Abstract
Classical cadherins have been proposed to mediate interactions between pre- and postsynaptic cells that are necessary for synapse formation. We provide the first direct, genetic evidence in favor of this model by examining the role of N-cadherin in controlling the pattern of synaptic connections made by photoreceptor axons in Drosophila. N-cadherin is required in both individual photoreceptors and their target neurons for photoreceptor axon extension. Cell-by-cell reconstruction of wild-type photoreceptor axons extending within mosaic patches of mutant target cells shows that N-cadherin mediates attractive interactions between photoreceptors and their targets. This interaction is not limited to those cells that will become the synaptic partners of photoreceptors. Multiple N-cadherin isoforms are produced, but single isoforms can substitute for endogenous N-cadherin activity. We propose that N-cadherin mediates a homophilic, attractive interaction between photoreceptor growth cones and their targets that precedes synaptic partner choice.
Axons can select synaptic targets with great precision, and understanding the molecular logic underlying this process has been a central goal since the chemoaffinity hypothesis was first articulated1. Implicit in this hypothesis are interactions between afferents and their prospective synaptic partners, informing targeting decisions. Indeed, many cell surface molecules are expressed by neurons and have a variety of roles in guiding axons2. However, little is known about how afferent axons interact with their targets to choose the appropriate synaptic partner. Here we investigate the contributions of one group of cell adhesion molecules, the classical cadherins, to these processes.
Classical cadherins are an evolutionarily conserved group of Ca2+-dependent cell adhesion molecules3. Many cadherins show restricted nervous system expression and are often found at specific synapses4-7. Diverse functions have been ascribed to classical cadherins during nervous system development. For example, mutations in N-cadherin disrupt neural tube development, retinal patterning and axonal projection patterns in zebrafish8-10. Reducing N-cadherin activity can also disrupt axon and dendrite outgrowth in both chick and frog retinal ganglion cells, as well as in mouse thalamocortical neurons11-13. Classical cadherins also influence the sorting of motor neurons into pools in the spinal cord14.
A central hypothesis describing cadherin function at the synapse posits that classical cadherins mediate homophilic interactions between pre- and postsynaptic cells that enable synapses to form4,15,16. However, the potential for redundancy within the vertebrate cadherin family, as well as the complex developmental functions of these genes, have made direct tests of this idea difficult.
By contrast, the Drosophila genome encodes only three classical cadherins: shg (E-cadherin), CadN (N-cadherin) and a third cadherin of unknown function, CadN2 (N-cadherin2)17. E-cadherin is widely expressed, and shg mutations affect epithelial integrity and neural proliferation18-20. The CadN locus contains three alternately spliced exons encoding portions of two cadherin repeats as well as the transmembrane domain, generating 12 isoforms21 (C.-H. Lee, personal communication). Embryos homozygous for a null CadN allele have complicated axon tract defects21.
In mosaic flies in which a subset of neurons lacks N-cadherin, specific defects emerge. In the olfactory system, mutant olfactory receptor neurons (ORNs) show defects in glomerulus formation, and projection neurons lacking N-cadherin fail to restrict their dendrites to the correct glomerulus22,23. These phenotypes do not reflect N-cadherin–mediated interactions between projection neurons and ORNs; rather, N-cadherin may regulate afferent-afferent interactions in ORNs and dendrodendritic interactions in projection neurons. Here, N-cadherin could attract ORN axons and projection neuron dendrites of the same type to one another or could repel axons and dendrites from different classes of ORNs and projection neurons from one another22.
Photoreceptor cells (R cells) require N-cadherin to choose synaptic targets24-26. In the retina, R cells form clusters, called ommatidia, each containing eight cells (R1–8). R1–6 axons travel to the first optic neuropil, the lamina, where they make lateral projections to specific targets. R7 and R8 axons make layer-specific connections in the second optic ganglion, the medulla. Both R cells and their target neurons express N-cadherin. Although N-cadherin mutant axons extend to the appropriate ganglia, both the lateral projections of R1–6 within the lamina and the layer-specific targeting of R7 depend upon N-cadherin function25.
Here we use mosaic analysis to determine where N-cadherin is required for R1–6 targeting. We show that N-cadherin mediates an attractive interaction between photoreceptors and lamina neurons in the target region.
RESULTS
N-cadherin is required for R1–6 target selection
To probe whether classical cadherin diversity contributes to R-cell target selection, we characterized the phenotypes associated with mutations in CadN, CadN2, and shg. E-cadherin, like N-cadherin, is expressed in R1–6 axons (Fig. 1a,b), suggesting that it, too, might play a role in target selection. As mutations in shg are embryonically lethal, and as E-cadherin function is required to maintain the integrity of the retinal epithelium during larval development, we generated single-cell mosaics by modifying the mosaic analysis with a repressible cell marker (MARCM) method, expressing the recombinase FLP under the control of the photoreceptor-specific GMR promoter25,27. This approach renders approximately 15% of R1 and R6 cells homozygous mutant in otherwise heterozygous and phenotypically wild-type animals24. The mitotic recombination events that produce these homozygous cells take place in the immediate progenitor to R1 and R6, bypassing the early requirement for E-cadherin. Under these conditions, R1 and R6 cells mutant for shg innervated the appropriate target neurons (25/25 axons, data not shown). Although we cannot exclude the possibility of E-cadherin perdurance, these results suggest that E-cadherin does not play a role in R1–6 target selection.
Figure 1.
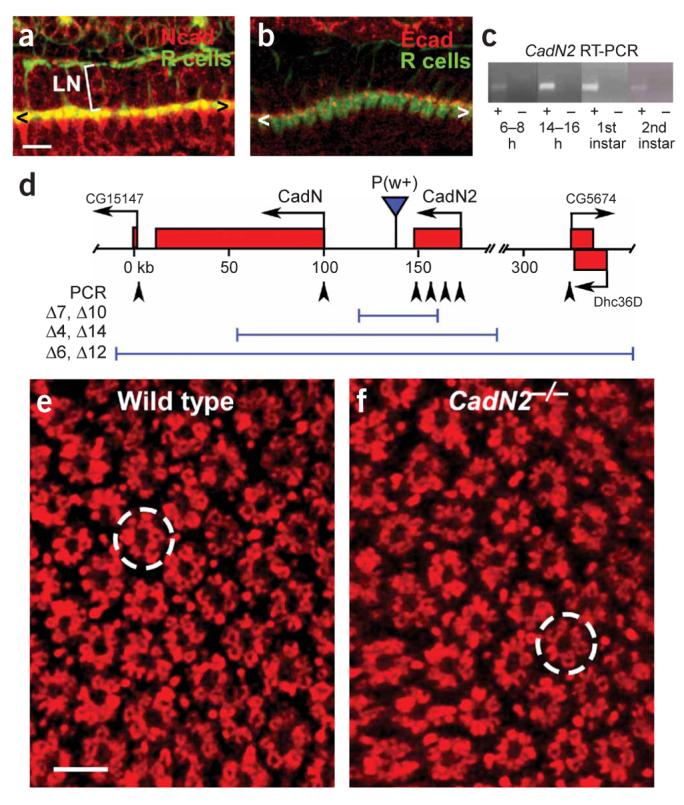
N-cadherin is the only classical cadherin involved in R-cell target selection. (a) N-cadherin expression in the lamina during mid-pupal development. N-cadherin (red) is expressed in R-cell axons (green) and their targets at this stage. < and > mark the lamina plexus. LN, lamina neurons. (b) E-cadherin expression in the lamina. E-cadherin (red) shows punctate expression in R-cell axons (green). (c) RT-PCR analysis of N-cadherin2 transcript expression at multiple developmental stages. N-cadherin2 is expressed during both embryonic and larval stages. (d) Schematic drawing of the CadN–CadN2 locus. Molecular null alleles of CadN2 and of both N-cadherins in combination were isolated by X-ray–mediated deletion of a P element inserted between the two loci. Deletions of three classes of different size, as defined using PCR-mediated amplification of known regions (arrowheads), were isolated. In all subsequent analyses, CadN2Δ7 and CadN–CadN2Δ14 were used as reference stocks. (e) Cross-section of the wild-type adult lamina, stained with an R cell–specific antibody. Individual cartridges are regularly arrayed; they comprise clusters of six R-cell axons surrounding an unlabelled space (which contains the processes of lamina neurons). A single cartridge is circled. (f) Cross-section of the lamina in a CadN2Δ7 homozygote. Neither the overall arrangement of cartridges nor the number of R-cell axons per cartridge are altered in this background. Scale bar: 10 μm in a and b;5 μm in e and f.
We observed N-cadherin2 expression at several developmental stages (Fig. 1c), raising the possibility that CadN2 might affect photoreceptor development. To isolate mutations affecting CadN and CadN2, we used X-ray irradiation to generate deletions in the locus (Fig. 1d). Molecular analysis of these identified two CadN2 deletions, two molecular nulls deleting both CadN and CadN2, and two large deletions that extended into neighboring loci. Homozygous CadN2 null mutants were viable and showed no defects in the spacing or composition of fascicles in the lamina (Fig. 1e,f). Although N-cadherin2 is not necessary for normal photoreceptor axon extension, it does have a partially redundant function with N-cadherin, as we describe below.
In all subsequent experiments, we analyzed N-cadherin function using a deletion disrupting both CadN genes (denoted CadNΔ).
N-cadherin is required cell autonomously in all R1–6 cells
R-cell axons extend from the eye to the brain in fascicles containing all eight R-cell axons from the same ommatidium. Within the lamina plexus, each R1–6 cell axon innervates a specific, spatially defined cartridge containing the processes of five monopolar lamina neurons, designated L1–L5 (ref. 28). To select a cartridge, each R-cell axon extends away from the fascicle containing all of the R-cell axons from the same ommatidium, following a trajectory that is both invariant and characteristic of each R-cell subtype. This trajectory connects a starting point, the cartridge of origin, with a second cartridge, the target, located at a precisely defined relative position. Synapse formation does not begin until R-cell axons reach their target cartridge. To address whether each R-cell axon requires cell-autonomous N-cadherin function, we used the MARCM method, expressing the FLP recombinase under the control of the heat-shock promoter to generate mosaic flies. In this way, sparsely distributed R cells of all subtypes were labeled and made homozygous for CadNΔ in an otherwise heterozygous animal. We identified each R cell in the retina by its distinctive rhabdomere morphology and position within the ommatidium, traced its axon into the brain and determined its cartridge of origin and its target (Fig. 2).
Figure 2.
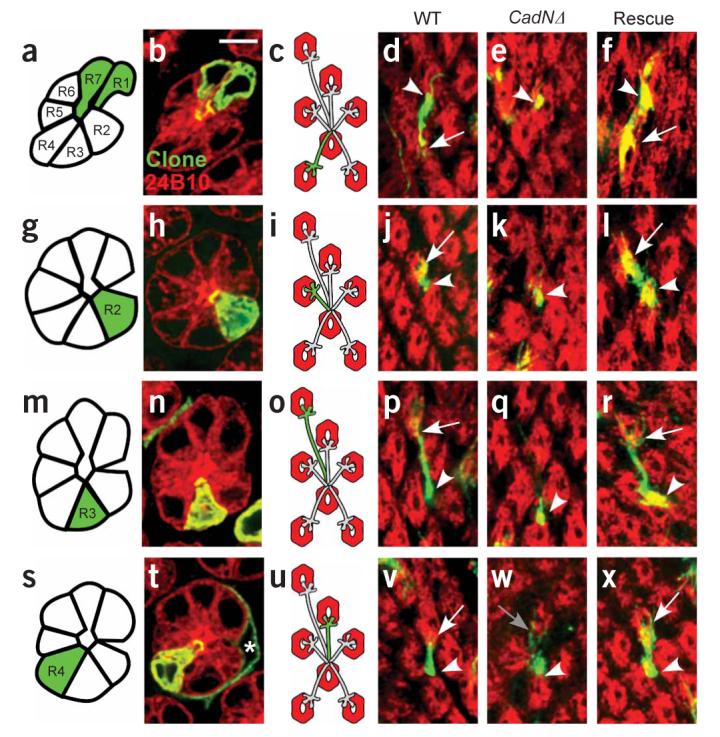
Single-cell MARCM analysis of N-cadherin function in R cells. Each row of the figure corresponds to a single R-cell subtype, and each column to a specific genetic background. (a,g,m,s) Schematic of the retina. Each row contains a single mutant R1–6 cell (green) surrounded by otherwise wild-type neighbors (white). (b,h,n,t) Confocal images of single ommatidia with one R1–6 cell labeled. These images were collected using a pan–R cell marker (red) and a marker specific to each mutant cell (green). (c,i,o,u) Schematic representation of labeled R-cell axons. (d–f,j–l,p–r,v–x) Confocal images of single R-cell axons. Single mutant R cells are green, whereas target cartridges containing all other R-cell axons are labeled red. a–f, R1. g–l, R2. m–r, R3. s–x, R4. a–d, g–j,m–p,s–v: wild type (WT). e,k,q,w: CadNΔ mutant R-cell axons. f,l,r,x: CadNΔ mutant R cells expressing a single isoform of N-cadherin, denoted ‘Rescue’. Arrowheads mark the start of each axon extension; arrows mark the end. Panel w shows an R4 axon that makes an aberrant extension of a few filopodia (gray arrow). Scale bar, 5 μm.
In control experiments, wild-type, single R-cell clones extended to target cartridges appropriate to their identity (n = 32/32; Fig. 2a-d, g-j,m-p,s-v; Fig. 3a,b). In addition, in clones containing multiple labeled R cells, individual axon extensions were resolved and targeted correctly (n = 68/68). In contrast, although the growth cones of CadNΔ R-cell clones invariably reached the lamina normally, their extensions within the lamina plexus were always highly abnormal (Fig. 2e,k,q,w; Fig. 3a,b). In particular, in approximately 59% (n = 132/222) of cases we observed no extension at all toward the target cartridge, and in the remaining 41% (n = 90/222) of cases only a few thin, highly branched filopodia were seen. These observations were consistent across all R1–6 cell subtypes. R-cell axons mutant for CadN (and not CadN2) showed qualitatively similar phenotypes, but milder extension defects were more frequent (Fig. 3a). Only 36% (n = 28/77) of CadN mutant R-cell axons did not extend, a proportion smaller than we observed in CadNΔ R cells (P < 0.001, Fisher's exact test). This suggests that CadN2 functions redundantly with CadN. We conclude that N-cadherin is required cell autonomously in all R-cell subtypes.
Figure 3.
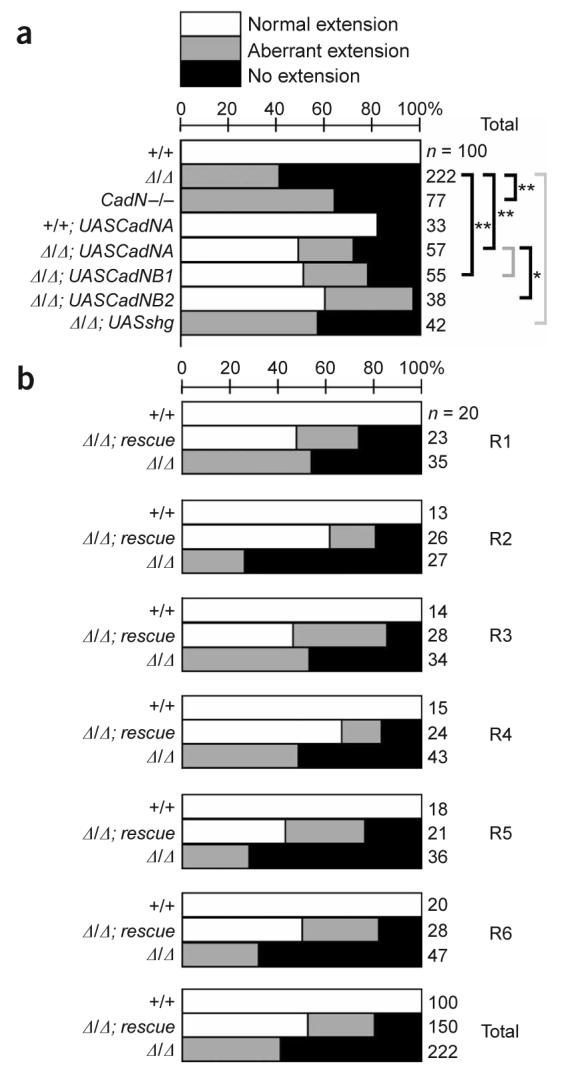
A single N-cadherin isoform can rescue extension in all R-cell subtypes. (a) The distribution of extension phenotypes, pooled across all R-cell subtypes. For each genotype, axons were classified as normal (white bars), aberrant (gray bars), or not extending (black bars). Number of axons indicated at right. Genotypes assessed were wild type (+/+), CadNΔ/Δ, CadN−/−, wild-type cells ectopically expressing CadNA (+/+; UASCadNA), CadNΔ/Δ expressing CadNA (Δ/Δ; UASCadNA), CadNΔ/Δ expressing CadNB1 (Δ/Δ; UASCadNB1), CadNΔ/Δ expressing CadNB2 (Δ/Δ; UASCadNB2), and CadNΔ/Δ expressing either one or two copies of E-cadherin (Δ/Δ; UASshg). Statistical comparisons (bracketed) used Fisher's exact test; *, P < 0.01, **, P < 0.001; gray brackets denote comparisons that were not statistically significant. (b) Each R-cell subtype depends on N-cadherin function. Data from the CadNA and CadNB transgenes are pooled as Δ/Δ; rescue.
These phenotypes were scored at the first developmental stage in which defects in extension could be evaluated. To confirm that the observed defects reflected permanent failures in R-cell target selection, we generated single mutant R1 and R6 cell axons and scored axon extension in otherwise wild-type adult animals. Wild-type axons extended to their targets normally (n = 37/38), whereas only 31% of CadNΔ mutant R cells reached their targets (n = 50/162). Compared with our observations in CadNΔ R cells at the early time point, complete failures of axon extension were less frequent in adults. This difference is likely to result from later stabilization of aberrant extensions that were observed early in development but could also be caused by differences in the strains and methods used to generate clones at each time point. We conclude that our assessment of R-cell extension during development largely reflects permanent deficits in target choice.
Isoform diversity does not instruct R1–6 target choice
To test whether N-cadherin isoforms might be functionally distinct, we replaced intrinsic N-cadherin activity with transgene-mediated expression of a single splice form and then observed R1–6 target selection. To do this, we used the MARCM method to drive transgene expression only in homozygous mutant neurons. We tested three transgenes for their rescuing capacity. One of these, designated CadNA, expressed one set of alternate exons (7B 13A 18A); the other transgene, CadNB, represented by two independent insertions (CadNB1 and CadNB2), expressed the complementary set (7A 13B 18B). Both transgenes effectively rescued R-cell axon extension: we observed 49% (n = 28/57), 51% (n = 28/55) and 61% (n = 23/38) of normal extension for mutant R cells expressing CadNA, CadNB1 and CadNB2, respectively (Fig. 3a). This compares with normal extension in 82% (n = 27/33) of wild-type cells ectopically expressing CadNA and 0% of CadNΔ mutant cells (n = 0/222, Fig. 3a). All R-cell subtypes were rescued with comparable efficacy (Fig. 2f,l,r,x; Fig. 3b). We attribute the difference in efficacy of rescue between CadNA and CadNB2 to differences in transgene expression, rather than to isoform-specific differences, because the CadNB1 transgene gives equivalent rescue to CadNA. As the strength of the rescue provided by either CadNA or CadNB was comparable across all R-cell subtypes, the data from these two experiments are pooled as Δ/Δ; rescue (Fig. 3b). To extend these experiments, we tested a third N-cadherin isoform (7B 13A 18B), and we obtained comparable results (S.P., T.R.C. and S.L. Zipursky, unpublished data). Therefore, expression of a single N-cadherin isoform in photoreceptors is sufficient to promote significant rescue of R-cell axon extension.
To address whether N-cadherin isoform diversity contributes to an R-cell axon's choice of cartridge, we determined whether mutant growth cones that express a single isoform, and that do extend, innervate the correct target. We observed that extending CadNΔ R cells expressing either the CadNA or the CadNB transgene rarely selected the wrong targets, with 89% (n = 25/28) and 94% (n = 48/51), respectively, of extending cells selecting targets appropriate to their identity (as compared with 96% of wild-type R cells ectopically expressing CadNA; n = 26/27). Therefore, N-cadherin isoform diversity is not required in photoreceptors for target choice.
N-cadherin cannot functionally substitute for E-cadherin
The absence of an instructive role for N-cadherin isoforms suggested that classical cadherins might be functionally interchangeable. We observed low E-cadherin expression in photoreceptor axons (Fig. 1b), and this level of expression was unaffected by the loss of N-cadherin (data not shown). We tested whether augmenting this basal expression could compensate for the loss of N-cadherin by expressing E-cadherin transgenes in >CadNΔ R cells. Under these conditions, E-cadherin staining became abundant in mutant growth cones (data not shown). We tested two different levels of E-cadherin expression, using either one or two transgene copies, and did not observe rescue in any case, with 0/18 and 0/24 R-cell axons extending, respectively. Therefore, E-cadherin and N-cadherin are not interchangeable.
Cartridge assembly requires N-cadherin in lamina neurons
As N-cadherin acts homotypically, and cadherins have been proposed to mediate interactions between pre- and postsynaptic cells, we tested whether N-cadherin is required in neurons in the target field. Indeed, lamina neurons, and not glia, express N-cadherin25 (Fig. 1a). We removed N-cadherin from lamina neurons using the MARCM method and observed targeting of wild-type R cells innervating patches of mutant lamina neurons (Fig. 4). Notably, removing N-cadherin from lamina neurons had no effect on their differentiation, morphology or projections out of the lamina (but did affect the layer-specific targeting of these cells in the lamina; Fig. 4e and data not shown).
Figure 4.
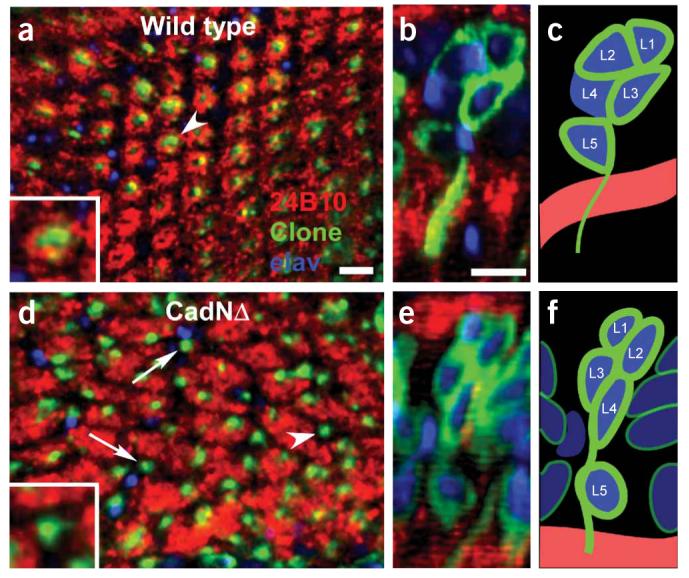
N-cadherin function is required in lamina neurons for cartridge assembly. (a) Cross section of the lamina in wild-type neurons. R-cell axons (red) form a regularly repeated array of cartridges, each surrounding the processes (green) from the column of lamina neuron cell bodies positioned above the plane of view. Inset corresponds to a high-magnification view of a single cartridge (white arrowhead). (b) Reconstruction of the column of cell bodies located above the marked cartridge in a. Each nucleus is labeled with a pan-neuronal marker, elav (blue), and the outlines of each homozygous cell are labeled with green fluorescent protein (GFP; green). R-cell axons (red) are also visible within the plexus, below the column. (c) Schematic reconstruction of the column in b. In this case, L1, L2, L3 and L5, but not L4, are homozygous for the control chromosome. (d) Cross-section of the lamina in CadNΔ neurons. R-cell axons form irregularly spaced cartridges of uneven size. Bundles of lamina neuron processes are frequently observed with no surrounding R-cell axons (white arrows), an arrangement that occurs when all R-cell axons initially associated with the column extend away, and no R-cell axons target to the column. Inset shows a high-magnification view of such a bare cartridge (arrowhead in d). (e) Column of nuclei above the single cartridge (arrowhead in d), (f) Schematic representation of e. In this case, L1–L5 are all homozygous for CadNΔ. Scale bar, 5 μm.
In such patches, cartridges were severely disrupted. In control patches, R-cell axons form uniform, circular cartridges consisting of six R1–6 termini surrounding a bundle of lamina neuron processes (4/4 optic lobes, Fig. 4a-c). In contrast, cartridges within or bordering a patch of lamina neurons homozygous for CadNΔ were eccentric, abnormal in size (reflecting abnormal numbers of R-cell termini innervating the cartridge) and frequently lacked all photoreceptor innervation (33/35 optic lobes; Fig. 4d-f). Therefore, N-cadherin activity is required in lamina neurons for R-cell axons to integrate into cartridges.
N-cadherin attracts R-cell axons to target cartridges
To define these defects with single-cell resolution, we specifically labeled wild-type R4 axons innervating mosaic patches of mutant lamina neurons (Fig. 5). The five lamina neurons extending processes to the same cartridge form a column above the cartridge in which each cell body occupies a characteristic position28 (Fig. 5a). As essentially all neuronal nuclei in the lamina cortex are lamina neurons, this conserved anatomy enabled us to serially reconstruct each column, assigning each lamina neuron to a cartridge and determining its subtype (L1–L5) and genotype (homozygous mutant or not). We then examined the connections made by R4 axons extending into or out of each mutant cartridge (Fig. 5b-h). These patches included 305 mutant lamina neurons belonging to 110 lamina cartridges. Projections of 124 R4 axons were scored. Of these, 20 R4 axons did not extend, 17 extended to the incorrect target and 87 extended normally. Therefore, N-cadherin function in lamina neurons affects R4 axon targeting.
Figure 5.
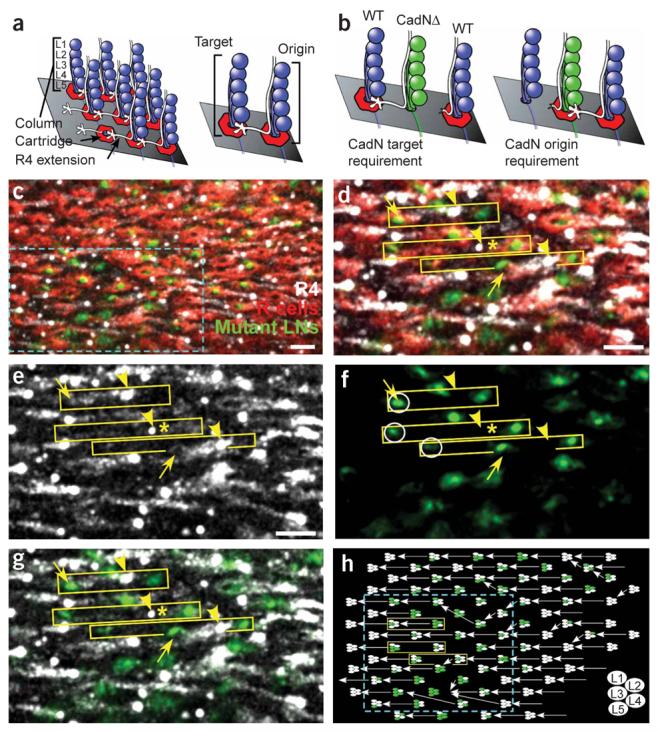
N-cadherin function is required in the target column, not the column of origin. (a) Lamina neurons form columns, each containing five cell bodies (blue circles, denoted L1–L5). Each R4 axon (white) is initially associated with a single column (origin); upon reaching the lamina plexus (grey plane) it extends laterally to a specific neighboring column (target). (b) The predicted phenotype if N-cadherin is required in the target column (left) or in the column of origin (right). (c) Cross-section of the lamina plexus in a region containing CadNΔ lamina neurons. Wild-type R-cell axons (red) form irregular cartridges surrounding mutant lamina neuron processes (green). Individual R4 axons (white) are seen as dots. Cyan box demarcates a subregion shown in d–g.(d) Each yellow box contains the lamina neuron processes from two columns that define the origin (right) and target (left) for a single R4 axon. Yellow arrowheads mark the start of the R4 axon extension; yellow arrows indicate the terminus. R4 axons were observed to extend normally (upper box), fail to extend (middle box, asterisk next to axon), and misproject to a neighboring cartridge (bottom box). (e)R4 axons. (f)Mutant lamina neuron processes. The correct target of the three R4 axons is circled. (g) Overlay of e and f.(h) Schematic reconstruction of the patch shown in a. Each column is represented by five ellipses; mutant lamina neurons are green, wild-type neurons are white. Each arrow is the projection of a single R4 axon. Scale bar, 5 μm.
N-cadherin–mediated interactions between R cells and lamina neurons could either repel R-cell axons away from inappropriate cartridges or attract them toward the correct target. If N-cadherin function is required in a particular column for R4 extension, there should be a correlation between column genotype and R4 axon targeting (Fig. 5b). If N-cadherin is required in the target column, the genotype of that column should correlate with the behavior of the R4 axon extending toward it. By contrast, if N-cadherin function is required in the column of origin, there should be a correlation between column genotype and the behavior of the R4 axon that extends away from it.
We observed a strong correlation between target column genotype and R4 axon extension (Fig. 6). R4 axons were much less likely to extend normally to a target cartridge with two or more mutant lamina neurons (53% of R cells misprojected, n = 62 axons) than to cartridges with only zero or one mutant cells (6% of R4 axons misprojected, n = 62, P < 0.0001, Fisher's exact test). Conversely, there was no correlation between the genotype of the column of origin and R-cell extension. R cells did not extend normally from a cartridge of origin with two or more mutant lamina neurons (28%, n = 79) as often as they misprojected from cartridges with fewer than two mutant cells (33%, n = 45). Therefore, N-cadherin is required in the target cartridge, not the cartridge of origin, for R-cell axon extension.
Figure 6.
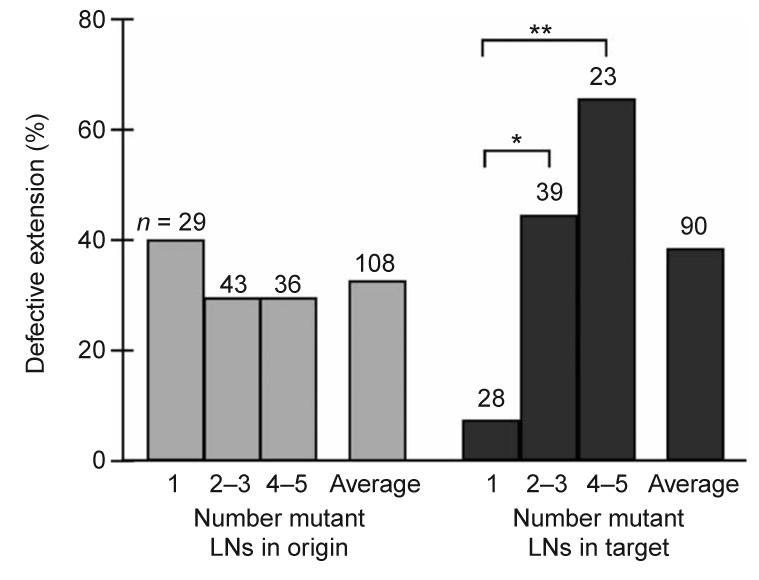
R-cell extension depends on the number of mutant lamina neurons in the target cartridge. The percentage of R4 axons that show defects in extension (either failing to extend or extending to the inappropriate cartridge) is plotted as a function of the number of mutant lamina neurons in the column of origin (gray bars) or target column (black bars). Cartridges are categorized by number of mutant cells per column (for example, a value of 2–3 indicates that columns containing either two or three mutant cells of five total were included). As the target column (but not the column of origin) contains an increasing proportion of mutant cells, the frequency of extension defects rises. *, P < 0.05. **, P < 0.01, Fisher's exact test. Average denotes the percentage of axon extension defects across all columns of each type. In some cases, we were unable to score either the R4 axon entering a given column, or the axon extending away from a given column, resulting in the differences in total number of columns scored in the two categories.
Why do R4 axons occasionally extend to inappropriate targets in these experiments? We reasoned that such projections could arise if R4 axons compare the relative N-cadherin activity between their normal target cartridge and an alternative cartridge close by. This hypothesis predicts that the genotype of the correct target cartridge (not innervated) should contain more mutant cells than the inappropriate, but chosen, cartridge. In the 17 such projections, the normal target cartridge contained significantly more mutant lamina neurons (2.9 mutant cells per cartridge) than the chosen, but incorrect, cartridge (1.8 mutant cells per cartridge, P = 0.048, Mann Whitney U). These results demonstrate that N-cadherin mediates attractive interactions between lamina neurons in the target cartridge and extending R-cell axons.
N-cadherin function is distributed among lamina neuron subtypes
The requirement for N-cadherin in target lamina neurons could be restricted to a specific neuron, limited to a subset of neurons or broadly distributed among the five cells in each column. Each scenario predicts a different distribution of mutant lamina neuron genotypes in target cartridges. If specific lamina neuron subtypes were particularly important for R-cell extension, we would expect those cells to be mutant more frequently than other lamina neurons when R4 axons fail to extend normally. We assessed only columns with at least one mutant cell and correlated the genotype of each individual lamina neuron subtype in the target column with the extension of the R4 axon (Fig. 7). As expected, the average number of mutant lamina neurons per column was greater in cases where R4 axons misprojected (3.2 mutant lamina neurons per column) than in cases in which the R4 axon projected normally (2.1 mutant lamina neurons per column, P < 0.0001, Mann-Whitney U-test). However, this enrichment was approximately uniform across lamina neuron subtypes, indicating that N-cadherin function in each lamina neuron in the target column contributes equally to R-cell axon extension.
Figure 7.
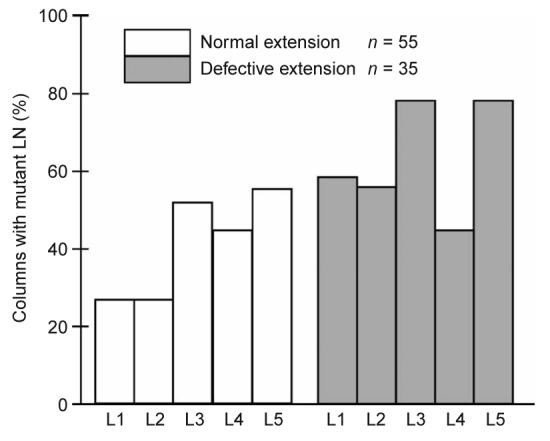
N-cadherin's role in R-cell extension is distributed among lamina neuron subtypes within the target column. The data is divided into two categories: cases where the R4 axon extended normally (white bars) and cases where R4 axons showed defects in extension (gray bars). Each bar shows the percentage of columns that contained a mutant cell of a particular lamina neuron subtype. Only cases in which at least one cell was mutant in each target column were included.
DISCUSSION
N-cadherin is the primary classical cadherin required for R1–6-cell target selection. This function is cell-autonomous to each R-cell subtype. Expression of a single N-cadherin isoform in a single mutant cell is sufficient to rescue the mutant phenotype, both for axon extension and for the choice of the correct group of target neurons. Elimination of N-cadherin from lamina neurons causes wild-type R-cell axons to show defects in extension. This requirement is restricted to target neurons toward which R-cell axons extend. We infer that N-cadherin mediates an attractive interaction between R-cell axons and their targets that stabilizes contact between them.
Cadherin diversity does not instruct R1–6 target choice
Classical cadherins are proposed to mediate homophilic interactions that specify connections between neurons16. The fly genome contains three classical cadherin loci and generates 12 isoforms of one of these, CadN, by alternative splicing. R1–6 cells express these molecules, and elaborate complex, genetically hard-wired connections, providing a suitable context to investigate such a specificity mechanism. Presynaptic manipulations of cadherin expression show, however, that cadherin diversity does not instruct R1–6 target choice. R-cell axons lacking either E-cadherin or N-cadherin2 target normally. R-cell axons lacking both N-cadherin and N-cadherin2 show defects qualitatively similar to those observed in axons lacking N-cadherin alone, but complete failures in axon extension are more common. We infer that N-cadherin2 can weakly augment N-cadherin activity in R1–6 axons. Rescue experiments in which N-cadherin function is replaced by ectopic E-cadherin expression show that these molecules are not functionally interchangeable. Analogous experiments show that N-cadherin isoform diversity in photoreceptors does not contribute to R-cell targeting: expression of a single isoform can rescue axon extension in single R cells homozygous for a deletion of the N-cadherin locus. Indeed, expression of a single isoform completely rescues the ability of extending N-cadherin mutant R-cell axons to choose the correct target. This result demonstrates that N-cadherin isoform diversity cannot uniquely label R-cell subtypes in a way required for target selection. Although we have not directly addressed the role of N-cadherin isoform diversity in target neurons, we infer that such diversity is unlikely to be necessary for normal R1–6 cell axon extension. We conclude that photoreceptor target choice depends on permissive, N-cadherin–mediated interactions. Recent biochemical experiments showing that N-cadherin isoforms bind in heterophilic fashion, as well as rescue experiments in other developmental contexts, are consistent with such a permissive role22,23 (C.-H. Lee, personal communication). Although classical cadherin gene and isoform diversity does not instruct R1–6 target choice, such diversity could still be important for targeting in the vertebrate nervous system, given the greater number of vertebrate cadherins and their complex expression patterns3,29.
N-cadherin mediates an attractive interaction in vivo
Although classical cadherins have been proposed to mediate interactions between afferent axons and their targets, no direct, in vivo evidence has, as yet, demonstrated such an interaction. We show that N-cadherin mediates an attractive interaction between R-cell axons and their targets. R-cell axons project along a precise trajectory beginning at one column of lamina neurons, the origin, and extend toward a second column, the target. To determine whether N-cadherin was required in the column of origin, the target column, or both, we have specifically removed N-cadherin from lamina neurons and examined the targeting of single wild-type R-cell axons. We have observed two types of defects: R-cell axons that do not extend, and R-cell axons that misproject, innervating the incorrect target. These defects correlate strongly with the genotype of the target column, not the origin. We infer that extension failures are caused by the absence of a stabilizing, homophilic attraction between the R-cell axon and its correct target that is mediated by N-cadherin. We interpret misprojection as resulting from R-cell axons comparing, perhaps through filopodial contact, the relative N-cadherin activity in two targets. We have observed that in these cases, R-cell axons typically choose the target with more adhesive activity, even if such a target is incorrect. In vitro experiments showing that N-cadherin can promote neurite outgrowth in cell culture are consistent with this view30,31.
Finally, we note that the phenotypes associated with removal of N-cadherin from lamina neurons are less severe than those associated with removal of N-cadherin from R-cell axons. We attribute this difference to the fact that it is not technically possible to generate clones in which all lamina neurons lack N-cadherin. Indeed, most of our target columns contain mixtures of mutant and wild-type cells. If we consider only those target columns in which all, or almost all, of the lamina neurons are mutant, the effects on R-cell axon extension are severe, with 65% (15/23 axons) of the projections showing defects. Thus, although it remains formally possible that N-cadherin may mediate additional interactions important for R-cell targeting, we favor a model in which the interaction between R cells and lamina neurons is the only one of functional import.
Synaptic partner choice: a two step process
To explore whether N-cadherin might match R1–6 cells with their synaptic partners, we have assessed whether the N-cadherin–mediated interaction between photoreceptors and their target neurons is restricted to the appropriate cells. After R1–6 axons reach their target cartridge, they make synaptic connections with a specific subset of the lamina neurons, L1–L3. We find that the N-cadherin requirement in lamina neurons for R-cell axon extension is not restricted to L1–L3, but rather includes L4 and L5. We infer that this N-cadherin–mediated interaction is distinct from the presumed interactions that determine which lamina neurons are suitable R-cell synaptic partners.
How, then, is synaptic specificity determined? One possibility is that N-cadherin acts at multiple steps in target selection. That is, although N-cadherin functions once to attract R-cell axons to their target cartridges, it may subsequently influence synaptic connectivity through a more specific interaction between R-cell termini and L1–L3. An alternate possibility is that the second step in selecting a synaptic partner after reaching the correct target cartridge is N-cadherin–independent. In this view, N-cadherin–mediated interactions enable R-cell axon termini to participate in these later, more specific signaling events. Either of these ideas is consistent with previously proposed models describing cadherin function at the synapse4.
N-cadherin regulation and photoreceptor targeting
R-cell axons are initially associated with one column of lamina neurons, the origin, but break this association to extend to a neighboring column, the target, where they will initiate synapse formation. N-cadherin is expressed by all lamina neurons in both columns. Why is N-cadherin function restricted to the target column, and why do photoreceptor axons not adhere to the column of origin?
One possibility is that N-cadherin adhesivity is temporally regulated post-translationally. That is, before photoreceptor axons project, N-cadherin in R cells, lamina neurons, or both, could be inactive. After R-cell axons project, adhesivity could increase, stabilizing the projections. Such temporal regulation could be at the level of post-translational changes in N-cadherin itself, as has been described for vertebrate N-cadherin32, or could occur through regulation of the molecules that couple N-cadherin to the cytoskeleton. Such mechanisms have been described for E-cadherin33,34. A second possibility is that N-cadherin function is spatially rather than temporally regulated. For example, perhaps spatial asymmetries in R-cell growth cones bias N-cadherin adhesion. Ultrastructural descriptions of the lamina during development show that R-cell growth cones are polarized, extending filopodia toward their presumptive target, and away from their origin28. Perhaps, then, the N-cadherin interaction between R cells and lamina neurons is inherently symmetric but, because R-cell growth cones extend more filopodia toward their presumptive targets (reflecting their polarization), an adhesive bias is created. Regardless of the precise mechanism involved, our results demonstrate that a ubiquitously expressed cell adhesion molecule can have very specific functions in controlling axon targeting.
METHODS
Genetics
N-cadherin locus deletions were generated by X-ray mutagenesis of a stock bearing P(w+) BG01221 (ref. 35). Rare F1 flies lacking the white+ marker were identified and individual deletions were molecularly defined.
Pupal MARCM was performed by expressing the FLP recombinase under control of a heat shock promoter; cells were positively labeled with elavGal4 controlling GFP expression. Axon projections were examined at 42% APF. Adult MARCM was performed by expressing the FLP recombinase under control of the GMR promoter; cells were positively labeled with Rh1Gal4 controlling β-galactosidase expression. R4 axons were labeled using mδlacZ36. Although this construct labels R3, R4 and R7 during larval development, during mid-pupal development, it is specific to R4.
Histological analyses
Brains were fixed and stained as described24. Photo-receptor axons were labeled with the monoclonal antibody 24b10, which recognizes chaoptin, and neuronal nuclei were visualized using rat antibody to elav (anti-elav; Developmental Studies Hybridoma Bank). Rat anti–N-cadherin and rat anti–E-cadherin were obtained from T. Uemura. GFP was either visualized directly or stained using rabbit anti-GFP (Molecular Probes). β-galactosidase was stained using mouse anti-lacZ (Promega). All secondary antibodies were obtained from Molecular Probes. Images were collected by confocal microscopy (Leica), deconvolved with Huygens Pro (Scientific Volume Imaging) and rendered using Imaris (Bitplane).
ACKNOWLEDGMENTS
The authors thank H.-T. Zhu and L. Luo for their generous donation of transgenes and stocks, C.-H. Lee and S.L. Zipursky for sharing reagents and for communicating results before publication, and T. Uemura for contributing his N-cadherin and E-cadherin antibodies to our work. We also thank J. Hatzidakis and B. Baker for their assistance with X-ray mutagenesis. L. Luo, J. Nelson, A. Katsov, M. Velez, K.-M. Choe, K. Clark, J. Mast and P.-L. Chen gave helpful comments on the manuscript. This work was supported, in part, by R01 EY015231-01A1 (T.R.C.). T.R.C. is a Sloan Fellow, a Searle Scholar and a recipient of a Burroughs-Wellcome Career Development Award.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 3.Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- 4.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 5.Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol. Cell. Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 7.Kohmura N, et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 8.Lele Z, et al. parachute/N-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- 9.Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev. Biol. 2003;259:95–108. doi: 10.1016/s0012-1606(03)00181-7. [DOI] [PubMed] [Google Scholar]
- 10.Masai I, et al. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- 11.Riehl R, et al. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuro. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 12.Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- 13.Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J. Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 15.Serafini T. Finding a partner in a crowd: neuronal diversity and synaptogenesis. Cell. 1999;98:133–136. doi: 10.1016/s0092-8674(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro L, Colman DR. The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- 17.Hill E, Broadbent ID, Chothia C, Pettitt J. Cadherin superfamily proteins in Caenorhabditis elegans and Drosophila melanogaster. J. Mol. Biol. 2001;305:1011–1024. doi: 10.1006/jmbi.2000.4361. [DOI] [PubMed] [Google Scholar]
- 18.Tepass U, et al. Shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- 19.Uemura T, et al. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996;10:659–671. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- 20.Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J. Neurosci. 2003;23:3325–3335. doi: 10.1523/JNEUROSCI.23-08-03325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwai Y, et al. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- 22.Hummel T, Zipursky SL. Afferent induction of olfactory glomeruli requires N-cadherin. Neuron. 2004;42:77–88. doi: 10.1016/s0896-6273(04)00158-8. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Luo L. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron. 2004;42:63–75. doi: 10.1016/s0896-6273(04)00142-4. [DOI] [PubMed] [Google Scholar]
- 24.Clandinin TR, et al. Drosophila LAR regulates R1–R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 26.Iwai Y, et al. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol. Cell. Neurosci. 2002;19:375–388. doi: 10.1006/mcne.2001.1081. [DOI] [PubMed] [Google Scholar]
- 27.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 28.Meinertzhagen IA, Hanson TE. The development of the optic lobe. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Press; Cold Spring Harbor, New York, USA: 1993. pp. 1363–1491. [Google Scholar]
- 29.Ranscht B. Cadherins: molecular codes for axon guidance and synapse formation. Int. J. Dev. Neurosci. 2000;18:643–651. doi: 10.1016/s0736-5748(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 30.Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J. Cell Biol. 1988;107:353–361. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka H, et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25:93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- 33.Fujita Y, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 34.Hogan C, et al. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand A, et al. Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics. 2001;158:1569–1595. doi: 10.1093/genetics/158.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]


