Abstract
OBJECTIVES
Severe burns are associated with a significant loss of muscle and strength. Studies have reported that oxandrolone improves lean body mass in muscle-wasting conditions. Also shown previously in burned children is that an exercise program increases lean body mass and muscle strength. We hypothesized that oxandrolone, in combination with exercise, would increase lean body mass and muscle strength in severely burned children more than oxandrolone alone or exercise alone.
METHODS AND PATIENTS
Fifty-one burned children (≥40% total body surface area burned) were randomly assigned to receive oxandrolone alone (0.1 mg/kg per day orally; n = 9), oxandrolone and exercise (n = 14), placebo and no exercise (n = 11), or placebo and exercise (n = 17). Administration of oxandrolone was started at discharge and continued until 1 year after burn. The 12-week exercise training program was started 6 months after burn. Serum hormones, lean body mass, muscle strength, and peak cardiopulmonary capacity were assessed at 6 (baseline) and 9 months after burn. Data were analyzed using a 1-way analysis of variance, and significance was set at P < .05.
RESULTS
The mean percentage of change or increase in weight and lean body mass in the oxandrolone and exercise group was significant compared with placebo and exercise, as well as with the oxandrolone alone group or placebo and no exercise group. Furthermore, lean body mass was significantly improved in the oxandrolone and exercise, oxandrolone alone, and placebo and exercise group compared with the group only receiving placebo. Muscle strength significantly increased in oxandrolone and exercise, placebo and exercise, and the oxandrolone alone group when compared with the placebo and no exercise group. The peak cardiopulmonary capacity was significantly higher in both exercise groups. Insulin-like growth factor 1 was significantly increased in the oxandrolone alone group compared with placebo and exercise and placebo and no exercise. Both exercise groups showed significant changes in insulin-like binding-protein-3 when compared with groups without exercise.
CONCLUSIONS
Oxandrolone, in combination with exercise, is beneficial in severely burned children, thus improving their rehabilitation.
Keywords: burns, exercise, hormones, lean mass, oxandrolone
Severe burns are physically and psychologically catastrophic. Severely burned children have an elevated resting energy expenditure, whole body catabolism, and muscle weakness, which are worsened by prolonged bed rest and physical inactivity.1,2
Various therapeutic approaches have been investigated in burn care in an attempt to ameliorate these adverse effects.3 One of these therapies is exercise, which has been reported to improve functional outcome of burn patients.3 Suman et al4 demonstrated in a prospective, controlled, and randomized study an increase in muscle strength and in lean body mass (LBM) in response to a 12-week exercise program in severely burned children. However, improvements in LBM were moderate, and it seemed to be clinically important and relevant to investigate additional therapeutic interventions.
Studies using anabolic agents to ameliorate the hypermetabolic response and catabolism after burns have produced varying beneficial effects.5 We have investigated previously the combination of recombinant human growth hormone (rhGH) and exercise in burned children. We found that the combination of rhGH and exercise failed to produce a significantly greater increase in LBM than exercise alone and that improvements in muscle strength (MStr) were associated only with an exercise program.6 This apparent lack of effect of rhGH on MStr, the high cost of rhGH, and that rhGH has to be given by daily injections, presenting a daily source of tension to parents and child, are reasons for the need to investigate more efficient, less costly, and less invasive therapies.
Another anabolic agent that has been studied is oxandrolone. Oxandrolone is a synthetic androgenic steroid and, when given acutely, has been shown to decrease posttraumatic catabolism and improve muscle protein synthesis in burned patients.7 Long-term administration of oxandrolone ≤12 months after injury significantly increased bone mineral content and LBM at the end of the observation period, when compared with severely burned children receiving placebo.8 However, the possible effects of an in-hospital-based, outpatient exercise program in combination with oxandrolone have not been specifically addressed. Therefore, we hypothesized that the combination of oxandrolone, given over a shorter time period, combined with exercise in severely burned children, would result in significantly greater beneficial effects in LBM, MStr, and aerobic capacity than oxandrolone alone or exercise alone.
PATIENTS AND METHODS
Patients
Pediatric burn patients, aged 7 to 17 years old and with ≥40% total body surface area (TBSA) burns, were enrolled in the study. Patients were excluded if they had ≥1 of the following: anoxic brain injury, psychological disorders, quadriplegia, severe behavior, or cognitive disorders. Informed consent was given by the parent or legal guardian during the first day of acute admission. Thereafter, child assent or child consent was obtained where applicable.
The study design has been described previously by our group.6 Briefly, patients were randomly assigned into 1 of 4 groups (Fig 1). Children in 2 of the 4 groups participated in a 12-week in-hospital physical therapy program supplemented with an individualized and supervised exercise training program. One of these exercise groups received administration of (0.1 mg/kg of body weight per day orally) oxandrolone (OXEX); the other group received placebo plus exercise (PLEX). The remaining 2 groups received standard of care. These patients in the standard of care groups were prescribed a home-based physiotherapy program. One of the standard of care groups received oxandrolone alone (OX). The other home-based group received placebo alone (PL). The time chosen to start drug or placebo administration was at hospital discharge, when the wounds were 95% healed.
FIGURE 1.
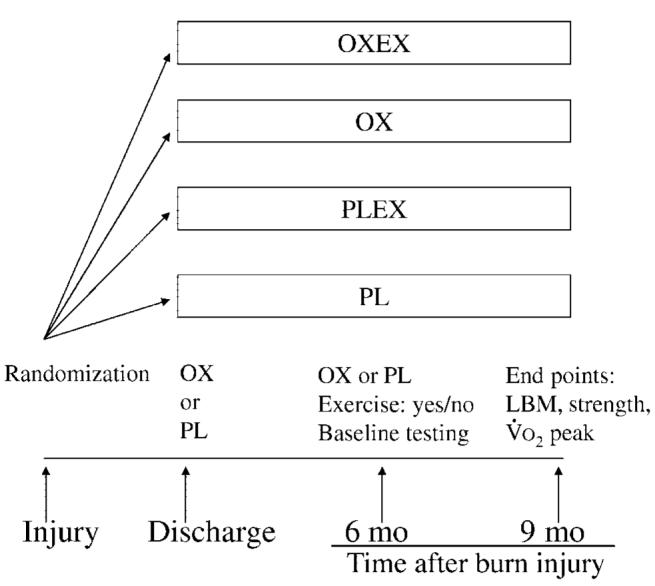
General design of the study. After randomization, patients received oxandrolone or placebo starting at hospital discharge. Patients then participated in an exercise program between 6 and 9 months after burn or in a home-based standard of care program.
All of the patients received similar standard medical care and treatment from the time of hospital admission at our institution and acute care of the burn injury until time of discharge. In addition, all of the groups were discharged with similar standard medical and rehabilitation care until the 6-month postburn injury time point.
At 6 months postburn, all of the patients returned to Shriners Hospitals for Children for baseline exercise testing and assessment of body composition. This time point of 6 months postburn for initial exercise assessment and exercise training represented a period when all of the patients were ambulatory and able to participate in strenuous exercise evaluations and training.
After completing the exercise tests, the OXEX and PLEX groups began participating in the 12-week in-hospital physical rehabilitation program supplemented with an individualized and supervised exercise training program. In contrast, the OX and PL groups continued participating in the standard of care, home-based physical rehabilitation program without a structured and supervised exercise program. The in-hospital physical rehabilitation program consisted of 12 weeks of conventional occupational therapy (OT) and physical therapy (PT) twice daily for ∼1 hour each session. Patients in the OX and PL groups did not receive an exercise prescription by an exercise physiologist at any time during the study. This study was approved by the institutional review board at our institution. For comparison, age- and gender-matched, healthy, nonburned children were used.
Compliance and Education on Drug or Placebo Administration
Education of the study participants and/or parents on the administration of oxandrolone or placebo was started early in the acute phase of treatment to allow sufficient time for understanding of the study, the importance of daily compliance, documentation on the calendar provided, safe drug handling, and storage requirements. Each participant performed return demonstrations on the proper way to administer the drug or placebo, and education was continued as needed. Compliance was determined via direct observation in the case of the OXEX and PLEX groups or by a patient/guardian questionnaire using a Self-reported Compliance Questionnaire and set at a minimum of 75% compliance to their daily study drug for all of the groups. Compliance percentage was chosen from estimates for children in the medical literature where improvement in chronic therapy was achieved with a minimal compliance of 70%.9
At each hospital visit (6 and 9 months after burn), a clinical research nurse met with all of the study participants who were asked to fill out a Self-reported Compliance Questionnaire for the time since their last clinic visit. The questions related to any problems they encountered with the study drug, adverse reactions, supplies, and how many doses of the study drug they missed. Calendars provided for documentation were reviewed, otherwise, the number of doses missed were an estimate of compliance by the participant.
Exercise Testing
Exercise assessments were conducted at the beginning of 6 months and at the end of 9 months after the burn injury. Before strength testing, the patient was familiarized with the exercise equipment and instructed on proper weight lifting techniques. The patient was asked to sit quietly for ∼15 minutes before resting measurements were recorded. After this time period, vertical height and body weight was measured.
Strength Measurements
Strength testing was conducted on day 1 of the 6-month and 9-month postburn period using a Biodex System-3 dynamometer (Shirley, NY). The isokinetic test was performed on the dominant leg extensors and tested at an angular velocity of 150°/s. This speed was chosen because it was well tolerated (compared with lower or higher angular speeds) by the children across all of the ages and all of the groups. The patients were seated and their position stabilized with a restraining strap over the midthigh, pelvis, and trunk in accordance to the Biodex System-3 Operator's Manual. All of the patients were familiarized with the Biodex test in a similar manner. First, the administrator of the test demonstrated the procedure. Second, the test procedure was explained to patients. Third, patients were allowed to practice the actual movement during 3 submaximal repetitions without load as warm-up. More repetitions were not allowed to prevent the possible onset of fatigue. The anatomic axis of the knee joint was aligned with the mechanical axis of the dynamometer before the test. After the 3 submaximal warm-up repetitions, 10 maximal voluntary muscle contractions (full extension and flexion) were performed. The maximal repetitions were performed consecutively without rest in between. Three minutes of rest were given to minimize the effects of fatigue, and the test was repeated.
Values of peak torque were calculated by the Biodex software system. The highest peak torque measurement between the 2 trials was selected. Peak torque was corrected for gravitational moments of the lower leg and the lever arm.
3-Repetition Maximum Test
After a 30-minute rest period, patients enrolled in the OXEX or PLEX groups were tested to determine the amount of weight or load that would be used during the first week (of the 12-week program) as baseline loads. They were tested in the following order of exercises: bench press, leg press, shoulder press, leg extension, biceps curl, leg curl, and triceps curl. The 3-repetition maximum load (3RM) was determined as follows: after an instruction period on correct weightlifting technique, the patient warmed up with lever arm and bar (or wooden dowel) and was allowed to become familiar with the movement. After this, the patient lifted a weight that allowed successful completion of 4 repetitions. If the fourth repetition was achieved successfully and with correct technique, a 1-minute resting period was allowed. After the resting period, a progressively increased amount of weight or load was instructed to be lifted ≥4 times. If the patient lifted a weight that allowed successful completion of 3 repetitions, with the fourth repetition not being volitionally possible because of fatigue or inability to maintain correct technique, the test was terminated, and the amount of weight lifted from the successful set was recorded as their individual 3RM. A 3RM was not done on the nonburned group of children or on the nonexercising groups (OX and PL), because they did not exercise train for 12 weeks.
Peak Oxygen Consumption
All of the subjects underwent a standardized treadmill exercise test using the modified Bruce protocol (2000a) as part of their standard clinical outpatient evaluation. Heart rate and oxygen consumption were measured and analyzed using methods described previously.4 Briefly, breath-by-breath analysis was continuously made of inspired and expired gases, flow, and volume using a Medgraphics CardiO2 Combined O2/ECG Exercise System (St Paul, MN). Speed and angle of elevation started at 1.7 mph and 0%, respectively. Thereafter, the speed and level of incline were increased every 3 minutes. Subjects were constantly encouraged to complete 3-minute stages, and the test was terminated once peak volitional effort was achieved. The peak oxygen consumption (V̇o2peak) and peak heart rate were additionally used to establish the intensity at which patients in the OXEX and PLEX groups initially exercised during the 12 weeks of training.
LBM Measurements
On day 2 (6 month and/or 9 month), LBM measurements were made for all 4 of the burned groups and in the nonburned group by dual energy radiograph absorptiometry using the QDR 4500A software (Hologics, Waltham, MA). Although assessment of body composition was made at discharge, this is a time point when some patients have staples or are undergoing fluid shifts in cellular and whole body water because of resuscitation or excisional therapy, thereby confounding assessment of LBM.7 Therefore, LBM was not reported for the discharge time period. Scans were taken in slow array mode, with the patient laying supine on the scanning table. The protocol for obtaining a whole body scan was done according to the manufacturer's instructions and has been described previously by our group.10 Briefly, dual energy radiograph absorptiometry with pediatric software was used to measure the attenuation of 2 radiograph beams: 1 high energy and the other low energy. These measurements were then compared with standard models of thickness used for bone and soft tissue. Subsequently, the calculated soft tissue was separated into LBM, bone mineral content, and fat mass.
Physical and Occupational Rehabilitation
Children in the OX and PL groups returned home to continue standard-of-care occupational and physical rehabilitation. The patient (parents or legal guardians) was instructed to continue standard OT or PT at home with or without supervision by an occupational or physical therapist. In contrast, children in the OXEX and PLEX groups remained as outpatients and received a supervised hospital-based OT/PT program and a structured exercise training program. The OT/PT program for burned children included range of motion exercises, specific limb or digit position, and splinting. In addition, scar management using pressure therapy and inserts were used. Finally, patient and caregiver education of the described OT/PT program was done.
Exercise Training Program
All of the burned subjects were sedentary before starting the exercise program and had never participated in an exercise training program. Children were considered sedentary if they did not participate in ≥30 minutes of exercise per day for 3 times per week or were not engaged in organized sports. Each exercise training session consisted of resistance and aerobic exercises, with aerobic exercise preceding resistance exercise. Eight basic resistance exercises were used: bench press, leg press, shoulder press, biceps curl, leg curl, triceps curl and toe raises. At no time did the OXEX and PLEX groups train using the Biodex dynamometer. All of the exercises were done using variable resistance machines or free weights. During the first week of training, the patients became familiarized with the exercise equipment and were instructed in proper weightlifting techniques. The weight or load lifted was set at 50% to 60% of their individual 3RM and performed for 4 to 10 repetitions for 3 sets. During the second week, the lifting load was increased to 70% to 75% (3 sets, 4–10 repetitions) of their individual 3RM and continued for weeks 2 to 6. After this, training intensity was increased to 80% to 85% (3 sets, 8–12 repetitions) of the 3RM and implemented from weeks 7 to 12. A rest interval of ∼1 minute was given between sets.
Each exercise training session also included aerobic conditioning exercises on a treadmill or cycle ergometer. This aerobic training was conducted 5 days per week. Each session lasted 20 to 40 minutes, and participants exercised at 70% to 85% of their previously determined individual V̇o2peak. All exercise sessions were preceded by a 5-minute warm-up period on the treadmill at an intensity of < 50% of each individual V̇o2peak. Heart rate and oxygen saturation were monitored using a Radical Signal Extraction pulse oximeter (Masimo Corp, Irvine, CA). Rated perceived exertion was obtained at regular intervals during aerobic exercise. All of the exercise sessions and exercise prescriptions were supervised by an exercise specialist and were conducted according to the guidelines set by the American College of Sports Medicine.11 No strength-training activities were permitted outside the supervised training session; however, both groups were allowed to pursue their normal daily activities. Patients randomly assigned to the exercise program were required to have participated in ≥33 workout sessions of the 36 total workout sessions to be considered compliant with the exercise program.
Hormone Panel
Briefly, whole blood was used to analyze serum insulin-like growth factor-1 (IGF-1), IGF binding protein-3 (IGFBP-3), insulin, cortisol, total thyroxine, tri-iodothyronine uptake, and free thyroxine index. All of the measures used enzyme linked immunosorbent assays from Diagnostic Systems Laboratory (Webster, TX).
Data Analysis
All of the data are expressed as mean ± sem in tables and figures. The mean percentage change of the dependent variables because of different interventions were analyzed using a 1-way analysis of variance and a Student-Newman-Keuls test for multiple comparisons for the OXEX, PLEX, OX, and PL groups before and after 12 weeks of intervention. A P < .05 was considered statistically significant.
RESULTS
Fifty-one patients met the age and burn size criteria, as well as the return visit requirements. The 4 study groups were similar regarding age, gender, TBSA burn, and area of third degree burn. The demographic results of patients per group are displayed in Table 1. We found no significant difference among the 4 groups in height, weight, LBM, MStr, and cardiopulmonary capacity at baseline (6 months after burn injury). No adverse effects, such as hirsutism or elevated liver enzymes, were noted. Absolute values of burned children, as well as nonburned, healthy children for comparison are displayed in Table 2.
TABLE 1.
Demographic Characteristics of the Study Groups
| Characteristic | OXEX (n = 14) |
OX (n = 9) |
PLEX (n = 17) |
PL (n = 11) |
|---|---|---|---|---|
| Gender, n | ||||
| Male | 13 | 6 | 13 | 9 |
| Female | 1 | 3 | 4 | 2 |
| Age | 12.1 ± 0.8 | 11.8 ± 1.1 | 10.9 ± 0.9 | 11.8 ± 1.0 |
| Burn (TBSA), % | 52.1 ± 3.4 | 54.7 ± 3.9 | 55.6 ± 3.6 | 51.6 ± 4.6 |
| Height, m | 147.3 ± 5.5 | 144.5 ± 8.8 | 138.8 ± 7.1 | 147.1 ± 7.4 |
| Weight, kg | 41.5 ± 4.2 | 40.2 ± 5.4 | 33.3 ± 5.9 | 39.8 ± 5.3 |
Values are mean ± SEM except where otherwise noted. Height and weight measured at baseline. All 4 groups of burned children were similar in demographics.
TABLE 2.
Absolute Values for LBM, Muscle Strength (Peak Torque), and Aerobic Capacity (V̇O2peak) of Burned and Nonburned children
| Variable | OXEX |
OX |
PLEX |
PL |
Nonburned | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | ||
| Lean mass, kg | 31.2 | 35.3 | 33.1 | 34.9 | 31.2 | 33 | 31.5 | 31.2 | 48.8 |
| SEM | 2.4 | 3 | 4.9 | 5 | 4.4 | 4.7 | 3.9 | 3.9 | 8.1 |
| Peak torque, Nm | 47 | 69.7 | 50.4 | 72.8 | 35.4 | 45.7 | 35.8 | 36.4 | 94.7 |
| SEM | 4.4 | 10.3 | 4.7 | 10.1 | 9.8 | 11.3 | 9.6 | 8.8 | 4.8 |
| V̇O2peak, mL O2/kg/min | 31.9 | 36.4 | 32.5 | 35.4 | 26.6 | 32.7 | 26.9 | 29 | 47.4 |
| SEM | 3.8 | 4.2 | 3.5 | 3.4 | 2.1 | 2.8 | 2.8 | 2.9 | 2.8 |
Values are mean ± SEM except where otherwise noted. Pretest is for baseline; posttest is for end point. Mean age for nonburned children (65% boys) is 12.2 years (SEM: 0.5).
Height and Weight
Standing height was increased in all of the groups, but there was no significant difference between groups. Body weight improved in all 4 of the groups and resulted in a significant increase in the OXEX group (14.0% ± 5.4%, SD) compared with OX (8.6% ± 5.0%, SD), PLEX (4.9% ± 7.6%, SD), and PL groups (2.5% ± 4.2%, SD; Fig 2).
FIGURE 2.
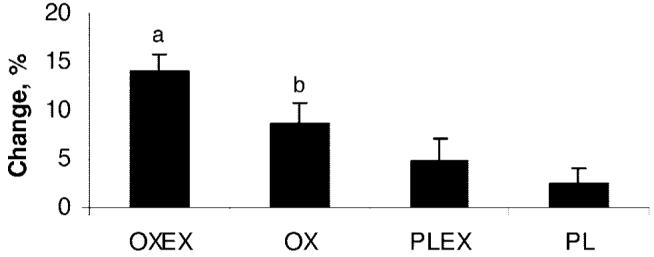
Mean percentage changes in weight. Data are presented as mean ± SEM. a Significant difference in weight compared with OX, PLEX, and PL (P < .05). b Significant difference in weight compared with PL (P < .05).
LBM
Three of the 4 groups showed an increase in LBM during the observation period, and only the PL group showed a mean decrease in LBM (−0.8% ± 6.3%, SD), indicating that children with massive burns are still losing muscle mass 6 months after the injury. The combination of oxandrolone and exercise, OXEX, showed the highest positive percentage change in LBM (12.8% ± 5.3%, SD). This change was significantly greater compared with all of other groups. OX (5.7% ± 2.7%, SD) showed a significant improvement in LBM compared with PL (−0.8% ± 6.3%, SD). Finally, the mean percentage of change in LBM in the PLEX group was significantly higher compared with PL (Fig 3) but was not different from OX.
FIGURE 3.
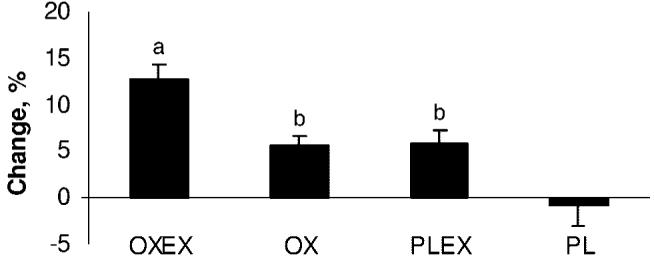
Mean percentage changes in lean mass from 6 to 9 months after burn. Data are presented as mean ± SEM. a Significant improvement compared with OX, PLEX, and PL (P < .05). b Significant difference compared with PL (P < .05).
MStr
Peak torque was chosen as the variable to assess MStr as in our previous publications.4,6 All of the groups had a mean percentage increase in peak torque. The lowest improvement was observed in the PL group (6.6% ± 15%, SD). Both exercise groups, OXEX (44.9% ± 34.6%, SD) and PLEX (47.3% ± 40.5%, SD), as well as the drug-only group, OX (44.3% ± 13.3%, SD) showed a significant increase in strength when compared with PL (6.6% ± 15%, SD; Fig 4).
FIGURE 4.
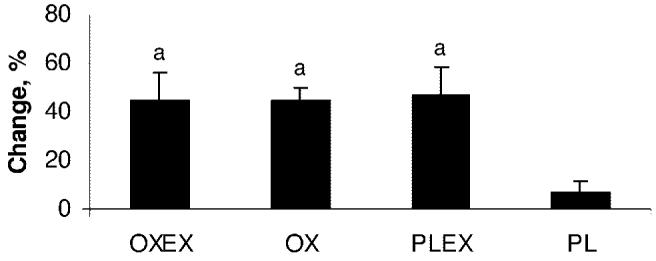
Mean percent changes in MStr. a Significant difference in MStr compared with PL (P < .05). Data are shown as mean ± SEM.
Cardiopulmonary Capacity
Cardiopulmonary capacity was significantly increased only in the groups that participated in the exercise program, OXEX (18.1% ± 36.1%, SD) and PLEX (23.2% ± 16.6%, SD), when compared with OX (8.9% ± 20%, SD) and PL (9.35% ± 16.8%, SD; P < .05; Fig 5).
FIGURE 5.
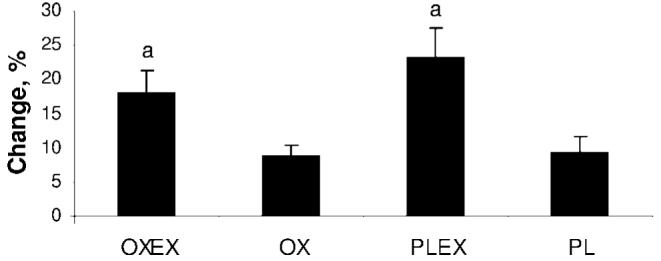
Mean percentage changes in peak aerobic capacity. Data are presented as mean ± SEM. a Peak aerobic capacity, which was significantly improved in both exercise groups, OXEX and PLEX, when compared with OX and PL (P < .05).
Hormone Analysis
A significant increase in IGF-1 serum concentrations was observed in the OX group compared with PLEX and PL. The second highest increase was seen in the OXEX group, but this increase was not significant when compared with PLEX or PL (Fig 6). Both groups with an exercise component had a significant increase in IGFBP-3 concentrations, whereas the OX and PL groups showed a decrease in IGFBP-3 levels (Fig 7). No significant changes between groups were observed for serum concentrations of insulin, cortisol, parathyroid hormone, tri-iodothyronine uptake, and the free thyroxine index.
FIGURE 6.
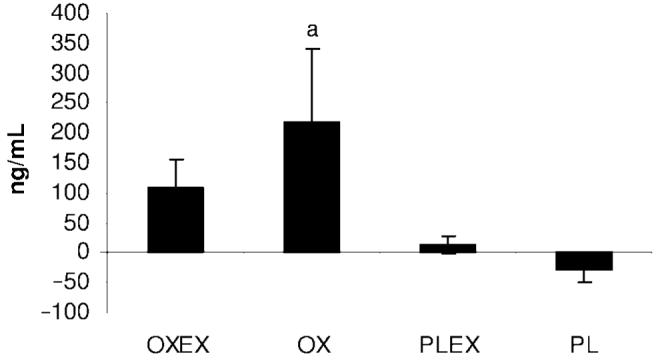
Mean percentage changes in IGF-1 from 6 months to 9 months after burn. Data are presented as mean ± SEM. a Significant difference in IGF-1 compared with OXEX, PLEX, and PL (P < .05).
FIGURE 7.
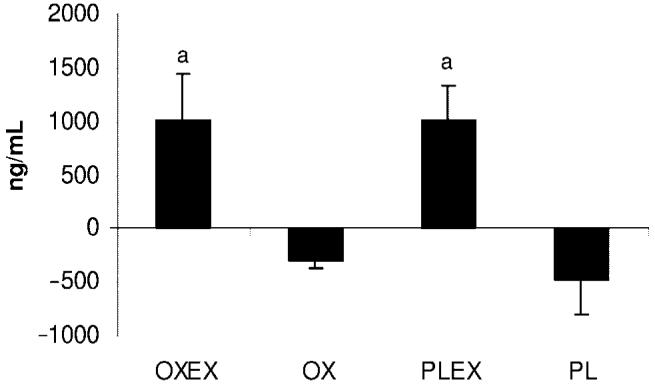
Mean percentage changes in IGFBP-3 from 6 months to 9 months after burn. a Significant difference between the exercise groups, OXEX and PLEX, relative to the groups without exercise, OX and PL (P < .05). Data are presented as mean ± SEM.
DISCUSSION
The current study tested the hypothesis that oxandrolone, in combination with exercise, would have an increase in LBM and MStr to a greater extent than OX, than exercise and placebo drug, or than placebo drug only. This is, to our knowledge, the first time that such a study has been performed in severely burned children. The results of our study demonstrate that OXEX improves LBM to a significantly greater extent than the other 3 groups (OX, PLEX, and PL). However, the mean percentage change in LBM of the OX and PLEX groups was significantly greater relative to the mean percentage change of the PL group. The PL group was the only group that showed a negative change for LBM 9 months after burn, which is consistent with our previous reports regarding the extent and duration of the muscle catabolism after massive thermal injury in children.2
Compared with the study of rhGH and exercise,6 in which the combination of exercise and rhGH was not superior to exercise alone with regard to an increase in LBM, the combination of oxandrolone as an anabolic agent and exercise showed a significantly greater increase in LBM compared with OX, PLEX, and PL. The observed significant gain in weight in the OXEX group, compared with PLEX and PL, is in accordance with the increase in LBM in this group.
Our results in MStr showed a different pattern than the LBM results. MStr was not only significantly increased with exercise but surprisingly also with the OX. This observation clearly differs from the rhGH exercise trial in which rhGH alone, without exercise, did not significantly improve MStr when compared with the placebo group.6 This finding is desirable, because children <7 years of age typically are not capable of participating in a strenuous exercise program.
With regard to the cardiopulmonary capacity, we observed a similar pattern as reported previously with the rhGH trial.6 The cardiopulmonary capacity could only be improved significantly with exercise. OX has no demonstrable effect on this parameter.
Additional studies investigating the efficacy of oxandrolone on muscle catabolism in severely burned children support our findings. Hart et al7 reported that oxandrolone given during the acute hospitalization phase after burn significantly improved muscle anabolism. Similarly, the administration of oxandrolone for ≤1 year after burn showed a significant attenuation of muscle catabolism in addition to a significant improvement in MStr in severely burned children when compared with burned children receiving placebo.8 Studies in burned adults have shown similar effects of oxandrolone treatment on lean mass and body weight. A randomized study of severely burned adults, reported by Demling and DeSanti,12 demonstrated the ability of oxandrolone to increase lean mass and weight in adults when compared with placebo treatment.
Furthermore, studies in body wasting conditions, such as AIDS/HIV-associated muscle and weight catabolism, have found comparable results with our findings.13 The administration of oxandrolone in combination with a progressive resistance exercise program resulted in significantly higher gains in LBM and strength compared with patients who participated in the exercise program alone.14 Berger et al15 found that HIV-positive men treated with oxandrolone showed significant improvements in weight, appetite, and activity when compared with the placebo group. Yet another area in which the beneficial effects of oxandrolone have been implicated is in spinal cord injuries, where similarities with burns, such as prolonged inactivity and muscle wasting, are found. In a study by Spungen et al,16 oxandrolone administration promoted weight gain and wound healing.
Alterations observed on serum hormone concentrations of IGF-1 and IGFBP-3 suggest that the increase in IGF-1 in the OX group (significantly larger compared with PLEX and PL) and the increased level in the OXEX group, although not significantly different from the PLEX and PL groups, is related to oxandrolone. Recent findings suggest the secretion of IGF-1 isoforms from muscle in response to stimulation, such as anabolic drugs or exercise, but following this hypothesis, the PLEX group should have resulted in a greater increase.17 However, we suggest that an increase in LBM because of OX or in combination with exercise has a positive feedback on serum IGF-1 concentrations. Elevated IGF-1 can itself promote anabolic effects on LBM.1 Interestingly, IGFBP-3 was significantly higher in groups receiving exercise, OXEX or PLEX, suggesting a positive feedback regulation, independent from oxandrolone. Although the relation of IGFBP-3 and exercise is still controversial, some studies report an association between elevated insulin-like binding proteins and exercise.18
Our study does not address mechanisms, therefore, we can only speculate. Insight into how oxandrolone may exert its effects may be found in a study by Sheffield-Moore et al.19 In their study, which used stable isotopes to assess protein metabolism, they found that in young men, oxandrolone significantly increased the fractional synthetic rate without an increase in muscle protein breakdown, resulting in a positive net protein balance, as well as an improved intracellular use of amino acids. In addition, their study found a significant increase in the number of androgen receptors, through which anabolic agents translate their action. It is possible that this scenario may be similar in burned children.
In all of these studies, oxandrolone has been proven to be relatively safe. However, patients have to be monitored closely for possible adverse effects such as hepatotoxicity, hirsutism/testicular atrophy, or behavioral changes specifically associated with the use of oxandrolone.15
The results of our study show that the combination of oxandrolone and exercise produced the largest increase in LBM and weight. In addition, this combination resulted in a significant improvement in MStr. OX showed a significant increase in LBM and in MStr when compared with placebo. This observation may be clinically relevant for children who are unable or too young to participate in a structured resistance and aerobic exercise program. However, burned children continue to present lower values in lean mass and exercise capacity relative to healthy, nonburned children (Table 2). Therefore, the value of continued treatment with oxandrolone (eg, 1 year) and/or exercise should be explored.
There are obstacles and challenges in applying an exercise program such as the one described in the present study to children 6 months after being severely burned. One of these challenges is that these children and caretakers typically require housing while participating in the outpatient exercise program. In our study, the children and caretakers live in hospital-sponsored housing outside of the hospital. These children are not rehospitalized but are brought in as residential outpatients. This may not be feasible for many medical care facilities. Another challenge, and perhaps the largest obstacle, is presented by the caretaker, as very often this type of program entails being away from work and also away from other family members that need their care. However, these obstacles are not impossible to overcome, and we make it known to everyone involved that this is part of their physical rehabilitation, and it is of utmost importance in their physical rehabilitation. One great importance and significance of the present study is that it presents evidence that oxandrolone, alone or in combination with exercise, is beneficial. As such, one can then envision modifications of the design of this study or program to fit non–Shriners Hospital settings. Modifications could include starting the exercise program immediately after discharge (typically 2–3 months after burn) versus waiting for the 6 months postburn time point. In addition, one can envision an exercise program similar to ours but done in a community-based facility or gym (with or without oxandrolone). These modifications would probably be less disruptive to the family of burned children undergoing exercise rehabilitation. These modifications are presently being studied by our group.
CONCLUSION
We recommend the combination of oxandrolone and exercise as a fundamental component of a multidisciplinary approach to improve the rehabilitation and re-integration of burned patients into society.
ACKNOWLEDGMENTS
The study was supported by the National Institute for Disabilities and Rehabilitation Research grant H133A020102, the National Institutes of Health grants P50-GM06338 and K01-HL70451, and Shriners Hospitals for Children grant 8760.
We thank Serina J. McEntire and Rebecca L. Whitlock for their valuable technical assistance.
Abbreviations
- LBM
lean body mass
- rhGH
recombinant human growth hormone
- MStr
muscle strength
- TBSA
total body surface area
- OXEX
oxandrolone plus exercise
- PLEX
placebo plus with exercise
- OX
oxandrolone alone
- PL
placebo alone
- OT
occupational therapy
- PT
physical therapy
- 3RM
3-repetition maximum load
- V̇O2peak
peak oxygen consumption
- IGF-1
insulin-like growth factor-1
- IGFBP-3
insulin-like binding-protein-3
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1900. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 3.Pereira CT, Herndon DN. The pharmacologic modulation of the hypermetabolic response to burns. Adv Surg. 2005;39:245–261. doi: 10.1016/j.yasu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 5.Hart DW, Herndon DN, Klein G, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–834. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suman OE, Thomas SJ, Wilkins JP, Mlcak RP, Herndon DN. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol. 2003;94:2273–2281. doi: 10.1152/japplphysiol.00849.2002. [DOI] [PubMed] [Google Scholar]
- 7.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–564. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Przkora R, Jeschke MG, Barrow RE, et al. Metabolic and hormonal changes of several burned children receiving long-term oxandrolone treatment. Ann Surg. 2005;242:384–389. doi: 10.1097/01.sla.0000180398.70103.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Costa IG, Rapoff MA, Lemanek K, Goldstein GL. Improving adherence to medication regimes for children with asthma and its effect on clinical outcome. J Appl Behav Anal. 1997;30:687–691. doi: 10.1901/jaba.1997.30-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein GL, Herndon DN. The role of bone densitometry in the diagnosis and management of the severely burned patient with bone loss. J Clin Densitom. 1998;2:11–15. doi: 10.1385/jcd:2:1:11. [DOI] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine . Exercise testing and prescription for children, the elderly, and pregnant women. In: Franklin BA, editor. ACSM's Guidelines for Exercise and Prescription. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. pp. 217–223. [Google Scholar]
- 12.Demling RH, DeSanti L. Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid. Burns. 2003;29:793–797. doi: 10.1016/j.burns.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64:725–750. doi: 10.2165/00003495-200464070-00004. [DOI] [PubMed] [Google Scholar]
- 14.Strawford A, Barbieri T, Van Loan M, et al. Resistance exercise and supraphysiologic androgen therapy in eugonadal men with HIV-related weight loss: a randomized controlled trial. JAMA. 1999;281:1282–1290. doi: 10.1001/jama.281.14.1282. [DOI] [PubMed] [Google Scholar]
- 15.Berger JR, Pall L, Hall CD, Simpson DM, Berry PS, Dudley R. Oxandrolone in AIDS-wasting myopathy. AIDS. 1996;10:1657–1662. doi: 10.1097/00002030-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Spungen AM, Koehler KM, Modeste-Duncan R, Rasul M, Cytryn AS, Bauman WA. Nine clinical cases of nonhealing pressure ulcers in patients with spinal cord injury treated with an anabolic agent: a therapeutic trial. Adv Skin Wound Care. 2001;14:139–144. doi: 10.1097/00129334-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32:181–186. doi: 10.3109/07853890008998825. [DOI] [PubMed] [Google Scholar]
- 18.Elloumi L, El Elj N, Zaouali M, et al. IGFBP-3, a sensitive marker for physical training and overtraining. Br J Sports Med. 2005;39:604–610. doi: 10.1136/bjsm.2004.014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheffield-Moore M, Wolfe RR, Gore DC, Wolf SE, Ferrer DM, Ferrando AA. Combined effects of hyperaminoacidemia and oxandrolone on skeletal muscle protein synthesis. Am J Physiol Endocrinol Metab. 2000;278:E273–E279. doi: 10.1152/ajpendo.2000.278.2.E273. [DOI] [PubMed] [Google Scholar]


