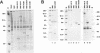Abstract
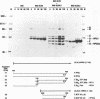
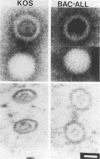
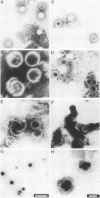
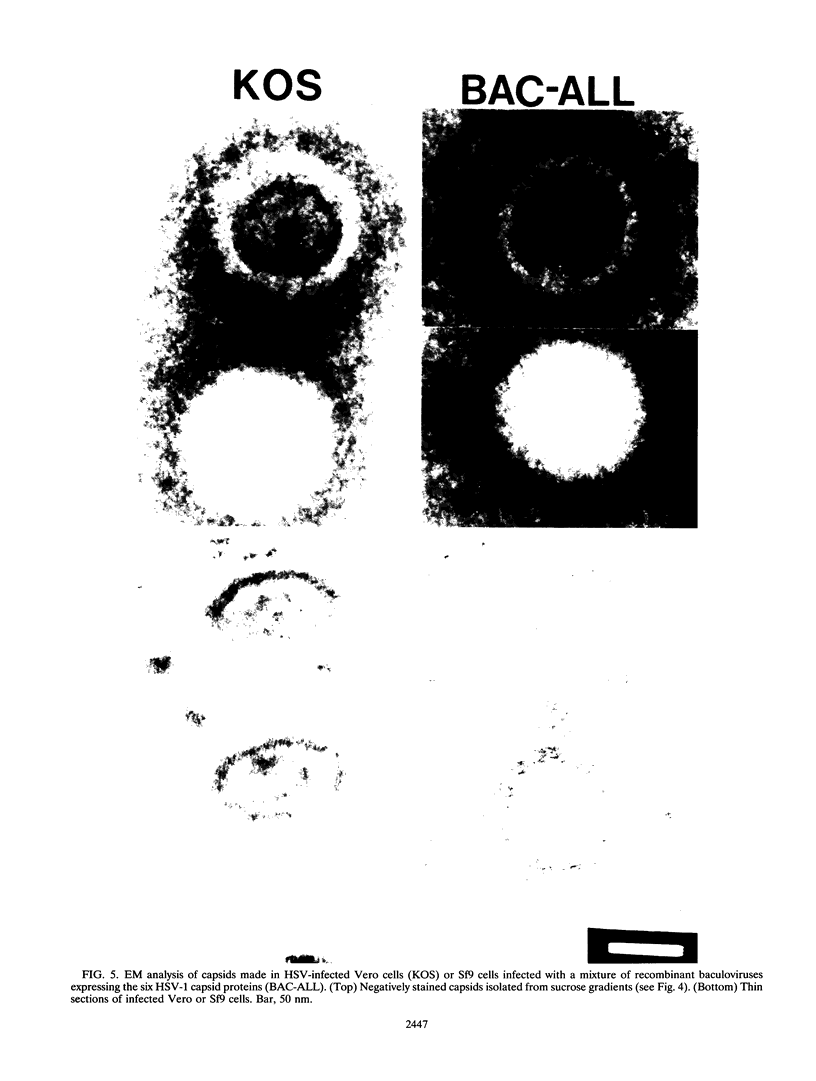
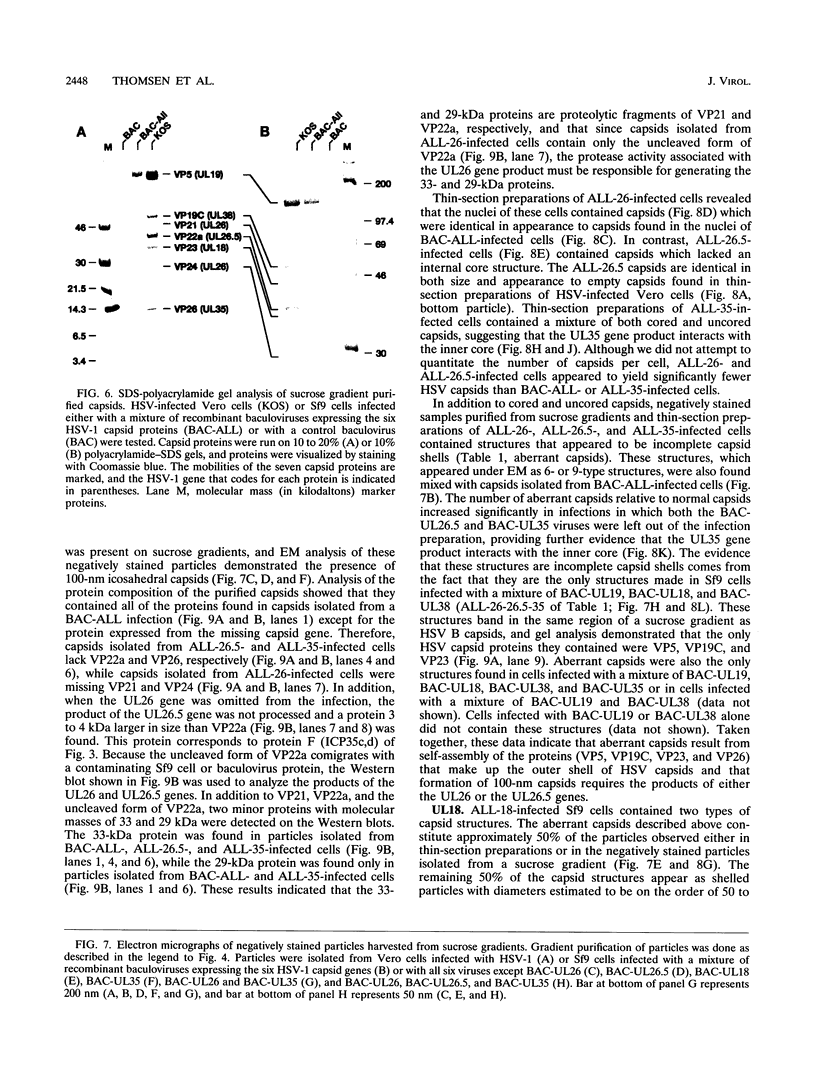
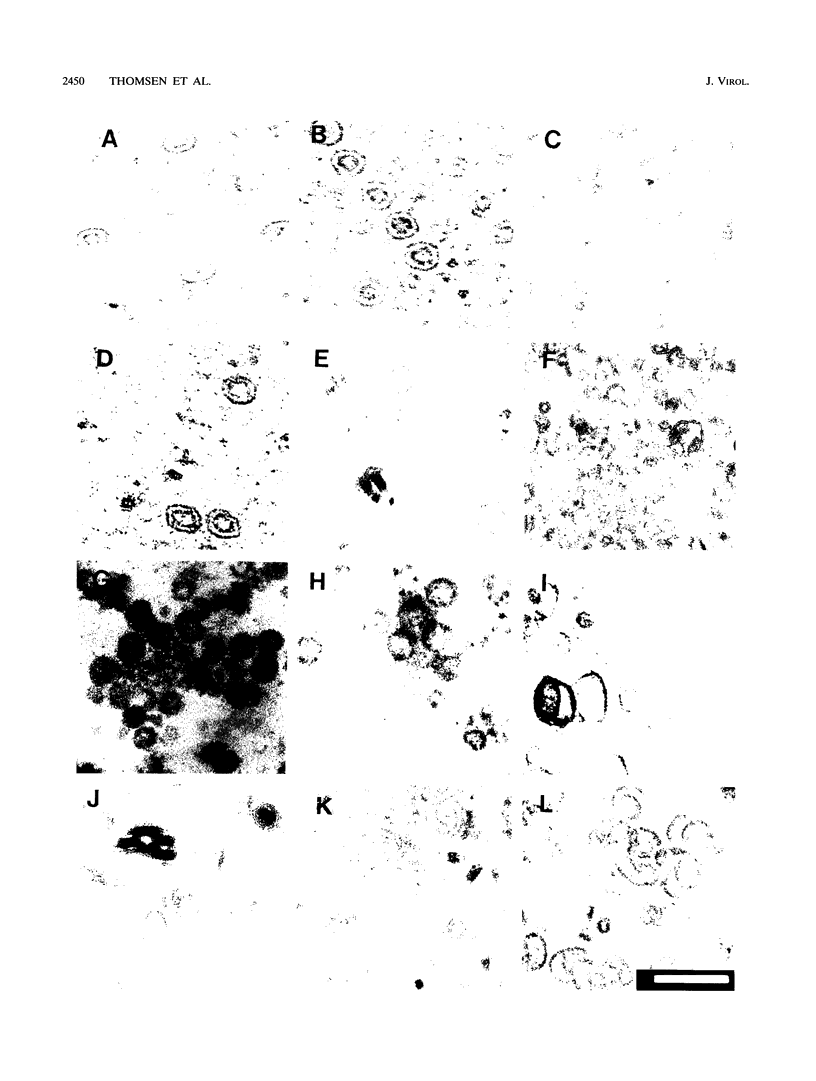
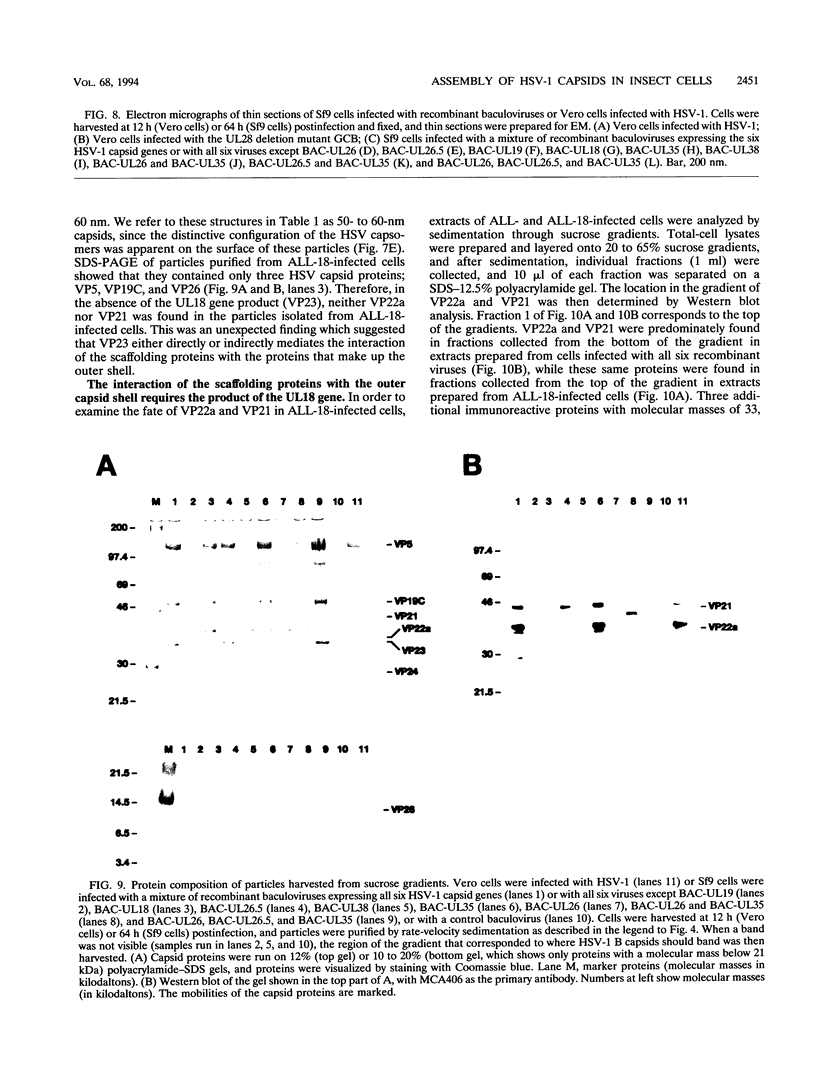
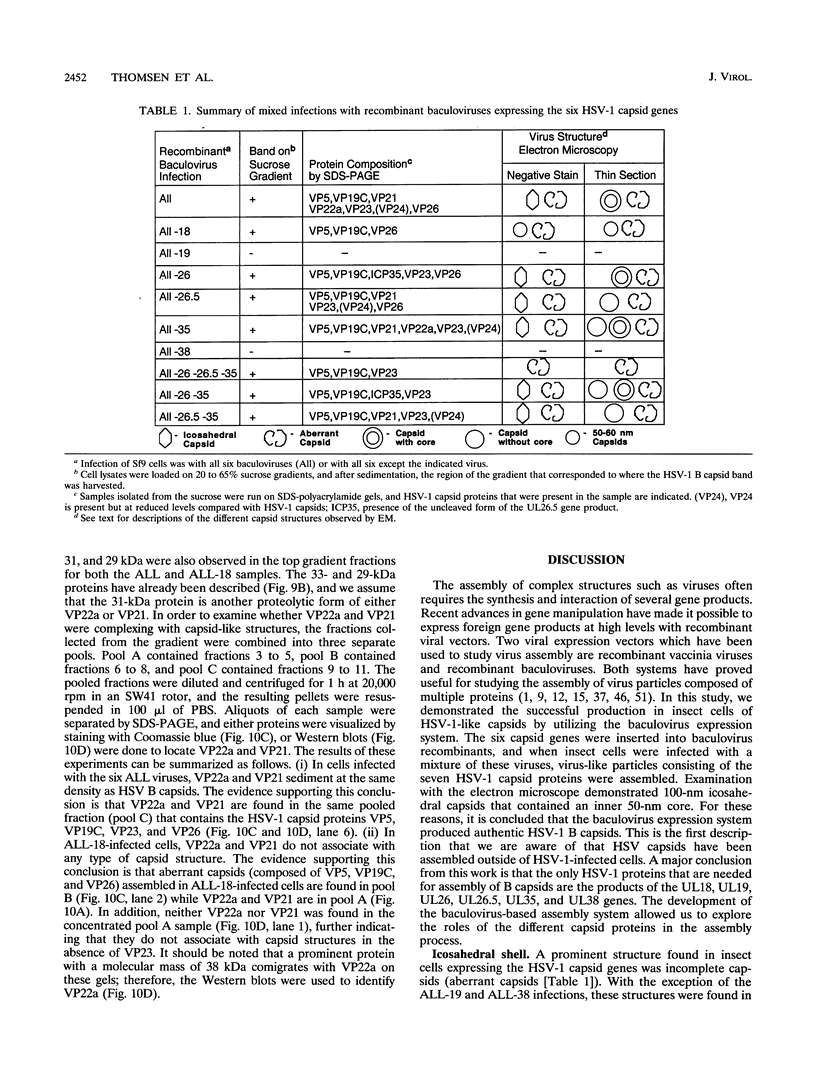
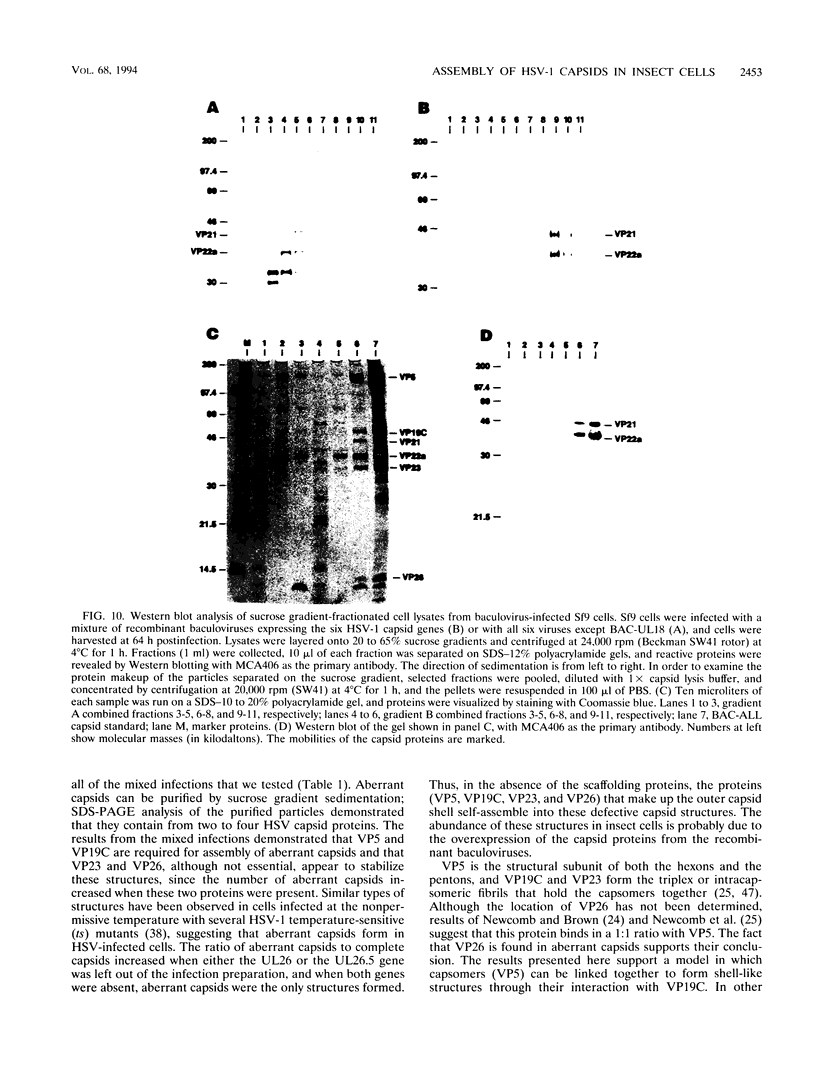
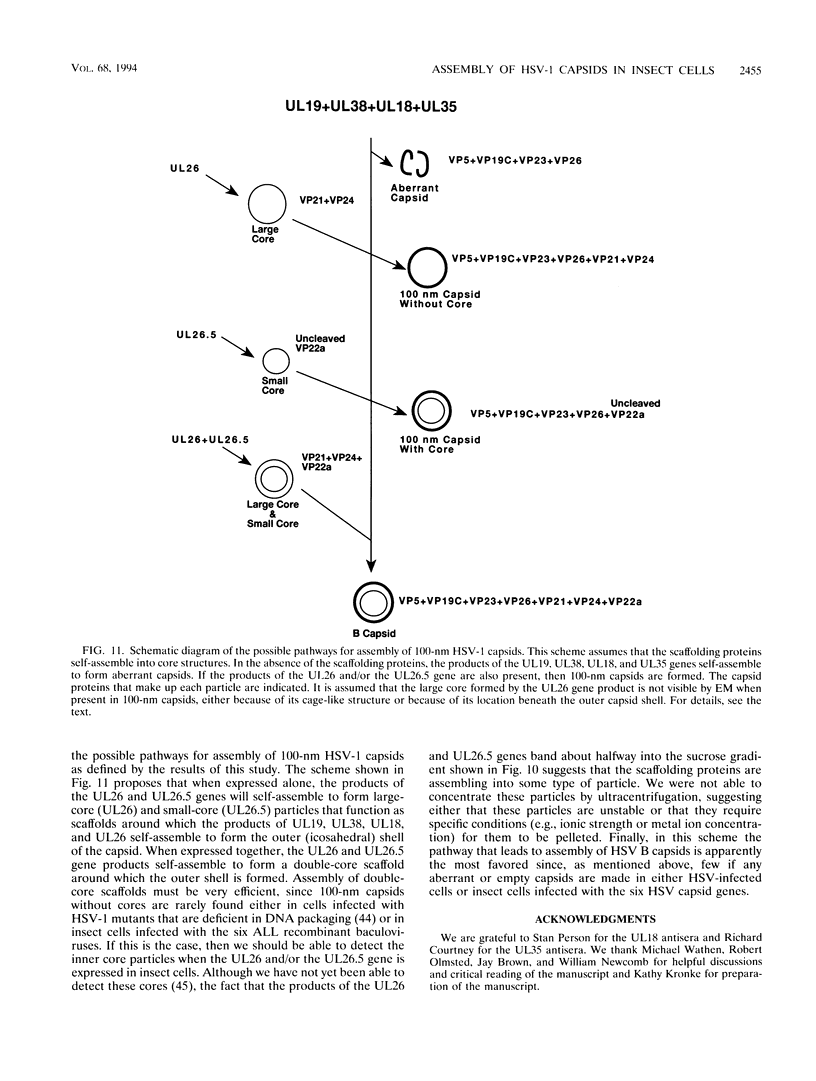
The capsid of herpes simplex virus type 1 (HSV-1) is composed of seven proteins, VP5, VP19C, VP21, VP22a, VP23, VP24, and VP26, which are the products of six HSV-1 genes. Recombinant baculoviruses were used to express the six capsid genes (UL18, UL19, UL26, UL26.5, UL35, and UL38) in insect cells. All constructs expressed the appropriate-size HSV proteins, and insect cells infected with a mixture of the six recombinant baculoviruses contained large numbers of HSV-like capsids. Capsids were purified by sucrose gradient centrifugation, and electron microscopy showed that the capsids made in Sf9 cells had the same size and appearance as authentic HSV B capsids. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis demonstrated that the protein composition of these capsids was nearly identical to that of B capsids isolated from HSV-infected Vero cells. Electron microscopy of thin sections clearly demonstrated that the capsids made in insect cells contained the inner electron-translucent core associated with HSV B capsids. In infections in which single capsid genes were left out, it was found that the UL18 (VP23), UL19 (VP5), UL38 (VP19C), and either the UL26 (VP21 and VP24) or the UL26.5 (VP22a) genes were required for assembly of 100-nm capsids. VP22a was shown to form the inner core of the B capsid, since in infections in which the UL26.5 gene was omitted the 100-nm capsids that formed lacked the inner core. The UL35 (VP26) gene was not required for assembly of 100-nm capsids, although assembly of B capsids was more efficient when it was present. These and other observations indicate that (i) the products of the UL18, UL19, UL35, and UL38 genes self-assemble into structures that form the outer surface (icosahedral shell) of the capsid, (ii) the products of the UL26 and/or UL26.5 genes are required (as scaffolds) for assembly of 100-nm capsids, and (iii) the interaction of the outer surface of the capsid with the scaffolding proteins requires the product of the UL18 gene (VP23).
Full text
PDF

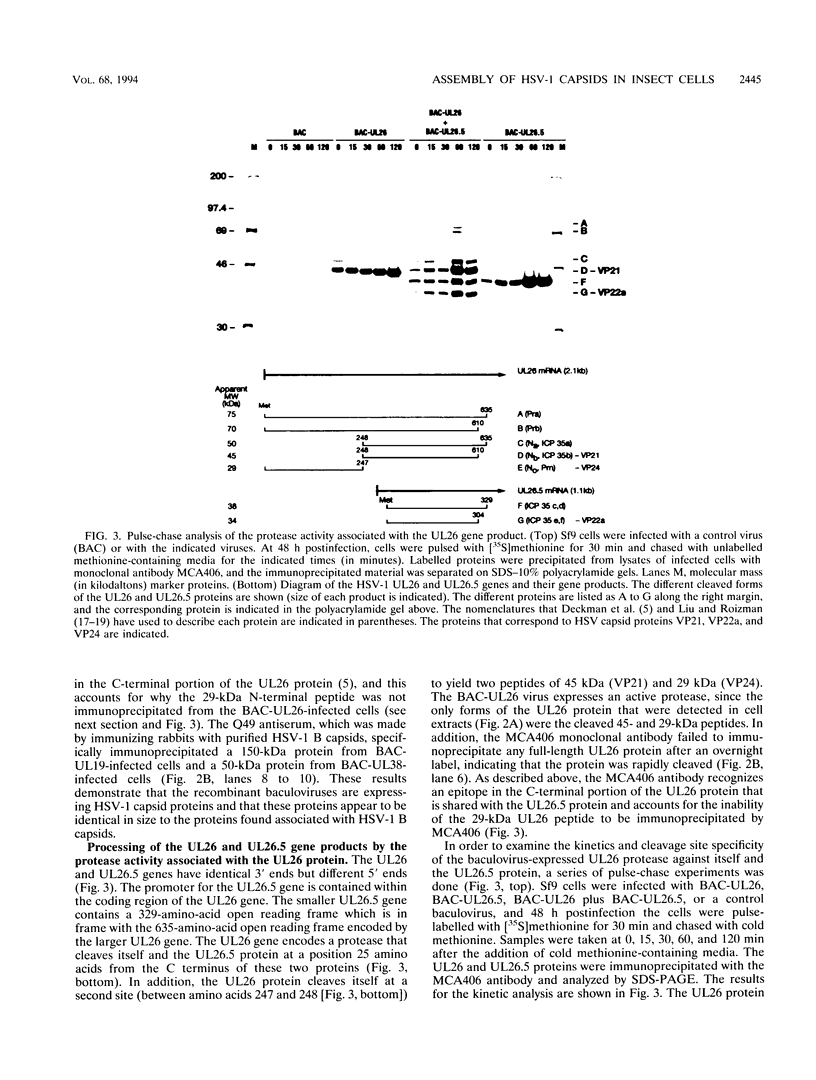





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belyaev A. S., Roy P. Development of baculovirus triple and quadruple expression vectors: co-expression of three or four bluetongue virus proteins and the synthesis of bluetongue virus-like particles in insect cells. Nucleic Acids Res. 1993 Mar 11;21(5):1219–1223. doi: 10.1093/nar/21.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassai E. N., Sarmiento M., Spear P. G. Comparison of the virion proteins specified by herpes simplex virus types 1 and 2. J Virol. 1975 Nov;16(5):1327–1331. doi: 10.1128/jvi.16.5.1327-1331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Diggelmann H., Lawrence W. C., Vernon S. K., Eisenberg R. J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980 May;34(2):521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M. D., Rixon F. J., Davison A. J. Identification of genes encoding two capsid proteins (VP24 and VP26) of herpes simplex virus type 1. J Gen Virol. 1992 Oct;73(Pt 10):2709–2713. doi: 10.1099/0022-1317-73-10-2709. [DOI] [PubMed] [Google Scholar]
- Deckman I. C., Hagen M., McCann P. J., 3rd Herpes simplex virus type 1 protease expressed in Escherichia coli exhibits autoprocessing and specific cleavage of the ICP35 assembly protein. J Virol. 1992 Dec;66(12):7362–7367. doi: 10.1128/jvi.66.12.7362-7367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P., DeLuca N. A., Glorioso J. C., Person S. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol. 1993 Mar;67(3):1357–1364. doi: 10.1128/jvi.67.3.1357-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiIanni C. L., Drier D. A., Deckman I. C., McCann P. J., 3rd, Liu F., Roizman B., Colonno R. J., Cordingley M. G. Identification of the herpes simplex virus-1 protease cleavage sites by direct sequence analysis of autoproteolytic cleavage products. J Biol Chem. 1993 Jan 25;268(3):2048–2051. [PubMed] [Google Scholar]
- French T. J., Marshall J. J., Roy P. Assembly of double-shelled, viruslike particles of bluetongue virus by the simultaneous expression of four structural proteins. J Virol. 1990 Dec;64(12):5695–5700. doi: 10.1128/jvi.64.12.5695-5700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagensee M. E., Yaegashi N., Galloway D. A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993 Jan;67(1):315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Marlin S. D., Levine M., Glorioso J. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J Virol. 1983 Feb;45(2):672–682. doi: 10.1128/jvi.45.2.672-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R., Booy F., Cheng N., Lowy D. R., Schiller J. T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman D. J., Roof L. L., Brideau R. J., Aeed P. A., Thomsen D. R., Elhammer A. P., Wathen M. W., Homa F. L. Comparison of soluble and secreted forms of human parainfluenza virus type 3 glycoproteins expressed from mammalian and insect cells as subunit vaccines. J Gen Virol. 1993 Mar;74(Pt 3):459–469. doi: 10.1099/0022-1317-74-3-459. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991 Oct;65(10):5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Roizman B. Characterization of the protease and other products of amino-terminus-proximal cleavage of the herpes simplex virus 1 UL26 protein. J Virol. 1993 Mar;67(3):1300–1309. doi: 10.1128/jvi.67.3.1300-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Roizman B. Differentiation of multiple domains in the herpes simplex virus 1 protease encoded by the UL26 gene. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2076–2080. doi: 10.1073/pnas.89.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McNabb D. S., Courtney R. J. Identification and characterization of the herpes simplex virus type 1 virion protein encoded by the UL35 open reading frame. J Virol. 1992 May;66(5):2653–2663. doi: 10.1128/jvi.66.5.2653-2663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb D. S., Courtney R. J. Posttranslational modification and subcellular localization of the p12 capsid protein of herpes simplex virus type 1. J Virol. 1992 Aug;66(8):4839–4847. doi: 10.1128/jvi.66.8.4839-4847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991 Feb;65(2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Use of Ar+ plasma etching to localize structural proteins in the capsid of herpes simplex virus type 1. J Virol. 1989 Nov;63(11):4697–4702. doi: 10.1128/jvi.63.11.4697-4702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Trus B. L., Booy F. P., Steven A. C., Wall J. S., Brown J. C. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993 Jul 20;232(2):499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Kemp M. C., Randall C. C., O'Callaghan D. J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974 May;59(1):201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- Person S., Laquerre S., Desai P., Hempel J. Herpes simplex virus type 1 capsid protein, VP21, originates within the UL26 open reading frame. J Gen Virol. 1993 Oct;74(Pt 10):2269–2273. doi: 10.1099/0022-1317-74-10-2269. [DOI] [PubMed] [Google Scholar]
- Pertuiset B., Boccara M., Cebrian J., Berthelot N., Chousterman S., Puvion-Dutilleul F., Sisman J., Sheldrick P. Physical mapping and nucleotide sequence of a herpes simplex virus type 1 gene required for capsid assembly. J Virol. 1989 May;63(5):2169–2179. doi: 10.1128/jvi.63.5.2169-2179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Rixon F. J., McDougall I. M., McGregor M., al Kobaisi M. F. Processing of the herpes simplex virus assembly protein ICP35 near its carboxy terminal end requires the product of the whole of the UL26 reading frame. Virology. 1992 Jan;186(1):87–98. doi: 10.1016/0042-6822(92)90063-u. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Cross A. M., Addison C., Preston V. G. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J Gen Virol. 1988 Nov;69(Pt 11):2879–2891. doi: 10.1099/0022-1317-69-11-2879. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Davison M. D., Davison A. J. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990 May;71(Pt 5):1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Bonnez W., Reichman R. C., Garcea R. L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993 Apr;67(4):1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Brunschwig J. P., McCombs R. M., Benyesh-Melnick M. Electron microscopic studies of temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1974 Dec;62(2):444–457. doi: 10.1016/0042-6822(74)90406-1. [DOI] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988 Apr;163(2):471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven A. C., Roberts C. R., Hay J., Bisher M. E., Pun T., Trus B. L. Hexavalent capsomers of herpes simplex virus type 2: symmetry, shape, dimensions, and oligomeric status. J Virol. 1986 Feb;57(2):578–584. doi: 10.1128/jvi.57.2.578-584.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengelsen L. A., Pederson N. E., Shaver P. R., Wathen M. W., Homa F. L. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993 Jun;67(6):3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen D. R., Meyer A. L., Post L. E. Expression of feline leukaemia virus gp85 and gag proteins and assembly into virus-like particles using the baculovirus expression vector system. J Gen Virol. 1992 Jul;73(Pt 7):1819–1824. doi: 10.1099/0022-1317-73-7-1819. [DOI] [PubMed] [Google Scholar]
- Trus B. L., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinheimer S. P., McCann P. J., 3rd, O'Boyle D. R., 2nd, Stevens J. T., Boyd B. A., Drier D. A., Yamanaka G. A., DiIanni C. L., Deckman I. C., Cordingley M. G. Autoproteolysis of herpes simplex virus type 1 protease releases an active catalytic domain found in intermediate capsid particles. J Virol. 1993 Oct;67(10):5813–5822. doi: 10.1128/jvi.67.10.5813-5822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Carmichael E. P., Aschman D. P., Goldstein D. J., Schaffer P. A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987 Nov;161(1):198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- Wildy P. Herpesvirus. Intervirology. 1986;25(3):117–140. doi: 10.1159/000149666. [DOI] [PubMed] [Google Scholar]
- Zhou J., Stenzel D. J., Sun X. Y., Frazer I. H. Synthesis and assembly of infectious bovine papillomavirus particles in vitro. J Gen Virol. 1993 Apr;74(Pt 4):763–768. doi: 10.1099/0022-1317-74-4-763. [DOI] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Hampar B. Identification of disulfide-linked protein complexes in the nucleocapsids of herpes simplex virus type 2. Virology. 1979 Apr 30;94(2):442–450. doi: 10.1016/0042-6822(79)90474-4. [DOI] [PubMed] [Google Scholar]