Summary
Objectives
This study examined the risk factors of mortality related to pregnancy for the first year post partum in a cohort of 25,580 pregnancies.
Study design
Longitudinal cohort follow-up.
Methods
Details of socio-economic status, mid-upper arm circumference (MUAC), diet, illness, work, substance use and previous pregnancy history were collected during early to mid-gestation, and these women were followed for 1 year post partum. All-cause mortality rates per 100,000 pregnancies were calculated for deaths during pregnancy or up to 42 days post partum (early period) and 43−364 days post partum (late period). Odds ratios (OR) of mortality were estimated using five groups of risk factors: biological; morbidity; dietary; lifestyle; and socio-economic. Significant factors within each group were included in a single risk model for each time period.
Results
Early and late pregnancy-related mortality rates were 469 [95% confidence interval (CI) 385−553] and 254 (95% CI 192−316), respectively. Maternal age ≥35 years was associated with a three- to four-fold increase in mortality, whereas increasing parity conferred increasing protection. In the final model, a larger MUAC and consumption of dark green leaves were associated with decreased risk of death in the early period (OR 0.76, 95% CI 0.67−0.87 and 0.64, 95% CI 0.41−0.99, respectively). A larger MUAC was also associated with a lower risk of death in the late period. Diarrhoea/dysentery and pre-eclampsia were associated with increased risk of death in the early period (OR 2.78, 95% CI 1.40−5.51 and 2.95, 95% CI 1.48−5.90, respectively). Factors weakly associated (P<0.1) with mortality in both periods included night blindness, strenuous work activity and cigarette smoking. No socio-economic factors were significant in the models.
Conclusions
Maternal age, parity, MUAC, diet and illness in early to mid-gestation were associated with risk of death during pregnancy and the first year post partum in rural Nepal.
Keywords: Maternal mortality, Risk factors, Nutrition, Pregnancy, Nepal
Introduction
Maternal mortality is unacceptably high in many regions of the world. Based on an estimate by the World Health Organization in 1990, 585,000 women die each year, more than one each minute, due to pregnancy-related causes.1 The fifth millennium development goal is to reduce maternal mortality by three-quarters between 1990 and 2015, leaving less than a decade to achieve this goal. By 1995, it was estimated that 515,000 deaths were occurring annually, based on mathematical modelling, indicating a worldwide maternal mortality ratio of 397/100,000 live births at that time.2 However, because maternal death remains a rare event and its actual measurement requires population-based data, precise measurement of maternal mortality poses a major challenge in many developing countries.3 Prospective community-based data on large numbers of pregnancies are generally not available for the estimation of maternal mortality rates in low-income countries. Consequently, there have been few opportunities to assess determinants and antenatal risk factors of maternal mortality. Rather, ecological analyses have been used to identify population risk factors for maternal mortality. For example, in an analysis of national data from countries in sub-Saharan Africa, factors such as gross national product per capita, average life expectancy, health expenditures and birth attendance by skilled personnel were correlated with maternal mortality rates.4 Some studies have examined individual-level risks, such as data from Matlab District, Bangladesh that found a positive exponential relationship between maternal age and risk of maternal mortality; the highest maternal mortality was observed among women aged 40−44 years.5 Parity, however, shared a J-shaped relationship with the maternal mortality ratio, with primiparous women being at lowest risk.5 In Ethiopia, on the other hand, neither parity nor age was significantly associated with the risk of maternal death, although multivariate analysis found that antenatal care, occupation and income were significant risk factors.6 Using a case–control approach in Guinea–Bissau, the odds of not receiving prenatal care, home delivery, female genital mutilation, and birth attendance by either a family member or traditional birth attendant were two to four times higher among women who died compared with women who survived.7 Illiteracy and rural residency were marginally associated with increased risk of mortality. In Kalimantan, Indonesia, delays in decision making and poor quality of care at health facilities contributed to 77% and 60% of maternal deaths respectively, and economic constraints contributed to 37% of deaths.8 Vulnerability of retrospective data to bias, low statistical power and inadequate study design raise questions about the validity of some of these study findings.
Two large collaborative field trials conducted in rural Nepal9,10 offer new opportunities to identify antecedent maternal risk factors in pregnancy that may be associated with mortality related to pregnancy during the first year post partum. This study examined the importance of maternal nutritional status, dietary intake, prevalent illness, work activity, substance use during early to mid gestation, previous pregnancy history and household socio-economic status as mortality risk factors during pregnancy and up to 42 days post partum (early period) and 43−364 days post partum (late period). A prospective cohort of 25,580 pregnancies was derived from the two field trials.
Methods
The data used in the present analysis come from two community trials conducted in Sarlahi, a district in the southern agricultural plains of Nepal, near the border of India. The study area, comprising 270 administrative wards in 30 subdistricts called ‘village development communities’, has been the site of consecutive community micronutrient and health intervention trials in women of reproductive age, infants and children since 1989.
The first of the two trials, conducted between 1993 and 1997, enrolled 44,000 women of reproductive age into a placebo-controlled, cluster-randomized trial of low-dose vitamin A and beta-carotene supplementation.9 Women were visited in their home each week, dosed, and asked about menstruation and pregnancy status. In total, 20,654 incident pregnancies were identified during the study period. Women informed the female field staff of their pregnancies at a mean (standard deviation) of 20.7 (11.8) weeks of gestation. At the time of pregnancy declaration, an enrolment interview was conducted by trained staff to ask about reproductive history, last menstrual period, and frequencies of morbidity symptoms, cigarette smoking, alcohol consumption, strenuous work activities and dietary intake during the previous 7 days. Family socio-economic status was assessed, and mid-upper arm circumference (MUAC) was measured as an indicator of malnutrition. Similar interviews on risk factors were repeated approximately 3 months later (while still pregnant) and 3 months following pregnancy outcome, at which time questions were added regarding labour and delivery. In the original trial, some women contributed more than one pregnancy (n = 2866). Since survival is an independent outcome and death can only occur once, all pregnancies in the trial were included in the present analysis.
The second trial was conducted in the same study area from late 1999 to April 2001. It assessed the efficacy of antenatal micronutrient supplementation in improving birth weight and reducing infant mortality.10 In total, 4926 pregnant women were identified prospectively using a 5-weekly community surveillance system that confirmed pregnancy at the first report of amenorrhoea with a urine-based human chorionic gonadotrophin test. As enrolment was limited to 1 year, only one pregnancy per woman was enrolled in this trial. After obtaining consent, women received a coded daily supplement containing folic acid, folic acid + iron, folic acid + iron + zinc, a combination of 14 micronutrients or a control. All supplements contained a daily allowance of vitamin A, which has been shown to reduce pregnancy-related mortality by 40%.9 Upon enrolment, at ∼11 (±5) weeks of gestation, women were interviewed using the same questions as in the previous trial regarding their reproductive history and 7-day frequencies of morbidity symptoms, cigarette smoking, alcohol consumption, strenuous work activity and dietary intake. A similar module on socio-economic status was administered, and MUAC was measured. At 32 weeks of gestation, a second interview was scheduled. During a home visit shortly after birth, questions pertaining to labour and delivery were posed. In this study, women were only eligible to be enrolled for one pregnancy, which was used for this study of risk factors.
In both studies, maternal deaths were ascertained through a combination of weekly home visits, monthly surveillance and postpartum interviews. All reports of maternal death initiated ‘verbal autopsy’ interviews with family members of the deceased to document events and illnesses preceding the death, including during labour and delivery. These data were reviewed by two physicians who independently assigned primary and contributory causes of death. The results were jointly reviewed and agreement was reached.
The consent rate was very high (∼99%) in both studies. While adherence to supplementation was approximately 80−90%, all enumerated women in the community were followed for pregnancy status and vital outcomes.9,10 In the second trial, women who were not incident pregnancies at the outset were not eligible for enrolment. However, all incident pregnancies during the enrolment period were included in the trial. All women who consented were included in the intent-to-treat analysis in both trials, and these women are also included in the present analysis.
Both studies received ethical approval by the Johns Hopkins Bloomberg School of Public Health Committee on Human Research, Baltimore, USA and the national Nepal Health Research Council, Nepal. Verbal informed consent was obtained from all study participants.
Statistical analysis
Common risk factors assessed in the two studies in early to mid-gestation were initially examined in a univariate analysis to explore their association with the risk of pregnancy-related mortality for each of the two time periods. Subsequently, these variables were grouped into five risk categories as follows. Group 1 included biological variables such as maternal age, parity, MUAC and gestational age at enrolment. Gestational age was not associated with mortality and was excluded from the model. Maternal age was initially examined as a continuous variable to explore its relationship with pregnancy-related mortality. The risk of mortality was found to increase exponentially at ≥35 years of age. Thus, maternal age was treated as a dichotomous variable (<35 vs ≥35 years) in all the models. Parity was categorized as 0, 1−4 or 5+. Group 2 comprised composite and individual morbidity variables from both studies, which were constructed based on reported frequency in the week prior to the interview: urinary or reproductive tract infection (i.e. vaginal discharge or lower abdominal pain or painful urination); upper gastrointestinal tract infection (i.e. poor appetite or nausea or vomiting); lower gastrointestinal tract infection (i.e. mucus/blood in stool or loose, watery stool for ≥4 days); pre-eclampsia/eclampsia (i.e. swelling of hands or face, or convulsions); acute lower respiratory tract infection (i.e. productive cough plus either high fever or blood in sputum); and night blindness (difficulty seeing after dusk). Morbidity variables were categorized as non-occurrence vs occurrence on at least 1 day. Lifestyle exposures (Group 3) in the previous week included non-exposure vs reported number of days in which the subject smoked (any filter or rolled cigarettes) or consumed alcohol. Strenuous work was defined as the total number of days in the past week in which the woman performed seven activities, namely carrying firewood, carrying rocks/dirt, grinding grain, cutting and carrying fodder, carrying grain, carrying heavy products to market, and working in the fields. A total score for strenuous work days in the past week was calculated (range 0−49) and treated as a continuous variable. Group 4 comprised dietary variables, including the number of times in the previous week that women reported consuming (separately) any meat, fish, milk, dark green leafy vegetables or yellow fruits/vegetables vs non-consumption. Household socio-economic factors (Group 5) included maternal literacy, land ownership (any land owned by the family vs no land ownership), any radios owned by the household, and roof construction material (tin, tile or cement vs thatch or grass).
Pregnancy-related mortality rates were calculated by dividing the number of deaths by the number of pregnancies, and expressed per 100,000 pregnancies. The goal was to identify, separately, risk factors for deaths occurring during pregnancy and up to 42 days post partum (early period) and deaths occurring during the remainder of the first year post partum (late period).11,12 A multivariate logistic regression was fit for variables within each group of risk factors and for each time period. Factors that were statistically significant (P<0.05) from these group regressions were entered into a single multivariate model for each of the two time periods. Although maternal supplementation in the two trials was associated with a reduction in mortality in the early period, this variable was not a confounder and was excluded in the regression models. All analyses were conducted in SAS version 8.1 (SAS Inc., Cary, NC, USA).
Results
In total, 25,580 pregnancies were identified and followed, and 22,975 ended in a live birth (Table 1). Approximately 16% of women (n = 2823) who took part in the first trial also took part in the second trial. In total, 120 women died during the early period, yielding a pregnancy-related mortality rate of 469/100,000 (95% CI 385−553) pregnancies. An additional 65 women died during the late period, yielding a mortality rate of 254/100,000 pregnancies. Mortality rates in both periods were higher among women whose pregnancies ended in fetal loss, either miscarriage or stillbirth. Mortality was somewhat elevated in pregnancies ending in pre-term deliveries compared with term deliveries (P = 0.13). Mortality rates varied by supplement type; rates in the groups where supplements contained vitamin A or beta-carotene were lower in the early period, but not in the late period.
Table 1.
Pregnancy-related mortality rates (MR) per 100,000 pregnancies by time of death post partum, outcome, gestational age at birth and study supplement type in rural Nepal.
| n | Died during pregnancy or 0−42 days post partum |
Died 43−364 days post partum |
Total deaths <364 days |
||||
|---|---|---|---|---|---|---|---|
| n | MR | n | MR | n | MR | ||
| Total | 25,580 | 120 | 469 | 65 | 254 | 185 | 723 |
| Pregnancy outcomea | |||||||
| Live birth | 22,975 | 56 | 244* | 57 | 248 | 113 | 492* |
| Fetal loss | 2247 | 18 | 801 | 6 | 267 | 24 | 1068 |
| Gestationb | |||||||
| Preterm | 6001 | 15 | 250 | 16 | 267 | 31 | 517 |
| Term | 15,415 | 25 | 162 | 32 | 208 | 57 | 370 |
| Study supplementc | |||||||
| Placebo | 6723 | 45 | 669** | 20 | 297 | 65 | 967** |
| Vitamin A | 7189 | 35 | 487 | 20 | 278 | 55 | 765 |
| Beta-carotene | 6742 | 29 | 430 | 14 | 208 | 43 | 638 |
| Vitamin A+other micronutrients | 4926 | 11 | 223 | 11 | 223 | 22 | 447 |
Three hundred and twelve women had missing data on pregnancy outcome, of which two died during the 43−364 days post partum period. Forty-six women died during pregnancy and had death as their pregnancy outcome.
One thousand five hundred and fifty-nine women had missing data on gestational duration at birth, of which 16 died during pregnancy and up to 42 days post partum, and nine died 43−364 days post partum; preterm refers to live births with <37 weeks of gestation.
Placebo, vitamin A and beta-carotene supplement groups were allocated at random in the first trial,9 while vitamin A+other micronutrients represents the five supplement groups in the second trial.10
P<0.01 and
P<0.001 using Chi-squared test to compare mortality rates among strata for each pregnancy-related period.
The majority of maternal deaths that occurred during the early period were due to obstetric causes (53%), while most deaths occurring in the late period were attributed to infectious causes (60%) (Table 2). The majority of births were attended by family members, friends or neighbours (74%), while approximately 15% were attended by untrained traditional birth attendants (Table 3). More women who died than survived during the early period (15.8% vs 7.2%) were attended at parturition by a doctor (usually assumed to be a physician), presumably reflecting an attempt to seek emergency care for a critically ill woman.
Table 2.
Causes of maternal deaths related to pregnancy in rural Nepal.
| Cause of death | Died during pregnancy or 0−42 days post partum |
Died 43−364 days post partum |
||
|---|---|---|---|---|
| n | % | n | % | |
| Obstetrica | ||||
| Haemorrhage | 28 | 23.3 | 2 | 3.1 |
| Eclampsia | 13 | 10.8 | 0 | 0 |
| Puerperal sepsis | 10 | 8.3 | 0 | 0 |
| Other obstetric | 13 | 10.8 | 0 | 0 |
| Total | 64 | 53.2 | 2 | 3.1 |
| Infection (other) | ||||
| Gastrointestinal infection | 9 | 7.5 | 6 | 9.2 |
| Sepsis | 5 | 4.2 | 8 | 12.3 |
| Respiratory infection, tuberculosis | 8 | 6.7 | 17 | 26.2 |
| Otherb | 5 | 4.2 | 8 | 12.3 |
| Total | 27 | 22.6 | 39 | 60.0 |
| Injuriesc | 4 | 3.3 | 7 | 10.8 |
| Miscellaneous | ||||
| Chronic illnessd | 6 | 5.0 | 4 | 6.1 |
| Uncertain illnesse | 11 | 9.2 | 6 | 9.2 |
| Verbal autopsy not done | 8 | 6.7 | 7 | 10.8 |
| Total | 25 | 20.9 | 17 | 26.1 |
| Total | 120 | 100 | 65 | 100 |
Haemorrhage includes antepartum, postpartum, reactionary and unspecified haemorrhage, and retained placenta. Eclampsia includes unspecified and postpartum eclampsia. Other obstetric includes obstetric shock, postpartum shock and obstructed labour.
Other infections include hepatitis, tetanus, leshmaniasis, meningitis and typhoid.
Injuries include snake bite, burns, hanging and drowning.
Chronic illness includes anaemia, asthma, epilepsy, pulmonary embolism, blood cancer, breast cancer and hepatic failure.
Uncertain illness includes causes of death that remained undetermined by the physician review process.
Table 3.
Attendance at the time of labour and delivery in rural Nepal.
| Type of birth attendanta | Alive (n = 22,532) |
Died during pregnancy or 0−42 days post partum (n = 120) |
||
|---|---|---|---|---|
| nb | % | nb | % | |
| No one | 806 | 2.3 | 1 | 0.6 |
| Family member | 16,426 | 46.2 | 60 | 39.5 |
| Neighbour/friend | 9901 | 27.9 | 44 | 28.9 |
| Traditional birth attendant | 5308 | 14.9 | 20 | 13.2 |
| Community health volunteer | 283 | 0.8 | 2 | 1.3 |
| Auxiliary nurse midwife | 214 | 0.6 | 1 | 0.6 |
| Doctor | 2568 | 7.2 | 24 | 15.8 |
| Traditional healer | 49 | 0.1 | 0 | 0.0 |
| Total | 35,555 | 100.0 | 152 | 100.0 |
Multiple types of attendance at birth were recorded.
Five thousand four hundred and seventy-one women who were alive and 53 women who died had missing data on attendance at birth.
Among Group 1 risk factors, maternal age and parity were independently associated with maternal death during the first year post partum (Table 4, Fig. 1). The odds of death increased dramatically in women aged ≥35 years, whereas parity was negatively associated with risk of maternal death in both the early and late periods (Table 4). Relative to nulliparous women, higher parity women had a lower risk of death; this continued to decline with increasing parity. Mortality risk during pregnancy and the first year post partum was 25% lower (OR 0.75, 95% CI 0.67−0.83) with each centimetre increase in MUAC at the time of pregnancy enrolment (∼19 weeks of gestation) (Table 4). Among Group 2 risk factors, symptoms of diarrhoea/dysentery, pre-eclampsia/eclampsia and night blindness were associated with a nearly three-fold increased risk of death in the early period. Evidence of an association between midgestation illness and mortality was weaker for maternal deaths that occurred during the late periods, reflected by wider 95% CI. Among Group 3 risk factors, cigarette smoking in early to midgestation, but not alcohol consumption, was associated with a modest but sustained increase in mortality risk during the first year post partum. Strenuous work was protective, reflected by a 6% (95% CI 2−10) decrease in mortality risk for the first year post partum for each day of strenuous work activity during the reported week in early to midgestation. Among Group 4 factors, intake of dark green leafy vegetables was associated with a 33% lower risk of maternal death. Suggested protective associations with milk and meat intakes were not statistically significant. While all Group 5 variables were protective (OR 0.67−0.80), none were statistically significant (Table 4).
Table 4.
Maternal risk factors in early to mid-gestation and relative odds of dying during pregnancy through 364 days postpartum in rural Nepal.
| Characteristics | Alive (N = 25,395) |
Died during pregnancy or 0−42 days postpartum (n = 120) |
Died 43−364 days postpartum (n = 65) |
Total deaths < 365 days (n = 185) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| na | %b | na | %b | ORc (95% CI) | na | %b | ORc (95% CI) | na | %b | ORc (95% CI) | |
| Group 1: Biological | |||||||||||
| Age < 35 years | 22,946 | 94.2 | 85 | 89.5 | 1.00 | 51 | 89.5 | 1.00 | 136 | 89.5 | 1.00 |
| ≥ 35 years | 1413 | 5.8 | 10 | 10.5 | 4.61 (1.95−10.90) | 6 | 10.5 | 3.34 (1.09−10.28) | 16 | 10.5 | 4.03 (2.02−8.03) |
| Parity 0 | 6019 | 25 | 32 | 36 | 1.00 | 18 | 32 | 1.00 | 50 | 35 | 1.00 |
| 1−4 | 14,734 | 61 | 49 | 56 | 0.48 (0.30−0.78) | 32 | 57 | 0.58 (0.31−1.11) | 81 | 56 | 0.52 (0.35−0.76) |
| 5+ | 3554 | 14 | 7 | 8 | 0.11 (0.04−0.32) | 6 | 11 | 0.26 (0.08−0.87) | 13 | 9 | 0.15 (0.07−0.35) |
| MUAC, mean (SD) cm | 22,123 | 21.4 (1.74) | 81 | 20.7 (2.4) | 0.74 (0.64−0.84) | 48 | 20.7 (2.2) | 0.76 (0.63−0.91) | 129 | 20.7 (2.3) | 0.75 (0.67−0.83) |
| Group 2: Morbidity | |||||||||||
| UTI/RTI | 22,280 | 31 | 83 | 35 | 0.87 (0.54−1.42) | 49 | 37 | 1.35 (0.74−2.48) | 132 | 36 | 1.03 (0.70−1.51) |
| Poor appetite/nausea and vomiting | 22,282 | 26 | 83 | 37 | 1.49 (0.93−2.37) | 49 | 22 | 0.73 (0.37−1.47) | 132 | 32 | 1.17 (0.79−1.71) |
| Diarrhoea/dysentery | 22,281 | 4 | 83 | 12 | 2.76 (1.39−5.50) | 49 | 6 | 1.51 (0.46−4.95) | 132 | 10 | 2.30 (1.27−4.17) |
| Pre-eclampsia/eclampsia | 22,285 | 4 | 83 | 12 | 2.62 (1.29−5.31) | 49 | 2 | 0.42 (0.06−3.14) | 132 | 8 | 1.77 (0.92−3.39) |
| ALRI | 22,285 | 2 | 83 | 4 | 1.71 (0.52−5.56) | 49 | 4 | 2.77 (0.66−11.70) | 132 | 4 | 2.03 (0.81−5.09) |
| Night blindness | 22,270 | 2 | 83 | 6 | 2.63 (1.02−6.76) | 49 | 4 | 2.57 (0.61−10.82) | 132 | 5 | 2.61 (1.18−5.75) |
| Group 3: Lifestyle exposures (previous 7 days) | |||||||||||
| Cigarette smoking | 22,303 | 25 | 83 | 30 | 1.38 (0.84−2.26) | 49 | 35 | 1.72 (0.92−3.20) | 132 | 32 | 1.49 (1.01−2.20) |
| Alcohol | 22,292 | 8 | 83 | 8 | 1.00 (0.44−2.26) | 49 | 10 | 1.17 (0.44−3.11) | 132 | 9 | 1.06 (0.57−1.99) |
| Strenuous work, mean (SD) days | 22,301 | 4.1 (4.8) | 83 | 3.2 (4.0) | 0.95 (0.90−1.00) | 49 | 2.8 (5.2) | 0.92 (0.86−0.99) | 132 | 3.0 (4.5) | 0.94 (0.90−0.98) |
| Group 4: Diet (previous 7 days) | |||||||||||
| Meat | 22,292 | 33 | 83 | 29 | 0.85 (0.53−1.37) | 49 | 27 | 0.74 (0.39−1.39) | 132 | 28 | 0.81 (0.55−1.18) |
| Fish | 22,295 | 28 | 83 | 28 | 1.01 (0.62−1.63) | 49 | 39 | 1.69 (0.95−3.01) | 132 | 32 | 1.23 (0.85−1.78) |
| Milk | 22,295 | 62 | 83 | 52 | 0.67 (0.44−1.04) | 49 | 57 | 0.85 (0.48−1.50) | 132 | 54 | 0.73 (0.52−1.03) |
| Dark green leaves | 22,286 | 57 | 83 | 47 | 0.67 (0.44−1.04) | 49 | 47 | 0.67 (0.38−1.18) | 132 | 47 | 0.67 (0.48−0.95) |
| Yellow fruits/vegetables | 22,294 | 27 | 83 | 28 | 1.08 (0.66−1.74) | 49 | 27 | 1.02 (0.54−1.93) | 132 | 27 | 1.05 (0.72−1.55) |
| Group 5: Socio-economic status | |||||||||||
| Literacy | 23,041 | 16 | 80 | 13 | 1.00 (0.50−2.03) | 58 | 5 | 0.32 (0.10−1.04) | 138 | 9 | 0.67 (037−1.23) |
| Land owned | 22,975 | 76 | 85 | 67 | 0.74 (0.45−1.20) | 59 | 64 | 0.65 (0.37−1.15) | 144 | 66 | 0.70 (0.48−1.02) |
| Radio owned | 23,037 | 29 | 85 | 21 | 0.63 (0.35−1.14) | 59 | 25 | 1.07 (0.57−2.01) | 144 | 23 | 0.80 (0.52−1.23) |
| Roof material | 23,046 | 70 | 85 | 61 | 0.77 (0.48−1.25) | 59 | 63 | 0.85 (0.49−1.48) | 144 | 62 | 0.80 (0.56−1.16) |
OR, odds ratio; CI, confidence intervals; MUAC, mid upper arm circumference; UTI/RTI, urinary/reproductive tract infection; ALRI, acute lower respiratory tract infection.
UTI/RTI was defined as any symptom of vaginal discharge, lower abdominal pain or painful urination; pre-eclampsia was defined as swollen hands or face or non-epileptic convulsions; ALRI was defined as any symptom of productive cough with high fever or blood in sputum; strenuous work was defined as the number of days on which each of the seven strenuous activities was performed in the past 7 days; range 0−49; yellow fruits/vegetables included any intake of ripe pumpkin, mango or papaya in the past 7 days; roof material included tin, tile and cement compared with thatch and grass.
n do not add to the total N in the first row due to missing data for different variables.
All values are percentages unless otherwise indicated.
OR and 95% Cl calculated using multiple logistic regression models that included the risk factors within each group as independent variables.
Figure 1.
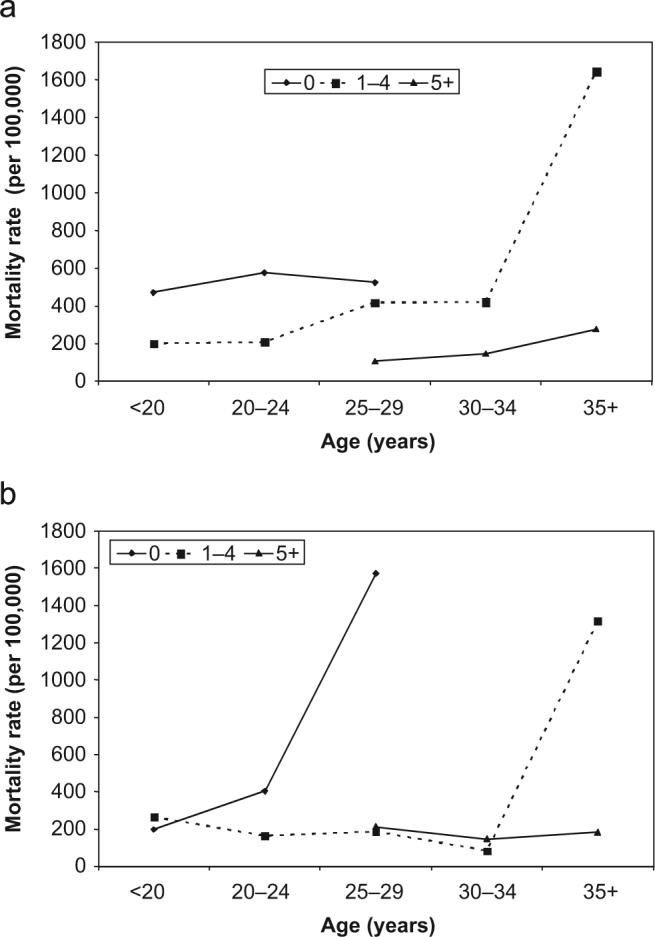
Maternal mortality by age and parity in rural Nepal: (a) mortality rate for deaths during pregnancy or 0−42 days post partum and (b) mortality rate for deaths 43−364 days post partum. Parity was classified as 0, 1−4 or 5+.
In the final multivariate models, biological factors such as maternal age and parity, and nutritional influences reflected by MUAC and dark green leafy vegetable intake remained associated with pregnancy-related mortality risk (Table 5). Mid-gestational morbidity symptoms of pre-eclampsia/eclampsia and diarrhoea/dysentery were associated with mortality risk during the early period. Night blindness, strenuous work activity and cigarette smoking were weakly associated with mortality risk in the final model.
Table 5.
Risk factors of mortality related to pregnancy in rural Nepal.
| Risk factors in early to mid-gestation | Died during pregnancy or 0−42 days post partum (n = 79) |
Died 43−364 days post partum (n = 47) |
||
|---|---|---|---|---|
| ORa | 95% Cl | OR1 | 95% Cl | |
| Age ≥35 years | 4.04 | 1.69−9.64 | 3.01 | 0.98−9.27 |
| Parity 1−4 | 0.49 | 0.30−0.80 | 0.58 | 0.30−1.12 |
| Parity 5+ | 0.10 | 0.03−0.31 | 0.24 | 0.07−0.83 |
| MUAC (cm) | 0.76 | 0.67−0.87 | 0.78 | 0.65−0.93 |
| History in previous week | ||||
| Diarrhoea/dysentery | 2.78 | 1.40−5.51 | - | - |
| Pre-eclampsia/eclampsiab | 2.95 | 1.48−5.90 | - | - |
| Night blindness | 2.35 | 0.90−6.11 | 2.17 | 0.52−9.06 |
| Performed strenuous workc | 0.97 | 0.92−1.02 | 0.93 | 0.86−1.00 |
| Smoked cigarettes | 1.38 | 0.83−2.31 | 1.66 | 0.87−3.17 |
| Consumed dark green leafy vegetables | 0.64 | 0.41−0.99 | 0.67 | 0.38−1.28 |
OR, odds ratio; CI, confidence intervals; MUAC, mid upper arm circumference.
OR and 95% Cl calculated using multiple logistic regression analysis. Diarrhoea/dysentery and pre-eclampsia were excluded in the model for late deaths.
Pre-eclampsia/eclampsia was defined as swollen hands or face or non-epileptic convulsions.
Strenuous work was defined as the number of days on which each of the seven strenuous activities was performed in the past 7 days; range 0−49.
Discussion
This prospective population-based study identified early to mid-gestation risk factors for pregnancy-related mortality. Maternal age, parity and nutritional status as assessed by MUAC were strong predictors of maternal survival in both the early and the late periods. Intake of green leafy vegetables and morbidity in pregnancy appeared to be good predictors for mortality risk in the early period but not in the late period. There was a weak association between night blindness, strenuous work and smoking and mortality risk. Socio-economic factors were not associated with mortality risk for the early or the late period.
Few previous investigations have examined, prospectively, the relationship of antenatal and lifestyle factors with maternal mortality risk. Instead, studies have focused on characteristics of labour and delivery. A major strength of this analysis is that risk factors were ascertained before the outcomes and hence do not suffer from recall bias or missing data. Also, the early ascertainment of pregnancy allowed the capture of many deaths in pregnancy that would be harder to identify in a retrospective study. These results are likely to be generalizable to environments of chronic malnutrition where few women deliver in facilities or with trained attendants, and where access to care is very limited. This study had limited power to detect certain associations and effect modifications. Specifically, the sample size to investigate effect modification with treatment groups was limited. However, the study sample size was large compared with other prospective, community-based maternal mortality studies. Also, verbal autopsies had limited ability to identify causes of death, and assignment of one primary cause simplifies the contribution of underlying causes to the deaths.13 Data were therefore analysed for all-cause mortality.
Age and parity
Maternal age and parity were independently associated with maternal mortality risk; an association examined in few previous studies.5 Risk of death increased with maternal age (9% per year, data not shown), especially above 35 years of age (Fig. 1). Similar to the findings in a study in Matlab District, Bangladesh, the risk of mortality increased exponentially at older ages, although the inflection was observed at an earlier age (≥35 years) in the Nepali cohort compared with the Matlab population, which experienced increased risk at 40−44 years.5 Also, unlike in Bangladesh, lowest mortality was not observed in primiparous women in the present study. In fact, the risk of mortality appeared to decline with parity, such that women with the highest parity were at the lowest risk. This may perhaps be explained by reverse causation, as only surviving women can bear more children.
Nutritional findings
Maternal malnutrition is chronic among women of childbearing age in this population, and low MUAC appeared to predispose women to adverse pregnancy outcomes. There is growing evidence about the importance of nutritional influences on maternal obstetric complications and mortality in under-nourished populations. Specifically, severe anaemia is associated with an increased risk of mortality due to its exacerbating effect on haemorrhage or due to an increased risk of cardiac shock.14,15 Stunting among women may increase the risk of obstructed labour and caesarean section deliveries.16 Calcium supplementation may reduce the risk of eclampsia, hypertension and maternal mortality.17 In the trial in Nepal, vitamin A supplementation decreased the risk of maternal mortality10,18 in a region where vitamin A deficiency is endemic.19,20 The current results reinforce the potential value of dietary quality and nutritional status in reducing the risk of potentially adverse events related to pregnancy. Dietary intake of dark green leafy vegetables reduced the risk of death (OR 0.67). Dark green leaves are known to be a good source of provitamin A carotenoids in the diet of poor rural populations of South Asia and have a high anti-oxidant potential.21 It is noteworthy that the ∼33% risk reduction observed in this analysis is comparable in magnitude to the ∼44% reduction in pregnancy-related mortality observed with weekly beta-carotene or vitamin A supplementation in this population.10 It is also likely that consumption of dark green leaves may be a proxy measure for better dietary practices or better health in general. Yellow fruits and vegetables, also high in their provitamin A content, were not associated with survival, perhaps because the main food in this group (mango) is only available from late May to early July, providing too brief an exposure for an effect to be detected in this study. In this analysis, a history of night blindness elicited earlier in gestation was associated with a nearly three-fold higher risk of mortality related to pregnancy during the first year post partum, as observed previously.18
Protein malnutrition had a clear and consistent risk for mothers in the study population. In this setting, where the MUAC was ∼21 cm, the risk of death was 25% higher for each 1-cm decrement in size, reinforced by a dose-responsive 1.7- and 5.9-fold increase in risk at conventional cut-offs of 21.5 and 18.5 cm, respectively (data not shown). A thinner arm is reflective of wasted lean mass, associated with weakened host resistance to infection,22 and has long been known to increase mortality risk in young children.23–25 However, to the authors' knowledge, a thinner arm has not previously been associated quantitatively with maternal mortality risk. Given the extreme simplicity and low cost, this study suggests that measuring arm circumference and obtaining a history of night blindness26 should be evaluated further and considered for standard antenatal assessment, especially in undernourished, rural settings.
Socio-economic status
In this rural setting in Nepal where access to antenatal and emergency care is uniformly limited, socio-economic attributes, such as literacy, land and smaller asset ownership and house construction, were associated with 20−30% lower mortality risk related to pregnancy, although 95% CI for each OR failed to exclude unity. The lack of strength in the association between maternal mortality risk and socio-economic status could be due to narrow socio-economic dispersion in this setting or a choice of socio-economic indicators that were insensitive to maternal and family care providing and seeking behaviours related to pregnancy. Alternatively, a universally low availability of emergency obstetric care in this setting could have affected all socio-economic strata equally. However, an equally compelling explanation lies in the fact that this study had insufficient power to discern protective ORs of ∼0.75 from chance with 95% confidence.
Lifestyle exposures
This analysis supports previous findings27 that women who smoke cigarettes during pregnancy incur an increased risk of mortality during the first year post partum. In this analysis, smoking pregnant women were at ∼40% and ∼70% increased risk of death during pregnancy or up to 42 days post partum, and from 43 to 364 days post partum, respectively, based on both the within-risk-factor-group and final logistic models. Although period-specific CIs included unity, the ∼50% increment in risk over the entire period was statistically significant, based on the group-based regression analyses.
Performance of strenuous work during pregnancy was found to be associated with a decreased risk of pregnancy-related mortality. If causal, the directional pathways are not clear and may reflect a ‘healthy worker effect’. While moderate activity may improve health during pregnancy, it is also plausible that healthy women are more likely to do hard work even during pregnancy, whereas sicker women are less likely to carry out heavy household chores. This is borne out by the fact that the work variable became non-significant in the model when morbidity was added.
Conclusion
Little is known about modifiable factors in early to mid-gestation that affect the risk of maternal mortality. Identification of potential factors or ‘flags’ could help target high-risk groups and patterns of behaviour which could inform educational programmes that seek to guide women to adopt healthy, risk-reducing lifestyles. Where antenatal and obstetric care is poor, evidence-based educational programmes that alter maternal behaviour may be worthwhile. In this undernourished, underserved, high-risk rural population of Nepalese women, maternal age ≥35 years, prima-parity, a thin arm, infrequent intake of leafy greens, prevalent illness, occurrence of night blindness and cigarette smoking during pregnancy were associated with an increased risk of mortality that continued for the first year post partum.
Acknowledgements
The study in Sarlahi was carried out under cooperative agreement HRN-A-00-97-00015-00 between the Office of Health, Infectious Disease and Nutrition, US Agency for International Development (USAID), Washington, DC, and the Center for Human Nutrition (CHN), Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA. It was a joint collaboration between the CHN and the National Society for the Prevention of Blindness, Kathmandu, Nepal, under the auspices of the Social Welfare Council of His Majesty's Government of Nepal. The supplements were provided by Roche, Brazil and manufactured by NutriCorp International, C E Jamieson, Canada. Support from NIH R03 HD049406-01 is also acknowledged. The funders had no role in the design of the study, data collection, analysis or interpretation. The authors would like to thank the study participants and the members of the study teams.
Ethical approval Johns Hopkins Committee on Human Research, Baltimore, MD, USA; Nepal Health Research Council, Kathmandu, Nepal.
Funding USAID, Washington DC, USA, UNICEF Country Office, Kathmandu, Nepal; Bill and Melinda Gates Foundation, Seattle, WA, USA; Sight and Life Research Institute, Baltimore, MD, USA.
Footnotes
Competing interests None.
References
- 1.World Health Organization and UNICEF . Estimates of maternal mortality. World Health Organization and UNICEF; Geneva: 1996. [Revised 1990] [Google Scholar]
- 2.Hill K, AbouZahr C, Wardlaw T. Estimates of maternal mortality for 1995. Bull WHO. 2001;79:182–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Graham WJ. Now or never: the case for measuring maternal mortality. Lancet. 2002;359:701–4. doi: 10.1016/S0140-6736(02)07817-0. [DOI] [PubMed] [Google Scholar]
- 4.Buor D, Bream K. An analysis of the determinants of maternal mortality in sub-Saharan Africa. J Women Health. 2004;13:926–38. doi: 10.1089/jwh.2004.13.926. [DOI] [PubMed] [Google Scholar]
- 5.Hill K, El Arifeen S, Koenig M, Al-Sabir A, Jamil K, Raggers H. How should we measure maternal mortality in the developing world? A comparison of household deaths and sibling history approaches. Bull WHO. 2006;84:173–80. doi: 10.2471/blt.05.027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwast BE, Liff JM. Factors associated with maternal mortality in Addis Ababa, Ethiopia. Int J Epidemiol. 1988;17:115–21. doi: 10.1093/ije/17.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Oosterbaan MM. Guinea–Bissau: maternal mortality assessment. World Health Stat Quart. 1995;48:34–8. [PubMed] [Google Scholar]
- 8.Supratikto G, Wirth ME, Achadi E, Cohen S, Ronsmans C. A district-based audit of the causes and circumstances of maternal deaths in South Kalimantan, Indonesia. Bull WHO. 2002;80:228–34. [PMC free article] [PubMed] [Google Scholar]
- 9.West KP, Jr., Katz J, Khatry SK, et al. The NNIPS study group. Low dose vitamin A or β-carotene supplementation reduces pregnancy-related mortality: a double-masked, cluster randomized prevention trial in Nepal. Br Med J. 1999;318:570–5. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian P, Khatry SK, Katz J, et al. Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal. A double-blind randomized community trial. Br Med J. 2003;326:571–6. doi: 10.1136/bmj.326.7389.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortney JA. Implications of the ICD-10 definitions related to death in pregnancy, child birth or puerperium. World Health Stat Quart. 1990;43:246–8. [PubMed] [Google Scholar]
- 12.Pradhan EK, West KP, Jr., Katz J, et al. Risk of death following pregnancy in rural Nepal. WHO Bull. 2002;80:887–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Sloan NL, Langer A, Hernandez B, Romero M, Winikoff B. The etiology of maternal mortality in developing countries: what do verbal autopsies tell us? WHO Bull. 2001;79:805–10. [PMC free article] [PubMed] [Google Scholar]
- 14.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131:604S–14S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- 15.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Vol. 1. World Health Organization; Geneva: 2004. pp. 163–209. [Google Scholar]
- 16.Rush D. Nutrition and maternal mortality in the developing world. Am J Clin Nutr. 2000;72:212S–40S. doi: 10.1093/ajcn/72.1.212S. [DOI] [PubMed] [Google Scholar]
- 17.Villar MD, Abdel-Aleem H, Merialdi M, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194:639–49. doi: 10.1016/j.ajog.2006.01.068. [DOI] [PubMed] [Google Scholar]
- 18.Christian P, West KP, Jr., Khatry SK, et al. Night blindness during pregnancy and subsequent mortality among women in Nepal: effects of vitamin A and β-carotene supplementation. Am J Epidemiol. 2000;152:542–7. doi: 10.1093/aje/152.6.542. [DOI] [PubMed] [Google Scholar]
- 19.Katz J, Khatry SK, West KP, Jr., et al. Night blindness is prevalent during pregnancy and lactation in rural Nepal. J Nutr. 1995;125:2122–7. doi: 10.1093/jn/125.8.2122. [DOI] [PubMed] [Google Scholar]
- 20.Christian P, West KP, Jr., Khatry SK, et al. Night blindness of pregnancy in rural Nepal—nutritional and health risks. Int J Epidemiol. 1998;27:231–7. doi: 10.1093/ije/27.2.231. [DOI] [PubMed] [Google Scholar]
- 21.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr Opin Lipidol. 2005;16:47–54. doi: 10.1097/00041433-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Keusch GT. Nutrition–infection interactions. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principles, pathogens & practices. Churchill Livingstone; Philadelphia: 1999. pp. 62–75. [Google Scholar]
- 23.Sommer A, Loewenstein MS. Nutritional status and mortality: a prospective validation of the QUAC stick. Am J Clin Nutr. 1975;28:287–92. doi: 10.1093/ajcn/28.3.287. [DOI] [PubMed] [Google Scholar]
- 24.West KP, Jr., Pokhrel RP, Katz J, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet. 1991;338:67–71. doi: 10.1016/0140-6736(91)90070-6. [DOI] [PubMed] [Google Scholar]
- 25.Katz J, West KP, Jr., Khatry SK, et al. Risk factors for early infant mortality in Nepal. WHO Bull. 2003;81:717–25. [PMC free article] [PubMed] [Google Scholar]
- 26.Christian P. Recommendations for indicators: night blindness during pregnancy—a simple tool to assess vitamin A deficiency in a population. J Nutr. 2002;132:2884S–8S. doi: 10.1093/jn/132.9.2884S. [DOI] [PubMed] [Google Scholar]
- 27.Christian P, West KP, Jr., Katz J, Kimbrough-Pradhan E, LeClerq SC, Khatry SK. Cigarette smoking during pregnancy in rural Nepal. Risk factors and effects of β-carotene and vitamin A supplementation. Eur J Clin Nutr. 2004;58:204–11. doi: 10.1038/sj.ejcn.1601767. [DOI] [PubMed] [Google Scholar]


