Abstract
Rift Valley fever virus (RVFV) is an emerging pathogen that maintains high biodefense priority based on its threat to livestock, its ability to cause human hemorrhagic fever, and its potential for aerosol spread. To define the range of human transmission during inter-epidemic and epidemic periods in Kenya, we tested archived sera from defined populations (N = 1,263) for anti-RVFV IgG by ELISA and plaque reduction neutralization testing. RVFV seroprevalence was 10.8% overall and varied significantly by location, sex, and age. In NW Kenya, high seroprevalence among those born before 1980 indicates that an undetected epidemic may have occurred then. Seroconversion documented in highland areas suggests previously unsuspected inter-epidemic transmission. RVFV seroprevalence is strikingly high in certain Kenyan areas, suggesting endemic transmission patterns that may preclude accurate estimation of regional acute outbreak incidence. The extent of both epidemic and inter-epidemic RVFV transmission in Kenya is greater than previously documented.
Introduction
Rift Valley fever virus (RVFV) represents a significant threat to human health in endemic countries of Africa and the Middle East because of its ability to cause retinitis, encephalitis, and hemorrhagic fever in intermittent epidemics.1,2 Epizootics and epidemics can result in massive loss of livestock, consequent export embargoes, and significant human morbidity and mortality, all of which can be economically devastating to affected areas.1,3,4 RVFV has been studied as a potential agent of biologic warfare both by the US and the former USSR, and it is adaptable to weaponization.5,6 Recent experience of inadvertent West Nile virus introduction into North America indicates that “exotic” arboviral pathogens can quickly become persistent in local ecosystems, provided that the necessary vectors and animals reservoirs are present. Because of the threat of natural or bioterrorist introduction of RVFV into new areas of the world, and the likelihood of its regional persistence once introduced, it is essential to learn more about how RVFV is spread (and contained) under natural circumstances.
Relatively little is known about the natural history of RVFV transmission and infection because natural outbreaks are sporadic and explosive.7,8 RVFV is maintained in nature at least in part by transovarial transmission in floodwater Aedes mosquitoes,9,10 and therefore, epizootic outbreaks do not occur at random. Instead, they are closely linked to excess rainfall,11 and particularly to El Niño/Southern Oscillation and sea surface temperature anomalies in the Indian and Pacific oceans. Excess rainfall anomalies occurred in many sections of Kenya during the 1990s, and although these have been associated with increased mosquito abundance and documented periods of significantly increased malaria and filaria transmission,12,13 they have not all been associated with obvious outbreaks of RVF. This may be explained on the basis of critical local differences in habitat and abundances of mosquito species, but it may also reflect our presently insensitive surveillance system for human RVF, which is primarily based on clinical symptom-based case-finding. Only a minority of patients who are infected with RVFV develop severe disease,3,14 and many “competing” pathogens are capable of causing acute febrile illness associated with bleeding.8,15,16 The resulting insensitivity of RVF detection and the remote location and inherent disruption of communications and transportation caused by extensive rainfall leading to RVF outbreaks means that the actual frequency of RVFV transmission to humans is not well defined and that the spatial extent of transmission during outbreak periods is not well known. The present study's objective was to refine understanding of the natural history, epidemiology, and ecology of RVF in a recurrently epizootic and epidemic region of East Africa.
In 1997–1998, the El Niño/Southern Oscillation (ENSO) resulted in extensive heavy rains and flooding in East Africa with epidemic RVF disease activity in Ethiopia, Sudan, Somalia, Tanzania, and Kenya.8 The epicenter of the Kenyan epidemic was Garissa District (see map, Figure 1), in Northeastern Province, where in December 1997, 170 hemorrhagic fever–associated deaths were reported.8 Systematic multistage cluster sampling across Garissa District in 1997–1998 indicated a 14% prevalence of acute (IgM-positive) cases, with an estimated 20–26% of the population having either recent or past infection with RVFV. Some populations had RVF IgG seropositivity as high as 32%. An estimated 27,500 infections occurred in Garissa District, making it the largest recorded outbreak of RVFV in East Africa. However, the nationwide extent of RVFV transmission during the 1997–1998 outbreak was not studied. In order for surveillance, prediction, and containment programs to be most effective, it is important that knowledge of RVFV transmission be determined both on the national as well as regional and district levels during inter-epidemic and epidemic periods.17 The goal of our project was to better define the regional extent of RVFV infection in Kenya prior to and during the 1997–1998 epidemic outbreak using samples from surveys originally undertaken for other reasons in three different areas of Kenya. Our hypothesis was that the regional extent of RVFV transmission in Kenya during the 1997–1998 ENSO event was greater than that detected by outbreak investigation of clinical cases in Garissa District.
Figure 1.
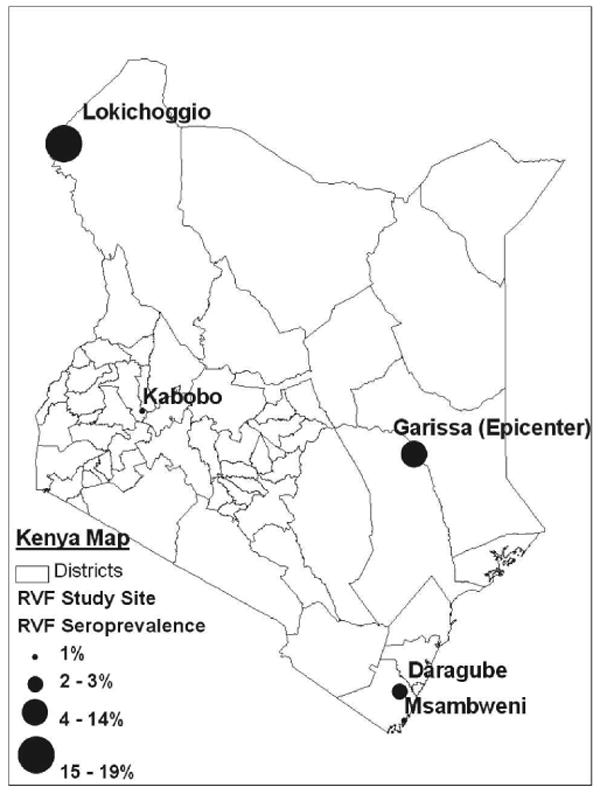
Locations and seroprevalence of RVF study sites in Kenya.
Materials and Methods
Study areas
This study tested archived anonymous human serum samples taken from well-defined cross-sectional studies performed during 1994, 1996, 1997, and 1998 in three distinct areas of Kenya outside the recognized RVF transmission zone. The primary focus of the official RVF outbreak investigation and the epicenter of the RVF outbreak in 1997–1998 was Garissa District, Northeastern Province,8 centered at 0°27.5′ S, 39°39′E (see map, Figure 1). For the present study we tested banked serum derived from populations in (1) Kabobo Division, Uasin Gishu District (located in the western highlands at 0°40′N, 35°30′E, 260 miles west of Garissa), (2) Msambweni and Daragube villages, Kwale District (located on the Indian Ocean at 4°28′S, 39°30′E, 260 miles south of Garissa), and (3) Lokichoggio area in Turkana District (located in NW Kenya, 4°13′N, 34°17′E, 230 miles north of Kabobo). These rural village sites, distributed across Kenya and separated by distances of at least 250 miles, represent a spectrum of different habitats, environmental conditions, and population lifestyles. The study sites span a range of climates from highlands (Kabobo), to monsoonal coastal plain (Msambweni and Daragube), to semi-arid (Lokichoggio).
Populations surveyed
The serum samples tested were from serum banks obtained as part of well-defined cross-sectional village or family surveys for infection and immune response to Echinococcus granulosus in 1994 (cross-sectional village survey of Lokichoggio; R. Blanton, unpublished), malaria in 1996 and 1997 (cross-sectional village survey of Kabobo12), and schistosomiasis and filariasis in 1996–1998 (matched case-control study of Msambweni and Daragube18). Serum samples were stored at −80°C prior to RVFV testing. Informed consent was obtained from all human adult participants and from parents or legal guardians of minors. Institutional review board approval was obtained from University Hospitals of Cleveland and the Kenya Medical Research Institute.
Seroepidemiology
To test for evidence of past RVFV infection, the serum specimens were screened for the presence of anti-RVF IgG via ELISA as previously described,8,19 using lysates of VERO cells infected with the MP-12 strain (vaccine strain) of RVF as the test antigen and mock-infected cells as the internal control antigen. This ELISA assay has been established and validated in previous survey studies.20 Serum samples diluted at 1:100 were read at 405 nm, and those scoring an OD value greater than the mean + 2 SD for control sera and an absolute value > 0.2 were deemed positive. Each sample was run in duplicate, and OD values were averaged. Any discrepancy in the ODs between duplicate tests was resolved by repeat testing. Pooled RVF-positive Lokichoggio sera were used as the positive control, and pooled RVF-negative North American sera were used as a negative control (for cross-contamination) to ensure accurate ELISA assay performance. All positive samples (N = 143) and an age- and location-matched set of negative samples (N = 142) had confirmatory testing via plaque reduction neutralization (PRN) testing, as previously described.21 RVFV PRN titers ≥ 1:40 were considered positive. The PRN results followed a Gaussian distribution with the majority of positive ELISAs clustered around the 1:320 PRN titer range.
Results
Serological outcomes for study subjects
Initially, 1263 samples were screened for anti-RVF IgG via ELISA (see Table 1). Of the 143 ELISA-positive samples, confirmatory PRN testing indicated that the overall anti-RVF seropositivity of the study sample was 10.8% (136 samples/1,263 total). This confirmatory PRN testing indicated a relatively low ELISA false-positive rate of 4.9% (95% CI: 2.02–10.08%, 7 PRN negative samples/143 ELISA-positive samples). All of the 142 matched ELISA-negative samples had negative PRN results (titers < 1:10 for 140 samples, and 1:10 for 2 samples); and thus no ELISA false-negatives were found in this relatively small sample (0%, 95% CI: 0–2.6%). It is possible that a few positives may have been missed by ELISA prescreening because all study samples did not undergo PRN testing (only the ELISA positives and a matched set of ELISA negatives had PRN testing). All the data presented below pertain to PRN results.
Table 1.
Collection year, number, sex, and seropositivity of RVF serum samples by study site location
| Location | Year | Total N | Positive N | Males | Females | Positive males | Positive females | Prevalence of anti-RVFV IgG (%) | 95% confidence intervals (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lokichoggio | 1994 | 675 | 129 | 286 | 384 | 42 | 86 | 19.1 | 15.76 to 21.66 |
| Kabobo | 1996 | 119 | 0 | 47 | 72 | 0 | 0 | 0 | 0 to 3.03 |
| Kabobo | 1997 | 101 | 1 | 31 | 69 | 0 | 1 | 1.0 | 0.03 to 5.45 |
| Daragube | 1996 | 169 | 5 | 81 | 75 | 4 | 1 | 3.0 | 0.94 to 6.78 |
| Msambweni | 1997 | 106 | 1 | 0 | 106 | N/A | 1 | 0.9 | 0.02 to 5.19 |
| Msambweni | 1998 | 93 | 0 | 0 | 93 | N/A | 0 | 0 | 0 to 3.89 |
| Total | 1263 | 136 | 445 | 799 | 46 | 89 | 10.8 | 9.13 to 13.11 |
Results by location
Seropositivity varied significantly according to study area, with the Lokichoggio area of Turkana District in Northeastern Province having the highest RVF seroprevalence of 19.1% (129 samples/675 total; see Table 1), which was significantly higher than all other locations (Fisher's exact test, P < 0.001). Daragube, located on the monsoonal coastal plain, had the second highest RVF seroprevalence of 3.0% (5 samples/169 total) with lower seroprevalence in the nearby coastal Msambweni area and in the highland Kabobo area.
Differences by age group
Seroprevalence also varied by age group, with those persons 15 years old and younger from Lokichoggio having a significantly lower RVF seroprevalence, 10.0% (26 samples/260 total) versus 25.0% (102 samples/408 total; χ2 = 23.067, P ≤ 0.001) among those 16 years and older (see Figure 2). The Daragube sample had similar age-group seroprevalence trends with those 15 years and younger having a seroprevalence of 0% (0 samples/71 total) versus 6.0% (5 samples/83 total; χ2 = 4.421, P ≤ 0.05) among those 16 years and older.
Figure 2.
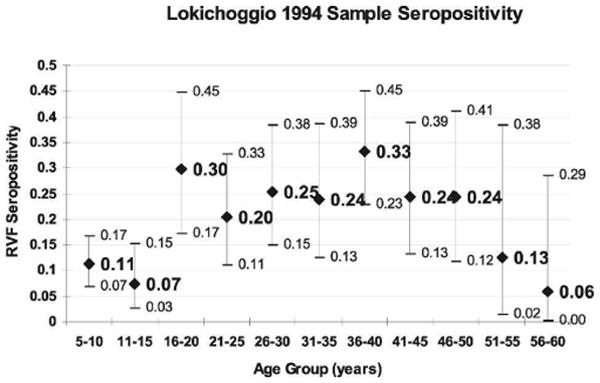
Lokichoggio RVF seropositivity by age group (N = 675) with 95% confidence intervals.
Differences by sex
Seropositivity varied significantly according to sex in our Lokichoggio sample. Women were at greater risk of infection than men, 22.4% (86 samples/384 total) versus 14.7% (42 samples/286 total; χ2 = 6.306, P ≤ 0.025). Sex differences from other regions could not be analyzed secondary to small sample numbers.
Differences over time
Msambweni, a coastal region, had one seropositive sample in March 1997 (1 sample/105 total) but had no seropositive samples in March 1998. This pattern is quite distinct from the large RVF outbreak in nearby Garissa that occurred in the interim period. Kabobo, in the central highlands, had no seropositive samples in August 1996 (0 samples/119 total), but had one seroconversion detected in April 1997, yielding an RVF seroprevalence rate of 1% (1 sample/100 total) prior to the observed period of epizootic/epidemic transmission in late 1997.
Discussion
RVFV is native to sub-Saharan Africa and natural epizootic/epidemic outbreaks of RVF have been detected 8 times during the second half of the 20th century in Kenya.22 After sheep and cattle were introduced into Africa, the disease pattern became one of apparently low-level enzootic and endemic activity punctuated by massive transmission during periods of very high rainfall.11 Introduction of RVFV to new areas has been linked to livestock movement,23 environmental modification,1,14,24 and weather changes.8 Thus far, our knowledge of human RVF epidemiology has been limited to outbreak investigation, and sparse data exist about seroprevalence in endemic areas, such as Kenya. As witnessed in prior outbreaks, detection of even low levels of human seroprevalence can have enormous significance for an RVF outbreak potential in a given area.24
By testing archived human serum samples collected as part of well-defined community surveys across Kenya, we were able to demonstrate that RVF was present in populations outside the Garissa District both before and during the 1997–1998 epidemic, with a collective seropositivity rate of nearly 11%. Past serosurveys of humans and primates from different parts of Kenya suggest that unrecognized transmission is occurring on a regular basis across the country.25–28 Our locations' seroprevalence rates parallel those recorded via a non-systematic sampling during an inter-epidemic period in the early 1980s, although the absolute rate values were dissimilar possibly secondary to temporal trends and testing procedures.26 Our study confirmed the highest prevalence of anti-RVFV antibodies in northwestern Kenya. Johnson, using the less sensitive indirect fluorescent antibody test, found a seroprevalence of 9% in Lodwar in 1983,26 which is significantly lower than the 19% prevalence we found in the same district in Lokichoggio in 1994. The coastal province and highland samples were significantly lower in anti-RVF seroprevalence than our northwestern samples.
The variation in location may be secondary to multiple factors, including environmental differences. RVFV transmission is closely related to flooding events, and transmission can be widespread during a period of excessive rainfall and flooding.22,29 Most regions of Kenya have two rainy seasons: the long rains falling between April and June and the short rains between October and December. Average annual rainfall varies from 5 inches a year in the most arid regions of the northern plains to 70 inches a year near Lake Victoria. The coast and highland areas have an average of 40 inches per year. In times of very heavy rainfall, many Kenyan regions could be at extensive risk for epidemic RVF. In ordinary times, climactic and terrestrial factors define area risk. Areas that are arid in the dry season, such as Turkana, often have extensive standing water during the rainy season. These conditions enable the hatching of transovarially infected floodwater Aedes and initiate local transmission. Thus, Lokichoggio's semi-arid climate allows for annual RVFV transmission and results in high human seropositivity rates, whereas the highland climate of Kabobo may permit RVFV transmission only during periods of dramatic rainfall and manifests as low RVF seropositivity.
Cultural differences can also modify RVF risk. The Turkana people are a nomadic pastoralist society that have substantial contact with their herds and depend on livestock for their survival and economics. Because the Turkana people live in such close proximity with their herds, they could easily be infected by mosquitoes or by blood/abortus from the infected animals. Although direct transmission from livestock is not thought to be important, shed blood and abortuses contain large amounts of virus and the high viremias may also foster mechanical transmission.30 Tissue from RVFV-infected animals that abort or die in the vicinity of mosquito larvae breeding habitats may contaminate the water and infect mosquito larvae, a cycle that propagates transmission.31 Local factors—such as proximity of populations to floodwater Aedes breeding sites, secondary vectors, and their biting habits, and the relationships of humans to their livestock—can foster RVFV transmission independent of the overarching seasonal climactic factors. Therefore, our strikingly high RVF seroprevalence in the Turkana population is logical and important, for it is this group who is most at risk for RVF infection because of the high local transmission and who would be most impacted by a RVF outbreak because of their community's reliance on livestock. Local factors may also help to explain the slight nonsignificant variation we saw across Kenyan regions with similar climates (3% in Daragube vs. 1% in Msambweni).
Lokichoggio and Daragube seroprevalence rates were greatest among persons 16 years and older. The increased seroprevalence rates among older populations in these two regions suggest increased transmission of RVF in these areas around 1980. The ages of the youngest seropositive subjects (5 years old in Lokichoggio and 22 years old in Daragube) provide a maximum estimate of the time since the last period of RVFV circulation. It is likely that inter-epidemic transmission is ongoing in Lokichoggio, given the high overall seroprevalence and the detection of many seropositive young children (see Figure 2). The seroprevalence spike in older Turkana children, both male and female, supports the idea that there was an intense episode of transmission occurring > 15 years before the samples were taken. Out of 77 samples tested of Daragube children of age ≤ 18 years, none was positive. This makes it less likely that significant inter-epidemic RVFV transmission was occurring there in the 2 decades prior to sample collection. Our community IgG age profiles give evidence of past RVFV transmission that is out of phase with the past outbreaks reported from these regions and suggest the existence of previously undetected but potentially significant local transmission. Climate data reveals that an ENSO event occurred around 1977 and may have contributed to increased RVF transmission leading to increased seroprevalence among study subjects born prior to that time.22
In our Lokichoggio sample population, women were more likely to be RVF seropositive than men. Turkana women stay near the hut tending to cattle, caring for children, and cooking, while men roam the pastures tending to their herds of sheep and goats. Direct contact with infected animals is a known risk factor for RVF.32 Prior studies, however, have also shown that being male, having contact with sheep blood, amniotic fluid, or milk, and sheltering livestock in the home were associated with RVF infection.8 We initially hypothesized that the Turkana men have more direct contact with livestock and are therefore, at greater risk for RVF. Our results contradict this hypothesis and underscore our current lack of understanding of the RVF risk factors. Household risks or certain animal exposures, such as cattle contact or birthing of animals, may prove in future studies to be independent risk factors for RVFV infection and explain the higher RVF seropositivity rates we detected among Turkana women.
The seroconversion in Kabobo of a 22-year-old female between 1996 and 1997 may reflect a very local RVF re-emergence or extension to the highlands region. The highlands people of Kabobo have limited travel or contact with other populations, and therefore the RVFV seroconversion we detected likely represents local exposure and infection. Studies conducted in this region have previously demonstrated significant variation in malaria transmission depending on total and seasonal rainfall effects.12,33 Our 1996 samples were collected in July and August of 1996 during a prolonged rainy season, whereas the 1997 samples were collected in March and April after the rainy season was complete. This isolated seroconversion, combined with the detection of other foci of transmission during the 1997–1998 outbreak, indicates that RVF transmission may be occurring sporadically in many non-arid agricultural areas of central Kenya, such as the highlands.
Recent evidence from the Kenya RVF outbreak in 1997–1998 indicates that case-finding based on clinical case-definition has poor specificity.8 At the same time, the low incidence of the dramatic clinical manifestations of RVFV infections has made it difficult to estimate the full extent of RVFV infection among humans during periods of lesser transmission. Having high endemic transmission can significantly alter the positive predictive value (PPV) of anti-RVF IgG detection by ELISA, reducing it to 50% in locations like Lokichoggio during an epidemic. Vaccine-efficacy estimations could also be flawed if endemic transmission and baseline seropositivity rates are not considered. It is important to note that although the PPV of ELISA IgG is modest in highly endemic areas during epidemic periods, the negative predictive value is excellent (100%, 142 negative ELISA samples/142 negative PRN samples). This suggests that RVF IgG testing may be more useful for surveillance than outbreak investigations in these endemic regions when outbreaks occur. In coastal or highland areas where baseline RVF seropositivity is low, ELISA IgG is both useful for surveillance and outbreak screening because the PPV will improve with increasing disease prevalence and the likelihood of a false-negative test result is low.
RVF IgG ELISA and PRN antibodies are believed to last decades after infection and so provide a reliable index of prior RVF exposure. In contrast, though less well studied, it appears that IgM is lost in 50% of patients by day 45 and is absent in 100% by 4 months after infection.34 Addition of IgM data in our study would have yielded important information about acute RVF infection in our populations, but such a study could not be performed. Although we recognize that ELISA positivity may prove to be “false-positive” due to cross-immunoreactivity for viruses in the same family, it was low (4.9%) in our study, and the addition of confirmatory PRN testing of ELISA positives is able to greatly improve viral specificity. New ELISA methods may prove to be even more sensitive and specific than PRN tests in the future.35
Our study may have limited generalizability because serum samples were tested in only 3 distinct regions of Kenya. Although we cannot comment on the RVF seroprevalence in other Kenyan regions, our study highlights the valid point that one cannot speak in general terms about RVF prevalence, even on a provincial basis in Kenya.
In conclusion, the extent and the inter-epidemic spread of RVF in Kenya were greater than previously documented. Our evidence expands the known range of RVFV circulation during the 1997–1998 ENSO era, with the implication that broader surveillance and control measures are required. Our finding that certain populations had baseline RVF IgG seroprevalence rates as high as 19% makes reliance on outbreak seroprevalence results problematic. More studies are needed to delineate these rates to reflect endemic transmission and improve the accuracy of serologic testing. Ongoing studies are assessing the continued enzootic/endemic presence of RVFV and the late outcomes of infection in the Garissa area of Kenya. The results will be used to develop and refine predictive algorithms for RVFV transmission based on environmental and remote sensing data, with the ultimate goal of providing improved early prediction of RVFV outbreaks.
Acknowledgments
The authors thank Dr. Ronald Blanton, Dr. Chandy John, and Dr. Christopher King for their donation of archived human sera for this study.
Financial support: This work was supported in part by NIH grants T32 AI52067, K12 RR023264, and 1U01AI45473-S1.
References
- 1.CDC. Viral hemorrhagic fevers: fact sheets. 2002. Rift Valley fever; pp. 1–3. [Google Scholar]
- 2.Isaacson M. Viral hemorrhagic fever hazards for travelers in Africa. Clin Infect Dis. 2001;33:1707–1712. doi: 10.1086/322620. [DOI] [PubMed] [Google Scholar]
- 3.WHO. WHO Fact Sheet No 207. 2000. Rift Valley fever; pp. 1–5. [Google Scholar]
- 4.Laughlin LW, Meegan JM, Strausbaugh LJ. Epidemic Rift Valley fever in Egypt: observations of the spectrum of human illness. Trans R Soc Trop Med Hyg. 1979;73:630–633. doi: 10.1016/0035-9203(79)90006-3. [DOI] [PubMed] [Google Scholar]
- 5.Peters CJ. The role of antivirals in responding to biological threats. In: Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing the Science and Response Capabilities. Washington: National Academy Press; 2002. pp. 119–130. [PubMed] [Google Scholar]
- 6.Peters CJ, Spertzel R, Patrick W. Aerosol technology and biological weapons. In: Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing the Science and Response Capabilities. Washington: National Academy Press; 2002. pp. 66–77. [PubMed] [Google Scholar]
- 7.CDC. Outbreak of Rift Valley fever—Yemen, August–October 2000. MMWR. 2000;49:1065–1066. [PubMed] [Google Scholar]
- 8.Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, Muchiri E, Dunster L, Henderson A, Khan AS, Swanepoel R, Bonmarin I, Martin L, Mann P, Smoak BL, Ryan M, Ksiazek TG, Arthur RR, Ndikuyeze A, Agata NN, Peters CJ. Hemorrhagic Fever Task Force WHO 2002. An outbreak of Rift Valley Fever in Northeastern Kenya. Emerg Infect Dis. 1997–98;8:138–144. doi: 10.3201/eid0802.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linthicum KJ, Davies FG, Kairo A, Bailey CL. Rift Valley fever virus (family Bunyaviridae, genus Phlebovirus): isolations from Diptera collected during an interepizootic period in Kenya. J Hyg (Lond) 1985;95:197–209. doi: 10.1017/s0022172400062434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies FG, Highton RB. Possible vectors for Rift Valley fever in Kenya. Trans R Soc Trop Med Hyg. 1980;74:815–825. doi: 10.1016/0035-9203(80)90213-8. [DOI] [PubMed] [Google Scholar]
- 11.Davies FG, Linthicum KJ, James AD. Rainfall and epizootic Rift Valley Fever. Bull World Health Organ. 1985;63:941–943. [PMC free article] [PubMed] [Google Scholar]
- 12.John CC, Sumba PO, Ouma JH, Nahlen BL, King CL, Kazura JW. Cytokine responses to Plasmodium falciparum liver-stage antigen 1 vary in rainy and dry seasons in highland Kenya. Infect Immun. 2000;68:5198–5204. doi: 10.1128/iai.68.9.5198-5204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogh C, Pedersen EM, Mukoko DA, Ouma JH. Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Med Vet Entomol. 1998;12:52–59. doi: 10.1046/j.1365-2915.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 14.Peters CJ. Emergence of Rift Valley fever. In: Saluzzo JF, Dodet B, editors. Factors in the Emergence of Arboviruses. Paris: Elsevier; 1997. pp. 253–264. [Google Scholar]
- 15.CDC. Update: Outbreak of Rift Valley Fever—Saudi Arabia, August–November 2000. MMWR. 2000;49:982–985. [PubMed] [Google Scholar]
- 16.Bowen MD, Trappier SG, Sanchez AJ, Meyer RF, Goldsmith CS, Zaki SR, Dunster L, Peters CJ, Ksiazek TG, Nichol ST, Task Force RVF. A reassortant Bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology. 2001;291:185–190. doi: 10.1006/viro.2001.1201. [DOI] [PubMed] [Google Scholar]
- 17.Shears P. Communicable disease surveillance with limited resources: the scope to link human and veterinary programmes. Acta Trop. 2000;76:3–7. doi: 10.1016/s0001-706x(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 18.King CL, Malhotra I, Mungai P, Wamachi A, Kioko J, Muchiri E, Ouma JH. Schistosoma haematobium-induced urinary tract morbidity correlates with increased tumor necrosis factor-alpha and diminished interleukin-10 production. J Infect Dis. 2001;184:1176–1182. doi: 10.1086/323802. [DOI] [PubMed] [Google Scholar]
- 19.Niklasson B, Peters CJ, Grandien M, Wood O. Detection of human immunoglobulins G and M antibodies to Rift Valley fever virus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984;19:225–229. doi: 10.1128/jcm.19.2.225-229.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niklasson B, Grandien M, Peters CJ, Gargan TP. Detection of Rift Valley fever virus antigen by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983;17:1026–1031. doi: 10.1128/jcm.17.6.1026-1031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meadors GF, Gibbs PH, Peters CJ. Evaluation of a new Rift Valley fever vaccine: safety and immunogenicity trials. Vaccine. 1986;4:179–184. doi: 10.1016/0264-410x(86)90007-1. [DOI] [PubMed] [Google Scholar]
- 22.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 23.Gad AM, Feinsod FM, Allam IH, Eisa M, Hassan AN, Soliman BA, el Said S, Saah AJ. A possible route for the introduction of Rift Valley fever virus into Egypt during 1977. J Trop Med Hyg. 1986;89:233–236. [PubMed] [Google Scholar]
- 24.Digoutte JP, Peters CJ. General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res Virol. 1989;140:27–30. doi: 10.1016/s0923-2516(89)80081-0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BK, Gitau LG, Gichogo A, Tukei PM, Else JG, Suleman MA, Kimani R, Sayer PD. Marburg, Ebola and Rift Valley Fever virus antibodies in East African primates. Trans R Soc Trop Med Hyg. 1982;76:307–310. doi: 10.1016/0035-9203(82)90175-4. [DOI] [PubMed] [Google Scholar]
- 26.Johnson BK, Ocheng D, Gichogo A, Okiro M, Libondo D, Tukei PM, Ho M, Mugambi M, Timms GL, French M. Antibodies against haemorrhagic fever viruses in Kenya populations. Trans R Soc Trop Med Hyg. 1983;77:731–733. doi: 10.1016/0035-9203(83)90216-x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BK, Ocheng D, Gitau LG, Gichogo A, Tukei PM, Ngindu A, Langatt A, Smith DH, Johnson KM, Kiley MP, Swanepoel R, Isaacson M. Viral haemorrhagic fever surveillance in Kenya, 1980–1981. Trop Geogr Med. 1983;35:43–47. [PubMed] [Google Scholar]
- 28.Morrill JC, Johnson BK, Hyams C, Okoth F, Tukei PM, Mugambi M, Woody J. Serological evidence of arboviral infections among humans of coastal Kenya. J Trop Med Hyg. 1991;94:166–168. [PubMed] [Google Scholar]
- 29.Anyamba A, Linthicum KJ, Tucker CJ. Climate–disease connections: Rift Valley fever in Kenya. Cad Saude Publica. 2001;17(Suppl):133–140. doi: 10.1590/s0102-311x2001000700022. [DOI] [PubMed] [Google Scholar]
- 30.Hoch AL, Gargan TP, 2nd, Bailey CL. Mechanical transmission of Rift Valley fever virus by hematophagous Diptera. Am J Trop Med Hyg. 1985;34:188–193. doi: 10.4269/ajtmh.1985.34.188. [DOI] [PubMed] [Google Scholar]
- 31.Turell MJ, Linthicum KJ, Beaman JR. Transmission of Rift Valley fever virus by adult mosquitoes after ingestion of virus as larvae. Am J Trop Med Hyg. 1990;43:677–680. doi: 10.4269/ajtmh.1990.43.677. [DOI] [PubMed] [Google Scholar]
- 32.Logan TM, Davies FG, Linthicum KJ, Ksiazek TG. Rift Valley fever antibody in human sera collected after an outbreak in domestic animals in Kenya. Trans R Soc Trop Med Hyg. 1992;86:202–203. doi: 10.1016/0035-9203(92)90571-s. [DOI] [PubMed] [Google Scholar]
- 33.John CC, Koech DK, Sumba PO, Ouma JH. Risk of Plasmodium falciparum infection during a malaria epidemic in highland Kenya, 1997. Acta Trop. 2004;92:55–61. doi: 10.1016/j.actatropica.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 34.van Velden DJ, Meyer JD, Olivier J, Gear JH, McIntosh B. Rift Valley fever affecting humans in South Africa: a clinicopathological study. S Afr Med J. 1977;51:867–871. [PubMed] [Google Scholar]
- 35.Paweska JT, Mortimer E, Leman PA, Swanepoel R. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J Virol Methods. 2005;127:10–18. doi: 10.1016/j.jviromet.2005.02.008. [DOI] [PubMed] [Google Scholar]


