Abstract
To achieve greater understanding of the brain mechanisms underlying nicotine craving in female smokers, we examined the influence of nicotine non-abstinence vs. acute nicotine abstinence on cue-elicited activation of the ventral striatum. Eight female smokers underwent an event-related functional magnetic resonance imaging (fMRI) paradigm presenting randomized sequences of smoking-related and non-smoking related pictures. Participants were asked to indicate by a key press the gender of individuals in smoking-related and non-smoking related pictures (gender discrimination task), to maintain and evaluate attention to the pictures. There was a significant effect of smoking condition on reaction times (RT) for a gender discrimination task intended to assess and maintain attention to the photographs—suggesting a deprivation effect of acute nicotine abstinence and a statistical trend indicating greater RTs for smoking cues than neutral cues. BOLD contrast (smoking vs. non-smoking cues) was greater in the non-abstinent vs. acutely abstinent conditions in the ventral striatum including the nucleus accumbens (VS/NAc). Moreover, a significant positive correlation was observed between baseline cigarette craving prior to scanning and VS/NAc activation (r=0.84, p=0.009), but only in the non-abstinent condition. These results may either be explained by ceiling effects of nicotine withdrawal in the abstinent condition or, may indicate reduced relative activation (smoking vs. neutral contrast) in the VS/NAc in the abstinent vs. non-abstinent conditions in this group of female smokers.
Keywords: fMRI, Smoking, Tobacco, Cue reactivity, Ventral striatum, Nucleus accumbens
Introduction
A major contributor to substance abuse is the development of craving or desire for a particular drug when people are confronted with environmental stimuli (visual, olfactory, tactile, and imaginal) associated with use of substances such as alcohol and tobacco (Niaura et al. 1988, 1998; Rohsenow et al. 1990). Presumably this occurs because the drug user selectively processes these stimuli over other environmental stimuli. Such processing biases are predicted by positive incentive models of addiction, including early conditioning models (Stewart 1983, 1984; Stewart et al. 1984) and, more recently, Robinson and Berridge’s incentive sensitization model (Robinson and Berridge 1993, 2000). Positive incentive models broadly suggest that drug-paired stimuli stimulate appetitive, motivational processes, which generate craving, drug-seeking behavior, and compulsive drug use (Stewart 1983, 1984; Stewart et al. 1984). The incentive-sensitization model extends that model, and suggests that repeated use of substances that enhance mesolimbic dopamine neurotransmission, such as nicotine, results in permanent or semi-permanent adaptations in these neural mechanisms, which assign incentive salience to stimuli (Robinson and Berridge 1993, 2000). Incentive sensitization subsequently results in stimuli associated with these substances being assigned high levels of incentive salience, which corresponds to the subjective “wanting” of these substances (i.e., craving) and related stimuli.
The ventral striatum (VS) includes the core and shell of the nucleus accumbens (NAc) and is a region of particular interest because of its dual role in processing the hedonic effects of nicotine administration and in signalling the presence of drug-related environmental stimuli (Balfour 2002; Balfour et al. 1998; Benwell et al. 1995; Stein et al. 1998). Dopaminergic projections to the shell signal the presence of rewarding (i.e., drug) stimuli, facilitate the acquisition of behaviours related to obtaining the reward, and become desensitized with repeated drug exposure (Benwell et al. 1995).
One hypothesized consequence of this mechanism is a processing bias towards drug-related stimuli, and several behavioral studies have demonstrated evidence for a processing bias for smoking-related stimuli in smokers compared to non-smokers (Munafò et al. 2003), and abstinent smokers compared to non-abstinent smokers (Gross et al. 1993; Waters and Feyerabend 2000). A further consequence is the extent to which cues associated with drug use elicit cravings for these drugs. Drug-related cue reactivity is demonstrated not only by modulating drug craving (Carter and Tiffany 1999), but also through dopaminergically-mediated reductions in inhibition of the acoustic startle reflex (Hutchison et al. 1999, 2000, 2003; Niaura et al. 1988), cardiovascular reactivity (Carter and Tiffany 1999; Niaura et al. 1988), and altered skin conductance (Niaura et al. 1988). Furthermore, there is growing evidence, that conditioned sensory stimuli play an important role in the maintenance of drug use behavior, including nicotine, from both animal (Caggiula et al. 2001) and human (Rose et al. 2000, 2003) studies.
Functional neuroimaging has demonstrated that environmental cues associated with drug use activate an integrated network of brain regions involved in the motivational and appetitive processes of addiction to drugs of abuse (Breiter and Rosen 1999; Koob and Le Moal 2001; Volkow et al. 2003). To date, however, relatively few studies have investigated the neural correlates of these behavioral effects with respect to nicotine addiction. Studies of nicotine-dependent smokers using positron emission tomography (Brody et al. 2002, 2004) and functional magnetic resonance imaging (fMRI) (Due et al. 2002) have demonstrated that smoking-related cues activate regions associated with dopamine-dependent incentive-sensitization processes in multiple cortical (ventral anterior cingulate gyrus, middle frontal gyrus, prefrontal cortex [inferior and middle frontal gyri], parietal cortex, fusiform gyri) and subcortical (amygdala, posterior hippocampus, medial thalamus, ventral striatum/nucleus accumbens, ventral tegmental area), limbic regions. Furthermore, amongst overnight abstinent smokers, our group recently demonstrated a difference between smokers and non-smokers in the VS including the nucleus accumbens (VS/NAc) associated with smoking-related cues compared to neutral cues using fMRI (David et al. 2005). Another fMRI study, by (McClernon et al. 2005), demonstrated greater activation for smoking-related cues than neutral cues in the ventral anterior cingulate gyrus and superior frontal gyrus. Moreover, the authors found that at the individual level, abstinence-induced changes in self-reported tobacco craving (abstinence minus satiety) were significantly correlated with fMRI-BOLD response in several frontal regions (inferior frontal gyrus, left anterior cingulate gyrus, middle frontal gyrus) and there was a significant trend for a correlation with the NAc.
Given this background, we sought to use fMRI to determine whether smoking abstinence would affect VS/NAc activation to smoking-related pictorial cues. First, based on our own (David et al. 2005) and other work (Heinz et al. 2004; McClernon et al. 2005; Smolka et al. 2006), we hypothesized that there would be a significant correlation between reactivity to smoking cues and VS/NAc activation in general. Second, we sought to determine whether such activation would differ according to smoking abstinence. Given the paucity of neuroimaging data on abstinence inductions in smokers, we considered the direction (relative activation or deactivation) of any abstinence effects to be uncertain, but, based on the information above; we hypothesized that VS/NAc activation associated with smoking-related vs. neutral pictorial cues (% signal change) would be higher during the condition of abstinence than non-abstinence, and that the % signal change for the smoking vs. neutral cue contrast would be significantly and positively correlated with tobacco craving and nicotine withdrawal symptoms.
Materials and methods
Design
The study employed a 2 (condition: non-abstinence vs. acute abstinence) × 2 (session: session 1 vs. session 2) within-subjects design to evaluate acute abstinence effects on smoking-related pictorial cue-elicited activation of the VS/NAc in addicted smokers. For brevity, when describing the two abstinence-related scanning sessions, we will use the terms ‘abstinent’ or ‘abstinence’ beyond this point in reference to acute nicotine abstinence (overnight abstinence verified by exhaled CO; details in “Procedure” subsection). Smokers were randomized in counterbalanced fashion, such that they would either attend a sequence comprising a non-abstinent day (N), followed by an abstinent (A) day, a second N day, and finally a second A day (i.e., NANA), or an alternate sequence (i.e., ANAN) of scanning sessions, in order to avoid potentially confounding results. Once randomized, all participants were asked to agree to attend four scanning sessions on four separate days consisting of two non-abstinent and two abstinent days.
The rationale for this design was four-fold. First, by averaging results from sessions on separate days we are able to increase the reliability of our inference of fMRI BOLD signal for a particular task and brain region (Heiervang et al. 2006; Johansen-Berg et al. 2002). Secondly, the use of multiple days makes more statistically powerful repeated-measures analysis possible. Third, we were interested in abstinence effects on ventral striatum activation associated with smoking-related pictorial cues and therefore required verified abstinent and non-abstinent within-subjects contrasts. Finally, and a 2-×-2 factorial [2 (condition: non-abstinent vs. abstinent) × 2 (session: session 1 vs. session 2)] within-subjects design made it possible to examine whether or not there were in fact main effects of abstinence on BOLD contrast or if the apparent differences between abstinent and non-abstinent scanning days were explained entirely by day-to-day random variation resulting from any number of confounds. Figure 1 presents a diagram illustrating the paradigm design.
Fig. 1.
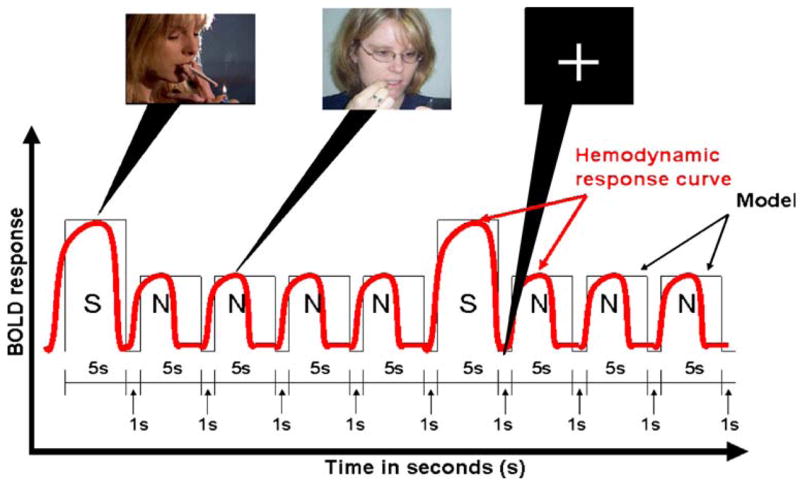
Illustration of predicted blood-oxygen-level-dependent signal during a smoking cue reactivity paradigm. S smoking-related picture, N non-smoking/neutral picture, the fixation cross is present during the 1 s inter-stimulus intervals
Participants
The study population consisted of 12 right-handed current smokers (67% female), aged ≥18 years, who had previously participated in the Patch II study (ICRF 1993; Yudkin et al. 2004) and had consented to be contacted for future research. Exclusion criteria included any unstable medical condition, contraindications to MRI scanning as determined through screening with a standard safety questionnaire, pregnancy, self-reported current Axis I psychiatric disorders other than nicotine dependence, use of psychotropic medications, or current use of nicotine replacement therapy. All study procedures were approved by the Central Oxfordshire Research Ethics Committee, and participants provided written, informed consent. Data on four participants was incomplete due to a failure to attend all scanning sessions. The final complete sample therefore consisted of eight smokers (100% female), aged 45–67 years (M=55 years, SD=9 years).
Cue paradigm
The cue exposure employed was the same as that employed previously (David et al. 2005), but consisted of a greater number of volumes (200 volumes) than in our earlier study (150 volumes) in order to improve statistical power. The paradigm consisted of 100 pictorial cues, randomized for presentation sequence and presented for 5 s at a frequency of one image every 6 s, with a fixation cross during rest periods (1 s inter-stimulus intervals), in an event-related design. Picture cues were smoking-related (31%) and neutral (69%) scenes from the International Smoking Image Series (Gilbert and Rabinovich 1999). Figure 1 illustrates the stimulus paradigm model.
Gender discrimination task
In order to encourage participants to maintain attention to the pictures and to assess potential attentional bias toward smoking-related photographs, participants were instructed to indicate with a keypress whether they thought the subject in the photograph was male or female, and reaction times for responses were recorded. In order to control for the potential confound of gender on neural responses to the smoking-related cues, the photographs in the paradigm were gender-balanced (i.e., same number of photographs with male and female faces).
Materials
Self-reported behavioral data
Questionnaire measures included the Fagerström Test of Nicotine Dependence (FTND) (Heatherton et al. 1991), the Shiffman-Jarvik Craving Scale (SJCS) (Shiffman et al. 1998) and the Minnesota Withdrawal Scale (MNWS) (Hughes and Hatsukami 1986; Hughes et al. 1986). The SJCS consists of five items: (1) “I have a desire for a cigarette right now,” (2) “If it were possible, I would smoke right now,” (3) “All I want right now is a cigarette,” (4) “I have an urge for a cigarette,” (5) “I crave a cigarette right now”). Subjects are asked to rate each item from 0–100 (total 500 maximum). We included seven items with the highest scale/subscale correlations (r=0.52–0.74) and high external construct validity with other scales of nicotine dependence severity (Etter and Hughes 2006), from the ten item MNWS (range 0–4 per item; total 28 maximum). The items include (1) “anger, irritability, frustration,” (2) “anxiety, nervousness,” (3) “difficulty concentrating,” (4) “impatience, restlessness,” (5) “hunger,” (6) “depression,” and (7) “desire to smoke.”
Pictorial stimuli were selected from the International Smoking Image Series (ISIS) as employed previously in fMRI studies (David et al. 2005; Due et al. 2002; Gilbert and Rabinovich 1999) and comprised of standardized color pictures of human male and female faces engaged in either cigarette smoking (smoking-related) or a behavior unrelated to smoking (neutral). Pictures from this series (ISIS) that did not include faces were excluded because of the potential confounds posed by differential visual processing of faces, other body parts, and inanimate objects by ventral extra-striate visual-spatial pathways (Ishai et al. 1999).
Procedure
‘Abstinent days’ required overnight abstinence from cigarette smoking or use of other tobacco or nicotine products for at least 12 h and participants were explicitly instructed to refrain from smoking any time after going to bed the evening prior to the scans. ‘Non-abstinent days’ involved instructions to participants to smoke according to their usual routines to the point of satiation with the last cigarette smoked prior to entering the scanning center. Prior to scanning, participants’ abstinence (or non-abstinence) status was confirmed by exhaled carbon monoxide monitoring, with a threshold of ≤10 parts per million (p.p.m.) to verify acute abstinence. Participants who failed to abstain from smoking prior to abstinent sessions were re-scheduled.
Pre- and post-scan self-reported withdrawal and craving
While the participants were situated within the magnet, both immediately prior to initiation of and immediately after the stimulus presentation and scanning, an investigator outside of the magnet chamber using a microphone asked the participants to rate from 0–4 the severity of their present experiences of each MNWS item and to rate from 0–100 their agreement with each item of the SJCS. The investigator in the scanner operator room recorded the participants’ verbal responses to each item.
We chose to include a greater frequency of neutral pictures than smoking pictures in order to minimize cue-induced BOLD latency during neutral and rest periods and as a standard approach to presenting target images as has been employed in odd-ball designs (Garreffa et al. 2004). Furthermore, we chose to randomize the sequence of picture cue type (i.e., smoking-related or neutral) to avoid potential bias arising from repetitive patterns of cue sequences. The sequence of images was determined by randomizing three sequences of picture blocks consisting of eight neutral (n) and two smoking (s) pictures (nnnnsnnnns), five smoking and five neutral pictures (nsnsnsnsns), and nine neutral pictures and one smoking picture (nnnnnnnnns).
Whole brain functional MRI data were acquired continuously through the period of visual stimulus presentation with a 3 Tesla whole-body scanner (Siemens, Erlangen, Germany) with a quadrature birdcage head coil. Before the first presentation of stimuli, sagittal localization (two-dimensional spoiled gradient recall acquisition; nine slices around midline; 5 mm thick) was performed. For functional imaging during stimulus presentation, echo planar T2*-weighted axial images were acquired (TR=3,000 ms, TE=30 ms, flip angle=90°, in-plane resolution 4×3.5 mm2, slice thickness=5 mm). Following functional imaging, three-dimensional, high resolution T1-weighted structural images were obtained with 128 axial slices (TE=5 ms, flip angle=120°, TR=15 ms, in-plane resolution=1.5 × 1 mm2).
Statistical analysis
Data pre-processing
Data pre-processing was conducted using FEAT (FMRI Expert Analysis Tool) Version 5.42 from the FMRIB Software Library http://www.fmrib.ox.ac.uk/fsl). Pre-statistical processing was as follows: motion correction using FMRIB’s Linear Registration Tool (MCFLIRT) (Jenkinson and Smith 2001); exclusion of non-brain areas using FMRIB’s Brain Extraction Tool (BET) (Smith 2002); spatial smoothing with a Gaussian kernel of 5 mm full-width half maximum; mean-based intensity normalisation to remove linear trends; and, non-linear high-pass temporal filtering to exclude low frequency confounds such as breathing (Gaussian-weighted least squares straight line fit, with sigma=25.0 s). Time series statistical analysis was carried out using FMRIB’s Improved Linear Model (FILM) with local autocorrelation correction (Woolrich et al. 2001).
Multilevel linear modelling for fMRI group analysis
A multilevel voxel-wise hierarchical analysis was performed with separate general linear model analyses for each of the four levels in the hierarchy (1: within-session level, 2: within-subject level, 3: group level within condition, 4: group between conditions level). Summary statistics from voxel-wise analyses at each level were entered as inputs into each higher level analysis, which in turn generated summary statistics. Effect sizes for the smoking vs. neutral contrast are expressed as percent (%) signal change for generalizable interpretation. This multilevel approach using Bayesian inference provided the means to assess the full uncertainty of the % signal change at the highest level; taking into account the unknown random and fixed variance components within each level in the model (Beckmann et al. 2003; Woolrich et al. 2004):
(1) In the first-level voxel-wise GLM analysis smoking vs. neutral conditions were modelled as explanatory variables. Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance level of p=0.05. Registration to high resolution T1 structural images of each individual was carried out using FMRIB’s Linear Registration Tool (FLIRT) (Jenkinson et al. 2002) and both were co-registered to standard (Montreal Neurological Institute) space. Contrasts at this level examined whether the parameter estimate (PE) of the hemodynamic response to smoking-related pictures was greater than the PE for the hemodynamic response to neutral pictures.
All higher-level analysis was carried out using FMRIB’s Local Analysis of Mixed Effects (FLAME) (Beckmann et al. 2003; Woolrich et al. 2004). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z>2.3 and an adjusted corrected cluster significance threshold of P=0.05, corrected for multiple comparisons (Worsley et al. 1992).
(2) Second-level, voxel-wise, fixed-effects GLM analyses were conducted separately for each participant to determine the average relative activation of both sessions in each condition (smoking and abstinent).
(3) The third-level, voxel-wise, random effects GLM analyses examined whether or not there were regions of significant activation associated with smoking-related picture cues in the group of subjects for both conditions; separate analyses were performed for the smoking condition and for the abstinent condition.
(4) The fourth-level voxel-wise GLM analysis examined whether or not there was a paired-difference in relative activation between smoking and abstinent conditions using FLAME.
In addition to voxel-wise comparisons, region-of-interest (ROI) analyses were conducted to test hypotheses regarding the effects of smoking condition on mean VS/NAc % signal change. ROI anatomical masks were defined using the averaged group T1 structural image normalised to standard space for the VS/NAc using FSL View software http://www.fmrib.ox.ac.uk/fsl). The brain atlas by Duvernoy (Duvernoy 1999) was used as a guide for defining anatomical landmarks. Criteria for defining the mesolimbic VS were those published by (Mawlawi et al. 2001) and, as previously described (David et al. 2005), (MNI coordinates X: ±4 to 10; Y: +6 to+18, 0 to −10) consistent with the landmarks of the “limbic-related” striatum as defined by (Fudge and Haber 2002).
Statistical methods for hypothesis testing
In order to test the hypotheses stated above, the following planned analyses were conducted. A repeated-measures analysis of variance (ANOVA) was performed with mean VS/NAc % signal change in voxels within the VS/NAc mask described above as the dependent variable and hemisphere, abstinence condition, and session as within-subjects variables.
Results
Participants
Participants smoked an average of 17.9 (SD=7.6; range 3–30) cigarettes per day, had smoked for a mean of 38.0 (SD=7.1; range=27–48) years, and had a mean FTND score of 6.5 (SD=1.3; range=3–5). The mean exhaled CO of participants on abstinent days was 4.6 (SD=2.2; range=1.0–7.5) p.p.m. was less than that of participants on non-abstinent days was 14.6 (SD=4.1; 8.5–21.5) p.p.m., which was statistically significant (paired t-test, t [1,7]=8.95, p<0.001).
FMRI data
Whole-brain mixed-effects group analysis with a z-statistic threshold of 2.3 (and corrected cluster p<0.05) for the smoking condition demonstrated two large clusters in the temporal-occipital regions bilaterally, inclusive of right and left posterior fusiform gyri and inferior temporal gyri, as seen in Fig. 2. Centers of gravity (COG), coordinates in MNI space, and maximum z-statistic (max z-stat) and p values are presented in Table 1. Furthermore, stratified correlational analyses were performed between mean craving scores pre- and post-scan for the two non-abstinent sessions and two abstinent sessions, respectively.
Fig. 2.
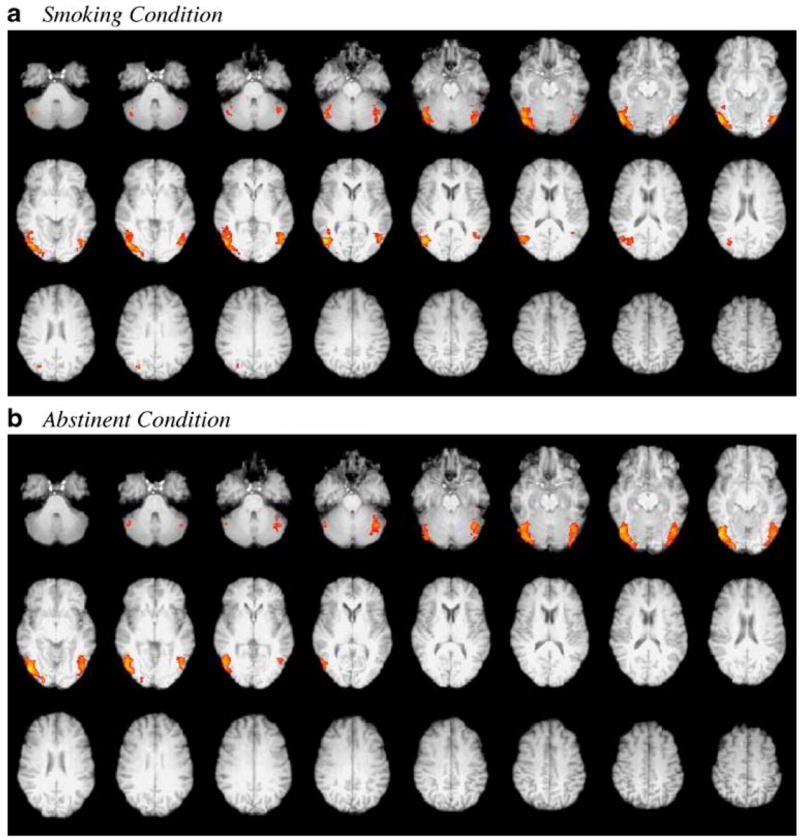
Mixed-effects group analyses of smokers in smoking and abstinent conditions. a Smoking condition, b Abstinent condition. Image depicts statistical maps using clusters determined by Z>2.3 and a corrected cluster significance level of p<0.05
Table 1.
Corrected mixed effects cluster analysis in smokers by condition
| Cluster | COG x | COG y | COG z | Max Z-statistic | % signal change | p-value |
|---|---|---|---|---|---|---|
| Smoking condition | ||||||
| Right posterior fusiform gyrus | 44.6 | −72.2 | −6.61 | 3.66 | 0.27 | <0.001 |
| Left posterior fusiform gyrus | −44.5 | −69.2 | −10.40 | 3.42 | 0.24 | <0.001 |
| Abstinent condition | ||||||
| Right inferior temporal gyrus | 45.3 | −79.3 | −11.8 | 3.63 | 0.29 | <0.001 |
| Left posterior fusiform gyrus | −43.9 | −69.5 | −16.1 | 3.17 | 0.25 | <0.001 |
Legend: Group mixed-effects analysis of smoking vs. neutral contrast demonstrated significant activation in three major clusters consisting of multiple local maxima. X, y, z are the coordinates of the centers of gravity (COG) for each cluster. P represents the p value corresponding to the maximum z-statistic within each cluster.
In addition to the COGs of each cluster, local maxima were observed in the left posterior fusiform gyrus (PFG) (−44, −70, −8, max z-stat=2.97), right inferior temporal gyrus (ITG) (52, −72, −12, max z-stat=3.66) and left ITG (−50, −74, −12, max z-stat=3.42). In a pattern similar to that observed in the smoking condition, mixed-effects group analysis of the same subjects in the abstinent condition revealed bilateral occipital-temporal clusters inclusive of right and left posterior fusiform gyri and inferior temporal gyri, respectively [Local maxima: right ITG (52, −64, −4, max z-stat=3.45), left ITG (−48, −74, −12, max z-stat=3.16), left PFG (−44, −68, −16, max z-stat=3.16)].
Next, as described above (fourth level analysis), whole brain, within-subjects, mixed-effects group contrasts using voxel-wise GLM analyses were conducted comparing the average activation between the abstinent and non-abstinent conditions. Specifically, the fourth level analysis examined whether or not there were within-subjects differences in the mean contrast of the PEs (COPE)(s) for the smoking vs. neutral cue contrasts (averaged across both scan sessions for each condition for each individual from second level analyses), between the average COPE for the group of eight smokers (from the third level analysis) in the abstinent vs. non-abstinent conditions. Using the fourth level abstinent vs. non-abstinent within-subjects group subtraction data, we tested our specific regional hypothesis that there are smoking-related changes in the VS/NAc in nicotine-dependent smokers. We found bilateral relative activation within the NAc proper (p<0.05) in the contrast of the non-abstinent with the abstinent condition. The maximum z-statistic in the right VS/NAc was 2.12 and in the left VS/NAc was 2.02, as shown in Fig. 3. To assess possible additional brain functional changes with smoking, differences in whole-brain activation were tested at a corrected cluster z-threshold of 2.3, but no significant changes were found in any brain region when we compared non-abstinent to abstinent conditions (non-abstinent minus abstinent) or abstinent to non-abstinent conditions (abstinent minus non-abstinent).
Fig. 3.
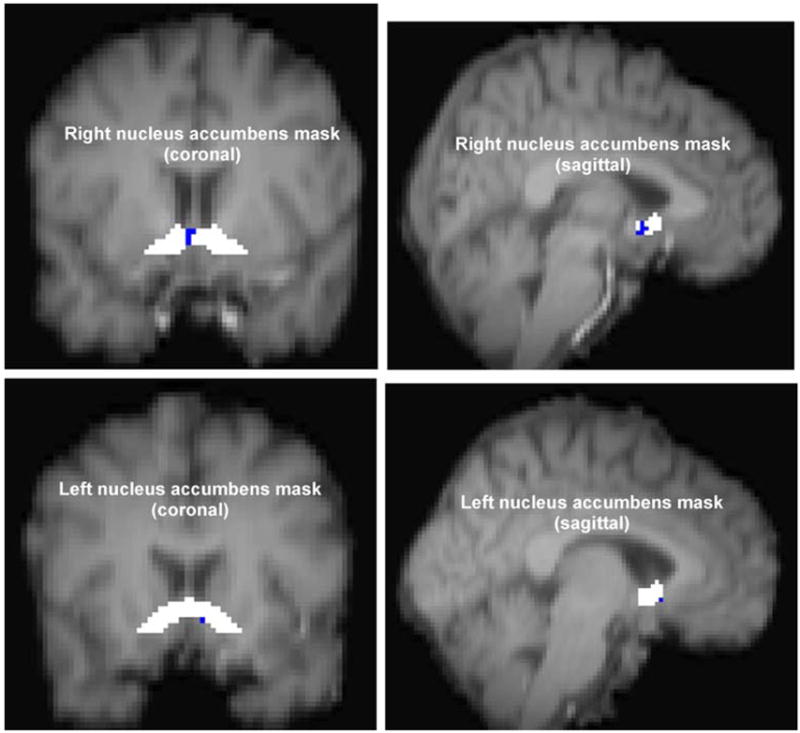
Activation within ventral striatum/nucleus accumbens anatomical mask. Statistical map of non-abstinent condition compared with abstinent condition within pre-defined VS/NAc anatomical masks. Activation is shown in blue. Activation clusters (in blue) represent group within-subjects mixed-effects analyses comparing average activation to smoking vs. neutral cues between non-abstinent and abstinent conditions. Thresholds were set at z-statistic of 2.0, p=0.05 uncorrected
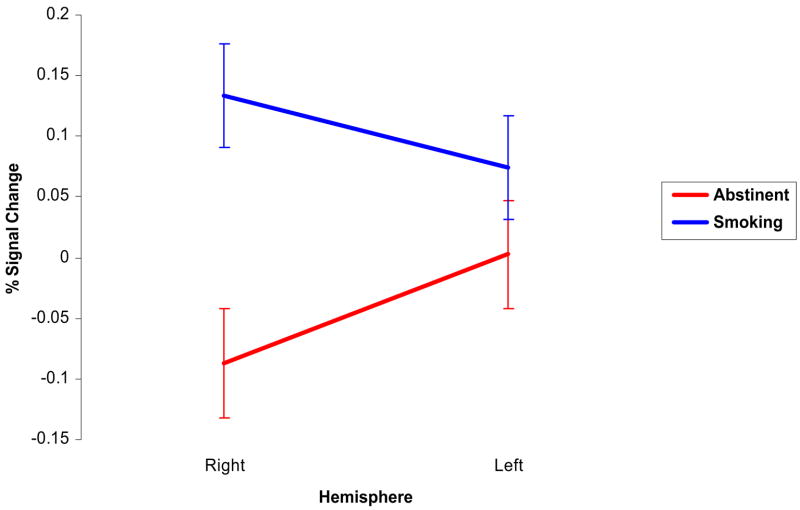
ROI analyses were then conducted by calculating the COPEs, expressed as percent (%) signal change, for the smoking vs. neutral contrast for the group of voxels within VS/NAc masks separately for each hemisphere for each scan. Therefore, there were four estimates of % signal change for both right and left VS/NAc hemispheres taken at the four scans (2 × abstinent; 2 × non-abstinent), respectively. A repeated-measures ANOVA was performed using % signal change in the (VS/NAc) ROI (measurements taken at all four scanning sessions) as the dependent variables, with condition (smoking vs. abstinent), hemisphere (right vs. left), and session number (first vs. second) as dichotomous within-subjects independent variables. We found a main effect of condition, with greater mean VS/NAc signal change in the non-abstinent than in the abstinent condition (F [1, 7]=7.87, p=0.026), and a significant abstinence condition by hemisphere interaction (F [1,7]=11.30, p=0.012). There were no main effects of session (F [1,7]=1.88, p=0.212) or hemisphere (F [1,7]=0.6, p=0.461).
In order to determine the nature of the hemisphere by abstinence condition interaction demonstrated in the repeated-measures ANOVA described above, a series of paired t-tests using the average VS/NAc across sessions were performed. Bi-hemispheric VS/NAc activation was significantly greater in the non-abstinent condition than in the abstinent condition (t [1, 7]=2.39, p=0.048). In addition, right hemisphere VS/NAc relative signal change was significantly greater in the non-abstinent than in the abstinent conditions (t [1, 7]=3.38, p=0.012). However, in the left hemisphere, the mean VS/NAc relative signal change was not significantly greater in the non-abstinent condition than in the abstinent condition (t [1, 7]=1.30, p=0.235), although the effect was in the same direction (i.e., non-abstinent condition greater than abstinent condition).
There were no significant differences in VS/NAc relative signal change between hemispheres for either condition. Figure 4 illustrates the mean VS/NAc relative activations for each hemisphere in both conditions.
Fig. 4.
Ventral striatum/nucleus accumbens relative signal change by hemisphere and condition. Mean COPE (smoking vs. neutral stimulus contrast) within the ventral striatum including the nucleus accumbens (VS/NAc) for right and left hemispheres and both conditions (smoking and abstinent). Error bars represent the standard error of the relative activation (expressed as % signal change) for each hemisphere. Relative activation values were calculated within an anatomically-defined VS/NAc mask created with FSLview software as described in the text
Non-imaging behavioral data
Self-reported craving and withdrawal data
Self-reported craving and withdrawal data are presented for in Table 2 for each session, and Fig. 5 illustrates total withdrawal and craving scores across sessions within both conditions. Average MNWS and SJCS scores were not significantly different before and after the scans. No other pre/post scan comparisons of craving or withdrawal were significantly different, although qualitatively both craving and withdrawal increased from before to after the non-abstinent scans and decreased from before to after the abstinent scans, as seen in Fig. 5. Averaged across sessions, total nicotine withdrawal and craving scores were higher in the abstinent condition than the non-abstinent condition: average withdrawal: t [1,7]=5.20, p=0.002; average craving: (t=[1, 7]=4.11, p=0.005).
Table 2.
Self-reported withdrawal and craving before and after scanning sessions
| Abstinent 1 Pre-scan | Abstinent 1 Post-scan | Abstinent 2 Pre-scan | Abstinent 2 Post-scan | |
|---|---|---|---|---|
| Withdrawal (MNWS) | ||||
| Anger, irritability, frustration | 1.5 (1.4) | 1.1 (1.1) | 1.9 (1.6) | 1.4 (1.7) |
| Anxiety, nervousness | 1.9 (1.4) | 1.1 (1.1) | 1.4 (1.5) | 1.4 (1.3) |
| Difficulty concentrating | 1.3 (1.4) | 1.8 (1.4) | 1.5 (1.1) | 2.3 (1.6) |
| Impatience, restlessness | 1.9 (1.4) | 1.3 (1.5) | 1.9 (1.2) | 2.0 (1.6) |
| Hunger | 0.75 (1.4) | 0.6 (1.1) | 1.3 (1.2) | 1.1 (1.35) |
| Depression | 1.0 (1.4) | 0.1 (0.4) | 1.0 (1.6) | 1.1 (1.6) |
| Desire to smoke | 3.3 (1.5) | 2.4 (1.5) | 3.1 (1.4) | 3.1 (1.5) |
| Total | 11.5 (7.7) | 8.4 (5.0) | 12.0 (7.1) | 12.4 (8.4) |
| Craving (SJCS) | ||||
| Desire to smoke | 74.9 (36.1) | 61.3 (40.2) | 68.6 (38.1) | 72.4 (39.8) |
| If possible | 92.2 (17.4) | 78.1 (36.4) | 81.1 (27.3) | 83.6 (27.7) |
| All I want | 56.9 (35.3) | 49.3 (44.8) | 63.6 (40.9) | 74.3 (38.4) |
| Urge | 63.1 (40.8) | 44.4 (39.4) | 65.5 (39.7) | 74.9 (38.5) |
| Crave | 76.3 (32.8) | 45.6 (41.2) | 64.3 (40.4) | 74.3 (38.4) |
| Total | 363.4 (132.5) | 286.1 (167.1) | 341.3 (177.4) | 379.4 (180.3) |
| Non-abstinent 1 Pre-scan | Non-abstinent 1 Post-scan | Non-abstinent 2 Pre-scan | Non-abstinent 2 Post-scan | |
| Withdrawal (MNWS) | ||||
| Anger, irritability, frustration | 0.0 | 0.25 (0.5) | 0.8 (1.0) | 1.25 (1.8) |
| Anxiety, nervousness | 1.3 (1.5) | 1.0 (1.4) | 1.1 (1.1) | 0.6 (1.2) |
| Difficulty concentrating | 0.6 (1.1) | 1.4 (1.8) | 0.3 (0.5) | 0.6 (1.4) |
| Impatience, restlessness | 0.1 (0.2) | 0.8 (1.4) | 0.5 (0.8) | 1.1 (1.6) |
| Hunger | 0.4 (1.1) | 0.9 (1.4) | 0.1 (0.4) | 0.5 (1.1) |
| Depression | 0.0 | 0.0 | 0.25 (0.5) | 0.35 (0.7) |
| Desire to smoke | 0.5 (0.8) | 1.9 (1.5) | 1.0 (1.4) | 1.5 (1.6) |
| Total | 2.8 (3.3) | 6.1 (6.3) | 4.0 (3.1) | 5.9 (6.0) |
| Craving (SJCS) | ||||
| Desire to smoke | 17.5 (21.9) | 32.8 (29.7) | 20.0 (27.8) | 35.8 (35.5) |
| If possible | 48.6 (36.2) | 54.9 (41.0) | 31.8 (37.2) | 52.4 (33.5) |
| All I want | 18.1 (23.9) | 22.6 (33.5) | 13.8 (22.5) | 30.0 (33.8) |
| Urge | 13.8 (20.0) | 20.0 (22.4) | 15.6 (27.8) | 31.3 (36.4) |
| Crave | 118.8 (31.8) | 27.0 (25.2) | 13.8 (27.6) | 30.0 (34.2) |
| Total | 116.8 (99.3) | 157.2 (122.6) | 94.9 (119.2) | 179.4 (164.7) |
Legend: Values are means and standard deviations (SD) for withdrawal as assessed with the Minnesota Nicotine Withdrawal Symptoms Scale (MNWS) (Hughes and Hatsukami 1986; Hughes et al. 1986) and Shiffman-Jarvik Craving Scale (SJCS) (Shiffman et al. 1998).
Fig. 5.
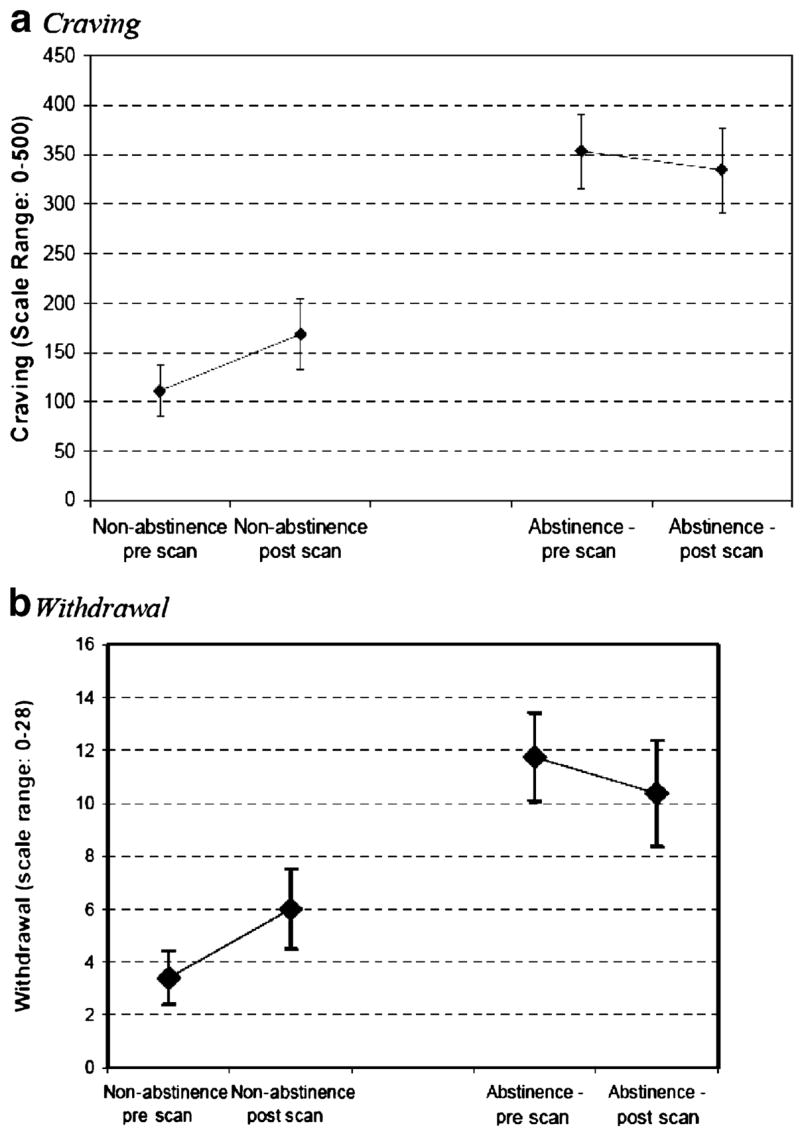
Tobacco craving and withdrawal during scanning. Craving (a) and withdrawal (b) prior to and following fMRI scans. Collapsed means ± SEs for both trials are provided
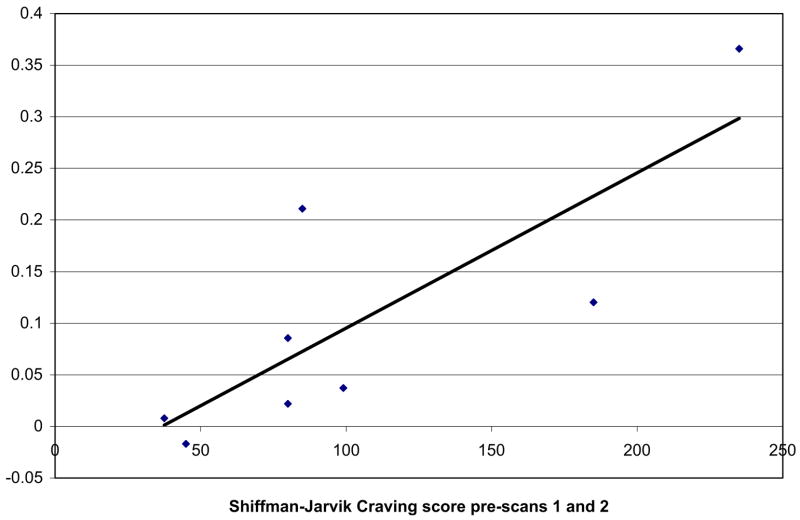
Bivariate non-parametric correlational analyses were performed separately for the two conditions, examining VS/NAc BOLD contrast with craving and withdrawal to better characterise the relationship between the fMRI activation (for the smoking minus neutral image contrast) and self-reported craving. There was a significant positive correlation between the average pre-scan craving score and the global bi-hemispheric VS/NAc activation (r=0.84, p=0.009) in the non-abstinent condition, as demonstrated in Fig. 6. There were no significant correlations between VS/NAc activation and craving in the non-abstinent condition post-scans, or in the abstinent condition (ps>0.4). Nor were there any significant correlations between post-scan or pre-minus post-scan self-reported craving scores. Nicotine withdrawal scores were not significantly correlated with VS/NAc relative activation for either condition before or after the scans.
Fig. 6.
Correlation of global VS/NAc relative signal change and tobacco craving in non-abstinent condition. Scatter plot of total score on the Shiffman-Jarvik Craving Scale prior to picture presentation averaged across both scanning sessions on the x-axis and mean bi-hemispheric relative activation (% signal change) averaged across both smoking sessions for the VS including the nucleus accumbens (VS/NAc) on the y-axis and best-fit regression line illustrating the correlation
Reaction times
In order to provide confirmation of the internal validity of our study and demonstrate external validity consistent with other published literature (Havermans et al. 2003; Trimmel and Wittberger 2004), a repeated-measures ANOVA was performed with reaction time (RT) as the dependent variable, and cue type (smoking-related vs. neutral), condition (non-abstinent vs. abstinent), and session (session 1 vs. session 2) as within-subjects independent variables. A significant main effect of session was observed, indicating faster reaction times in the second session for both conditions (F [1,7]=5.89, p=0.046). Statistical trends were observed for a main effect of cue type, suggesting that reaction times might be greater for smoking-related pictures than neutral pictures (F [1,7]=0.94; p=0.094), and for a cue type by condition interaction, suggesting a greater relative interference for smoking-related cues compared to neutral cues in the abstinent condition compared to the non-abstinent condition (F [1,7]=3.81, p=0.092).
Given the potential confound of time within each of the smoking sessions on RT because of the short half-life of nicotine, which may have resulted in a change in nicotine levels over the course of a testing session, we performed the same analysis including a within-subjects factor of time (first half vs. second half). There was a significant main effect of time (F [1,7]=5.63, p=0.049), but no significant interactions involving the time factor (ps>0.4), and the other effects and trends observed in the primary analysis were retained. Mean reaction times by cue type, condition and session are presented in Table 3.
Table 3.
Reaction times by condition, session and cue type
| Session | Condition | Reaction Time (ms)
|
p-value | |
|---|---|---|---|---|
| Cue Type
|
||||
| Smoking-Related | Neutral | |||
| 1 | Non-abstinent | 1079.23 (161.04) | 1069.59 (287.47) | 0.903 |
| 2 | 708.60 (443.41) | 673.26 (426.49) | 0.363 | |
| 1 | Abstinent | 1164.82 (403.14) | 1052.30 (346.56) | 0.010 |
| 2 | 903.85 (407.21) | 811.46 (362.29) | 0.001 | |
| Average Reaction Times | Non-abstinent | 1050.67 (148.34) | 1040.19 (289.30) | 0.892 |
| Abstinent | 1098.58 (283.91) | 992.10 (241.06) | 0.002 | |
Legend: Mean and standard deviations for reaction times in milliseconds (ms) for smoking and neutral picture cues. Data are presented for each of two sessions for two conditions (smoking and abstinent days). Subjects are asked to indicate whether the picture displayed is male or female with a forced key press.
Discussion
The current study used an fMRI approach to examine the effects of abstinence and cue exposure on tobacco dependent individuals, with mixed findings. As anticipated, abstinence resulted in greater craving and withdrawal, however, contrary to our hypothesis, greater VS/NAc activation was associated with smoking-related (vs. neutral) cues when smokers were in the non-abstinent state compared to when they were abstinent. This effect was remarkably consistent in this sample of eight female smokers, and was evident in both whole brain and ROI analyses. We had predicted greater activation in these brain regions during nicotine abstinence, reflecting increased nicotine craving. The rationale underlying this hypothesis was that the craving following deprivation would be a more salient catalyst for VS/NAc brain activation than the actual reinforcing properties of nicotine, particularly among chronic smokers. This was not the case, as smokers exhibited greater relative activation associated with smoking cues when they were satiated with nicotine. That VS/NAc activation was greater in the non-abstinent condition than the abstinent condition may seem surprising given a previously published report of abstinent smokers who demonstrated differentially greater VS/NAc activation (David et al. 2005). However, the two studies are not comparable because of substantial differences in methodology (i.e., use of enforced abstinence, within-subjects vs. between-subjects designs). In addition, additional sample characteristics, such as the different sex composition, age, and years of smoking between smokers make the two studies even more difficult to compare.
Despite being unanticipated, a potential explanation for these findings can be drawn from the previous literature on the neurobiological effects of nicotine abstinence on smokers. Given that substance cue reactivity appears to be mediated by dynamic changes in mesolimbic dopamine (Katner et al. 1996; Katner and Weiss 1999; Melendez et al. 2002; Weiss et al. 1993) and given extensive evidence that nicotine abstinence results in deficits in accumbal dopamine release [for a review, see Kenny and Markou (2001)], it is possible that acute nicotine abstinence reduced the capacity of the mesolimbic dopamine system to produce acute phasic responses to cigarette stimuli, resulting in greater reactivity to smoking cues in the non-abstinent condition.
At first glance, this may seem paradoxical, suggesting that deprived individuals would be less reactive to environmental stimuli, and therefore less motivated to smoke. However, this is not our contention. Rather, these data suggest that abstinent smokers may be less reactive to cues relative to when they are satiated, but smoking behavior is not motivated by cue reactivity alone and self-reported craving does not exclusively reflect reactions environment cues, so much as the individual’s overall motivational state. Smoking is driven by both positive and negative reinforcement (Tiffany and Drobes 1991), and, during abstinence, negative reinforcement plays a particularly prominent role (Willner et al. 1995). Thus, a potential interpretation of current data is that during abstinence, smokers may experience less VS/NAc cue reactivity resulting from relatively reduced burst firing of dopamine in the nucleus accumbens, but may nonetheless be highly motivated to smoke to alleviate aversive withdrawal symptoms (i.e., negative reinforcement). In contrast, under normal smoking conditions, substance-related cues may play a more prominent role because of minimal need for negative reinforcement due to ongoing smoking.
Such an interpretation is consistent with studies by Teneggi et al. who demonstrated that tobacco craving and withdrawal are modulated via distinct neural circuits (Teneggi et al. 2002, 2005). In addition, it is consistent with human laboratory studies that have not demonstrated potentiation of cue-elicited craving by nicotine enforced abstinence (e.g., Palfai et al. 2000; Tidey et al. 2005). Finally, it is consistent with a recent study by (Waters et al. 2004) who found that when nicotine replacement alleviated withdrawal symptoms, cue-elicited craving appeared to play a more prominent role in relapse.
Alternatively, a second explanation for the unanticipated finding of greater VS/NAc signal in the non-abstinence condition is that these findings may be related to a ceiling effect in terms of relative activation. A high-magnitude effect of acute abstinence on craving was observed, resulting in craving levels at approximately the 25% and 70% of the scale maximum for the satiated and acutely abstinent conditions (see Fig. 5), respectively. BOLD imaging is fundamentally a measure of relative activation during the abstinence condition, high levels of craving may have reflected high levels of absolute VS/NAc activation, which itself may have prevented substantial increases in VS/NAc activation. Thus, with more possible variation, the participants during the non-abstinence scanning may have simply exhibited greater relative VS/NAc change. This explanation is also consistent with the studies cited above reporting greater relative increases in craving in response to a smoking cue exposure under non-deprived conditions compared to after abstinence (e.g., Tidey et al. 2005).
However, we acknowledge that this finding was in contrast to our original hypothesis and these explanations are a posteriori and speculative. As such, they should be considered provisional interpretations, and a basis for further neuroimaging examinations of cue reactivity and nicotine abstinence. Determining that greater VS/NAc cue reactivity is reliably evident during satiation in subsequent studies will be essential to bolster the validity of these findings. It is also important to acknowledge that these effects may be related to the methodology used. An argument could be made that our approach to ROI analysis of aggregating across functional voxels of normalized data on ROI masks that were generated from an average, normalized anatomical image may not be as accurate as using individually-tailored ROI masks. However, of equal concern to the investigators was the need to avoid bias in determining anatomical boundaries according to the statistical maps of each individual (Warfield et al. 2004).
Of note, a number of additional findings also warrant discussion. Cue reactivity was associated with bilateral temporal-occipital activation. The most pronounced activation was in the posterior fusiform gyri and inferior temporal gyri. These findings are consistent with previous reports of craving-related posterior fusiform gyrus activation (Due et al. 2002; McClernon et al. 2005; Smolka et al. 2006; Wilson et al. 2005) and provides further confirmation of the notion that extrastriate visual pathways are integrated with the mesolimbic system to process information during motivational states (David et al. 2005; Due et al. 2002). In addition, a significant hemisphere by abstinence condition interaction was detected, however, given the sample size, it is premature to assert that there is clear lateralization of the effect of abstinence condition on VS/NAc activation. There were no significant differences in VS/NAc activation between hemispheres in either condition. Qualitatively, it appears that the mean activation is greater in the right hemisphere than the left hemisphere in the smoking condition and greater in the left hemisphere than the right hemisphere in the abstinent condition, which is consistent with animal studies (Bortolozzi et al. 2003; Schneider et al. 1982; Stein et al. 1998).
The observation of statistical trends for cue type and cue type by condition interactions is reassuring, as post-hoc testing demonstrated that, as expected, reaction times to smoking-related cues were significantly greater than to neutral cues in the abstinent condition, which is consistent with results of other studies and suggestive of incentive sensitization to smoking-related stimuli (Havermans et al. 2003; Trimmel and Wittberger 2004). Furthermore, the observation of faster reaction times in the second session is expected, as subjects have become accustomed to the task. Although not statistically significant, the difference in reaction times in the non-abstinent condition was in the expected direction and does not obviate the possibility of a smaller effect of cue type not reflecting nicotine deprivation.
There are also a number of limitations of the current study that need to be considered. The sample used was small, and although multiple sessions were used to partially address this, the study had relatively low statistical power. In addition to the sample size, one of the weaknesses of the study was that, as a result of three male subjects not attending all scanning sessions, complete data was only available for females. Thus, the observations can only be generalized to females.
A recent systematic review of the literature by (Carpenter et al. (2006) demonstrated a lack of consistency of findings with regard to sex differences in cue reactivity, but did find evidence of an effect of menstrual cycle phase on the severity of nicotine withdrawal. Specifically, in 13 studies, women in the luteal phase demonstrated heightened experiences of withdrawal or craving. The degree to which variation in menstrual cycle phase for the three of eight women in our sample who were pre-menopausal might have affected our results but this cannot be evaluated in the present study because precise dates regarding last menstrual periods or biomarkers were not available. We acknowledge, however, that menstrual cycle phase could present a potential confound.
Another limitation to the study is that without subtraction data using methods such as arterial spin labeling, we cannot ascertain the degree to which changes in regional cerebral blood flow resulting from nicotine vs. neural activation associated with smoking-related cues. However, we have no reason to expect that the BOLD contrast (signal during smoking vs. neutral images) would be differentially affected, and our data reflect relative activation rather than absolute activation. Theoretically, the known protective effects of endogenous estrogen on the cardiovascular system in women might indirectly affect fMRI BOLD signal comparisons of male and female subjects due to differences in regional cerebral blood flow. However, we were not able to compare our female smokers to male counterparts and thus cannot dray any conclusions regarding the influence of endogenous estrogen on our fMRI results—particularly because none of the participants reported oral contraceptive use nor use of hormone replacement therapy.
Yet another potential limitation is that the unbalanced nature of the design, with less frequent presentation of smoking-related pictures, might result in evoking a greater response to smoking pictures given the novelty of the target (smoking) pictures and that this could pose a confound to the contrast. However, we were interested in constructing a paradigm that might reflect the occasional smoking-related cues that occur on a daily basis, and thus be able to evaluate potentially less frequent but more salient cues. One of the reasons for including a reaction time task was to evaluate attentional bias toward smoking-related pictures and the effects of acute abstinence on reaction times to a simple gender discrimination task. Our reaction time data do suggest, with statistical trends, that attention to smoking-related cues was relatively impaired in the acute abstinence condition, and suggest greater attentional bias toward smoking-related cues given the longer reaction times associated with smoking vs. neutral cues. In summary, we believe that the unbalanced design presents advantages and disadvantages but acknowledge the distinct possibility that it could present a potential confound.
Despite these limitations, these data provide additional evidence implicating the VS/NAc as an important locus in the processing of salient drug-associated stimuli and revealed an unexpected attenuation of such reactivity following nicotine deprivation. As such, these results contribute to the sparse literature on the neurobiological effects of abstinence on motivation for tobacco in dependent smokers. If the current findings can be replicated, they may considerably clarify the differential processes that contribute to the maintenance of ongoing smoking and relapse following cessation.
Acknowledgments
This research was funded by Public Health Service grant K08 DA14276 from the National Institute on Drug Abuse/National Institutes of Health, and by Cancer Research UK. Work in the Centre for Functional Neuroimaging of the Brain (FMRIB) and personal support to PMM come from the Medical Research Council. We would like to acknowledge Peter Hobden for assistance in protocol development and scanner operation.
Footnotes
The research reported in this manuscript was performed at the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Department of Clinical Neurology, The University of Oxford, John Radcliffe Hospital, Oxford, UK.
Contributor Information
Sean P. David, Department of Clinical Pharmacology, University of Oxford, Radcliffe Infirmary, Oxford, UK Center for Primary Care & Prevention, The Warren Alpert Medical School of Brown University, 111 Brewster Street, Pawtucket, RI 02860, USA, e-mail: Sean_David@Brown.Edu.
Marcus R. Munafò, Department of Experimental Psychology, University of Bristol, Bristol, UK
Heidi Johansen-Berg, Department of Clinical Neurology, John Radcliffe Hospital, The University of Oxford, Oxford, UK.
James MacKillop, Center for Alcohol and Addiction Studies, The Warren Alpert Medical School of Brown University, Pawtucket, USA.
Lawrence H. Sweet, Centers for Behavioral and Preventive Medicine, The Warren Alpert Medical School of Brown University, Pawtucket, USA
Ronald A. Cohen, Centers for Behavioral and Preventive Medicine, The Warren Alpert Medical School of Brown University, Pawtucket, USA
Raymond Niaura, Department of Psychiatry & Human Behavior, The Warren Alpert Medical School of Brown University, Pawtucket, USA.
Robert D. Rogers, Department of Psychiatry, University of Oxford, Oxford, UK
Paul M. Matthews, GlaxoSmithKline Clinical Imaging Centre, London, UK
Robert T. Walton, Medical Research Council Laboratories, Fajara, The Gambia
References
- Balfour DJ. Neuroplasticity within the mesoaccumbens dopamine system and its role in tobacco dependence. Current Drugs Targets CNS and Neurological Disorders. 2002;1(4):413–421. doi: 10.2174/1568007023339076. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacology, Biochemistry and Behavior. 1998;59(4):1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fmri. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. British Journal of Pharmacology. 1995;114(2):454–460. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Duffard R, de Duffard AM. Asymmetrical development of the monoamine systems in 2,4-dichlorophenoxy-acetic acid treated rats. Neurotoxicology. 2003;24(1):149–157. doi: 10.1016/s0161-813x(02)00156-0. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Annals of the New York Academy of Sciences. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59(12):1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacology, Biochemistry and Behavior. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine & Tobacco Research. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- David SP, Munafò MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58(6):488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159(6):954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The human brain. 2. London: Springer; 1999. [Google Scholar]
- Etter JF, Hughes JR. A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction. 2006;101(3):362–372. doi: 10.1111/j.1360-0443.2005.01289.x. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Defining the caudal ventral striatum in primates: Cellular and histochemical features. Journal of Neuroscience. 2002;22(23):10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreffa G, Bianciardi M, Hagberg GE, Macaluso E, Marciani MG, Maraviglia B, et al. Simultaneous eeg-fmri acquisition: How far is it from being a standardized technique? Magnetic Resonance Imaging. 2004;22(10):1445–1455. doi: 10.1016/j.mri.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International smoking images series (with neutral counterparts), (version 1.2 Carbon-dale) Southern Illinois University: Integrative Neuroscience Laboratory, Department of Psychology; 1999. [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific stroop interference. Psychopharmacology (Berl) 1993;110(3):333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Havermans RC, Debaere S, Smulders FT, Wiers RW, Jansen AT. Effect of cue exposure, urge to smoke, and nicotine deprivation on cognitive performance in smokers. Psychology of Addictive Behaviors. 2003;17(4):336–339. doi: 10.1037/0893-164X.17.4.336. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heiervang E, Beherens TE, Mackay CE, Robson MD, Johansen-Berg H. Brain session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33(3):867–877. doi: 10.1016/j.neuroimage.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser-Sinopoli SM, et al. Correlation between dopamine d(2) receptors in the ventral striatum and central processing of alcohol cues and craving. American Journal of Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Pickens RW, Gust SW, Hatsukami DK, Svikis DS. Smoking behavior of type a and type b smokers. Addictive Behaviors. 1986;11(2):115–118. doi: 10.1016/0306-4603(86)90035-3. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. Smoking cues decrease prepulse inhibition of the startle response and increase subjective craving in humans. Experimental and Clinical Psychopharmacology. 1999;7(3):250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. The effects of smoking high nicotine cigarettes on prepulse inhibition, startle latency, and subjective responses. Psychopharmacology (Berl) 2000;150(3):244–252. doi: 10.1007/s002130000399. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, et al. Olanzapine reduces craving for alcohol: A drd4 vntr polymorphism by pharmacotherapy interaction. Neuropsychopharmacology. 2003;28(10):1882–1888. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- ICRF. Effectiveness of a nicotine patch in helping people stop smoking: Results of a randomised trial in general practice. Imperial cancer research fund general practice research group. Builders Merchants Journal. 1993;306(6888):1304–1308. doi: 10.1136/bmj.306.6888.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125(12):2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (p) but not wistar rats. Behavioural Pharmacology. 1996;7(7):669–674. [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcoholism, Clinical and Experimental Research. 1999;23(11):1751–1760. [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacology, Biochemistry and Behavior. 2001;70(4):531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of d(2) receptor parameter measurements in ventral striatum. Journal of Cerebral Blood Flow and Metabolism. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related fmri responses to smoking cues. Neuropsychopharmacology. 2005;30(10):1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (p) rats during anticipation and operant self-administration of ethanol. Alcoholism, Clinical and Experimental Research. 2002;26(3):318–325. [PubMed] [Google Scholar]
- Munafò M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified stroop task. Journal of Psychopharmacology. 2003;17(3):310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97(2):133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: Effects of gender and cue type. Addictive Behaviors. 1998;23(2):209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Monti PM, Ostafin B, Hutchison K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. Journal of Abnormal Psychology. 2000;109(1):96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: Theoretical and treatment implications. International Journal of the Addictions. 1990;25(7A–8A):957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacology, Biochemistry and Behavior. 2003;76(2):243–250. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology, Biochemistry and Behavior. 2000;67(1):71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Murphy RB, Coons EE. Lateralization of striatal dopamine (d2) receptors in normal rats. Neuroscience Letters. 1982;33(3):281–284. doi: 10.1016/0304-3940(82)90385-8. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kheryallah M, Niaura R, Shadel WG, Jorenby D, Ryan C, et al. Efficacy of acute administration of nicorette gum against cue-provoked craving. Fourth annual scientific conference of the society for research on nicotine and tobacco, new orleans, la., society for research on nicotine and tobacco. Paper presented at the Fourth Annual Scientific Conference of the Society for Research on Nicotine and Tobacco; New Orleans, LA. 1998. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184(3–4):577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: A functional mri study. American Journal of Psychiatry. 1998;155(8):1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Progress in Neuropsychopharmacology and Biological Psychiatry. 1983;7(4–6):591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacology, Biochemistry and Behavior. 1984;20(6):917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91(2):251–268. [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72 h: Effect of nicotine patches on craving and withdrawal. Psychopharmacology (Berl) 2002;164(2):177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Effect of sustained-release (sr) bupropion on craving and withdrawal in smokers deprived of cigarettes for 72 h. Psychopharmacology (Berl) 2005;183(1):1–12. doi: 10.1007/s00213-005-0145-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine & Tobacco Research. 2005;7(3):421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Trimmel M, Wittberger S. Effects of transdermally administered nicotine on aspects of attention, task load, and mood in women and mean. Pharmacology, Biochemistry and Behavior. 2004;78(3):639–645. doi: 10.1016/j.pbb.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. Journal of Clinical Investigation. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (staple): An algorithm for the validation of image segmentation. IEEE Transactions on Medical Imaging. 2004;23(7):903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14(2):111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72(6):1136–1143. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. Journal of Pharmacology and Experimental Therapeutics. 1993;267(1):250–258. [PubMed] [Google Scholar]
- Willner P, Hardman S, Eaton G. Subjective and behavioural evaluation of cigarette cravings. Psychopharmacology (Berl) 1995;118(2):171–177. doi: 10.1007/BF02245836. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine & Tobacco Research. 2005;7(4):637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fmri group analysis using bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fmri data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for cbf activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yudkin P, Munafo M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: Randomised controlled trial. Builders Merchants Journal. 2004;328(7446):989–990. doi: 10.1136/bmj.38050.674826.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]