Abstract
Background
Research in animal models has demonstrated that elevated levels of glucocorticoids can inflict damage within the hippocampus. In adult humans, elevated cortisol levels have been associated with reduced hippocampal volumes; however, normative data in children are not available. The objective of this study was to examine possible associations of serum cortisol levels with hippocampal volumes and morphology in healthy children.
Methods
Morning serum cortisol levels and hippocampus magnetic resonance imaging were measured in 17 healthy children (8 girls, 9 boys) between 7 and 12 years of age.
Results
Cortisol levels were not associated with total hippocampal volumes; however, with an analysis of surface morphology, significant associations were found for regionally specific portions of the hippocampus. Positive associations were detected for the anterior segment of the hippocampus and inverse associations along the lateral aspects of the hippocampus.
Conclusions
Associations of cortisol levels with regionally specific variations in hippocampal morphology were detected during early development in healthy preadolescent children.
Keywords: Cortisol, hippocampus, structural alteration, children, stress, brain development
The hypothalamic–pituitary–adrenal (HPA) axis regulates the secretion of glucocorticoids including cortisol. Chronic stress produces a prolonged activation of the HPA axis and sustained elevations in glucocorticoid levels (Johnson et al 1992). Excess glucocorticoids have damaging effects in the nervous system and can lead to adverse structural and functional changes in the brain (Sapolsky 2000). Chronically elevated glucocorticoid levels can inflict neuronal damage within the hippocampus, apparently through stimulation of glucocorticoid receptors in this region (Young and Vazquez 1996). More specifically, studies in experimental animals have demonstrated that glucocorticoids can decrease the length and branching of dendrites, inhibit neurogenesis, increase vulnerability to neurotoxicity, and induce cell death in the hippocampus (McEwen 1999; Sapolsky 2000).
These preclinical findings have led to studies assessing the influence of elevated cortisol on the morphology of the human hippocampus. Healthy elderly subjects whose cortisol levels increased over a period of 5 years had reduced hippocampal volumes, with the magnitude of reduction correlating with the increase in cortisol (Lupien et al 1998). Much of the research on the association of cortisol with hippocampus morphology has focused on patients with depression or other syndromes associated with hypercortisolism. Although results have not been entirely consistent (Vythilingam et al 2004), patients with major depression seem to have elevated cortisol levels and smaller hippocampal volumes compared with healthy control subjects (Bremner et al 2000; Campbell et al 2004; Sheline et al 1996). Still debated, however, is how the duration of the illness and hypercortisolism relates to hippocampal atrophy. Hippocampal volumes were not smaller in adult patients experiencing a first episode of depression, but volumes were reduced in patients who had multiple past episodes (MacQueen et al 2003). In contrast, Frodl et al (2002) found that hippocampal volumes of adult patients with major depression were reduced during the first episode compared with volumes in healthy control subjects, and a reduction in hippocampal volume was already detectable in children and adolescents with early onset depression (MacMaster and Kusumakar 2004; MacMillan et al 2003). The most direct association of cortisol level and hippocampal size with mood disorder has been demonstrated in two cohorts of patients with asthma and rheumatic diseases (Brown et al 2004). Non-psychiatric patients with asthma and rheumatic disease who received chronic corticosteroid treatment had a smaller hippocampus and more severe symptoms of depression than patients with the same medical conditions who had not received steroid therapy (Brown et al 2004).
In contrast to this large body of research in adults, little is known about the association of glucocorticoids with hippocampus size in children. It has been proposed that glucocorticoids could affect the development of the hippocampus (Cicchetti and Rogosch 2001; Goodyer et al 2001) and that such neural alterations could either increase the risk of developing mood and anxiety disorders later in life or make a child more vulnerable to subsequent stress (Bremner and Vermetten 2001; Heim and Nemeroff 2001; Penza et al 2003). The aim of the present study was to provide normative data on the relationship between cortisol and hippocampus size in healthy preadolescent children. We hypothesized that cortisol levels would correlate inversely with hippocampal volumes. Hippocampus surface morphology has previously been shown to be more sensitive than volumetric measures for detecting differences (Posener et al 2003). We therefore also used surface morphometry to determine associations between cortisol levels and shape of the hippocampus surface.
Methods and Materials
Seventeen children between 7 and 12 years of age were included in the present study (8 girls, 9 boys). They were recruited from random mailings and follow-up telephone calls to randomly selected families on a list purchased from a telemarketing company. Subjects did not meet the DSM-IV criteria for a current Axis I disorder and did not have a lifetime diagnosis of tic disorder, obsessive-compulsive disorder, attention-deficit/hyperactivity disorder, psychotic illness, or a history of developmental delay, seizure, head trauma with loss of consciousness, any current or prior substance abuse, or IQ below 80. Written informed consent was obtained for all participants. The human investigation committees at Yale School of Medicine, New Haven, Connecticut, and New York State Psychiatric Institute, New York, New York, approved the study.
Blood was obtained in the morning between 9:30 and 10:30 am from the antecubital vein. Serum or plasma was prepared by centrifugation and the samples stored at −70°C to −80°C until analyzed with a radioimmunometric assay kit (Diagnostics Products, Los Angeles, California). Cortisol levels of high- and low-concentration quality assessment samples were determined with within-day coefficients of variation (CVs) of 2.1% and 5.2%, respectively, and with day-to-day CVs of 4.5% and 7.2%, respectively.
The study included only subjects who were scanned within 3 months of blood collection to ensure temporal association between glucocorticoid levels and hippocampal size. The high-resolution magnetic resonance imaging scans were obtained with a single 1.5-T scanner (GE Signa, Milwaukee, Wisconsin). Head positioning was standardized with canthomeatal landmarks. Brain scans were acquired with a sagittal three-dimensional volume spoiled gradient echo sequence (repetition time, 24 msec; echo time, 5 msec; flip angle, 45°; frequency encoding superior/inferior; no wrap; 256 × 192 matrix; field of view, 30 cm; two excitations, slice thickness, 1.2 mm; and 124 contiguous slices encoded for sagittal slice reconstruction; voxel dimensions, 1.17 × 1.17 × 1.2 mm).
Region Definition
Morphometric analyses were performed on Sun Ultra 10 workstations with ANALYZE 7.5 software (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minnesota). The hippocampus was manually traced while blind to participant characteristics and hemisphere (images were randomly flipped in the transverse plane). Large-scale variations in image intensity were removed, and images were reformatted to standardize for head flexion, rotation, and tilt before region definition. Methods for definition of the hippocampus in this orientation were performed as described previously (Kates et al 1997). Intrarater and interrater reliabilities of the morphometric measurements were .94 and .91.
Statistical Analyses of Conventional Volumetric Measures
To control for the generalized scaling effects within the brain, whole brain volume was calculated for use as a covariate in the statistical analysis. This measure included gray and white matter, ventricular cerebrospinal fluid, and cerebrospinal fluid spaces within the brain. We tested our a priori hypothesis that basal cortisol levels would correlate inversely with hippocampal volumes with multiple linear regressions in which hippocampus volume was the dependent variable and cortisol level was the independent variable. To assess their influence on hippocampus volume, age and gender were used as covariates and whole brain volume was included to control for scaling effects.
Analysis of Surface Morphology
We next assessed whether focal variations within the surface of the hippocampus were associated with cortisol. This analysis used multivariable linear regression to correlate cortisol levels with deviations in the contours of the surfaces of the hippocampi from a canonical reference hippocampus, while covarying statistically for age and gender.
The distance of each point on the surface of the hippocampus of each subject from the corresponding point on the hippocampus of a reference subject was computed as follows (see Bansal et al 2005):
The entire cerebrum of each subject was co-registered with the cerebrum of the reference subject with a similarity transformation, in which the parameters of transformation (three translations, three rotations, and global scaling) were estimated under the constraint that the mutual information (Viola and Wells 1995) in gray scale values across the two brains was maximized. Thus, in this step, subject brains were transformed into the general coordinate space of the reference brain.
These estimated transformation parameters were used to transform the manually delineated hippocampus from each subject into this general coordinate space. Note that the global scaling parameter in the registration process for the entire cerebrum in Step 1 has been applied to each hippocampus, thereby accounting for scaling differences in this structure and obviating any need to covary for overall brain size in these analyses.
The transformed hippocampus of each subject was then individually and rigidly co-registered to the corresponding (left- or right-sided) hippocampus of the reference brain to further refine and improve the rigid body registration of these structures.
The principles of fluid dynamics, which minimize the differences in the gray scale values across two sets of images (Bansal et al 2005), were then invoked to impose a high-dimensional, nonlinear (and nonrigid) warping of the hippocampus from each subject to the corresponding hippocampus of the reference brain. A warped subject hippocampus will be exactly the same size and shape as the reference hippocampus, permitting identification of precisely corresponding points on the surfaces of the subject and reference hippocampi.
The warped hippocampi were unwarped into the refined coordinate space identified in Step 3 by simply reversing the high-dimensional, nonlinear warping performed to identify point correspondences in Step 4 but bringing along with the surface the labels that identified corresponding points on the surfaces of the subject and the reference hippocampi.
Finally, we calculated the signed Euclidean distances between the corresponding points of the hippocampi in the brains of our subjects and the corresponding hippocampus of the reference brain. Distances were either positive (outward deviations) or negative (inward deviations). To control for the effects of covariates (age, gender) on surface morphology, we performed a multivariate, linear-regression analysis (Rosner 1995) at each point on the reference surface:
where di was the set of signed Euclidean distances. We computed the correlation between the distances and cortisol levels, and we evaluated the p-value of this correlation with a Student's t test. Color-encoded p-values were then displayed across the entire surface of the reference structure.
Note that detecting, localizing, and interpreting the statistically significant differences between groups of subjects in these surface analyses could conceivably depend on the choice of subject to be designated as the reference. Therefore, in the steps outlined above to determine point correspondences between subject and reference hippocampi, we first selected as a reference a subject who demographically was as representative as possible of the children being studied. The brains for all remaining subjects were co-registered to this preliminary reference. The point correspondences on the surfaces of their hippocampi were determined, and we computed distances between the corresponding points. Then the brain for which all points across surface of its subcortical structure were closest, in terms of least squares, to the average of the computed distances was selected as the final reference brain. The registration process, the determination of point correspondences, and the calculation of distances across surfaces were then repeated for all subjects in the sample relative to this final reference brain.
Results
Serum cortisol levels and volumes of the hippocampus varied between children, but in this sample neither variable correlated significantly with age (r = 23, p < .4; r = 28, p < .3, respectively; Figure 1). Regression analyses detected no significant association between cortisol levels and total hippocampal volumes (β = − .24, p < .4; Figure 2). Cortisol levels were also not associated significantly with left (β = −.20, p < .4) or right (β = −.26, p < .3) hippocampal volumes. No significant gender influences were found.
Figure 1.
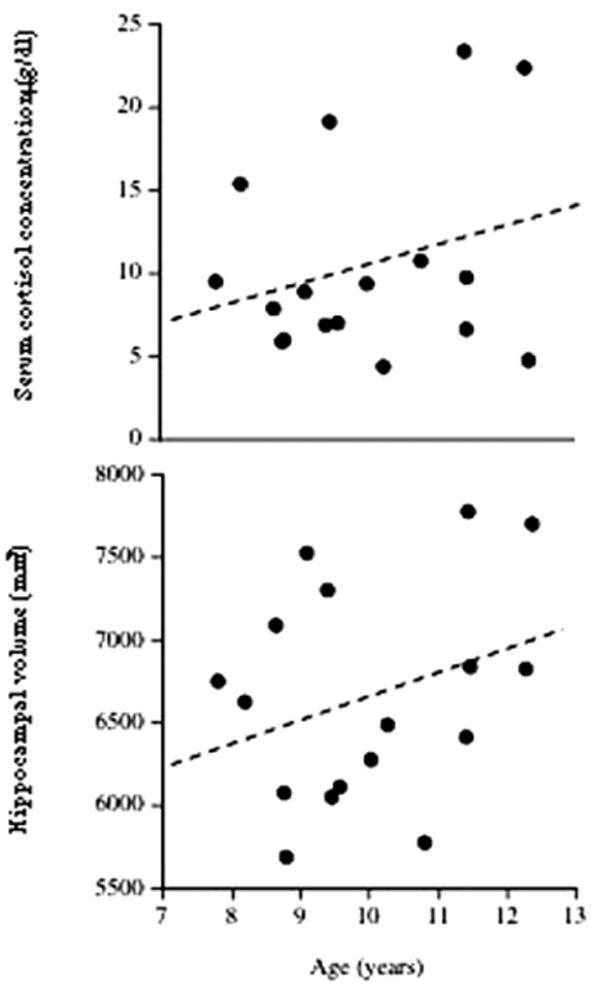
Serum cortisol concentrations (top) and hippocampal volumes (bottom) in healthy children. Levels and volumes did not correlate significantly with age [r = .23, p > .4, and r = 28, p > .3, respectively].
Figure 2.
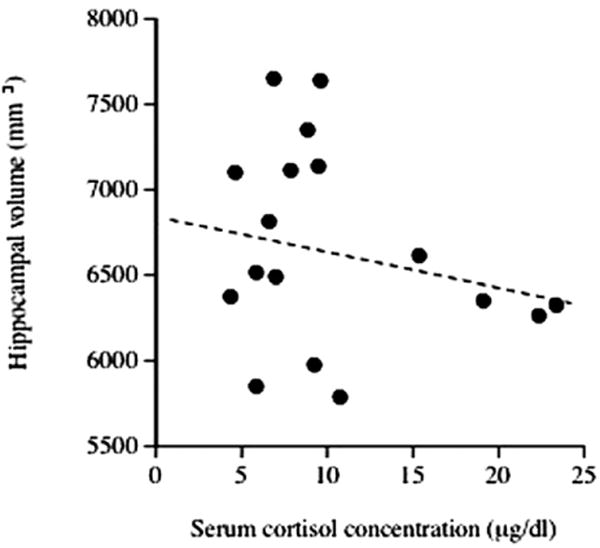
Serum cortisol concentrations and hippocampal volumes in healthy children, adjusted for the covariates age, gender, and whole brain volume. Cortisol levels were not associated significantly with hippocampal volumes (β = −.24, p > .4).
Analyses of surface morphometry, however, detected significant associations between cortisol levels and regions within the hippocampus, mainly for the right hippocampus. Significance levels are depicted in Figure 3 with a color scale. Positive associations indicate outward deformations, and inverse associations indicate inward deformations with increasing cortisol levels. Positive associations were found focally for the anterior segment of the hippocampus (Figure 3, in red), corresponding to the CA3 and dentate gyrus subfields. Inverse associations were found along the lateral aspects of the anterior, medial, and posterior segments of the hippocampus and were most pronounced in the medial segment (Figure 3, in purple), corresponding particularly to the CA1 subfield. No significant influences of gender or age were detected.
Figure 3.
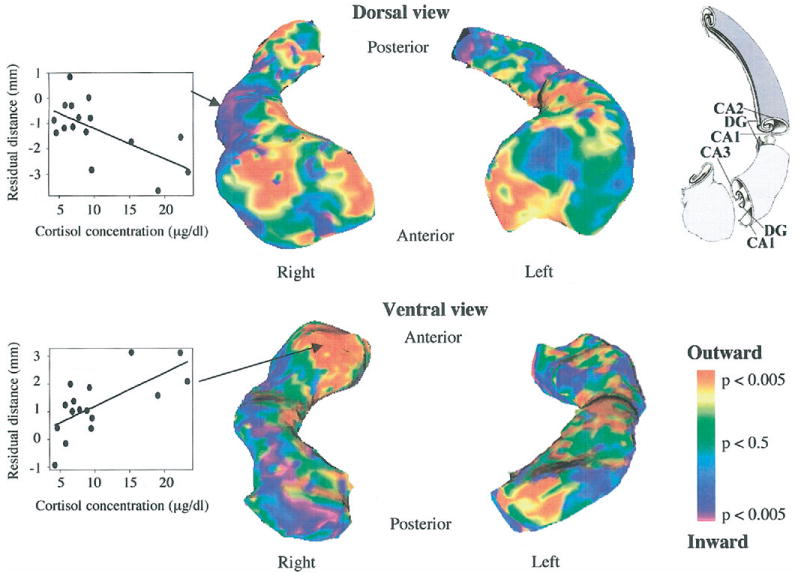
Statistical map showing focal associations between serum cortisol levels and structural alterations of the hippocampus, visualized as significant levels for outward and inward deformations. Scatterplots for two representative surface points are shown for the lateral aspect of the medial segment (inverse association, top) and for the anterior segment (positive association, bottom). The drawing of the internal structure of the hippocampus is adapted with permission from Duvernoy 2005. DG, dentate gyrus; CA1, CA2, CA3, fields of the cornu ammonis.
Discussion
Serum cortisol levels were not associated with overall volumes of the hippocampus in healthy children 7–12 years of age with conventional volumetric measurements; however, when using a more detailed analysis of surface morphology, significant regionally specific associations were detected, indicating that such associations can occur within more localized subregions of the hippocampus in healthy children.
Levels of morning serum cortisol and overall volumes of the hippocampus varied considerably between subjects, but they did not correlate with age. The few studies on serum cortisol in healthy children between the ages of 2 and 18 years reported similar large interindividual variability and also failed to find correlations with age (Guazzo et al 1996; Knutsson et al 1997). In contrast, studies that measured cortisol in saliva samples in children have found age-related increases (Lupien et al 2001), with the most prominent age associations occurring between years 10 and 12 (Lupien et al 2001) and during adolescence (Netherton et al 2004; Walker et al 2001). Given the high intercorrelation of serum and saliva cortisol levels reported in children (Goodyer et al 2001), the reason for this discrepancy in findings of age associations across studies remains unclear. Similar to our study, no age effect on hippocampal volume was detected in 13–18-year-old healthy children and adolescents (MacMaster and Kusumakar 2004). Another study that measured changes in hippocampal volume in children found a sharp increase until 2 years of age, followed by a slow increase until adolescence (Utsunomiya et al 1999). This age effect disappeared when age-related increases in overall volume of the temporal lobes were taken into account (Utsunomiya et al 1999). The absence of significant correlations of age with cortisol levels and overall hippocampal volumes in our study might be attributed to our use of a cohort of exclusively preadolescent children with a relatively narrow range of ages.
Convergent evidence suggests the presence of an inverse association of circulating cortisol levels and overall hippocampal volumes in both healthy and psychiatrically ill adults, although the underlying mechanisms responsible for these associations are debated (Lee et al 2002; Lupien et al 2005; Sapolsky 2000). Herein we report for the first time that basal cortisol levels are associated with local variation in surface morphology of the hippocampus in healthy children. Cortisol levels correlated inversely with focal volumes in lateral aspects of the anterior, medial, and posterior segments of the hippocampus, corresponding to the CA1 subfield (Duvernoy 2005); however, contrary to adult findings and our prediction, cortisol levels correlated positively with local volumes in the anterior hippocampus, corresponding to the CA3 and dentate gyrus subfields (Duvernoy 2005). Glucocorticoid receptor distribution has been described in adult hippocampus, with the dentate gyrus expressing higher receptor messenger RNA levels than CA subfields (Webster et al 2002). But associations of cortisol levels or receptor distribution with surface morphology have not been reported in adults, and so we do not know whether this pattern of correlations in children is similar to patterns that might be discerned in adults.
Findings from animal studies have been used to explain the reduction of hippocampus volumes in humans. Elevated glucocorticoid levels reduce neurogenesis and cell survival in the dentate gyrus and remodel CA3 pyramidal cells by decreasing the length and branching of apical dendrites (McEwen 2000; Sapolsky 2000). These results from preclinical studies stand in contrast to our findings of positive correlations of surface morphology with cortisol levels in human children (i.e., greater protrusion over the CA3 and dentate gyrus subfields accompanying more cortisol). CA1 dendrites seem to be less vulnerable to glucocorticoids, and stress-induced remodeling in this subfield has been described only occasionally (Sousa et al 2000), in modest agreement with our findings in human children. The cellular glucocorticoid-related processes that contribute to differential changes in volumes of the human hippocampus remain to be identified.
Normal childhood development is characterized by extensive maturation of the forebrain, including changes in the numbers of synapses, gray matter density, and myelination (Gogtay et al 2004; Huttenlocher and Dabholkar 1997; Paus et al 1999; Sowell et al 2003). These maturational and plastic features of brain development might help to compensate for the adverse effects of stress hormones on neural architecture. Severe early life trauma, for example, is associated with hypercortisolism (Cicchetti and Rogosch 2001; De Bellis et al 1999; Delahanty et al 2004), and yet children with posttraumatic stress disorder after maltreatment do not seem to have smaller hippocampi (Carrion et al 2001; De Bellis et al 2002). Alternatively, developmental changes could render the growing hippocampus more vulnerable to glucocorticoid-induced injury (Bremner and Vermetten 2001; Cicchetti and Rogosch 2001; Goodyer et al 2001), as evidenced by findings from imaging studies that adult women who were sexually abused as children had a smaller hippocampus compared with women without a history of abuse (Stein et al 1997; Vythilingam et al 2002). Similarly, adolescents with bipolar disorder had a similar or greater reduction in hippocampus volumes compared with adults with this illness (Blumberg et al 2003). Whether the associations of cortisol with local deformations of the hippocampus in the children of the present study reflect compensatory processes, markers for early neurodevelopmental insult, or predispositions to future illness remains to be determined.
Our findings have several implications. First, surface morphometry might provide a more detailed assessment of associations between cortisol and local features of hippocampus morphology than do more conventional measures of overall volume. Previous studies that examined only overall volumes and failed to detect significant associations with glucocorticoid levels should be reconsidered. Second, the hippocampus is not a unitary structure. Anatomically, the hippocampus can be divided into at least three different segments: an anterior head, a medial body, and a posterior tail (Duvernoy 2005). These subdivisions process information through topographically organized parallel pathways (Witter et al 2000). Recently, these subdivisions have been proposed to underlie different functions of the hippocampus, particularly memory-related and emotional processes (Bannermann et al 2004). Supporting this view, functional imaging studies in healthy adults have demonstrated that the anterior and posterior hippocampus is differentially activated during episodic learning (Strange et al 1999; Zeineh et al 2003). Other studies have found associations of regionally specific increases in volume with memory performance, with the size of the posterior hippocampus correlating with spatial learning (Maguire et al 2000), and the size of the anterior portion correlating with verbal memory performance (Hackert et al 2002). The subdivisions of the hippocampus also seem to be differentially affected in psychiatric disorders, with the anterior but not the posterior hippocampus being smaller in schizophrenic patients compared with healthy adults (Lee et al 2004; Narr et al 2004; Pegues et al 2003), even though the overall volume of the hippocampus was normal (Pegues et al 2003). Whether the hippocampus of children can also be segregated into subdivisions that subserve differing cognitive functions is unknown.
In summary, cortisol levels were associated with regionally specific variations in the surface morphology of the hippocampus but did not seem to be associated with changes in overall volume of this structure in healthy preadolescent children. Our study has several limitations. Serum cortisol was collected at a single time point in the morning. Interindividual differences in healthy children, however, are sometimes only detectable when circadian profiles of cortisol levels are assessed (Gunnar and Donzella 2002; Knutsson et al 1997). Another limitation is the small sample size, and our conclusions should thus be considered preliminary. Further research is needed to replicate and characterize the relationship between cortisol level and surface topology of the hippocampus in children and adults and to determine the functional consequences of the variations observed.
Acknowledgments
This work was supported by a grant from the Ruane Foundation (to CW); National Institute of Mental Health grants MH01232, MH59139, MH068318, and K02-74677; grants from the National Alliance for Research in Schizophrenia and Affective Disorders, the Tourette Syndrome Association; and funding from the Thomas D. Klingenstein & Nancy D. Perlman Family Fund and the Suzanne Crosby Murphy Endowment at Columbia University (to RB, GA, HZ, JA, RW, BP).
References
- Bannermann DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, et al. Regional dissociations within the hippocampus - memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bansal R, Staib LH, Whiteman R, Wang YM, Peterson BS. ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage. 2005;24:150–162. doi: 10.1016/j.neuroimage.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: Behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Brown ES, Woolston DJ, Frol A, Bobadilla L, Khan DA, Hanczyc M, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol Psychiatry. 2004;55:538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. Developmental traumatology part I: Biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: A sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinol. 2004;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus. 3rd. Berlin: Springer; 2005. [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Br J Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: Relation to blood levels and the effects of age. J Clin Endocrinol Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinol. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MMB. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: A dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev. 1992;16:115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Brönnegård M, Stierna P, et al. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Lee JM, Kim SH, Jang DP, Ha TH, Kim JJ, Kim IY, et al. Deformable models with surface registration for hippocampal shape deformity analysis in schizophrenia. NeuroImage. 2004;22:831–840. doi: 10.1016/j.neuroimage.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinol. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Medicine. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, et al. Increased amygdala: Hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinol. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and escents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophr Res. 2003;60:105–115. doi: 10.1016/s0920-9964(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: Implications for the pathophysiology of depression and anxiety. Arch Women Ment Health. 2003;6:15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160:83–89. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. Belmont, California: Duxbury; 1995. [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atropy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RNA, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: Analysis by MR-based volumetry. Am J Neuroradiol. 1999;20:717–723. [PMC free article] [PubMed] [Google Scholar]
- Viola P, Wells WM. Alignment by maximization of mutual information. Fifth Int Conf Computer Vision. 1995:16–23. [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7:985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Vazquez D. Hypercortisolemnia, hippocampal glucocorticoid receptors, and fast feedback. Mol Psychiatry. 1996;1:149–159. [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]


