Summary
Short-course antiretroviral drug regimens reduce the risk of mother-to-child transmission of HIV-1, but mechanisms affording protection of such interventions remain poorly defined. Because T-cell activation is an important factor in productive HIV-1 infection, we tested the hypothesis that single-dose nevirapine (NVP) reduces immune activation, which in turn reduces the likelihood of transmission. We compared concentrations of cord and maternal blood plasma immune activation markers, neopterin, β2-microglobulin, and soluble l-selectin, in 2 groups of HIV-1–exposed newborns whose mothers either received NVP at the onset of labor or who only received NVP as postexposure prophylaxis within 72 hours of birth and among HIV-unexposed controls. In utero exposure of the infant to HIV-1, regardless of NVP exposure, led to demonstrable increases in immune activation markers, this being most notable in the presence of preexisting infection. Contrary to what was hypothesized, immune activation was increased by prebirth exposure to single-dose NVP, with this effect being enhanced in infants already infected at birth. Our data suggest that reductions in immune activation do not explain transmission prevention effects of single-dose NVP. Our data also suggest a biological explanation for why HIV-1–infected infants exposed perinatally to antiretroviral drugs might experience hastened disease progression, namely, in some HIV-1–infected individuals, NVP may synergize with HIV-1 to enhance an environment that favors increased HIV-1 replication.
Keywords: plasma immune activation markers, newborn, nevirapine, HIV-1–seropositive mothers
Antiretroviral drugs, even if given in short and simple regimens, can dramatically reduce the risk of mother-to-child HIV-1 transmission.1-5 The simplest regimen involves only 1 dose of nevirapine (NVP) to the mother at the onset of labor and 1 dose to the infant within 72 hours of birth.2,6 Although consistently observed, the efficacy of these regimens is surprising because short regimens of monotherapy are expected to have, at best, minor effects on maternal viral load. The reduction in maternal plasma viral load achieved with even the longest of these regimens explained only a fraction of the transmission reduction caused by the regimen.7
There is general consensus that productive replication of the HIV-1 viral genome in CD4+ T lymphocytes depends on cellular activation.8,9 However, exposure to HIV-1 in the absence of seroconversion has been shown to induce HIV-specific, cell-mediated immune responses,10 a process which, in its own right, would require T-cell activation. If HIV-specific, cell-mediated immune responses are truly protective, then we have the paradoxical situation that protective immunity can successfully occur in an immune environment that ordinarily may be considered to favor HIV-1 replication. Some antiretroviral drugs, for example, the protease inhibitor indinavir,11 have been shown to have immunomodulatory consequences distinct from benefits attributable to control of viral replication. One consequence observed in vivo after a short-course zidovudine-lamivudine (AZT-3TC) regimen was reduced memory T-cell responses to HIV.12 These findings raise the question of whether there may be immune-suppressive effects of antiretroviral drugs given to prevent maternal-infant HIV transmission. Characterization of immunomodulatory influences of antiretroviral drugs may help explain why drugs are effective to reduce transmission.
In this study, we tested the hypothesis that the efficacy of NVP to reduce maternal-infant HIV-1 transmission may in part be a consequence of its ability to reduce immune activation as measured by decreased levels of 1 or more of the immune activation markers, neopterin, β2-microglobulin (β2-m), and soluble l-selectin (sl-selectin), all soluble factors which have been used to quantify levels of T-cell activation in vivo.13,14
METHODS
Study Population
The study population included HIV-seropositive and HIV-seronegative women delivering live-born infants at Chris Hani Baragwanath Hospital, Soweto, South Africa. Women who had not received any antiretroviral drugs before the infant’s birth either for prevention of mother-to-child HIV-1 transmission or for HIV treatment (n = 124) were recruited as part of a postexposure prophylaxis (PEP) trial.15 Women were eligible for the trial if they tested HIV positive for the first time after delivery and were drug naive usually because they received no prenatal care or had attended prenatal clinics where HIV testing was not available at the time. Their infants were randomized to receive either AZT or NVP to reduce the risk of vertical transmission. Drug-exposed, HIV-positive women (n = 78) were recruited among women delivering at the hospital around the same time who had attended prenatal clinics at Chris Hani Baragwanath Hospital, had received HIV counseling and testing there, and had accepted NVP as part of a demonstration of antiretroviral therapy (DART) initiative. NVP was given, as previously described,2 as a single maternal 200-mg oral dose at the onset of labor and a single 0.6-mL infant dose within 72 hours of birth. A control group of pregnant, HIV-seronegative women (n = 30) was recruited at the same site over the same period.
The infants of these mothers were followed up prospectively after birth to determine their HIV status until 3 months or until at least 4 weeks after all breast-feeding had stopped. HIV-1 DNA polymerase chain reaction (PCR) was performed on infant peripheral blood samples collected at 6 weeks of age (Amplicor, Roche Diagnostic Systems, Inc, Branchburg, NJ). Samples collected on the day of birth were tested if the 6 weeks’ PCR was positive to establish the timing of infection. A positive result on the day of birth was used to infer intrauterine (IU) transmission, and a negative result at birth with a positive result at 6 weeks or later was used to infer intrapartum transmission (IP). Children negative at 6 weeks or older were included as exposed-uninfected (EU). Children born to HIV-seronegative women (control group) were not followed up after birth.
We selected for this nested, case-control study all 18 HIV-infected infants (4 IU and 14 IP) and a random sample of 63 uninfected infants from the cohort of 124 infants born to HIV-seropositive mothers who did not receive any antiretroviral drugs before delivery (PEP). We also selected all 7 HIV-infected infants (4 IU and 3 IP) and a random sample of 54 uninfected infants from the cohort of 78 infants born to HIV-seropositive mothers who received single-dose NVP before delivery (DART). Samples were insufficient for this analysis for 1 of the IP infected infants (PEP group).
Laboratory Methods
Small aliquots (3–5 mL) of cord blood were obtained by cordocentesis immediately after delivery of the placenta, and 10 mL peripheral blood was obtained from each mother within 24 hours of delivery. Blood samples were drawn into EDTA tubes, and plasma samples were processed by standard procedures within 24 hours of collection and stored at −70°C until testing.
As levels of the plasma immune activation markers, neopterin, β2-m, and sl-selectin, are considered early and reliable markers of immune activation and response reflecting the activation of a variety of different cell types (neopterin: T cells/monocytes, β2-m: CD8+ T cells, and sL selectin: T lymphocytes, monocytes, and neutrophils) as well as being commonly used as diagnostic/prognostic tools of HIV-1 disease, a reduction in any of these markers was thought to best demonstrate an associated reduction in cellular immune activation. Maternal and infant (cord blood) plasma levels of the immune activation factors, β2-m and sl-selectin, were determined using the commercially available Quantikine ELISA assay kits obtained from R&D Systems, Inc (Minneapolis, MN), as described by the manufacturer. β2-m levels were determined using undiluted plasma, whereas sl-selectin determination required a 100-fold dilution of the samples. Neopterin was quantitated in undiluted plasma using an Immunotech ELISA system (Beckman Coulter, Marseille, France). The minimum detectable dose of β2-m is less than 0.2 μg/mL; for sl-selectin, less than 0.3 ng/mL; and for neopterin, 0.2 ng/mL.
Plasma HIV RNA levels were measured using the Roche Amplicor RNA Monitor assay (Roche Diagnostic Systems, Inc) with a lower detection limit of 400 RNA copies/mL. CD4 T-cell counts were determined using the commercially available FACSCount System from Becton Dickinson (San Jose, CA).
Statistical Analysis
Concentrations of the activation markers in plasma were compared between the groups using the nonparametric Mann-Whitney U test. Spearman rank correlation coefficient was used to describe associations between continuous variables. Differences between mothers’ and infants’ activation markers were tested using the Wilcoxon signed rank test for paired data. Statistical analyses were performed using SPSS software (version 11.0, SPSS, Inc, Chicago, IL). All statistical tests were 2-tailed and considered significant at P values of less than 0.05. No statistical corrections were applied for the multiple comparisons.
RESULTS
Clinical Characteristics of HIV-1–Seropositive Mothers and Their Infants
Clinical characteristics of the mother-infant participants who were included in this nested, case-control study are presented in Table 1. Viral loads were significantly higher among mothers who had not received any antiretroviral drugs before delivery (PEP) compared with mothers given NVP at the onset of labor (DART).
TABLE 1.
Clinical Characteristics of the HIV-Seropositive Mothers and Their Infants
| No Antiretroviral Drugs Given Before Birth (PEP) | Single-Dose NVP Given Before Birth (DART) | Total (PEP and DART) | |
|---|---|---|---|
| N | 80 | 61 | 141 |
| Mean (SD) | |||
| Mothers’ CD4+ T-cell count | 477 ± 259 | ND | 477 ± 259 |
| Mothers’age(y) | 26 ± 5 | 26 ± 5 | 26 ± 5 |
| Infant birth weight (g) | 2919 ± 453 | 3039 ± 409 | 2964 ± 438 |
| Median (IQR*) | |||
| Mothers’ viral load (log10)† | 4.8 (4.1 – 5.2) | 4.4 (3.7 – 4.9) | 4.6 (3.9 – 5.1) |
| % (n/N) | |||
| Infant sex (male) | 56 (40/72) | 50 (29/58) | 53 (69/130) |
| Primiparity | 34 (25/74) | 23 (13/56) | 29 (38/130) |
| Vaginal delivery | 100 (74/74) | 91 (49/54) | 96 (123/128) |
| Preterm (<37 wk) | 24 (17/71) | 9 (5/55) | 17 (22/126) |
| Infants HIV infected IP‡ | 11 (14/124) | 4 (3/78) | 8 (17/202) |
| Infants HIV infected IU‡ | 3 (4/124) | 5 (4/78) | 4 (8/202) |
| Infants ever breast-fed | 49 (37/75) | 12 (7/58) | 33 (44/133) |
25th and 75th percentiles.
Significant differences between PEP and DART groups are indicated (P = 0.016).
Transmission rates for PEP and DART study.
IQR indicates interquartile range; ND, not determined.
Infants Exposed to or Infected With HIV-1 Demonstrate Greater Immune Activation at Birth Than Control Uninfected Infants
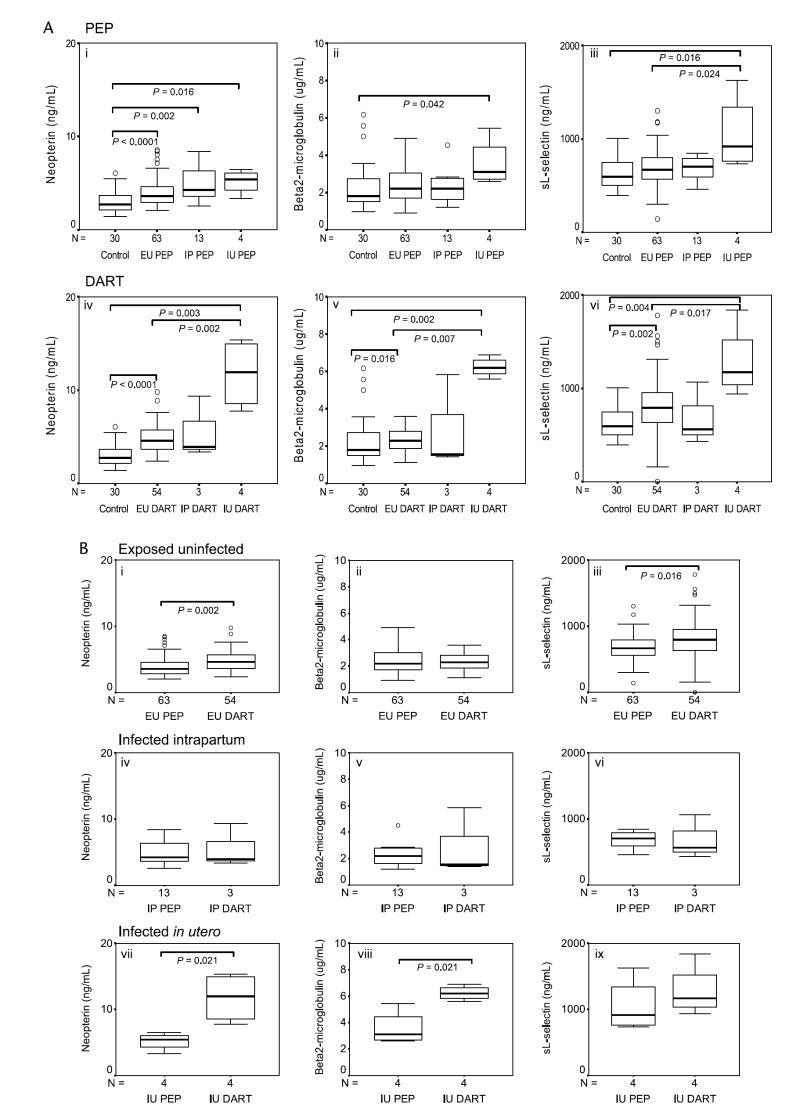
To establish if exposure to and/or infection with HIV-1 leads to increased immune activation as evidenced by raised levels of cord-blood plasma activation markers, infants born to HIV-1–infected mothers were stratified on the basis of their infection outcomes as EU, IP (acquired infection IP, birth PCR negative, and 6-week PCR positive), and IU (acquired infection in utero and birth PCR positive) and compared with control infants born to HIV-1–uninfected mothers. As markers are measured in cord blood, the IP infants are uninfected at delivery and so do not indicate consequences of productive infection at this time point.
Neopterin levels were elevated to more than control levels among infected (IP and IU) and uninfected infants born to HIV-positive mothers regardless of whether mothers received antiretroviral drugs (ie, in both the DART and PEP groups) (Fig. 1A, i and iv). Exposure to HIV-1 resulted in moderately elevated levels compared with HIV-seronegative controls of β2-m and sl-selectin that attained significance among EU infants whose mothers received NVP (Fig. 1A, v–vi). Infants who became infected during delivery (IP) did not show any difference from EU infants in immune activation markers. In contrast, in utero infection (IU) resulted in substantial and significant increases in all the activation markers (Fig. 1A, i–vi).
FIGURE 1.
Levels of soluble immune activation markers in plasma of infants born to HIV-seronegative and HIV-seropositive mothers. A, Levels of neopterin (ng/mL) (i and iv), β2-m (μg/mL) (ii and v), and sl-selectin (ng/mL) (iii and vi) of infants born to HIV-1 seropositive mothers in the absence (PEP) and in the presence (DART) of a single-dose of NVP, respectively. Immune activation marker levels measured in uninfected (control) infants are included. B, This panel depicts the effects of single-dose NVP exposure (DART) versus absence of NVP exposure (PEP) on the levels of neopterin (ng/mL), β2-m (μg/mL), and sl-selectin (ng/mL) of infants who remained uninfected (EU) (i, ii, and iii, respectively) or who become infected IP (iv, v, and vi, respectively) or in utero (IU) (vii, viii, and ix, respectively). Data are presented as medians (horizontal bar), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between groups and sample numbers per group are indicated.
Single-Dose Nevirapine Contributes to Immune Activation in Infants Born to HIV-1 Seropositive Mothers, Particularly Among Infants Infected In Utero
Having established that infants born to HIV-positive mothers have increased immune responsiveness as evidenced by raised levels of plasma activation markers, we next questioned if single-dose NVP might reduce immune reactivity in the presence of exposure or infection with HIV-1. Figure 1B shows a direct comparison of plasma immune activation markers of PEP and DART infants within each of the infection outcome groups (EU, IP, and IU). Contrary to our hypothesis, NVP exposure significantly increased neopterin (P = 0.002) and sl-selectin (P = 0.016) levels of EU infants (Fig. 1B, i and iii) as well as increased neopterin (P = 0.021) and β2-m (P = 0.021) levels of infants who were infected in utero (IU) (Fig. 1B, vii and viii).
Immune Activation in the Infant Is Modulated Independently of the Mother’s Viral Load and CD4 T-Cell Count and Is Not Influenced By Gestation Period
Given that infants exposed to HIV-1 or infected with HIV-1 demonstrated enhanced immune activation, we wanted to ascertain whether the mother’s viral load would directly impact on the levels of the immune activation factors of the PEP and DART infants. Only neopterin from the EU DART infants was weakly correlated to maternal viral load (P = 0.028, r = 0.298). Given that PEP mothers had significantly higher viral loads compared with DART mothers (log10 4.78 and log10 4.35, respectively; P = 0.016), we might have expected that activation markers would be lower in the NVP-exposed group (DART) in contrast to what we observed. After adjusting for maternal viral load, the association between NVP exposure and higher levels of neopterin (P = 0.035) and sl-selectin (P = 0.016) remained statistically significant in the EU group, indicating that differences in viral load between the groups did not account for the increased immune activation that we observed. No statistically significant correlations were demonstrated between maternal CD4+ T-cell counts and infant (CB) neopterin, β2-m, and sl-selectin levels for the PEP infants (EU, IP, or IU). It would be expected that the same would hold true for relationships between DART mothers and infants, although this was not tested, given that maternal CD4 T-cell counts were not determined for this group.
Infants born before 37 weeks’ gestation (preterm infants) might be expected to have a reduced ability to respond to antigen compared with term infants; however, no significant differences in levels of neopterin, β2-m, and sl-selectin levels were observed between preterm or term infants of either the PEP or DART group (data not shown).
Single-Dose Nevirapine Does Not Modulate Immune Activation Markers of IP Transmitting and Nontransmitting HIV-1–Infected Mothers
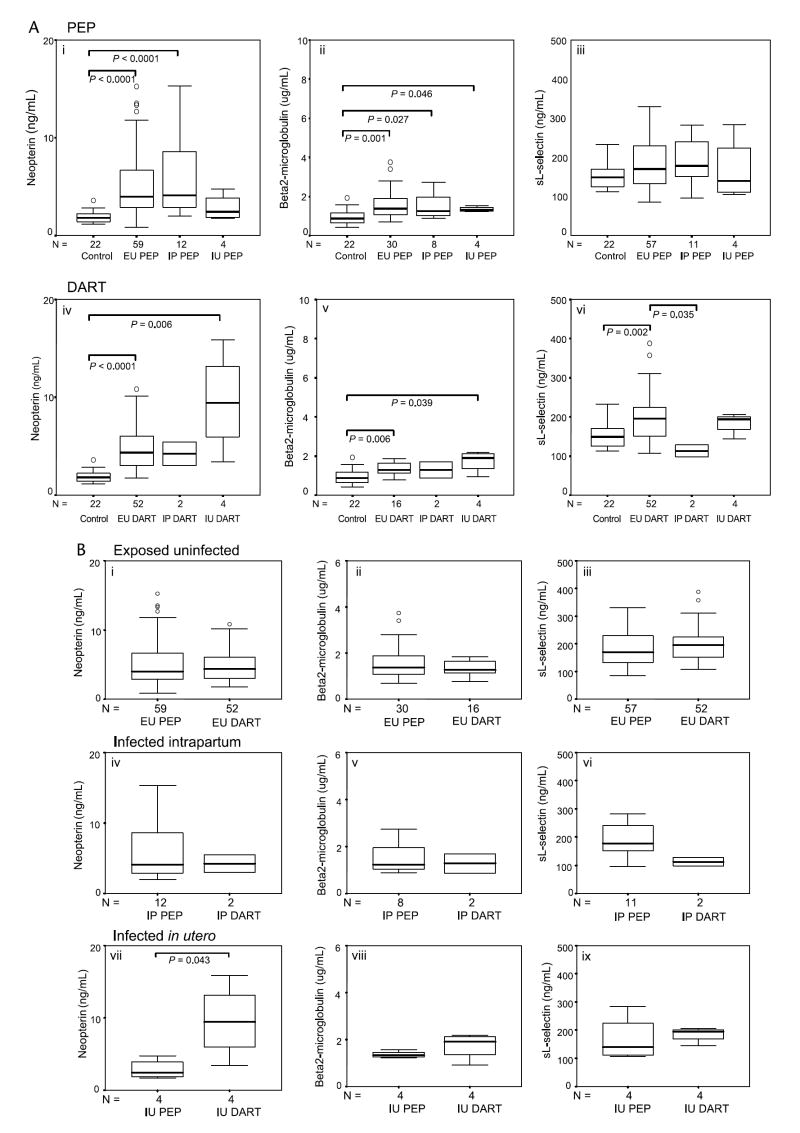
The immune activation markers tended to be higher among HIV-1–infected mothers than among uninfected control mothers (Fig. 2A). However, in contrast to the findings among the children, NVP exposure was not associated with differences in activation markers among nontransmitting mothers or mothers who transmitted IP (Fig. 2B). There was an intriguing increase in neopterin among IU transmitting mothers given NVP (Fig. 2B, vii).
FIGURE 2.
Levels of soluble immune activation markers of HIV-1–infected mothers grouped according to infection status of the infant and NVP exposure. A, Levels of neopterin (ng/mL), β2-m (μg/mL), and sl-selectin (ng/mL) in uninfected (control) mothers and HIV-1–seropositive mothers who did not receive NVP (PEP group) and those who did receive NVP at the start of labor (DART group), grouped according to infection outcome of the infant (EU, IP, and IU). B, Comparison of the effects on levels of neopterin (ng/mL), β2-m (μg/mL), and sl-selectin (ng/mL) of single-dose NVP exposure (DART) versus absence of NVP exposure (PEP) in HIV-1–seropositive mothers grouped according to infection status of the infant (EU, IP, or IU). Data are presented as medians (horizontal bar), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between groups and sample numbers per group are indicated.
Immune Activation in the Infant Is Modulated Independently of that in the Mother
As it is known that a range of low-molecular-weight compounds (<500 d) is actively or passively transferred through the placenta to the fetus,16 we sought to examine whether immune activation in the infant might be modulated independently of that in the mother by determining differences between mothers and infants in their immune activation markers. Interestingly, whereas levels of neopterin did not differ appreciably between infants and mothers, mother-child differences are notable for β2-m and sl-selectin with infants presenting with significantly higher levels of these markers in all groups, including the controls. The differences between mothers and infants were largest for infants infected in utero in both the PEP and DART groups. Within the control group, there were no significant correlations between mothers’ and infants’ (n = 22) levels of neopterin (r = 0.401, P = 0.064), β2-m (r = 0.150, P = 0.504), or sl-selectin (r = 0.068, P = 0.764), and infants had significantly increased levels of neopterin (P = 0.013), β2-m (P = 0.001), and sl-selectin (P < 0.001) compared with their mothers, further supporting independent immune activation modulation in the infant.
DISCUSSION
Previous studies have described nonspecific and HIV-1–specific immune responses in uninfected infants born to HIV-seropositive mothers,17-25 indicative of some immune challenge in utero. Based on previous demonstration of reduced T-helper cell reactivity to HIV envelope peptides in cord blood of infants born to HIV-1–seropositive mothers in the presence of AZT-3TC12 and single-dose NVP26 given to the mother, we hypothesized that other markers of immune activation would accordingly be reduced. This current study was therefore designed using the plasma markers, neopterin, β2-m, and sl-selectin, to assess the extent of immune activation in infants born to HIV-1–seropositive mothers in the presence (DART group) and absence (PEP group) of single-dose NVP administered to the mother at the start of labor.
Our results have shown that (i) in support of earlier studies, there was substantial immune activity in response to exposure to virus in utero (that did not result in infection) or as a result of exposure to other immune consequences of having an HIV-infected mother, and as might be expected, this was most elevated in infants infected in utero; (ii) in contrast to what we had hypothesized, there was evidence of further increased immune activation in the cord blood of infants exposed to NVP when compared with their drug-unexposed counterparts, and most notably, existing infection at birth was marked by substantially elevated levels of all immune activation markers in the presence of NVP; (iii) levels of peripheral blood immune activation markers were higher in infants than their mothers (HIV negative and HIV positive), particularly for β2-m and sl-selectin, indicating that their immune systems develop independently of their mothers; (iv) immune activation in the infant was modulated independently of maternal viral load; and (v) elevated immune activation in NVP-exposed, HIV-infected infants relative to drug-unexposed, HIV-infected infants (IU group) suggests an apparent synergy between HIV-1 and NVP in increasing overall immune activation. This latter phenomenon was also apparent among the IU mothers.
How does one explain attenuated HIV-specific, T-helper cell activity26 yet increased levels of immune activation markers in plasma through a brief exposure (the period of labor and delivery) to NVP? That NVP shows consequences of immune stimulation in such a short period would suggest that it may mediate its effects predominantly on cells of the innate immune response as these respond rapidly to stimuli. Plasma levels of immune activation markers are indicative of events that have already occurred, whereas detecting recall to HIV peptides (T-helper cell reactivity) involves activation of T cells after exposure in vivo to NVP. Because NVP has already increased activation of cells in vivo, as evidenced by increased levels of soluble immune activation markers, T cells may be more anergic on subsequent stimulation with peptides and therefore unable to respond in vitro. This is reminiscent of what occurs in HIV-infected individuals where spontaneous release of cytokines is often enhanced because of in vivo priming, whereas induced release of cytokines is impaired relative to uninfected controls. Because T-cell responses in newborns are very weak, even a slight reduction in responsiveness would reduce most responses to less than the level of detection. Alternatively, among T cells, immune responsiveness in the presence of NVP may be different, for example, increased levels of immune activation markers may be derived from CD8 T cells, whereas CD4 T cells are deactivated under these same circumstances. Although inflammatory markers may also be increased in infants born to HIV-infected mothers as a result of exposure and possible infection with agents other than HIV, it is unlikely that infections would be increased in the NVP-exposed group as opposed to the drug-unexposed group that could account for our results. Our 2 HIV groups would be expected to be comparable in this regard, and the increase in immune activation demonstrated is clearly caused by single-dose NVP exposure.
Levels of immune activation markers, neopterin, β2-m, and sl-selectin, reflect physiologic and pathologic conditions, and in HIV-1 infection, the former two are correlates of stage of disease and prognosis,13,14,27 whereas increased levels of sl-selectin have been reported in HIV-infected infants.28 One could question the extent of maternal transfer of these factors to the infants because studies concur that substances can be transferred from maternal blood to the fetus. There is no documented evidence that passive or active transfer of neopterin (253 d) occurs. Furthermore, fetal neopterin29 and β2-m30 concentrations have been reported to change during gestation with neopterin levels being reported to be substantially greater than maternal levels and not being correlated significantly with paired maternal levels, demonstrating that, during gestation, there is a progressive increase in fetal cell-mediated immunity and monocyte-macrophage activation.29,30 The immune activation marker levels measured in control uninfected infants of HIV-uninfected mothers in this study suggest an association to neonatal immune system development.
Neopterin production is associated with early T-cell responses14 and serves as an indirect measure of oxidative stress and therefore apoptosis (T-cell anergy).31 Elevated levels of β2-m have been associated with an increased turnover of immune cells, especially lymphocytes; however, high concentrations can trigger a cascade of signaling events that exert a negative effect on the immune system, including impaired antigen-presentation capacity of dentritic cells.32 Enhanced apoptosis has been described in cord-blood T lymphocytes of HIV-exposed newborns, with the one infected newborn tested in this study demonstrating the highest levels of CD4+ and CD8+ apoptosis.33 It stands to reason that raised levels of these markers would have consequences on the immune capability of newborn infants because increased apoptosis of T cells and reduced functional capacity of dendritic cells with a diminished ability to activate T cells would compromise antigen-specific, T-cell responses. Thus, although exposure to HIV-1 may result in priming of the immune system (nonspecific and HIV-1–specific immune responses), the presence of single-dose NVP may be sufficient to further drive the immune system into an anergic/immunodeficient state. Single-dose NVP can mediate its antiviral activity on the one hand by directly binding to the HIV-1 reverse transcriptase and, on the other hand, induce an anergic state in cells that may also be beneficial in preventing replication of HIV-1.
It is intriguing that levels of immune activation markers are higher in infants than in their mothers among all groups, even the HIV-seronegative controls. This would support the idea that (i) immune development is accompanied by signs of immune activation; (ii) levels of activation markers in plasma may serve to dampen immune responsiveness early in life and may account for the general T-cell anergy that exists in early life; and (iii) these factors are likely to play very specific roles in immune responsiveness in the infant and are thus unlikely to merely be waste products of immune responses. Furthermore, on the one hand, there appeared to be an independence of the infant’s developing immune system from the mother, but on the other, there were similarities in how infants and mothers responded to common factors in both environments. The mothers’ HIV-1 status appears to influence the infants’ ability to counteract infectious agents either (i) through altered maturation of the infant’s immune responsiveness through exposure to HIV-1 from the mother or (ii) through deficient signaling that may occur through lack of provision of essential factors to the fetus that may be necessary for early immune development.
Our findings challenge, in particular, the way one might view the role of immune activation in promoting the establishment and augmentation of HIV-1 replication in the infant. Our data do not argue against the antiviral effects of short-course treatment, rather we propose, in addition, that not all immune activation is necessarily deleterious and, if caused in vivo by NVP, may well assist infants to prevent establishment of infection. However, for infants already infected when exposed to NVP (ie, those with in utero–acquired infection), the augmented immune activation that occurs in the presence of both NVP and already established infection at birth may well be deleterious. This brings to mind those studies that have indicated that infants who become infected despite perinatal AZT prophylaxis may have a more rapid course of the HIV-1 disease and higher mortality compared with infants who become infected without drug exposure.34-37 However, this has not been observed in all studies38,39 and may be confounded by the severity of maternal disease. Our data would provide a biological explanation for how this may occur, and whether disease progression is more rapid among infected children with NVP exposure should be investigated. What is further thought provoking is the effect of NVP on levels of activation markers in the IU transmitting mothers, a phenomenon that did not occur in the absence of NVP (IU PEP mothers). This would suggest the existence of some factor/s unique to this group of mothers that may also relate to why these mothers were more likely to transmit HIV-1 to their fetuses during pregnancy. We propose that NVP is able to synergize with HIV-1 to increase immune activation either through a mechanism whereby NVP directly acts to activate cells harboring HIV-1 or indirectly by NVP acting on bystander uninfected cells which in turn produce cytokines/factors that increase immune responsiveness or increase HIV-1 replication which in turn generates elevations in levels of activation markers. A question that is raised is what is different about these IU mothers compared with other transmitting (IP) and nontransmitting mothers. The HIV-1 strain infecting these mothers may be much more conducive to increased replication through immunomodulatory effects of NVP, or alternatively, NVP may be metabolized differently in these women, resulting in different immunomodulatory effects to the other women. It will be important to establish if other antiretroviral drugs or drug combinations used to prevent mother-infant HIV-1 transmission also enhance immune activation in some individuals. Given that immune factors such as neopterin are excreted renally,40 we cannot exclude the possibility that reduced clearance may occur in the presence of NVP and so give rise to increased levels when compared with drug-unexposed individuals.
In conclusion, this study has demonstrated that HIV-1 exposure and short-course antiretroviral prophylaxis impact on the developing immune system of the infant. Short-course NVP exposure may be beneficial with respect to driving immune activation before the establishment of an infection in an infant exposed to HIV-1, but may have some detrimental consequences in the case of existing infection acquired in utero. Understanding the synergistic interaction between HIV-1 infection and NVP in substantially enhancing immune activation in some individuals but not in others will provide important insights that could lead to a means to identify mothers who present with this phenomenon, as these are the mothers who are likely to transmit HIV-1 during pregnancy.
Acknowledgments
This study was supported in part by the Poliomyelitis Research Foundation Major Impact grant of South Africa and by grants from NICHD (HD 42402, HD 36177).
References
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353(9155):773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 4.Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d’Ivoire: a randomised trial. Lancet. 1999;353(9155):781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 5.Dabis F, Msellati P, Meda N, et al. 6-Month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d’Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet. 1999;353(9155):786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362(9387):859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 7.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335(22):1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita S, Su L, Amano M, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6(3):235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 9.Oswald-Richter K, Grill SM, Leelawong M, et al. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur J Immunol. 2004;34(6):1705–1714. doi: 10.1002/eji.200424892. [DOI] [PubMed] [Google Scholar]
- 10.Rowland-Jones S, Tan R, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 11.Chavan S, Kodoth S, Pahwa R, et al. The HIV protease inhibitor indinavir inhibits cell-cycle progression in vitro in lymphocytes of HIV-infected and uninfected individuals. Blood. 2001;98(2):383–389. doi: 10.1182/blood.v98.2.383. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn L, Meddows-Taylor S, Gray G, et al. Reduced HIV-stimulated T-helper cell reactivity in cord blood with short-course antiretroviral treatment for prevention of maternal-infant transmission. Clin Exp Immunol. 2001;123(3):443–450. doi: 10.1046/j.1365-2249.2001.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322(3):166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs D, Hausen A, Reibnegger G, et al. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988;9(5):150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- 15.Gray GE, Urban M, Chersich MF, et al. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS. 2005;19(12):1289–1297. doi: 10.1097/01.aids.0000180100.42770.a7. [DOI] [PubMed] [Google Scholar]
- 16.Saji F, Samejima Y, Kamiura S, et al. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4(2):81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 17.Borkowsky W, Krasinski K, Moore T, et al. Lymphocyte proliferative responses to HIV-1 envelope and core antigens by infected and uninfected adults and children. AIDS Res Hum Retroviruses. 1990;6(5):673–678. doi: 10.1089/aid.1990.6.673. [DOI] [PubMed] [Google Scholar]
- 18.Cheynier R, Langlade-Demoyen P, Marescot MR, et al. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1–infected mothers. Eur J Immunol. 1992;22(9):2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 19.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341(8849):860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 20.Aldhous MC, Watret KC, Mok JY, et al. Cytotoxic T lymphocyte activity and CD8 subpopulations in children at risk of HIV infection. Clin Exp Immunol. 1994;97(1):61–67. doi: 10.1111/j.1365-2249.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Maria A, Cirillo C, Moretta L. Occurrence of human immunodeficiency virus type 1 (HIV-1)–specific cytolytic T cell activity in apparently uninfected children born to HIV-1–infected mothers. J Infect Dis. 1994;170(5):1296–1299. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS. 2001;15(1):1–9. doi: 10.1097/00002030-200101050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Wasik TJ, Bratosiewicz J, Wierzbicki A, et al. Protective role of beta-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J Immunol. 1999;162(7):4355–4364. [PubMed] [Google Scholar]
- 24.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96(12):3866–3871. [PubMed] [Google Scholar]
- 25.Levy JA, Hsueh F, Blackbourn DJ, et al. CD8 cell noncytotoxic antiviral activity in human immunodeficiency virus-infected and -uninfected children. J Infect Dis. 1998;177(2):470–472. doi: 10.1086/517378. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn L, Meddows-Taylor S, Gray G, et al. HIV-stimulated IL-2 production among exposed-uninfected infants of HIV-infected mothers given nevirapine prophylaxis. Presented at: 10th Conference on Retroviruses and Opportunistic Infections; February 10–14, 2003; Boston, MA. [Google Scholar]
- 27.Lifson AR, Hessol NA, Buchbinder SP, et al. Serum beta 2-microglobulin and prediction of progression to AIDS in HIV infection. Lancet. 1992;339(8807):1436–1440. doi: 10.1016/0140-6736(92)92030-j. [DOI] [PubMed] [Google Scholar]
- 28.Kourtis AP, Nesheim SR, Thea D, et al. Correlation of virus load and soluble l-selectin, a marker of immune activation, in pediatric HIV-1 infection. AIDS. 2000;14(16):2429–2436. doi: 10.1097/00002030-200011100-00003. [DOI] [PubMed] [Google Scholar]
- 29.Radunovic N, Kuczynski E, Rebarber A, et al. Neopterin concentrations in fetal and maternal blood: a marker of cell-mediated immune activation. Am J Obstet Gynecol. 1999;181(1):170–173. doi: 10.1016/s0002-9378(99)70455-2. [DOI] [PubMed] [Google Scholar]
- 30.Cejka J, Kithier K, Belamaric J, et al. Feto-specific features of human beta2-microglobulin. Experientia. 1974;30(5):458–459. doi: 10.1007/BF01926290. [DOI] [PubMed] [Google Scholar]
- 31.Widner B, Wirleitner B, Baier-Bitterlich G, et al. Cellular immune activation, neopterin production, tryptophan degradation and the development of immunodeficiency. Arch Immunol Ther Exp (Warsz) 2000;48(4):251–258. [PubMed] [Google Scholar]
- 32.Xie J, Wang Y, Freeman ME, 3rd, et al. Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood. 2003;101(10):4005–4012. doi: 10.1182/blood-2002-11-3368. [DOI] [PubMed] [Google Scholar]
- 33.Economides A, Schmid I, Anisman-Posner DJ, et al. Apoptosis in cord blood T lymphocytes from infants of human immunodeficiency virus– infected mothers. Clin Diagn Lab Immunol. 1998;5(2):230–234. doi: 10.1128/cdli.5.2.230-234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapid disease progression in HIV-1 perinatally infected children born to mothers receiving zidovudine monotherapy during pregnancy. The Italian register for HIV Infection in Children. AIDS. 1999;13(8):927–933. [PubMed] [Google Scholar]
- 35.Kuhn L, Abrams EJ, Weedon J, et al. Disease progression and early viral dynamics in human immunodeficiency virus– infected children exposed to zidovudine during prenatal and perinatal periods. J Infect Dis. 2000;182(1):104–111. doi: 10.1086/315678. [DOI] [PubMed] [Google Scholar]
- 36.de Souza RS, Gomez-Marin O, Scott GB, et al. Effect of prenatal zidovudine on disease progression in perinatally HIV-1– infected infants. J Acquir Immune Defic Syndr. 2000;24(2):154–161. doi: 10.1097/00126334-200006010-00010. [DOI] [PubMed] [Google Scholar]
- 37.Sutthent R, Chokephaibulkit K, Piyasujabul D, et al. Effect of perinatal short-course zidovudine on the clinical and virological manifestations of HIV-1 subtype E infection in infants. J Clin Virol. 2002;25(1):47–56. doi: 10.1016/s1386-6532(01)00258-x. [DOI] [PubMed] [Google Scholar]
- 38.Rich KC, Fowler MG, Mofenson LM, et al. Maternal and infant factors predicting disease progression in human immunodeficiency virus type 1 – infected infants. Women and Infants Transmission Study Group. Pediatrics. 2000;105(1):e8. doi: 10.1542/peds.105.1.e8. [DOI] [PubMed] [Google Scholar]
- 39.Dabis F, Elenga N, Meda N, et al. 18-Month mortality and perinatal exposure to zidovudine in West Africa. AIDS. 2001;15(6):771–779. doi: 10.1097/00002030-200104130-00013. [DOI] [PubMed] [Google Scholar]
- 40.Estelberger W, Weiss G, Petek W, et al. Determination of renal clearance of neopterin by a pharmacokinetic approach. FEBS Lett. 1993;329(1–2):13–16. doi: 10.1016/0014-5793(93)80182-t. [DOI] [PubMed] [Google Scholar]