Abstract
Purpose
To investigate the effects of acquired superior oblique palsy (SOP) and corrective strabismus surgery on torsional optokinetic nystagmus (tOKN) in monkeys.
Methods
The trochlear nerve was severed intracranially in two rhesus monkeys (M1 and M2). For each monkey, more than 4 months after the SOP, the ipsilateral inferior oblique muscle was denervated and extirpated. For M2, 4 months later, the contralateral inferior rectus muscle was recessed by 2 mm. tOKN was elicited during monocular viewing of a rotating stimulus that was rear projected onto a screen 43.5 cm in front of the animal. Angular rotation of the stimulus about the center was 40 deg/s clockwise or counterclockwise.
Results
The main findings after trochlear nerve sectioning were (1) the amplitude and peak velocity of torsional quick and slow phases of the paretic eye was less than that in the normal eye for both intorsion and extorsion, and (2) the vertical motion of the paretic eye increased during both torsional slow and quick phases. After corrective inferior oblique surgery, both of these effects were even greater.
Conclusions
Acquired SOP and corrective inferior oblique–weakening surgery create characteristic patterns of change in tOKN that reflect alterations in the dynamic properties of the extraocular muscles involved in eye torsion. tOKN also provides information complementary to that provided by the traditional Bielschowsky head-tilt test and potentially can help distinguish among different causes of vertical ocular misalignment.
Superior oblique.palsy (SOP) is a common cause of cyclo-vertical strabismus. The primary action of the superior oblique muscle is intorsion, and its secondary action is depression. Consequently, the paretic eye of a patient with a weakness of the SO muscle is elevated and extorted relative to the normal eye. The diagnosis of SOP often rests on the results of the Bielschowsky head-tilt test: In SOP, the vertical deviation increases when the head is tilted toward the side of the paretic eye. This response is due to the vestibulo-ocular reflex induced by a static roll tilt of the head. Normally, when the head is tilted laterally, the superior rectus (SR) muscle and SO muscles cooperate to intort the lower eye.1 When the head is tilted toward the side of an SOP, however, the upward action of the SR muscle is unopposed by the (paretic) SO muscle, leading to an increased vertical deviation.
As part of a study of the ocular motor signature of acquired SOP in monkeys,2–4 we sought to assay the function of the SO muscle by measuring the torsional response to an optokinetic stimulus that rotated around the line of sight (tOKN). By eliciting torsional nystagmus, we could examine the effect of the SOP on the primary action of the SO muscle. In addition, we asked if examining the changes in the dynamic characteristics of eye movements during tOKN might give information complementary to that from the Bielschowsky head-tilt test. Both monkeys also received corrective strabismus procedures (a weakening procedure of the ipsilateral inferior oblique [IO] muscle in both animals and a subsequent weakening procedure of the contralateral inferior rectus [IR] muscle in one animal). This gave us the opportunity to examine the effects of weakening of muscles with different primary muscle actions on the response to tOKN.
Methods
The general experimental methods have been described previously in the descriptions of the effects of trochlear nerve sectioning on static and dynamic alignment of the eyes.2–4 The protocol was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
In brief, two female juvenile rhesus monkeys (M1 and M2) weighing from 4 to 6 kg were studied. Three-dimensional eye movements were measured with the magnetic-field search-coil method. Two eye coils made from Teflon-coated stainless steel wire were implanted in each eye of the monkeys. The direction coil (measuring horizontal and vertical eye position) was sutured on the sclera under the conjunctiva just in front of the insertion of the rectus muscles. The torsion coil was implanted superotemporally between the lateral and superior rectus muscles and centered on the equator of the globe. During the experiments, the animal was seated in a primate chair that was placed in the center of a cubic frame in which three orthogonal magnetic fields of different frequencies (55.5, 83.3, and 42.6 kHz) were generated. The output signal of the coil system was band-pass filtered (0 –90 Hz), digitized at 1000 samples per second, and saved on a computer for later analysis.
Surgical Procedures
With the monkeys under inhalation anesthesia, the SOP was induced by intracranial sectioning of the trochlear nerve (left eye for M1 and right eye for M2). Through a subtemporal approach, posterior to the cavernous sinus, the trochlear nerve was visualized and severed, leaving a gap of approximately 10 mm. Subsequently, in M1 (at 7.5 months after the SOP) and in M2 (at 4.5 months after the SOP), the ipsilateral IO muscle was denervated and extirpated. For M2, 4 months after the IO-weakening procedure, the contralateral IR muscle was recessed by 2 mm. All the corrective surgeries were performed under pentobarbital anesthesia (30 mg/kg, intravenously). For the IO procedure, the IO muscle was isolated through an inferotemporal fornix incision by using muscle hooks. It was disinserted from the sclera and put on a stretcher, and then the nerve supplying the muscle was isolated separately and divided with cautery. The available muscle belly was drawn forward, clamped, and amputated with cautery.
For the recession of the IR, the muscle was isolated with muscle hooks through an inferonasal fornix incision. The tendon was secured 2 mm from the insertion point on the globe by a 6-0 Vicryl suture in a locking stitch. The muscle was disinserted from the scleral and reinserted 3 mm posterior to the original insertion point. The suture was adjusted so that the corneal light reflexes indicated a modest overcorrection. The final position of the muscle was 2 mm posterior to the original insertion point.
Stimulus Presentation
Torsional optokinetic nystagmus (tOKN) was elicited during monocular viewing of a rotating stimulus that was rear projected onto a tangent screen in front of the animal. The center of the stimulus was positioned 43.5 cm in front of the midpoint of the interpupillary axis. The stimulus consisted of a pattern of 20 alternating white and black radial sectors (Fig. 1) covering 74° of the visual field. The angular rotation of the stimulus about its center was 40 deg/s, clockwise (CW) or counterclockwise (CCW), which was optimal in our animals for eliciting tOKN. The periods of stimulation were 90 seconds. Each day, before collecting the tOKN data, reference positions were obtained. A red square (0.3 × 0.3°) was rear projected on the screen at the center of the stimulus (the apices of the sectors), and the animal was rewarded with water for fixing on the target, first with one eye viewing and then with the other eye viewing. These positions were defined as the reference positions for the two eyes. Using these new reference positions, we calculated the relative torsion and vertical deviations between the two eyes or the relative difference between torsion and vertical rotation in the individual eye. During the tOKN stimulation, the target was extinguished, but the animal was still rewarded when the viewing eye was fixing on the center of the stimulus.
Figure 1.
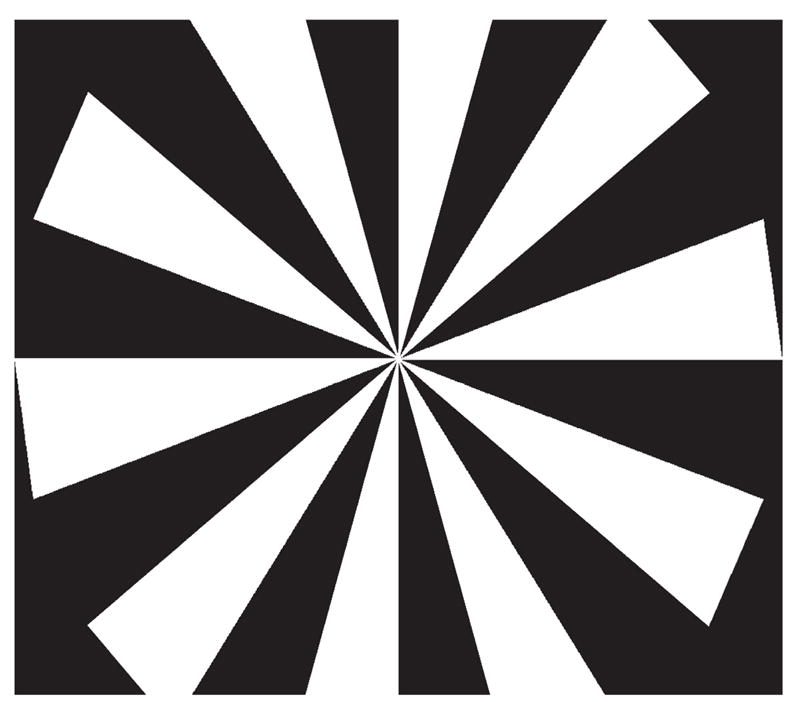
A tOKN stimulus.
Experimental Protocol
Pre-SOP data during monocular viewing were obtained 2 to 3 weeks before sectioning of the trochlear nerve. After SOP, the paretic eye (PE) was patched for 6 days in M1 and 9 days in M2, and then habitual binocular viewing was allowed. tOKN was tested 24 days after SOP in M1 (which was 18 days after habitual binocular viewing was allowed), and 35 days after SOP for M2 (which was 26 days after habitual binocular viewing was allowed). After the subsequent IO corrective surgery, the surgical eye was habitually patched, as after the SOP. This time, however, the surgical eye was allowed to view monocularly for an hour or so on each testing day. tOKN data were recorded 10 to 13 days after the corrective surgery, but before sustained habitual binocular viewing was allowed. Unless otherwise stated, the data presented are with the normal eye viewing.
Data Analysis
Data were analyzed with custom-developed software (MatLab; The MathWorks, Inc., Natick, MA). A rotation vector representation of eye orientation was calculated from the recorded coil signals, with the reference position being straight ahead. In the presentation of the data, up for vertical, right for horizontal and clockwise for torsion are positive. An FIR1 filter (bandwidth 30 Hz; MatLab, The MathWorks, Inc.) was used to smooth eye position, and this signal was differentiated, to produce eye velocity with a fifth-order Remez filter (MatLab, The MathWorks, Inc.) designed to differentiate up to 40 Hz and to low-pass filter above 80 Hz. The beginning and end of the quick (QP) and the slow (SP) phases of tOKN were identified manually. Data were included for analysis when the viewing eye fell within a window of ±1.5° horizontally and vertically about the center of the tOKN stimulus. Data segments containing blinks were discarded. The amplitude of the SP or QP was determined by subtracting the position of the eye at the beginning from the position at the end of the individual SP or QP. The ratio of the amplitude of the PE to the amplitude of the normal eye (NE) over the same period was calculated before and after SOP, to describe the relative change of PE to NE. A Kruskal-Wallis test was used to test for significance. The relationships between the torsional and vertical amplitudes of the series of individual SPs and QPs were determined by least-squares linear regression. A one-tailed t-test was used to evaluate the significance of changes in the slopes of the regression lines (before and after SOP, and after corrective surgery),5 because our initial hypothesis was that weakness of the SO muscle must increase the amount of vertical motion relative to torsion. Data from M2 were mirrored, so in the following text, in both monkeys the left eye is considered the PE and the right eye the NE.
Results
First, we will recapitulate briefly the main effects of trochlear nerve sectioning on vertical, torsional, and horizontal alignment. Then, we will present the effects on tOKN, emphasizing the changes in the relative amount of torsion made by the two eyes and the amount of inappropriate vertical rotation of the PE.
Before trochlear nerve sectioning, M1 and M2 both showed little ocular misalignment. After the animals habitually viewed out of one eye for 4 to 7 days, they developed small vertical (1°–2°), torsional (intorsion, 1°–3°, or extorsion, 1°), and horizontal (exo, 1°–3°) deviations. After the trochlear nerve was sectioned, the monkeys showed the expected pattern of ocular misalignment in SOP with a vertical and torsional deviation, but little change in horizontal alignment.2
Comparing the Torsion Response to tOKN between the Paretic and the Intact Eyes
In both monkeys, the rotating stimulus elicited a tOKN composed of SP in the direction of rotation and QP in the opposite direction. Before the SOP, with a 40-deg/s rotating stimulus the average slow-phase gain in M1 was 0.05 to 0.07 for both CW and CCW, whereas in M2 the gain was 0.04 to 0.05 for CW and 0.03 for CCW. Although average SP velocities were low, in the range of 1.5 to 3 deg/s, the SP and QP of tOKN were easily discernible.
The top panels of Figure 2 show the vertical and torsional eye positions of each eye in M1 during CW and CCW rotation, with the NE viewing. Responses in M2 were similar. Figures 2A and 2D show the pre-SOP responses, Figures 2B and 2E show the responses 24 days after trochlear nerve sectioning, and Figures 2C and 2F show the responses 2 weeks after the IO denervation and extirpation. The bottom panels show the vertical (VD) and torsional (TD) deviations (PE minus NE) during each rotation. In this figure a positive value for TD is a relative intorsional deviation, whereas a negative value is a relative extorsional deviation. There was a slight asymmetry between the two eyes before SOP, with the to-be-paretic eye rotating slightly faster than the intact eye.
Figure 2.
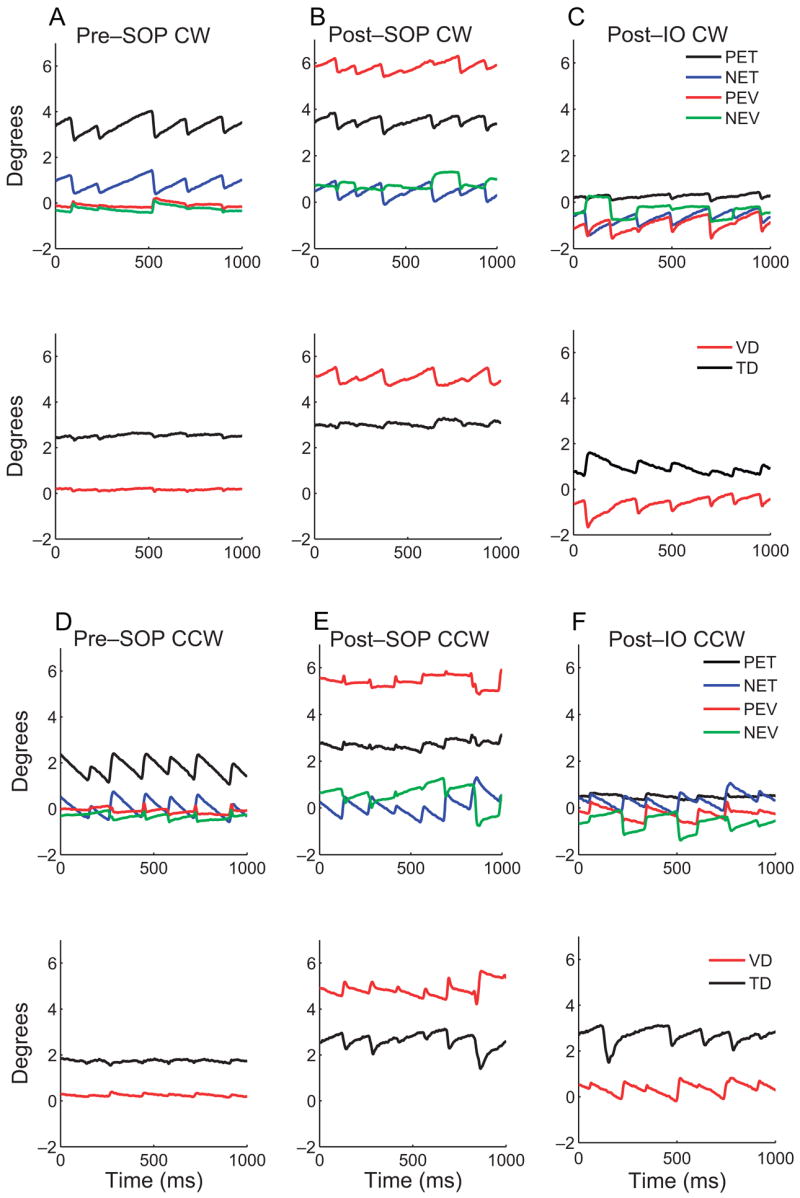
Top: typical tOKN recordings from M1, NE viewing, before (A, D) and after (B, E) SOP and after IO surgery (C, F). The left eye was paretic, and CW rotation (positive) produced PE intorsion and CCW rotation (negative), PE extorsion. PET, paretic eye torsion; NET, normal eye torsion; PEV, paretic eye vertical; NEV, normal eye vertical. Bottom: VD and TD (PE-NE). A positive number refers to a relative intorsional deviation or to a vertical misalignment, with the PE higher. A new reference position was taken each day so that torsion was arbitrarily set to 0 at straight-ahead gaze. Note the asymmetry in the torsional SP velocity after SOP and the changes in the TD and VD during SP after SOP and after IO surgery.
After both trochlear nerve sectioning and the IO procedure, both monkeys showed similar changes in tOKN. First, the velocity of the torsional SP made by the PE was less than that by the NE for both CW (Fig. 2B, top) and CCW (Fig. 2E, top) rotation. Consequently, there was an increased extorsional deviation during CW SP (Fig. 2B, bottom panel, black trace) and an increased intorsional deviation during CCW SP (Fig. 2E, bottom panel, black trace). Second, before trochlear nerve sectioning, there was a relatively small vertical deviation (VD) that changed little during tOKN (Figs. 2A, 2D, bottom panels, red traces). After trochlear nerve sectioning, however, the VD increased from its baseline during intorsion (CW SP) of the PE and decreased during extorsion (CCW SP) of the PE (Figs. 2B, 2E, bottom panels, red traces). Third, after the IO muscle was denervated and extirpated, the velocities of both intorting and extorting SP of the PE were reduced further (Figs. 2C, 2F, top panels, compare black and blue traces). Thus, there was an even greater change in the TD during both CW and CCW SP (Figs. 2C, 2F, bottom panels, black traces). Fourth, after IO surgery, the VD decreased even more during extorting torsional SP by the PE (Fig. 2F, bottom panel, red trace).
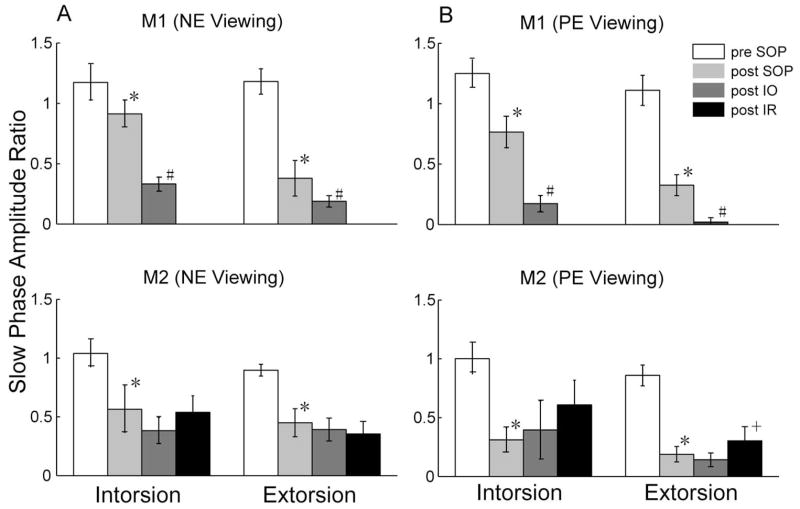
Figure 3 shows the mean (±SD) of the amplitude ratios (PE/NE) for torsional SP during NE and PE viewing for both M1 and M2, before and after SOP and after corrective surgery. Before SOP there were slight asymmetries between the two eyes in each monkey. In M1 the to-be-paretic eye had larger amplitudes for both torsional directions (amplitude ratios,> 1.0). After SOP, in both monkeys the amplitude ratio for SP (PE/NE) decreased significantly in both directions and for both NE and PE viewing (P < 0.001, Kruskal-Wallis). Note that the decreases were larger with PE viewing, especially for M2.
Figure 3.
Mean (±SD) of torsional SP amplitude ratios (PE/NE) with NE (A) and PE (B) viewing in both monkeys. Intorsion or extorsion refers to the PE. *Post-trochlear nerve sectioning values that were significantly decreased compared with pre-SOP values; #post-IO surgery values that were significantly decreased compared with post-SOP values; 3post-IR surgery values that were significantly changed compared with post-IO values (Kruskal-Wallis, P < 0.001).
After IO surgery, for M1 the amplitude ratios for SP decreased further in both directions (P < 0.001, Kruskal-Wallis). For M2, however, the amplitude ratios for SP showed no statistically significant changes (P > 0.05, Kruskal-Wallis) though with NE viewing the trend was similar to that of M1. After the IR recession (in M2) there were no significant changes in the amplitude ratios for SP except for extorsional SP with PE viewing.
We also examined the peak velocity of the torsional QP and computed a peak-velocity ratio, comparing the PE to the NE, for both NE (Figs. 4A, 4B) and PE viewing (Figs. 5A, 5B). The peak-velocity ratio may be a more consistent indicator of muscle palsy than the amplitude ratio.2 These figures also show peak-velocity ratios for vertical saccades for comparison (Figs. 4C, 4D, 5C, 5D, see the Discussion section). For torsion, as for the amplitude ratios, pre-SOP there were slight asymmetries between the two eyes for each monkey. In M1 the to-be-paretic eye had a higher peak velocity for both torsional directions (peak-velocity ratios, > 1.0). After SOP in both monkeys with NE viewing, the peak-velocity ratios were significantly decreased in both directions (P < 0.001, Kruskal-Wallis). As for the SP amplitude ratios, the decrease was greater with the PE viewing than with the NE viewing, in particular in M2. After IO surgery, in both monkeys with NE viewing, the peak-velocity ratios for the QP decreased further in both directions (P < 0.001, Kruskal-Wallis), except for the intorsional QP made by M2 (P > 0.05, Kruskal-Wallis). After the IR recession, there were no significant changes in the peak-velocity ratios in the QP.
Figure 4.
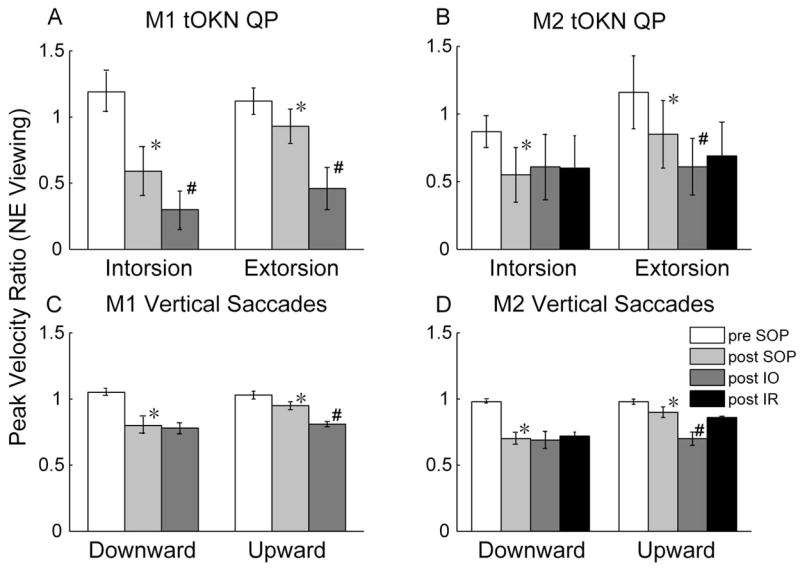
(A, B) Mean (±SD) of torsional QP peak-velocity ratios (PE/NE) in NE viewing in both monkeys. Intorsion or extorsion always refers to the direction of rotation of the PE. Bottom: mean value (±SD) of peak-velocity ratios for 20° vertical saccades (−10° to +10°) from NE viewing for both monkeys. (Vertical saccade data was averaged over several sessions occurring before and after the tOKN data were taken). *Significantly decreased post-SOP values (Kruskal-Wallis, P < 0.001); #significantly decreased post-IO surgery values (Kruskal-Wallis, P < 0.001).
Figure 5.
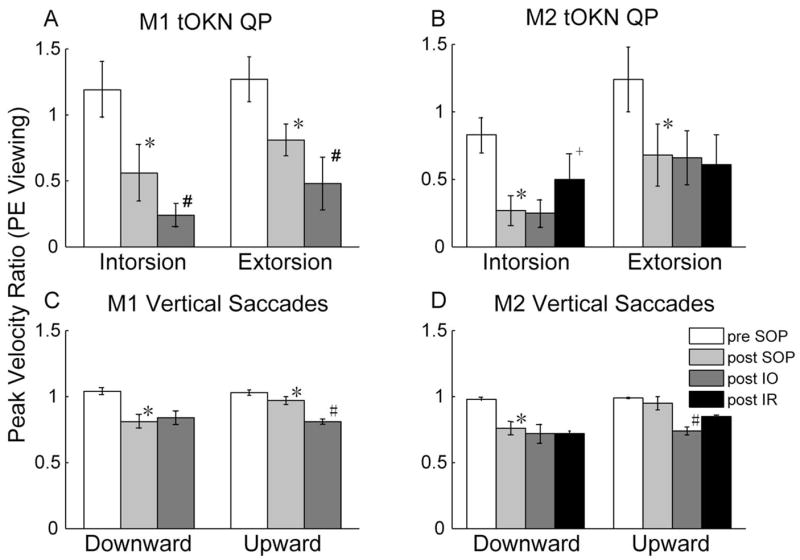
Same as Figure 4 but with the PE viewing. +Significantly changed post-IR surgery values (Kruskal-Wallis, P < 0.05).
Inappropriate Vertical Response to tOKN
We next examined the amount of “inappropriate” vertical rotation that appeared during tOKN stimulation after SOP. Figure 6 depicts the relationship between the amplitude of the torsional and vertical components of the QP made by the PE with the PE viewing in both monkeys. We fit the data with a linear regression to look for changes in the slope, which reflect changes in the amount of vertical rotation relative to torsion (see the Methods section). The slopes and coefficients of determination (r2) for the QP (PE from PE viewing and NE from NE viewing) are summarized in Table 1. Before the SOP, there was no pattern of consistent vertical motion during either the intorsional or extorsional QP. Correspondingly, the r2 values were small. In both monkeys after the SOP, the PE showed more elevation during the intorsional QP and more depression during the extorsional QP. The regression slopes changed significantly (P < 0.001) except for the extorsional QP in M2. After the IO-weakening surgery, M1 had an even smaller torsional amplitude and relatively more vertical motion (r2 = 0.94 for intorsion and 1.0 for extorsion), and the slopes also changed significantly (P < 0.001) in both directions. In M2 after IO surgery, the slope of the intorsional (P < 0.05) but not the extorsional QP changed significantly. We also examined the relationship between torsional and vertical motion of the NE during NE viewing (NEV). Not surprisingly, there were no consistent changes after SOP or IO surgery.
Figure 6.
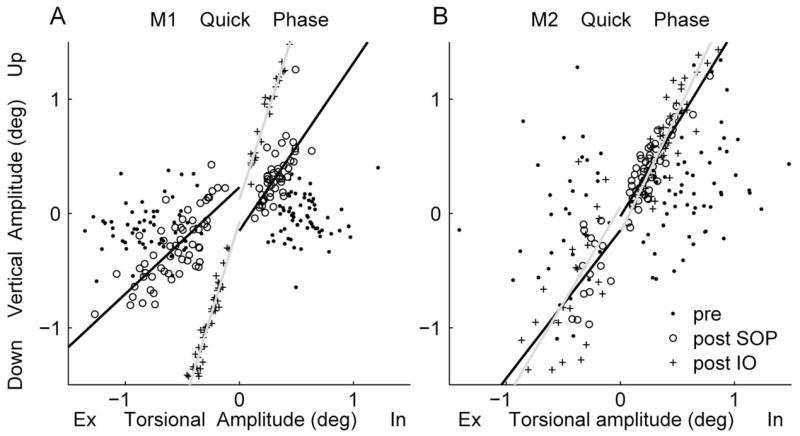
Linear regression of torsional versus vertical amplitude of quick phases of the PE during PE eye viewing from M1 (A) and M2 (B). In, intorsion; Ex, extorsion. The lines (separated by direction, CW, CCW) are regression equations fitting post-SOP (black line) and post-IO surgery (gray line) data.
Table 1.
Regression Slope and Coefficient of Determination (in Parentheses) of QP Torsional Amplitude versus Vertical Amplitude
| PE (from PE Viewing)
|
NE (from NE Viewing)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-SOP | Post-SOP | Post-IO | Post-IR | Pre-SOP | Post-SOP | Post-IO | Post-IR | |
| Intorsion | ||||||||
| M1 | −0.004 (0.00) | 0.79* (0.46) | 3.10† (0.94) | −0.03 (0.00) | 0.10 (0.02) | 0.11 (0.01) | ||
| M2 | 0.38 (0.08) | 1.63* (0.76) | 2.10† (0.72) | 1.49 (0.37) | 1.68 (0.19) | 0.15* (0.01) | 0.44 (0.10) | 0.75 (0.17) |
| Extorsion | ||||||||
| M1 | 0.08 (0.01) | 1.07* (0.59) | 3.29† (0.96) | 0.32 (0.14) | 0.41 (0.43) | 0.58 (0.58) | ||
| M2 | 0.55 (0.05) | 1.30 (0.15) | 1.67 (0.43) | 2.84 (0.67) | −0.09 (0.00) | 0.59* (0.51) | 0.39 (0.10) | 0.60 (0.35) |
Extorsion and intorsion refer to the direction of motion of the viewing eye.
Significantly changed, compared with pre-SOP data (P < 0.05).
Significantly changed, compared with post-SOP data (P < 0.05).
We performed a similar analysis of the SPs of tOKN (Fig. 7, Table 2). In M1, there was little vertical motion during intorsional and extorsional SPs before SOP, as was the case for the QP. After SOP, there was significantly increased elevation during the intorsional SP of the PE (P < 0.05) relative to that before SOP. Although the extorsional SP amplitude also decreased significantly, there was no change in the relative amount of coincident vertical motion (when compared with before SOP; P > 0.05). After the IO-weakening procedure, the torsional amplitude decreased further, and there was relatively more elevation or depression coincident with both intorsional and extorsional SP (slope change, P < 0.05). In M2, the slopes of torsion against vertical rotation increased significantly after SOP in both directions (P < 0.05), but after the corrective surgeries, there were no further changes (P > 0.05). There were no significant changes in the NE when it was viewing (Table 2).
Figure 7.
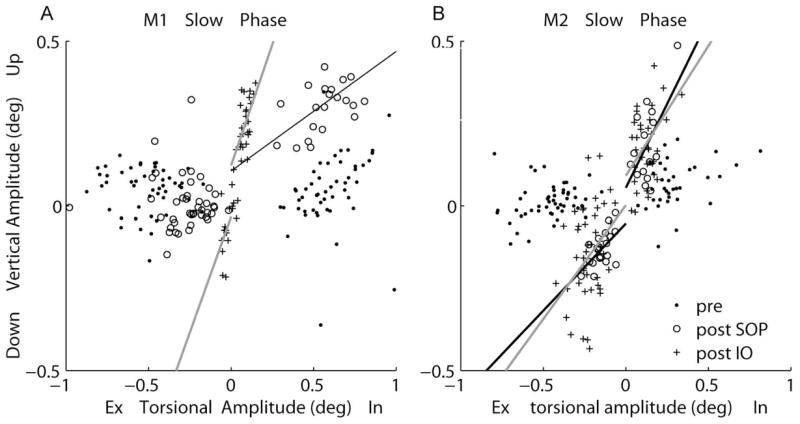
Linear regression of torsional versus vertical amplitude of PE slow phases during PE viewing from M1 (A) and M2 (B). In, intorsion; Ex, extorsion. The lines (separated by direction, CW, CCW) are equations fitting post-SOP (black line) and post-IO (gray line) data.
Table 2.
Regression Slope and Coefficient of Determinations of SP Torsional versus Vertical Amplitude
| PE (from PE Viewing)
|
NE (from NE Viewing)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pre-SOP | Post-SOP | Post-IO | Post-IR | Pre-SOP | Post-SOP | Post-IO | Post-IR | |
| Intorsion | ||||||||
| M1 | 0.09 (0.03) | 0.36* (0.41) | 1.47† (0.36) | 0.28 (0.45) | 0.28 (0.23) | 0.25 (0.43) | ||
| M2 | 0.16 (0.14) | 1.02* (0.34) | 0.79 (0.19) | 1.22 (0.57) | 0.06 (0.02) | 0.08 (0.07) | −0.003 (0.00) | 0.19‡ (0.23) |
| Extorsion | ||||||||
| M1 | −0.03 (0.01) | −0.02 (0.001) | 1.40† (0.31) | −0.01 (0.00) | −0.06 (0.03) | −0.39 (0.61) | ||
| M2 | 0.07 (0.04) | 0.52* (0.34) | 0.69 (0.13) | 0.13‡ (0.03) | −0.06 (0.02) | −0.19 (0.09) | −0.08 (0.09) | −0.02 (0.00) |
Extorsion and intorsion refer to the direction of motion of the viewing eye.
Significantly changed, compared with pre-SOP data (P < 0.05).
Significantly changed, compared with post-SOP data (P < 0.05).
Significantly changed, compared with post-IO surgery data (P < 0.05).
Discussion
We investigated the effects of acquired SOP on torsional eye movements. Our experiments were designed around two specific goals. First, we reasoned that the most sensitive way to identify the effects of a paralysis would be to analyze the changes in the primary action on the globe of the SO muscle—in this case, torsion. Hence, we compared the torsional response of the intact and paretic eyes by using an optokinetic stimulus that rotated around the line of sight (tOKN). Second, because the oblique muscles have a relatively strong secondary action—vertical rotation of the globe—we sought to quantify tOKN similar to the method in the Bielschowsky head-tilt test (i.e., to measure the vertical rotation of the PE in response to a pure torsional stimulus). We will discuss our findings related to these two goals and also consider some of the effects of corrective strabismus surgery on tOKN.
Effects of SOP on Torsion: Peak-Velocity Ratios for QP of tOKN
The effects of trochlear nerve sectioning on tOKN were similar in both animals. Using the peak-velocity ratio between the two eyes (Fig. 4, top panels), we showed that the torsional QP made by the PE was relatively slower than that made by the intact eye. The deficits were present during extorsion and intorsion, because extorsion is the result of an increase in active force in the agonist and a decrease in active force in the antagonist muscle. When the paretic muscle is the antagonist, this decrement in active force is missing, and so the acceleration is less than normal. Nevertheless, the relative decrease was greater for intorsion, because for QP (and saccades) the increment in active forces when a muscle is acting as the agonist is greater than the decrement in active forces when a muscle is relaxing as an antagonist. It is also possible that adaptive mechanisms contribute to the decrease in response when the paretic muscle is acting as an antagonist, to prevent the eye from reaching an extreme position of contraversive (to the intact side) deviation.
We have reported a comparable analysis of vertical saccades with SOP in the same animals by using the peak-velocity ratios3 and found a deficit that was greater for downward than for upward saccades (Fig. 4, bottom). When the change in peak-velocity ratios for vertical saccades was compared with that for the torsional QP, however, the relative deficit was more marked for torsion (compare Fig. 4, top and bottom). This finding is not surprising, because the primary action of the SO muscle is torsion, so that any weakness of this muscle may be expected to be more obvious when testing the muscle in its primary direction of action, where the requirement for a change in force is highest. The SPs of tOKN were also affected in both directions, although the asymmetry between extorsion and intorsion was less consistent. (In M1, for example, extorsional SPs were more affected than intorsional SPs; Fig. 3). Similarly, for upward saccades (when the SO muscle acts as an antagonist) we also found that some dynamic characteristics were affected by the same amount or even more than for downward saccades (e.g., pulse–pulse ratios, pulse–step ratios of the PE, especially in M1).3 Finally, we emphasize that these deficits and asymmetries in tOKN were quite clear, even though the amplitude and velocities of tOKN were low.
We also noted that in M2 the changes in peak-velocity ratios of the QP and the amplitude ratios of the SP were both considerably greater when measured with the PE viewing than with the NE viewing. M2 had the larger vertical deviation, so that when she was fixing with the NE, the PE was much higher in the orbit where its torsional action would be relatively less. Hence, it is not surprising that the effect of switching to fixing with the PE, which moves the PE into a position in the orbit where a loss of its normal contribution to torsion becomes more obvious, brought out the difference in the dynamic properties between the two eyes. In some ways, this is analogous to the finding that the secondary deviation (with the PE viewing) is greater than the primary deviation (with the NE viewing).
The IO denervation and extirpation also produced changes in tOKN, although there were differences between the two animals. In both animals, there was a further decrease in the peak-velocity ratios of the extorting QP; and, in M1 but to a lesser extent, there was a decrease in peak-velocity ratios of the intorting QP. There was a comparable change in the peak-velocity ratios for upward but not downward saccades after the IO procedure (Fig. 4). These findings presumably reflect the fact that the IO muscle was extirpated and denervated, minimizing its normal effect. On the other hand, the recession of the contralateral IR muscle, which led to an improvement in vertical ocular misalignment, produced no significant changes in the peak-velocity ratios for torsion or vertical saccades. This result is consistent with the few reports in the literature showing that recession of a rectus muscle produces no reduction in saccade velocities,6,7 presumably because a recession procedure only modestly decreases the generation of active muscle forces during rapid eye movements, and neuromuscular adaptation may also occur.
Abnormal Vertical Motion in Response to Torsional Stimuli: a Possible Complement to the Bielschowsky Head-Tilt Test
Our second goal was to quantify the amount of vertical motion by the PE in response to an optokinetic stimulus that would normally induce pure torsion. Inappropriate vertical motion arises naturally in SOP, because the secondary action of the oblique muscles is vertical rotation of the globe. Hence, because the oblique and rectus muscles have opposite actions with respect to torsion and vertical rotation—that is, the SO and the SR muscles are synergistic with respect to torsion (both intort the eye) but antagonistic with respect to vertical rotation (the SR muscle elevates the eye; the SO muscle depresses it)—paresis of an oblique muscle also leads to a vertical imbalance. Thus, in SOP, when a stimulus calls for a conjugate torsion, the unopposed SR muscle of the PE intorts and elevates the globe, leading to an increased vertical deviation between the two eyes. This phenomenon, of course, is the basis for a positive Bielschowsky head-tilt test when a patient tilts the head toward the side of the PE. The head tilt calls for a conjugate ocular torsion in the opposite direction. Hence, the PE intorts but also elevates (because of the unopposed action of the SR muscle), increasing the vertical deviation. In contrast when the head is tilted away from the side of the PE, extorsion of the PE is called for (by the IO and IR muscles) which is accompanied by inhibition of the SR and SO muscles in that eye. Hence, there is less inappropriate vertical rotation associated with torsion and the vertical deviation lessens. Such a pattern of changing vertical alignment also occurs during dynamic head roll, which is reflected in a change in the three-dimensional axis of eye rotation.8
As expected, during tOKN we found inappropriate vertical motion of the PE when a pure torsional response was called for. There was a large change after the trochlear nerve was sectioned, more so for intorsion than for extorsion and more so for M2 than for M1. These values during tOKN showed the same general pattern as those during actual head tilt (greater change for tilt toward the paretic side [intorsion] and greater change in M2; Fig. 7 2).
The results after the IO corrective surgeries were less consistent between animals though both showed a further decrease in the amount of torsion by the PE relative to the NE. Differences in the actual surgical effects as well as longer-term adaptive changes may contribute to these differences between animals. Finally, adaptive changes in otolith–ocular responses induced by head tilt may also add to the complexities of interpreting head tilt responses and tOKN.9
In sum, we report characteristic patterns of change in tOKN that provide additional information about the ocular motor signature of acquired SOP. tOKN is also a way to probe the dynamic function of the oblique muscles in relative isolation, and the pattern of changes in tOKN may help to distinguish true SOP from other causes of cyclovertical strabismus that often masquerade as SOP. These predictions remain to be tested.
Acknowledgments
The authors thank Corena Bridges, Dale Roberts, and Adrian Lasker for technical support.
Supported by National Eye Institute Grants R01-EY001849 (DSZ), K12EY015025 (HSY), and K23EY00400 (MFW); an Abe Pollin Scholarship (MFW); the Arnold-Chiari Foundation (MFW); and the Albert Pennick Fund (MFW).
Footnotes
Disclosure: X. Shan, None; J. Tian, None; H.S. Ying, None; M.F. Walker, None; D. Guyton, None; C. Quaia, None; L.M. Optican, None; R.J. Tamargo, None; D.S. Zee, None
References
- 1.Scott AB. Extraocular muscles and head tilting, electromyographic measurement of activity of individual muscles. Arch Ophthalmol. 1967;78:397–399. doi: 10.1001/archopht.1967.00980030399024. [DOI] [PubMed] [Google Scholar]
- 2.Shan X, Tian J, Ying HS, et al. Acute superior oblique palsy in monkeys: I. changes in static eye alignment. Invest Ophthalmol Vis Sci. 2007;48:2602–2611. doi: 10.1167/iovs.06-1316. [DOI] [PubMed] [Google Scholar]
- 3.Shan X, Ying HS, Tian J, et al. Acute superior oblique palsy in monkeys: II. changes in dynamic properties during vertical saccades. Invest Ophthalmol Vis Sci. 2007;48:2612–2620. doi: 10.1167/iovs.06-1318. [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Shan X, Zee DS, et al. Acute superior oblique palsy in monkeys: III. relationship to Listing’s Law. Invest Ophthalmol Vis Sci. 2007;48:2621–2625. doi: 10.1167/iovs.06-1319. [DOI] [PubMed] [Google Scholar]
- 5.Glantz SA. Primer of Biostatistics. 4. New York: McGraw-Hill; 1997. pp. 238–245. [Google Scholar]
- 6.Metz HS. Saccadic velocity measurements in strabismus. Trans Am Ophthalmol Soc. 1983;81:630–692. [PMC free article] [PubMed] [Google Scholar]
- 7.Bucci MP, Kapoula Z, Yang Q, Roussat B, Bremond-Gignac D. Binocular coordination of saccades in children with strabismus before and after surgery. Invest Ophthalmol Vis Sci. 2002;43:1040–1047. [PubMed] [Google Scholar]
- 8.Weber KP, Landau K, Palla A, Haslwanter T, Straumann D. Ocular rotation axes during dynamic Bielschowsky head-tilt testing in unilateral trochlear nerve palsy. Invest Ophthalmol Vis Sci. 2004;45:455–465. doi: 10.1167/iovs.02-1223. [DOI] [PubMed] [Google Scholar]
- 9.Kommerell G, Klein U. Adaptive changes of the otolith-ocular reflex after injury to the trochlea. Neuro-ophthalmology. 1986;6:101–107. [Google Scholar]