to give very similar hysteresis curves, suggesting that path-dependent hysteresis effects are dominant. Depending on the prior stimulus, both the skin cooling and skin heating curves differ even after 20 min, up to the limit of complete vasodilation or vasoconstriction.
These hysteresis phenomena in the homeostatic response should be taken into account when comparing blood flow and skin temperature under thermal perturbations.
Acknowledgments
We thank Andriy Bandrivskyy, Alan Bernjak, and Peter V. E. McClintock for their permission to use data for the analysis.
The research was supported by the Royal Society International Short Visit Grant, the Wellcome Trust, and the Engineering and Physical Sciences Research Council.
Editor: Herbert Levine.
References
- 1.Bruck, K. 1989. Thermal balance and the regulation of body temperature. In Human Physiology. R. Schmidt and G. Thews, editors. Springer-Verlag, Berlin, Heidelberg, New York. 624–644.
- 2.Kytney, R. I. 1974. The analysis and simulation of the human thermoregulatory control system. Med. Biol. Eng. 12:57–65. [DOI] [PubMed] [Google Scholar]
- 3.Nuzzaci, G., A. Evengelisti, D. Righi, G. Giannico, and I. Nuzzaci. 1999. Is there any relationship between cold-induced vasodilatation and vasomotion? Micrvasc. Res. 57:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Bandrivskyy, A., A. Bernjak, P. McClintock, and A. Stefanovska. 2004. Wavelet phase coherence analysis: application to skin temperature and blood flow. Int. J. Cardiovasc. Eng. 4:89–93. [Google Scholar]
- 5.Kytney, R. I. 1975. An analysis f the nonlinear behaviour of the human thermal vasomotor control system. J. Theor. Biol. 52:231–248. [DOI] [PubMed] [Google Scholar]
- 6.Kellogg, D. L. 2006. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J. Appl. Physiol. 100:1709–1718. [DOI] [PubMed] [Google Scholar]
- 7.McCord, G. R., J. L. Cracowski, and C. T. Minson. 2006. Prostanoids contribute to cutaneous active vasodilation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291:R596–R602. [DOI] [PubMed] [Google Scholar]
- 8.Hafner, H.-M., K. Brauer, M. Eichner, I. Koch, H. Heinle, M. Rocken, and A. Strolin. 2007. Wavelet analysis of skin perfusion in healthy volunteers. Microcirculation. 14:137–144. [DOI] [PubMed] [Google Scholar]
- 9.Hodges, G. J., Z. Kun, W. A. Kosiba, and J. M. Johnson. 2006. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J. Physiol. 574:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leahy, M. J., F. F. M. de Mul, G. E. Nilsson, and R. Maniewski. 1999. Principles and practice of the Laser-Doppler perfusion technique. Technol. Health Care. 7:143–162. [PubMed] [Google Scholar]
- 11.Schmidt, R. F. 1989. Somatovisceral sensibility: cutaneous senses, proprioception, pain. In Human Physiology. R. Schmidt and G. Thews, editors. Springer-Verlag, Berlin. 202–203.
- 12.Artmann, G. M., C. Kelemen, D. Porst, G. Büldt, and S. Chien. 1998. Temperature transitions of protein properties in human red blood cells. Biophys. J. 75:3179–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]