Abstract
Pregnancy during adolescence is associated with adverse birth outcomes, including preterm delivery and low birthweight. The nutrient availability to the fetus may be limited if the mother is still growing. This research aims to study the effects of pregnancy during adolescence in a nutritionally poor environment in rural Nepal. This study utilized data from a randomized controlled trial of micronutrient supplementation during pregnancy in south‐eastern Nepal. Women of parity 0 or 1 and of age ≤ 25 years who gave birth to a singleton liveborn infant who was measured within 72 h of delivery were included (n = 1393). There was no difference in the risk of low birthweight (OR = 0.96; 95% CI = 0.90–1.02) or small for gestational age (OR = 1.01; 95% CI = 0.94–1.08) per year of increasing maternal age among primiparae. Young maternal age did not affect the anthropometry or gestational age of the offspring of parity 1 women. Each year of increasing maternal age among primiparae was associated with increases in birth length (0.07 cm; 95% CI = −0.01 to 0.16), head (0.05 cm; 95% CI = 0.01–0.09) and chest circumference (0.07 cm; 95% CI = 0.01–0.12), but not weight (9.0 g; 95% CI = −2.1 to 21.8) of their offspring. Young maternal age was associated with an increased risk of preterm delivery among primiparae (OR = 2.07; 95% CI = 1.26–3.38) that occurred at an age cut‐off of ≤18 years relative to those 19–25 years. Thus, we conclude that young maternal age (≤18 years) increased the risk of preterm delivery, but not intrauterine growth retardation, for the first but not second liveborn infant.
Keywords: adolescent pregnancy, parity, preterm delivery, low birthweight, intrauterine growth retardation, Nepal
Introduction
Worldwide, approximately 25% of women have their first child before the age of 20 years, with even higher rates in developing countries (Delisle et al. 2005). At a time when the mother may still be growing, there may be an additional strain to meet the nutrient demands of both the mother and the fetus. In developed countries, adolescents are more than twice as likely as older women to have adverse pregnancy outcomes, such as low birthweight (LBW) and preterm delivery, and have a nearly three‐fold greater neonatal mortality rate (Lenders et al. 2000). Maternal growth during pregnancy may be a primary factor leading to the increased risk of these adverse outcomes (Frisancho et al. 1985; Scholl & Hediger 1993; Hediger et al. 1997a), but few studies have been conducted in regions with high rates of malnutrition. Nearly 45% of achieved adult weight and 15% of adult height are gained during adolescence (Spear 2002). In areas in which malnutrition is prevalent, puberty is delayed (Rogol et al. 2000) and may be prolonged (Scholl et al. 1993), potentially overlapping with the age at first pregnancy.
The degree to which many of these adverse pregnancy outcomes are due to the biological effects of a young maternal age and maternal growth, or to socio‐economic and lifestyle factors associated with adolescence, has been debated. Adolescents are more likely to be socio‐economically disadvantaged, have poorer education, have fewer social supports and are less likely to seek out prenatal care (Worthington‐Roberts & Rees 1996). However, a number of studies have found that young maternal age is indeed associated with adverse pregnancy outcomes, even after controlling for these factors (Fraser et al. 1995; Lee et al. 1998; Smith & Pell 2001). Age and parity are also highly correlated, and it is generally agreed that primiparity is associated with greater risk of LBW than multiparity (Kramer 1987).
LBW is an important risk factor in neonatal and infant mortality, as well as for health, growth and developmental problems through childhood. LBW is due to two factors: intrauterine growth retardation (IUGR) and preterm delivery. In developing countries, IUGR is the primary cause of LBW, whereas in developed countries, preterm delivery is a more important cause (Kramer 1987). Maternal malnutrition, either through poor pre‐pregnancy nutritional status or through inadequate weight gain during pregnancy, is an important cause of IUGR in developing countries (Kramer 1987).
Early marriage, early motherhood and chronic malnutrition are common in Nepal. More than two‐thirds of rural women marry by the age of 20 years, and only 54% of women report having ever used contraception (Choe et al. 2005). More than 15% of women are stunted (height < 145 cm), and more than 25% are thin [body mass index (BMI) < 18.5 kg m−2] (Ministry of Health [Nepal], New ERA, and ORC Macro 2002).
The aims of this study were: (1) to determine the association between young maternal age and the risk of LBW, small for gestational age (SGA) and preterm delivery in both parity 0 and parity 1 women, and (2) to determine the maternal age at which these adverse risks are no longer evident, thus determining the age beyond which the risk of adverse infant outcomes is reduced in a nutritionally deprived environment.
Materials and methods
This study utilized data from the Nepal Nutritional Intervention Project – Sarlahi, carried out in the rural Sarlahi District of Nepal from 1999 to 2001, that consisted of a cluster‐randomized controlled trial of micronutrient supplementation provided to pregnant women from early pregnancy through 3 months postpartum. Sarlahi is a poor district within the Terai region of Nepal, dependent upon a rural subsistence economy. The main results have been described previously (2003a, 2003b). In this trial, 426 sectors of 30 village development communities were assigned daily vitamin A alone (control) or with folic acid, iron–folic acid, folic acid–iron–zinc, or a multiple micronutrient supplement containing the former plus an additional 11 vitamins and minerals. The overall nutritional status of women in this population is poor. The women were stunted (with an average height of 150.5 cm) and thin (with an average BMI of 19.3 kg m−2), and also suffer from multiple concurrent micronutrient deficiencies in early pregnancy (Jiang et al. 2005).
To enrol pregnant women early in pregnancy, female project workers visited all married women of reproductive age. Those who were currently pregnant, breastfeeding a baby <9 months old, menopausal, sterilized or widowed were excluded. Thereafter, women were visited every 5 weeks and were asked about menses since the last visit. If the woman said that she had not menstruated since the last visit, she was offered a urine‐based pregnancy test (human chorionic gonadotrophin; Clue, Orchid Biomedical Systems, Goa, India). If the woman was found to be pregnant, she was invited to participate in the study and, upon consent, was provided supplements and visited weekly. Pregnant women were interviewed at baseline, during the third trimester, at birth, and at 6 weeks postpartum.
Women were interviewed at the time of enrolment to ascertain age, pregnancy history, date of last menstrual period, alcohol and tobacco use, diet, socio‐economic status, ethnic group (Pahadi or Madeshi), caste, educational level and morbidity history. Maternal age was ascertained by questionnaire. If there was uncertainty, local and national event calendars were used to estimate the year of birth and the data were checked for digit preference. Height, weight and mid‐upper arm circumference (MUAC) were also measured at baseline, and weight and MUAC were repeated at the third‐trimester and 6‐week postpartum interviews. Pregnant women reporting to be ≤25 years old and parity 0 (no previous live birth) or parity 1 (one previous live birth) were included in this analysis.
Most women delivered their babies at home, assisted by a traditional birth attendant, relative or neighbour. Once a birth was reported, an anthropometrist visited the home and recorded infant weight using a digital scale (Seca 727, Hamburg, Germany), recumbent length using a length board (Shorr Infant Measuring Board, Shorr Productions, Rhode Island, USA), and head and chest circumference using an insertion tape (Ross Laboratories, Columbus, OH, USA). All measurements, except weight, were recorded in triplicate and the median was used for analysis. Only liveborn singleton infants who were measured within 72 h after birth were included in this analysis.
Gestational age was calculated from the reported first date of the last menstrual period, obtained at the baseline interview, and then checked against the week of the positive pregnancy test and prospectively collected histories of menstruation. If the date of the last menstrual period was not known, the midpoint between the previous reported menstrual period and the date of the positive pregnancy test was used. Preterm was defined as delivery before 37 weeks of gestation. No clinical parameters were used to determine gestational age. SGA infants were defined as those whose weight was below the 10th percentile of the gestational age‐ and sex‐specific US reference for fetal growth (Alexander et al. 1996). LBW was defined as <2.5 kg. Ponderal index (kg/m3) was calculated by using birthweight and length measurements.
The primary outcome of the original trial was birthweight, and it was observed that the treatment arms had a differential effect on birthweight. The iron plus folic acid and the multiple micronutrient groups reduced the incidence of LBW by 16% (95% CI = 0.72–0.99) and 14% (95% CI = 0.72–0.99), respectively (Christian et al. 2003a). Maternal age, parity, ethnicity and other characteristics potentially associated with birthweight were found to be comparable across treatment groups. There also appeared to be no difference in the relationship between maternal age and birthweight within the different treatment arms, and thus all treatment groups were pooled for this analysis. To determine the association between maternal age and the risk of adverse outcomes, we first examined the linear associations between age and the continuous variables of birthweight and length, head and chest circumference, ponderal index and gestational age. Scatter plots with lowess curves, which employ a locally weighted regression smoothing method, were used to examine the shapes of the distributions. Crude and adjusted estimates were calculated using linear regression. Logistic regression was used to calculate crude and adjusted odds ratios for risk of LBW, SGA or preterm delivery by maternal age. P‐values and confidence intervals were calculated using robust variance estimation, accounting for the potential clustering of covariates within sectors. The interaction between age and parity was examined by including an interaction term in the regression models and testing its significance. When the interaction terms were found to be significant, stratified models are presented to show the different relationships between parity 0 and 1. Potential confounders were included in the models if they were significantly (P < 0.05) associated with both maternal age and either birthweight or gestational age in bivariate analyses. Regression models were checked by examining plots of leverage versus the squared residual. Sensitivity analyses were conducted by removing points with high leverage or residuals and comparing the new model with the original.
To determine if there was a threshold age beyond which the risk of adverse outcomes substantially decreased among parity 0 women, a logistic regression model was fit with preterm delivery as a function of six categories of maternal age. Confounders were included as either continuous, as in the case of maternal height and BMI in early pregnancy, or binary for smoking, literacy and ethnicity. The regression equation from this model was used to estimate the probability of preterm delivery for each of the six separate age categories using the regression coefficients and the means of the confounders for all parity 0 women in the dataset. In the case of the binary variables, the ‘mean’ was the proportion of 1’s for that variable. For example, literacy was coded as 0 if illiterate and 1 if literate, so the ‘mean’ in the equation was the proportion of all the women who were literate. For the continuous variables, the mean was the actual mean for all age groups of women. These estimates were then plotted to examine the differences in incidence of preterm delivery by maternal age group. Data were analysed using stata version 8 (StataCorp LP, College Station, TX, USA).
The study was approved by the National Health Research Council of the Ministry of Health of Nepal and the Committee for Human Research at the Johns Hopkins Bloomberg School of Public Health. Informed consent was obtained from the participants.
Results
There were 1805 mother–infant pairs who met the selection criteria. Of these participants, we were able to examine 1359 (75%) infants within 72 h of delivery, of whom 759 and 600 were born to parity 0 and 1 women, respectively (Fig. 1).
Figure 1.
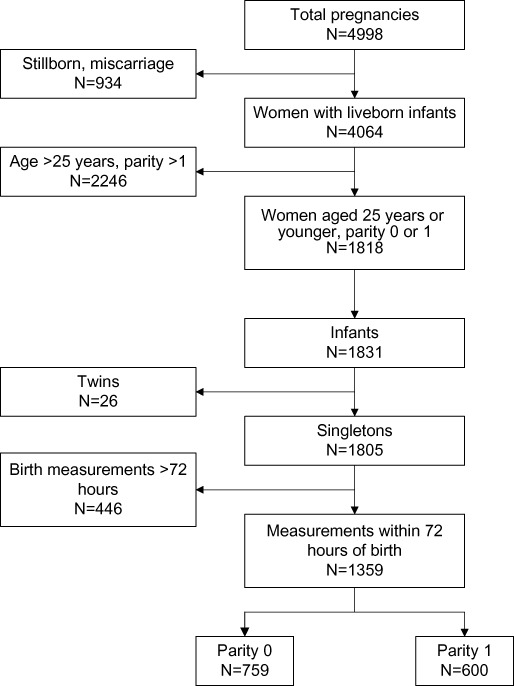
Overview of selection of mothers and infants included in the study.
Maternal age ranged from 12 to 25 years, with a mean of 18.8 years (SD = 2.6) (Table 1). Approximately 23.5% of the women smoked at some time during their pregnancy. Only 33% of women were literate and their households owned an average of <1 hectare of land. The mean BMI in early pregnancy was 19.7 kg/m2 (SD = 2.0). The mean birthweight was 2573 g (SD = 417), and 43.5% of infants were born LBW (<2500 g). The mean gestational age at birth was 38.8 weeks (SD = 2.9). About 19% were preterm and 63% were classified as SGA. A total of 66 infants (4.9%) were classified as both preterm and SGA. Maternal age, ethnicity, caste, literacy, height, weight, BMI, and MUAC and several infant outcomes differed by parity (Table 1). The mean age of parity 0 and 1 women differed by about 2 years (17.9 versus 20.0 years; P < 0.001). Infants born to parity 1 mothers were on average 89 g heavier than those born to parity 0 mothers (P < 0.001). There was no difference in gestational age by parity (P = 0.14).
Table 1.
Characteristics of mothers and infants by maternal parity*
| Characteristics | Mothers | |||
|---|---|---|---|---|
| All mothers (n = 1359) | Parity 0 (n = 759) | Parity 1 (n = 600) | P‐value † | |
| Age (years) | 18.8 (2.6) | 17.9 (2.4) | 20.1 (2.3) | <0.001 |
| Supplement group (%) | ||||
| Control | 19.9 | 20.6 | 19.0 | 0.164 |
| Folic acid | 19.0 | 20.4 | 17.2 | |
| Folic acid–iron | 20.5 | 18.6 | 23.0 | |
| Folic acid–iron–zinc | 20.5 | 19.6 | 21.7 | |
| Multiple micronutrient | 20.1 | 20.8 | 19.2 | |
| Ethnicity (%) | ||||
| Pahadi | 39.6 | 45.9 | 31.7 | <0.001 |
| Madeshi | 60.4 | 54.2 | 68.3 | |
| Caste (%) | ||||
| Brahmin | 9.9 | 12.0 | 7.3 | 0.001 |
| Chhetri | 11.0 | 12.7 | 8.8 | |
| Vaiysha | 63.1 | 60.2 | 66.7 | |
| Shudra | 11.1 | 11.3 | 10.8 | |
| Non‐Hindu | 5.0 | 3.8 | 6.3 | |
| Smoking (%) | 23.5 | 22.8 | 24.3 | 0.537 |
| Literacy (%) | 33.0 | 39.5 | 24.8 | <0.001 |
| Land ownership (hectares) | 0.9 (1.6) | 1.0 (1.6) | 0.8 (1.6) | 0.058 |
| Height (cm) | 150.7 (5.5) | 150.9 (5.4) | 150.5 (5.7) | 0.158 |
| Weight (kg) | 44.6 (5.5) | 45.6 (5.4) | 43.4 (5.4) | <0.001 |
| Body mass index (BMI; kg m−2) | 19.6 (2.0) | 20.0 (2.0) | 19.2 (1.9) | <0.001 |
| Mid‐upper arm circumference (cm) | 22.2 (1.8) | 22.4 (1.8) | 21.8 (1.8) | <0.001 |
| Thin (% with BMI < 18.5) | 27.9 | 20.8 | 36.8 | <0.001 |
| Infants | ||||
|---|---|---|---|---|
| All infants | Parity 0 | Parity 1 | P‐value † | |
| Sex (% male) | 52.0 | 52.0 | 51.8 | 0.938 |
| Age at the time of birth measurements (days) | 0.10 (0.37) | 0.13 (0.43) | 0.06 (0.29) | 0.002 |
| Birthweight (g) | 2573 (417) | 2534 (407) | 2623 (426) | <0.001 |
| Birth length (cm) | 47.11 (2.38) | 46.97 (2.46) | 47.28 (2.27) | 0.019 |
| Head circumference (cm) | 32.51 (1.47) | 32.42 (1.46) | 32.62 (1.47) | 0.011 |
| Chest circumference (cm) | 30.31 (1.90) | 30.14 (1.85) | 30.52 (1.94) | <0.001 |
| Ponderal index (kg m−3) | 24.42 (2.40) | 24.26 (2.30) | 24.62 (2.49) | 0.010 |
| Low birthweight (<2500 g) (%) | 43.2 | 47.0 | 38.3 | 0.001 |
| Small for gestational age (%) | 62.8 | 66.4 | 58.3 | 0.001 |
| Preterm (<37 weeks) (%) | 18.2 | 18.7 | 17.5 | 0.562 |
Estimates represent mean (SD) for continuous variables and percentage for categorical variables.
P‐values were calculated using linear regression for continuous variables and logistic regression for categorical variables using a robust estimation of the variance.
Infant size and preterm delivery
There was a modest trend of increasing birthweight and gestational age, with increasing maternal age among parity 0 but not parity 1 women (2, 3). The unadjusted age–parity interaction term was significant in both models (birthweight model: P = 0.006; gestational age model: P = 0.030). In an unadjusted analysis, there was a positive association between maternal age and birthweight, length, head circumference and chest circumference for parity 0 but not parity 1 women (Table 2). There was no association between age and ponderal index for either parity group. After adjusting for ethnicity, maternal height and BMI in early pregnancy, literacy and smoking status, there was no longer a significant association between maternal age and birthweight for parity 0 women. However, even after adjustment for these potential confounders, a positive association between maternal age and birth length (0.07 cm), head circumference (0.05 cm) and chest circumference (0.07 cm) remained (Table 2).
Figure 2.
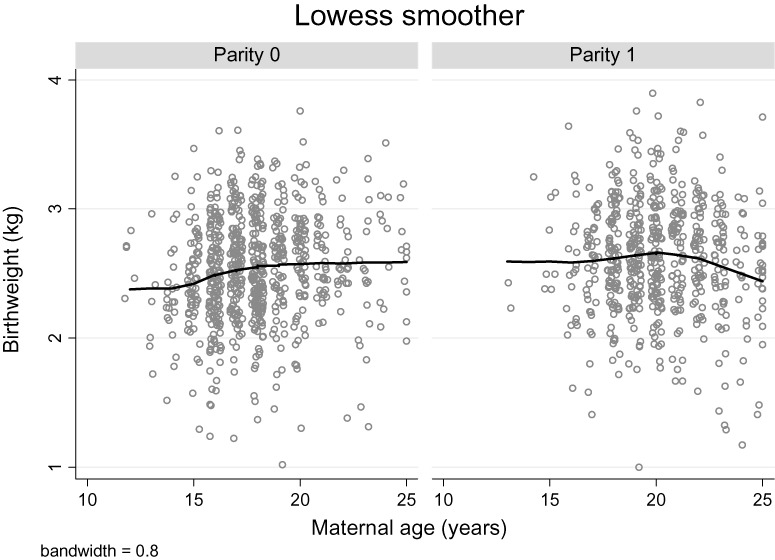
Lowess smoothed curves and scatter plots of infant birthweight for women aged 12–25 years by parity.
Figure 3.
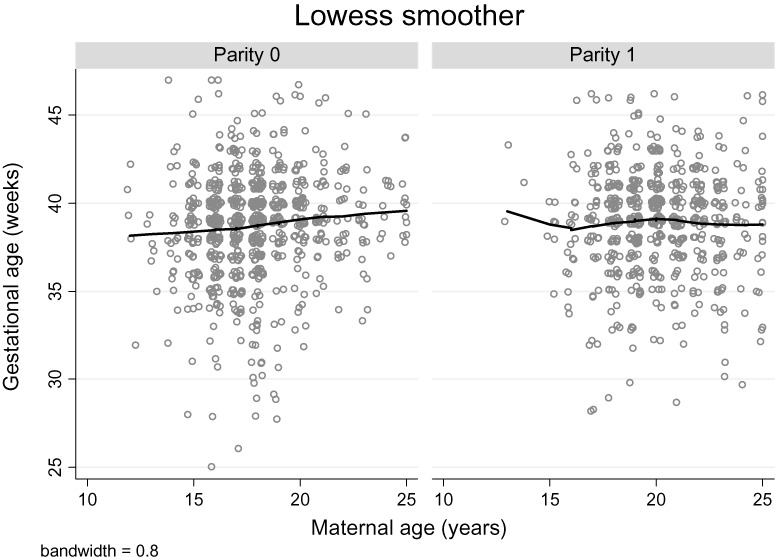
Lowess smoothed curves and scatter plots of infant gestational age at delivery for women aged 12–25 years by parity.
Table 2.
Unadjusted and adjusted differences* (95% CI) in infant anthropometry per year of increasing maternal age
| Parity 0 | Parity 1 | |||
|---|---|---|---|---|
| Unadjusted | Adjusted † | Unadjusted | Adjusted † | |
| Birthweight (g) | 19.8 (7.6, 31.9) | 9.0 (−2.1, 21.8) | −8.7 (−23.4, 6.0) | −10.3 (−25.2, 4.5) |
| Birth length (cm) | 0.13 (0.05, 0.20) | 0.07 (−0.01, 0.16) | −0.02 (−0.10, 0.06) | −0.04 (−0.12, 0.04) |
| Head circumference (cm) | 0.07 (0.03, 0.12) | 0.05 (0.01, 0.09) | −0.03 (−0.08, 0.02) | −0.04 (−0.09, 0.02) |
| Chest circumference (cm) | 0.11 (0.05, 0.16) | 0.07 (0.01, 0.12) | −0.04 (−0.11, 0.02) | −0.05 (−0.12, 0.03) |
| Ponderal index (kg m−3) | −0.02 (−0.09, 0.05) | −0.04 (−0.11, 0.03) | −0.05 (−0.14, 0.03) | −0.05 (−0.14, 0.03) |
Differences (95% CI) calculated using linear regression.
† Adjusted estimates control for maternal BMI and height in the first trimester, ethnicity, smoking and literacy. Confidence intervals were calculated using a robust estimation of the variance. BMI, body mass index.
After adjusting for potential confounders, younger maternal age among parity 0 women was associated with an increase in the odds of preterm delivery (Table 3). Women aged 12–18 years had nearly three‐fold greater odds of preterm delivery than those aged 21 years or over (OR = 2.90; 95% CI = 1.34–6.26) after controlling for potential confounders. Women who were 19–20 years old had a 71% increase in the odds of preterm delivery that was not statistically significant (OR = 1.71; 95% CI = 0.73–4.00). This was not the case for SGA, which showed no trend with each year of increasing maternal age (adjusted OR = 1.01; 95% CI = 0.94–1.08). The association between maternal age and LBW was no longer statistically significant after adjustment for confounding (OR = 0.96; 95% CI = 0.90–1.20).
Table 3.
Unadjusted and adjusted odds ratios* (95% CI) of preterm delivery among parity 0 women
| Age group (years) | N | Preterm n (%) | Crude OR (95% CI) | Adjusted † OR (95% CI) |
|---|---|---|---|---|
| 12–14 | 33 | 8 (24.4) | 2.80 (0.72–10.93) | 2.34 (0.57–9.53) |
| 15–16 | 196 | 46 (23.5) | 2.68 (0.89–8.13) | 2.74 (0.94–8.03) |
| 17–18 | 288 | 61 (21.2) | 2.35 (0.77–7.17) | 2.56 (0.87–7.50) |
| 19–20 | 144 | 19 (13.2) | 1.33 (0.41–4.27) | 1.54 (0.49–4.81) |
| 21–22 | 59 | 4 (6.8) | 0.64 (0.15–2.72) | 0.82 (0.20–3.44) |
| 23–25 | 39 | 4 (10.3) | Reference | Reference |
Odds ratios calculated using logistic regression with confidence intervals calculated using a robust estimation of the variance.
† Adjusted for ethnicity, BMI, height, literacy and smoking. BMI, body mass index.
Characterizing an age threshold for improved first pregnancy outcomes
There was no difference between the proportion of women in their first pregnancy who delivered preterm between the ages of 12 and 18 years (Fig. 4). Between the ages of 19 and 20 years, there was a notable decline in the proportion of preterm births. There was more than a twofold greater risk of preterm delivery for women who had their first live birth at 12–18 years of age, as compared with women who were 19 years or older in an unadjusted (OR = 2.28; 95% CI = 1.45–3.57) and adjusted (OR = 2.07; 95% CI = 1.26–3.38) analysis. Using younger age cut‐offs yielded dampened risk estimates. The adjusted odds ratio of preterm delivery comparing mothers <16 years versus 16+ years was 1.23 (95% CI = 0.70–2.16); for mothers <17 years versus 17+ years, it was 1.39 (95% CI = 0.90–2.16); and for mothers <18 years versus 18+ years, it was 1.69 (95% CI = 1.12–2.58).
Figure 4.
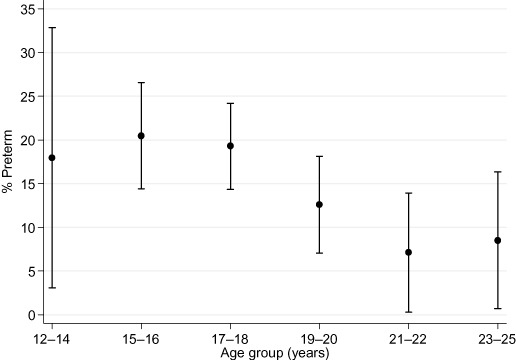
Per cent of parity 0 women stratified by age group that delivered preterm, adjusted for ethnicity, smoking, literacy, and maternal height and BMI in early pregnancy. All covariates set to their mean. Confidence intervals were calculated using a robust estimation of the variance. BMI, body mass index.
Ethnicity
Upon close examination of the other covariates, it became clear that ethnic group was the strongest confounder to the relationship between maternal age and infant outcomes. In our sample, Madeshis were more likely to experience their first pregnancy at a younger age. The mean age of first pregnancy was 17.6 and 18.3 years for Madeshis and Pahadis, respectively. Madeshi women were also likely to deliver lower‐birthweight babies in their first pregnancy that weighed an average of 243 g less (95% CI = −302 to −184) than babies of Pahadi women. Madeshi women in this sample were on average 1.4 cm shorter (95% CI = 0.8–2.0), 2.7 kg lighter (95% CI = 2.1–3.2), and had a BMI that was 0.8 kg m−2 less (95% CI = 0.6–1.0) than Pahadi women. However, there were no significant differences in haemoglobin concentration (0.04 g dL−1; 95% CI = −0.42 to 0.35) or serum retinol (−0.05 µmol L−1; 95% CI = −0.03 to 0.13). Even after controlling for maternal age differences, BMI, height, literacy and smoking, the Madeshi mothers in their first pregnancy delivered infants who were an average of 180 g lighter (95% CI = −256 to 103) than Pahadi mothers. They also had a greater risk of LBW (adjusted OR = 1.98; 95% CI = 1.33–2.96) and preterm delivery (adjusted OR = 1.68; 95% CI = 1.07–2.63).
Discussion
Among primiparous women, young maternal age was associated with an increased risk of preterm delivery. This relationship was not observed for parity 1 women. Nor was there a relationship between maternal age and the risk of LBW or SGA among either parity 0 or 1 women. The greatest risk of preterm delivery was in primiparous women aged ≤18 years, who had a more than a twofold greater risk of preterm delivery than women 19 years or older.
Age–parity interaction
Our results of an age–parity interaction are comparable to those of da Silva et al. (2003). They noted an association between maternal age and the risk of preterm delivery for parity 0 women, but not for multiparous women (da Silva et al. 2003). Similarly, in the Camden, New Jersey, study, there was an effect of age on the duration of gestation for primiparae, but not for multiparae (Scholl et al. 1992). This contrasts with the findings from a population‐based cohort study in Scotland in which the authors noted no increased risk of adverse pregnancy outcomes for adolescents in their first birth, but an approximately 2.5 greater risk of preterm delivery and stillbirth for those in their second birth (Smith & Pell 2001).
There are a number of differences between the parity 0 and 1 women in our study area. Infants born to parity 0 women were weighed about 1.7 h later after birth than those born to parity 1 women. Although it is common practice in this region of Nepal for women to return to their parental house to deliver their first baby, the restriction of weights to those taken within 72 h likely minimized the parity difference in time since birth when anthropometry was obtained. The parity 1 women in our study also had a lower weight and BMI, and were an average of 2 years older than parity 0 women, in spite of restricting the upper age limit to 25 years. Although the parity 1 women were older, after adjusting for age, the risk of an adverse outcome was not apparent in these women, compared with parity 0 ones.
Preterm delivery
The association between maternal age and the risk of preterm delivery among primiparae has been controversial, with some studies finding an association even after controlling for potential socio‐economic and reproductive factors (Fraser et al. 1995; Hediger et al. 1997b; Olausson et al. 1997; Lee et al. 1998; da Silva et al. 2003), while other studies have failed to find an association after controlling for confounding (Ekwo & Moawad 2000; Beydoun et al. 2004). In a study from Utah, young women, 13–17 years old, had a higher risk of preterm delivery (RR = 1.5; 95% CI = 1.4–1.6) than older women aged 20–24 years (Fraser et al. 1995). Similarly, young adolescents (<16 years) in Camden, New Jersey, had a twofold increased risk of preterm delivery with preterm labour (adjusted OR = 2.08; 95% CI = 1.08–4.00) as compared with women aged 18–29 years (Hediger et al. 1997b). In a large multinational cross‐sectional study from Latin America which included all parity groups, young women aged ≤19 years had more than a 20% increased risk of preterm delivery than women aged 20–24 years (adjusted OR = 1.22; 95% CI = 1.19–1.25). This pattern remained consistent when only primiparous women were examined separately (Conde‐Agudelo et al. 2005). Among parity 0 women in north‐eastern Brazil, young maternal age (<18 years) was associated with a 77% increased risk of preterm delivery (adjusted OR = 1.77; 95% CI = 1.02–3.08) compared with women aged 25–29 years (da Silva et al. 2003).
Intrauterine growth restriction
We were unable to observe an association between maternal age and LBW or SGA for either parity group, which would indicate that there was no greater risk of IUGR with young maternal age. This is similar to a Swedish study in which the risk of LBW or SGA for adolescents aged ≤17 years or 18–19 years was no greater than for women aged 20–24 years (Olausson et al. 1997). It does, however, contrast with the observations of a number of other studies that found a 25–60% increased risk of LBW or SGA for adolescents (Frisancho et al. 1985; Scholl & Hediger 1993; Fraser et al. 1995; Lee et al. 1998; Conde‐Agudelo et al. 2005). In Camden, growing adolescents (age 12–19 years) delivered infants who were an average of 153 g lighter than adolescents who had completed their growth, and 93 g less than mature women aged 18–29 years, after controlling for confounders (Scholl & Hediger 1993). Likewise, among a Peruvian cohort, young maternal age (≤16 years) was associated with lower birthweight even after controlling for maternal nutritional status, height and weight gain. These results were partially explained by the growth status of the mothers, whereby there was a greater competition for nutrients to meet the mother’s growth needs with those of her fetus (Frisancho et al. 1985). Our study was unable to discern the growth status of the mothers; yet, it is likely that in this malnourished population, menarche and puberty were delayed. Among a similar population in Bangladesh, it was found that age at menarche was delayed by about 3 years, and growth in weight and stature continued until the age of 20 years (Riley et al. 1989). A study from India found that girls of a lower socio‐economic status experienced a 3‐year delay in age at menarche compared with those of a higher socio‐economic status (Rao et al. 1998). Because birthweight for gestational age appeared not to be compromised among young women in our study, it is possible that they may have stopped growing in order to direct more nutrients to their fetus in this severely maternally deprived nutritional environment. Unfortunately, we are unable to discern the growth status of the mothers to test this hypothesis.
Characterizing an age threshold for improved first pregnancy outcomes
Many studies have found that the risk of preterm delivery is greater for adolescents than for older women, but the age cut‐points vary across studies, making it difficult to identify an age below which pregnancy would not be recommended on the basis of adverse infant outcomes. Biologic immaturity has also been implicated as a possible cause of the increased risk of preterm labour. In the Camden study, women who were young (<16 years) and classified as having a low gynaecologic age (<2 years since menarche) had 2.64 greater odds of preterm labour and delivery than women aged 18–29 years. Women who were young but whose gynaecologic age was >2 years were at no increased risk (Hediger et al. 1997b). We observed a similar magnitude of risk with an age cut‐off of ≤18 rather than <16 years. In our data, first pregnancy between 12 and 18 years of age resulted in similarly high proportions of preterm deliveries, while the risk for those aged 19 years or over was substantially lower. In a resource‐poor setting like Nepal, the risks to the health and survival of preterm infants are substantial. Allal and colleagues have utilized an evolutionary model to estimate the optimal age for a first pregnancy by balancing trade‐offs between an early initiation of reproduction with the need for maternal growth. The former would yield a greater number of offspring, whereas the latter would lead to an improvement in the odds of offspring survival (Allal et al. 2004). They estimated that the age at which reproductive success was achieved without a cost to the mother was 18 years, similar to our findings in Nepal.
Strengths and weaknesses
This study has the strength of utilizing data from a large population‐based study and therefore benefits from a large sample size. However, a potential weakness is that the women participating in this study had the benefit of receiving a daily Recommended Dietary Allowance (RDA) of micronutrient supplements throughout pregnancy, and thus may have had a better micronutrient status than women not participating in the study. All of the women received vitamin A at a minimum, and some received various combinations of other micronutrients. Even though the mothers may have been better off nutritionally, the supplementation did not interact with the association between age and birthweight or preterm delivery. Therefore, we believe that the study population is generalizable to women <25 years of parity 0 or 1 in South Asia with a calorie‐restricted diet and mild to moderate micronutrient deficiencies.
Conclusion
In many developing countries, the age at marriage and age at first pregnancy is younger than in developed countries. In places with chronic malnutrition, the period of pubertal growth may overlap considerably with the timing of first pregnancy. More research is needed in these regions of the world to better understand the risks to both the mother and the infant. As evident in our data, by delaying their age at first pregnancy to 19 years or older, young women may substantially reduce their risk of preterm delivery, an important cause of infant morbidity and mortality. In settings such as Nepal in which access to health care is limited, the risks to the health of a preterm infant are great. Efforts to reduce adolescent pregnancy have the potential to substantially improve infant health and survival.
Acknowledgements
The authors would like to thank the many other people that contributed to this study, especially those from the Nepal study team, including Tirtha Raj Shakya, Rabindra Shrestha, Uma Shankar Sah, Arun Bhetwal, Gokarna Subedi, Dhrub Khadka, Jaibar Shrestha as well as the area coordinators, team leader interviewers, drivers and administrative support staff. The authors would also like to thank Lee Wu for her help in the data management and preparation of datasets, Elizabeth K. Pradhan and Gwendolyn Clements for computer programming and data management, and Ravi Ram, Seema Rai and Sunita Pant for data cleaning and supervision. This project was supported by: the US Agency for International Development (USAID) under Cooperative Agreement HRN‐A‐00‐97‐00015‐00 between the Office of Health and Nutrition, USAID, Washington, DC, and the Center for Human Nutrition (CHN), Department of International Health; the Bill and Melinda Gates Foundation, Seattle, WA; the National Institutes for Health grant R03 HD049406‐01; UNICEF Country Office, Kathmandu, Nepal; and the Site and Life Research Institute, Johns Hopkins University, Bloomberg School of Public Health, Baltimore. The study was a joint undertaking between the CHN and the National Society for the Prevention of Blindness, Kathmandu, Nepal. Supplements were provided by Roche, Brazil, and were manufactured by NutriCorp International, CE Jamieson & Company Ltd, Windsor, Canada. The authors do not have any conflicts of interest associated with this work.
References
- Alexander G.R., Himes J.H., Kaufman R.B., Mor J. & Kogan M. (1996) A United States national reference for fetal growth. Obstetrics and Gynecology 87, 163–168. [DOI] [PubMed] [Google Scholar]
- Allal N., Sear R., Prentice A.M. & Mace R. (2004) An evolutionary model of stature, age at first birth and reproductive success in Gambian women. Proceedings of the Royal Society of London, Series B, Biological Sciences 271, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun H., Itani M., Tamim H., Aaraj A., Khogali M. & Yunis K. (2004) Impact of maternal age on preterm delivery and low birthweight: a hospital‐based collaborative study of nulliparous Lebanese women in Greater Beirut. Journal of Perinatology 24, 228–235. [DOI] [PubMed] [Google Scholar]
- Choe M.K., Thapa S. & Mishra V. (2005) Early marriage and early motherhood in Nepal. Journal of Biosocial Science 37, 143–162. [DOI] [PubMed] [Google Scholar]
- Christian P., Khatry S.K., Katz J., Pradhan E.K., LeClerq S.C. & Shrestha S.R. et al. (2003a) Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. British Medical Journal 326, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P., West K.P., Khatry S.K., LeClerq S.C., Pradhan E.K., Katz J. et al. (2003b) Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomized trial in Nepal. American Journal of Clinical Nutrition 78, 1194–1202. [DOI] [PubMed] [Google Scholar]
- Conde‐Agudelo A., Belizan J.M. & Lammers C. (2005) Maternal–perinatal morbidity and mortality associated with adolescent pregnancy in Latin America: cross‐sectional study. American Journal of Obstetrics and Gynecology 192, 342–349. [DOI] [PubMed] [Google Scholar]
- Delisle H., Chandra‐Mouli V. & De Benoist B. (2005) Should Adolescents Be Specifically Targeted for Nutrition in Developing Countries? To Address Which Problems, and How? World Health Organization: Geneva. [Google Scholar]
- Ekwo E.E. & Moawad A. (2000) Maternal age and preterm births in a black population. Paediatric and Perinatal Epidemiology 14, 145–151. [DOI] [PubMed] [Google Scholar]
- Fraser A.M., Brockert J.E. & Ward R.H. (1995) Association of young maternal age with adverse reproductive outcomes. New England Journal of Medicine 332, 1113–1117. [DOI] [PubMed] [Google Scholar]
- Frisancho A.R., Matos J., Leonard W.R. & Yaroch L.A. (1985) Developmental and nutritional determinants of pregnancy outcome among teenagers. American Journal of Physical Anthropology 66, 247–261. [DOI] [PubMed] [Google Scholar]
- Hediger M.L., Scholl T.O. & Schall J.I. (1997a) Implications of the Camden study of adolescent pregnancy: interactions among maternal growth, nutritional status, and body composition. Annals New York Academy Sciences 817, 281–291. [DOI] [PubMed] [Google Scholar]
- Hediger M.L., Scholl T.O., Schall J.I. & Krueger P.M. (1997b) Young maternal age and preterm labor. Annals of Epidemiology 7, 400–406. [DOI] [PubMed] [Google Scholar]
- Jiang T., Christian P., Khatry S.K., Wu L. & West K.P. Jr (2005) Micronutrient deficiencies in early pregnancy are common, concurrent and vary by season among rural Nepali pregnant women. Journal of Nutrition 135, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Kramer M.S. (1987) Determinants of low birth weight: methodological assessment and meta‐analysis. Bulletin of the World Health Organization 65, 663–737. [PMC free article] [PubMed] [Google Scholar]
- Lee M.C., Suhng L.A., Lu T.H. & Chou M.C. (1998) Association of parental characteristics with adverse outcomes of adolescent pregnancy. Family Practice 15, 336–342. [DOI] [PubMed] [Google Scholar]
- Lenders C.M., McElrath T.F. & Scholl T.O. (2000) Nutrition in adolescent pregnancy. Current Opinion in Pediatrics 12, 291–296. [DOI] [PubMed] [Google Scholar]
- Ministry of Health [Nepal], New ERA, and ORC Macro (2002) Nepal Demographic and Health Survey 2001. Family Health Division, Ministry of Health, New ERA, and ORC Macro: Calverton, MD. [Google Scholar]
- Olausson P.M., Cnattingius S. & Goldenberg R.L. (1997) Determinants of poor pregnancy outcomes among teenagers in Sweden. Obstetrics and Gynecology 89, 451–457. [DOI] [PubMed] [Google Scholar]
- Rao S., Joshi S. & Kanade A. (1998) Height velocity, body fat and menarcheal age of Indian girls. Indian Pediatrics 35, 619–628. [PubMed] [Google Scholar]
- Riley A.P., Huffman S.L. & Chowdhury A.K. (1989) Age at menarche and postmenarcheal growth in rural Bangladeshi females. Annals of Human Biology 16, 347–359. [DOI] [PubMed] [Google Scholar]
- Rogol A.D., Clark P.A. & Roemmich J.N. (2000) Growth and pubertal development in children and adolescents: effects of diet and physical activity. American Journal of Clinical Nutrition 72, 521S–528S. [DOI] [PubMed] [Google Scholar]
- Scholl T.O. & Hediger M.L. (1993) A review of the epidemiology of nutrition and adolescent pregnancy: maternal growth during pregnancy and its effect on the fetus. Journal of the American College of Nutrition 12, 101–107. [DOI] [PubMed] [Google Scholar]
- Scholl T.O., Hediger M.L., Huang J., Johnson F.E., Smith W. & Ances I.G. (1992) Young maternal age and parity. Influences on pregnancy outcome. Annals of Epidemiology 2, 565–575. [DOI] [PubMed] [Google Scholar]
- Scholl T.O., Hediger M.L., Cronk C.E. & Schall J.I. (1993) Maternal growth during pregnancy and lactation. Hormone Research 39(Suppl. 3), 59–67. [DOI] [PubMed] [Google Scholar]
- Da Silva A.A., Simoes V.M., Barbieri M.A., Bettiol H., Lamy‐Filho F. & Coimbra L.C. (2003) Young maternal age and preterm birth. Paediatric and Perinatal Epidemiology 17, 332–339. [DOI] [PubMed] [Google Scholar]
- Smith G.C. & Pell J.P. (2001) Teenage pregnancy and risk of adverse perinatal outcomes associated with first and second births: population based retrospective cohort study. British Medical Journal 323, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B.A. (2002) Adolescent growth and development. Journal of the American Dietetics Association 102, S23–S29. [DOI] [PubMed] [Google Scholar]
- Worthington‐Roberts B.S. & Rees J.M. (1996) The pregnant adolescent: special concerns In: Nutrition in Pregnancy and Lactation (eds Worthington‐Roberts BS. & Williams SR.), 6th edn, pp. 292–318. McGraw‐Hill, Boston, MA. [Google Scholar]


