Abstract
Substantial experimental evidence exists suggesting a critical role for dopamine in reinforcer-related processes, such as learning and drug addiction. Dopamine receptors, and in particular D1 receptors, are widely considered as modulators of synaptic plasticity. The amygdala contains both dopamine terminals and dopamine D1 receptors and is intimately involved in motivation and learning. However, little is known about the involvement of D1 receptor activation in two subnuclei of the mammalian amygdala, the central nucleus and basolateral complex in instrumental learning. Following recovery from surgery and preliminary training, rats with bilateral indwelling cannulae aimed at the central nucleus or basolateral complex were trained to lever-press for sucrose pellets over 12 sessions. Infusion of the selective D1 antagonist R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (0.3 nmol and 3.0 nmol) prior to the first five training sessions dose-dependently impaired instrumental learning when compared with vehicle-infused controls. All rats were then exposed to five sessions drug-free; lever-pressing quickly reached equal levels across groups. A drug infusion prior to an 11th session revealed no effect on performance. Control experiments indicated that basic motivational processes and general motor responses were intact, such as spontaneous feeding and locomotor activity. These results show an essential role for D1-receptor activation in both the central nucleus and basolateral complex on the acquisition of lever pressing for sucrose pellets in rats, but not the performance of the behavior once conditioned. We propose that instrumental learning is dependent on plasticity in the central nucleus and basolateral complex amygdala, and that D1 receptor activation participates in transcriptional processes that underlie this plasticity.
Keywords: instrumental learning, amygdala, dopamine, D1, plasticity, rats
Biologically important events, such as procurement of food, escape from predation, and access to sexual partners, often require flexible patterns of behavior in order to produce them—foraging, vigilance, and defense, for example. Important biological reinforcers (Reinf), in turn, change the behavior that produced them, illustrating a form of adaptability termed “instrumental learning.” Many Reinf-related processes are mediated by the mesocorticolimbic dopamine (DA) system, consisting of DA neurons in the ventral tegmental area and their projections to the nucleus accumbens, medial prefrontal cortex, striatum, amygdala and other forebrain structures (Beninger and Miller, 1998; Cardinal et al., 2002; Kelley and Berridge, 2002). Specifically, studies have shown that D1 receptor activation throughout this corticolimbicstriatal network plays a critical role in learning (Beninger and Gerdjikov, 2004). Indeed, D1 receptor activation is thought to serve a crucial modulatory function in the induction of intracellular transcriptional and translational cascades, leading to changes in gene expression and synaptic plasticity, thereby reconfiguring neural networks, and ultimately behavior (Abel and Lattal, 2001; Hyman and Malenka, 2001; Koob and Le Moal, 2001; O’Donnell, 2003; Wickens et al., 2003). Mesocorticolimbic DA function also maintains a prominent position in computational-error-correction models of learning (Romo and Schultz, 1990; Schultz, 2002; Schultz and Romo, 1990). Thus, recent evidence points to an important role for D1 receptor activation in a distributed network in the neural basis of learning.
As a prominent structure in this corticolimbic network, studies have shown that the amygdala is crucial for acquisition and expression of emotional, attentional, and Reinf-related behaviors (Davis, 1992; Gallagher and Holland, 1994). For example, lesions of two of the major subdivisions of the amygdala, the central nucleus of the amygdala (CeA) and the basolateral portion (BLA), disrupt distinct aspects of Pavlovian and instrumental learning (Balleine et al., 2003; Everitt and Robbins, 1992; Gallagher and Holland, 1992; Hall et al., 2001; Holland and Gallagher, 2003). However, these studies involved lesions that are non-selective with regard to neurotransmitter coding. Convincing evidence exists that both the CeA and BLA are innervated by midbrain DA neurons and contain moderate to high basal levels of both DA and DA D1 receptors (Asan, 1997; Boyson et al., 1986; Dawson et al., 1986; Young and Rees, 1998). Yet, only a few studies have investigated the role of DA receptor activation in the amygdala in learning, especially D1 receptor activation (Caine et al., 1995; Stevenson and Gratton, 2004; Zarrindast et al., 2003), although significant work on drug-related processes suggests important amygdalar DA involvement (Hitchcott and Phillips, 1998a,b; See et al., 2001).
The relative paucity of research on DA D1 function in the amygdala in instrumental learning, therefore, appears to be an important gap in our knowledge. Given the key functions of the BLA and CeA in a variety of learning models, as well as their DA innervation, the current experiments explored the role of D1 receptor activation the amygdala on food-reinforced lever-pressing, as well as locomotor activity and feeding-related behaviors.
EXPERIMENTAL PROCEDURES
Subjects
Male Sprague–Dawley rats (Harlan, Madison, WI, USA) were housed in pairs in polyethylene cages in colony room with a 12-h light/dark cycle. They were approximately 90 days old at the start of experimentation and weighed approximately 300 g each. They were weighed and handled daily and provided with food and water ad libitum prior to surgery. Following recovery from surgery, each rat was reduced to 85% of their ad libitum weight. During food restriction, and prior the start of testing, rats were given approximately 3 g of sucrose pellets in their home cages per day; the 85% weight was maintained for the remainder of the experiment. Care of the rats was in accordance with the guidelines of the University of Wisconsin–Madison animal care committee and with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to limit the number of rats used and to limit their suffering.
Apparatus
Instrumental learning chambers
Instrumental learning sessions were conducted in eight identical commercially constructed chambers (Coulbourn Instruments, Allentown, PA, USA) enclosed in sound attenuating, ventilated chests. Fans provided some masking noise continuously throughout the session. Two retractable levers, approximately 6 cm apart, could be projected into the chamber on the right-side wall. Spaced equally between the two levers was a feeder trough into which 45 mg Bio-Serv® sucrose pellets could be delivered. The feeder trough was equipped with a photo sensor, such that the number and timing of nose pokes (NP) into the tray could be recorded. Above the feeder trough was a row of three stimulus lights (red, yellow, and green) and a 28-V house light. Experimental events were arranged and recorded via a personal computer in the same room as the chambers, running Graphic State Notation™ (version 1.013–00), interfaced with L91-04S Habitest Universal Lincs (Coulbourn Instruments).
Locomotion and feeding experiments
Clear polycarbonate cages (24 cm×45 cm×21 cm) with a wire mesh floor and wire lid with a water bottle served as test cages for the unconditional effects of drug infusions. Two small ceramic dishes filled with sucrose pellets were affixed to the mesh floor with pliable putty. Data were obtained via an event recorder connected to a PC. Measures included the latency to eat, amount of sucrose eaten, amount of time spent of feeding, number of feeding bouts, number of center crossings and number of rears.
Surgery
Rats were anesthetized with a ketamine/xylazine mixture (100/10 mg/kg) and placed in a standard stereotaxic surgery device (incisor bar at −5.0 mm; flat skull). Indwelling stainless steel cannulae (23 gauge) were implanted bilaterally and secured to the skull with stainless steel screws and dental cement. Cannulae were aimed 2.5 mm above the injection targets: the CeA, or the BLA. Stainless steel stylets prevented occlusion of the cannulae. Coordinates (flat skull, in mm) were: CeA, −2.0 AP from bregma, ±4.0 LM from midline, and −5.7 DV from the skull surface, BLA, −2.8 AP, ±4.8 LM, and −5.8 DV.
Drugs and microinfusions
The selective D1 antagonist, R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH-23390 Research Biochemicals International, Natick, MA, USA), was dissolved in isotonic sterile saline. Doses of 0.1 μg (0.3 nmol), 1 μg (3 nmol) SCH-23390 or vehicle (saline) were administered via bilateral intracerebral microinfusions in a volume of 0.5 μl. After removing the stylets, injectors (30 gauge) were inserted 2.5 mm below the tips of the guide cannulae to the site of the infusion (−8.2 mm DV for CeA and −8.3 mm DV for BLA). A Harvard Apparatus (Holliston, MA, USA) pump, set at a rate of 0.32 μl/min, infused drug or vehicle for 1 min 33 s, followed by 1 min of diffusion time. The injectors were removed and the stylets replaced. Rats were immediately placed in the instrumental learning chambers or locomotor and feeding cages after microinfusions. The effects of SCH-23390 (0 nmol, 0.3 nmol, and 3.0 nmol) infused into the CeA were investigated in experiment 1. Experiment 2 explored the effects of SCH-23390 (0 nmol, 0.3 nmol, and 3.0 nmol) infusions into the BLA.
Instrumental learning
All sessions lasted 15 min. Rats were habituated to the chamber and trained to retrieve pellets from the trough (“magazine training”) for 3 sessions on consecutive days before testing. Prior to the first 2 habituation sessions, rats were given a mock infusion, in which an injector was lowered to the end of the cannulae, but not into brain tissue, the microdrive pump was turned on, but no drug was infused. Rats were then immediately placed into the chambers, with both levers retracted. With the house light on, sucrose pellets were delivered into the food trough on a random time 15” (RT-15”) schedule during the first habituation session, and on a random time 30” (RT-30”) on subsequent habituation sessions. The number and timing of NP into the tray were recorded. Prior to the third habituation session, rats were given a vehicle (saline) infusion as specified above. Again, they were placed in the chambers immediately after the infusion with both levers retracted and the house light on. Rats were matched on the basis of the frequency of nose poking and randomly assigned to the various groups (vehicle, 0.3 nmol, or 3.0 nmol). Prior to the next five training sessions (sessions 1–5), rats were infused with drug or vehicle, depending on group assignment, and placed immediately into the operant chamber. The right lever was projected into the chamber and lever-presses were immediately reinforced with one pellet (a Fixed Ratio-1 or FR-1 schedule). After 50 Reinf were earned, in any one session, the contingencies changed to a Random Ratio-2 schedule (RR-2); each lever press (LP) was reinforced with a probability of 0.5. The RT-30” schedule was maintained for the first two infusions sessions (sessions 1 and 2). This conjoint RT schedule remained operative to ensure some degree of arousal and exploration, and to approximately equate reinforcement rate during those first two sessions allowing for comparisons between drug groups on pellet-retrieval. Prior to sessions 6–10, no infusions were given, but prior to session 11, an infusion was given to test for performance effects. A 12th session with no infusion concluded experimentation.
Locomotion and feeding
Locomotion and feeding control experiments investigated the unconditional effects of drug infusions. Surgery, handling, and deprivation conditions were identical to those of operant learning experiments. Using a within-subjects design, each rat received each dose of SCH-23390 (vehicle, 0.3 nmol, and 3.0 nmol) immediately prior to being placed in the locomotor and feeding cages. Experimentation was conducted on consecutive days, in a randomized order for each rat, in which the experimenter was blind. For the CeA, eight naïve rats were used while the eight vehicle-treated rats in the operant learning portion of experiment 2 were used to investigate the effects of SCH-23390 in the BLA. The latency to eat, amount of sucrose eaten, amount of time spent feeding, number of feeding bouts, number of center crossings, number of rears, number of drinking bouts, and length of drinking were recorded and serve as the dependent variables in this portion of experimentation.
Histological analysis
After the completion of the experiment, all rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with isotonic saline followed by 10% formalin. Brains were stored in 10% sucrose–10% formalin mixture before sectioning. Sixty-micrometer sections were stained with Cresyl Violet and examined under light microscopy for location of infusion sites.
Statistical analysis
LP and nose-pokes were analyzed by multivariate ANOVA, with treatment as the between-subjects variable, and sessions as within-subjects variable. Lever-presses and NP were analyzed in three phases: sessions 1–5 (infusion sessions), sessions 6–10 (no infusion sessions), and performance test session 11. Data from performance tests were analyzed with the results from the prior session (session 10). Tukey honestly significant difference (HSD) post hoc comparisons were used following significant effects to reveal between group differences. Data from the locomotion and feeding control experiments were analyzed using univariate dependent-samples ANOVA.
Analysis of the microstructure of behavior
Statistical analyses were supplemented by detailed, microstructural behavioral analyses of operant learning experiments by exporting raw data files from the Colbourn system into Excel®. The purpose of these analyses was to gain further insight into the sequencing and organization of behavior under the influence of amygdala drug infusions. We have recently presented similar analyses (Andrzejewski et al., 2004). LP, NP, Reinf that occurred during each rat’s session were time-stamped by Graphic State Notation. The order of events and their temporal relation were analyzed by counting all the dyads of events that occurred. For example, a NP could be followed by a LP, a Reinf (if the conjoint RT schedule was operative) or another NP, yielding three types of dyads (NP-LP, NP-Reinf, or NP-NP). The dyads were used to compute conditional probabilities: for example, the probability of an LP given that a NP had just occurred, was the number of NP–LP dyads divided by the total number of NP, or NP–LP+NP–Reinf+NP–NP. These conditional probabilities were averaged across rats per session and differentiated by group assignment. Data were included in the computation of group averages only if at least five of the dyads in question occurred to limit the effects of small samples on the group mean.
RESULTS
Histology
Cannulae placements for all rats with cannulae aimed at the CeA are shown in the left side of Fig. 1. An example of a Cresyl Violet-stained section is also shown. Note that in the stained section that the anterior portion of the BLA is some distance lateral to the injector tip. Placements were deemed appropriate for seven rats in each of the three groups (n=7 each group, or N=21 in the CeA). For the locomotor and feeding portion of the present experimentation, eight rats had placements in the CeA.
Fig. 1.
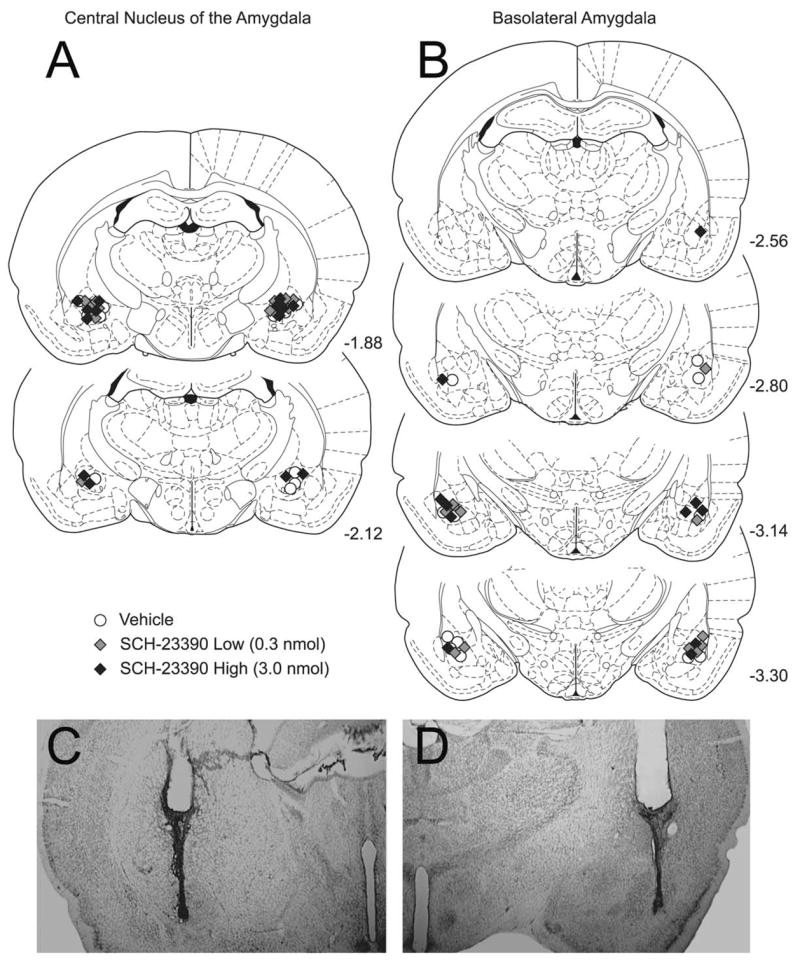
Histological reconstructions of cannula placements in rats in the instrumental learning experiments. Histological sections were examined under light microscope and the site of infusion was estimated. Placements in the CeA are represented in schematic form in A, while placements in the BLA are represented in B. Symbols are consistent with other figures; black circles are vehicle treated (CeA: n=7 and BLA: n=8), light gray diamonds represent 0.3 nmol SCH-23390 treated (n=7 and n=7) and dark gray diamonds represent 3.0 nmol SCH-23390 treated (n=7 and n=7). The schematic reconstructions show that there was no systematic bias in placement based on group assignment. Note that CeA placements were anterior to the BLA placements. Two examples of Cresyl Violet-stained pictomicrographs are shown in C (CeA) and D (BLA). Placements in the CeA for the locomotor portion of experiment 1 were consistent with those in panel A. The vehicle-treated rats in the instrumental learning portion of experiment 2 were used in the locomotor and feeding control portion. Schematic diagrams are from The Rat Brain in Stereotaxic Coordinates. (4th ed.) (Paxinos and Watson, 1998).
Cannulae placements for all rats with cannulae aimed at the BLA are shown on the right side of Fig. 1, with an example of a Cresyl Violet-stained section presented below. Note that the injector tip is some distance from the posterior portion of the CeA. Placements were deemed appropriate for 22 rats (n=7 each drug group and n=8 in the vehicle group). For the locomotor and feeding portion of the present experimentation, the eight rats in the vehicle group were used.
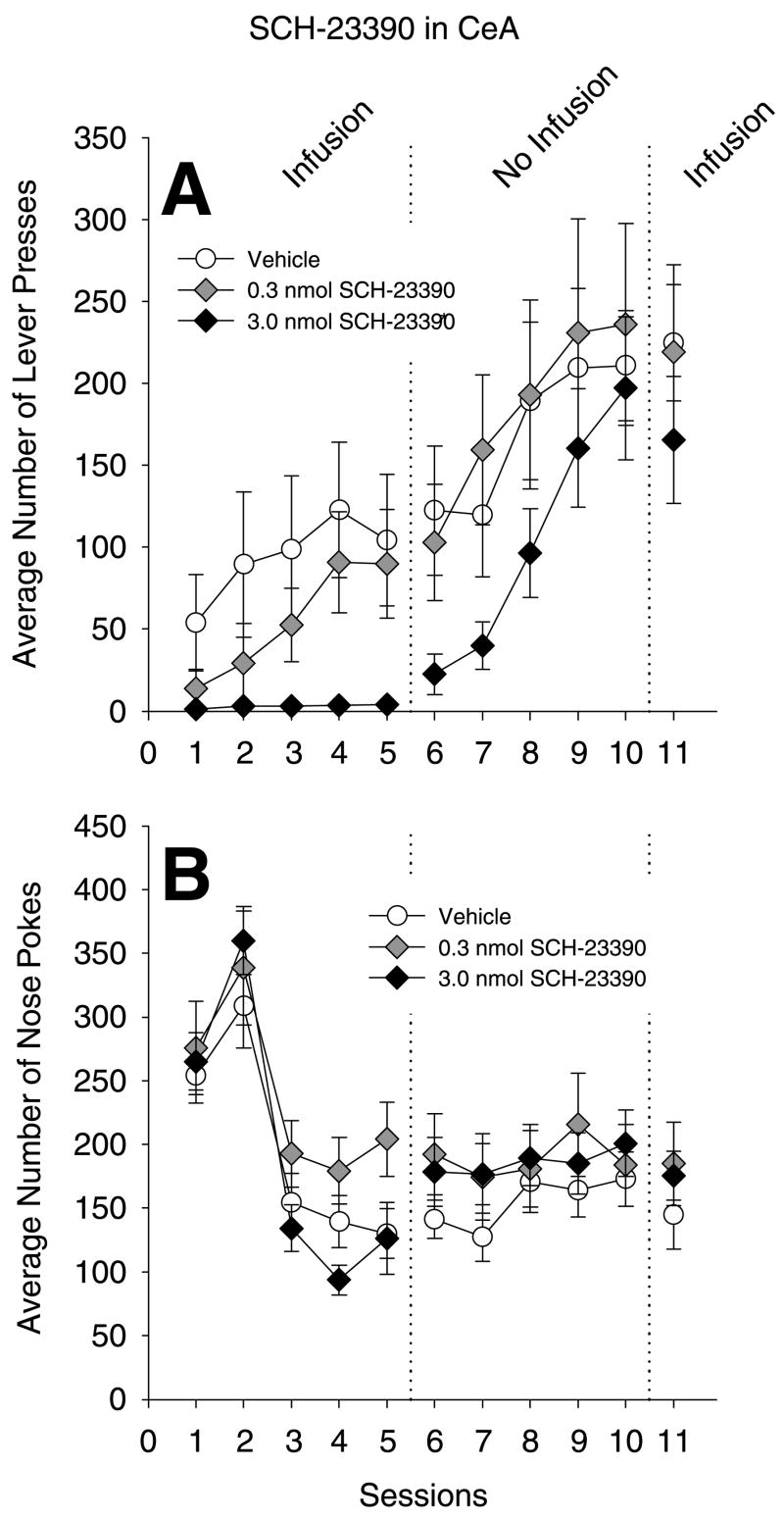
The effects of SCH-23390 infusions into the CeA on instrumental learning
Fig. 2A shows that SCH-23390 infusions into the CeA impaired instrumental conditioning in a dose dependent fashion. Analyses of LP from sessions 1–5 confirmed a main effect of treatment (F(2, 18)=3.50, P=0.05), a main effect of sessions (F(4, 72)=6.60, P<0.01), however the treatment×session interaction (F(8, 72)=1.89, P=0.07) only approached statistical significance. Tukey HSD post hoc test revealed that the 3.0 nmol SCH-23390-treated grouped differed from the vehicle-treated group, but that the 0.3 nmol did not differ from vehicle.
Fig. 2.
Effects of infusions the D1 antagonist SCH-23390 in the CeA on instrumental learning, performance and nosepoking. Rats received infusions of SCH-23390 or vehicle prior to sessions 1–5, and prior to session 11, but not prior to sessions 6–10. (A) Mean number of LP per session per group for sucrose pellets (±S.E.M.). * P<0.05 main effect of treatment. (B) Mean number of NP per session per group (±S.E.M.). No statistical reliable main effect of treatment or interaction on nosepoking was found.
Prior to sessions 6–10, no infusions were given and the average number of correct LP for previously drug-treated rats quickly increased, reaching a level roughly the same as vehicle-treated rats. Analysis of LP from sessions 6–10 revealed no main effect of previous treatment (F(2,18)=1.95, P =0.17), or treatment×session interaction (F(8, 72)=0.88, P =0.54). A main effect of sessions was confirmed (F(4, 72)=19.52, P<0.01). SCH-23390 infusions prior to session 11, when contrasted with LP during session 10, had no discernible effect (F=0.53, P=0.60). The main effect of sessions and the session×treatment interaction were not statistically reliable, either (F=1.64, P=0.21; F=2.19, P=0.14).
The number of NP into the food trough are shown in Panel 2B. For all rats, rates of nosepoking were high on the first two days and subsequently decreased to between 100 and 200 by session 3. The high rates of nosepoking during the first two days reflects the presence of the conjoint RT-30” food delivery. Analyses of NP from sessions 1–5 revealed no main effect of treatment (F(2, 18)=1.90, P=0.18), or treatment×session interaction (F(8, 72)=1.38, P=0.22); a main effect of sessions (F(4, 72)=35.64, P<0.01) was found. Analyses on nose poking for sessions 6–10 revealed no statistically significant differences or interactions; NP during session 11, when analyzed with those of session 10, were not statistically differentiated.
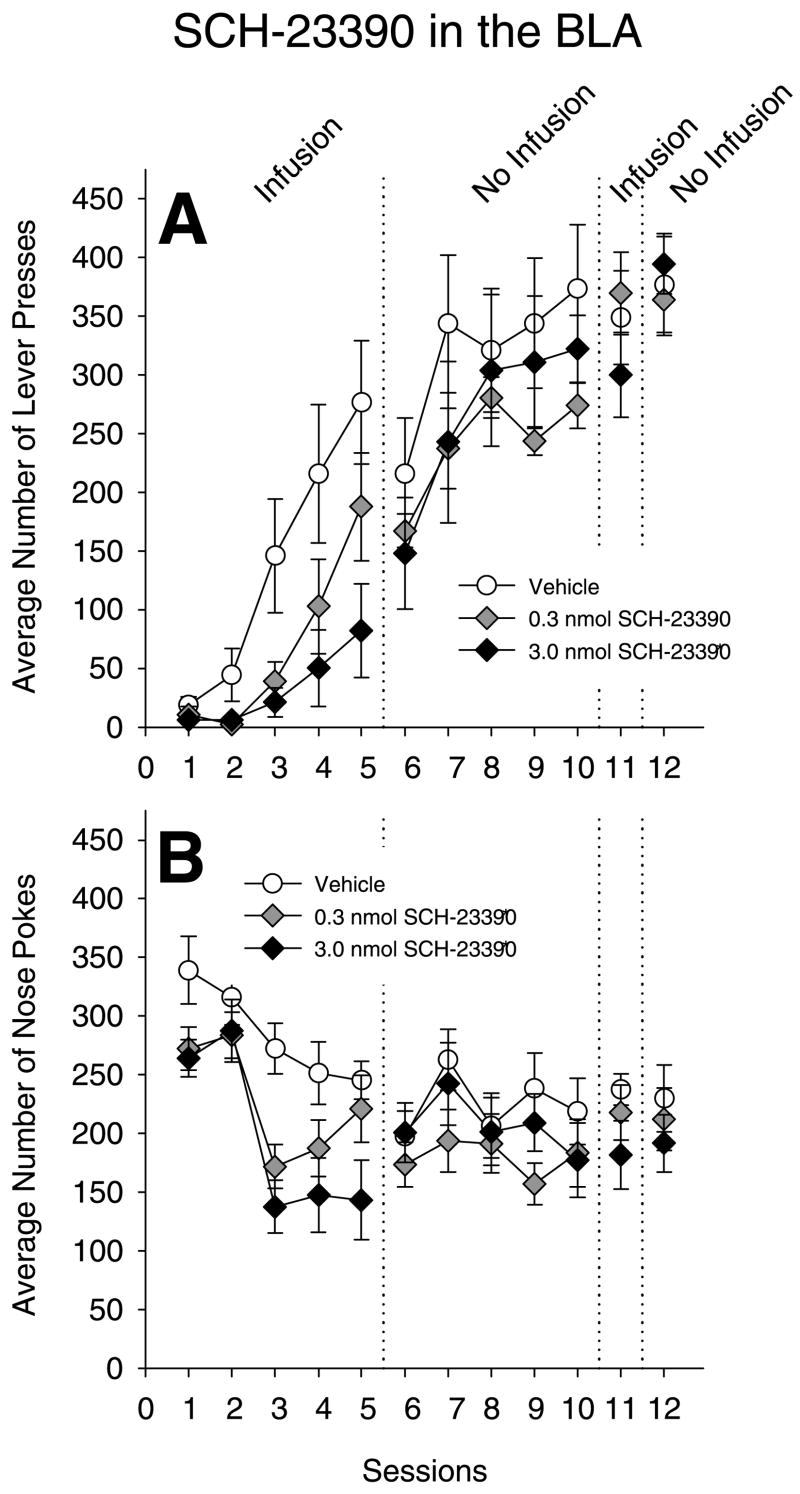
The effects of SCH-23390 infusions into the BLA on instrumental learning
SCH-23390 infusions into the BLA also impaired instrumental conditioning in a dose-dependent fashion, as shown in Fig. 3A. ANOVA on LP during sessions 1–5 revealed significant main effects of treatment (F(2, 19)=4.26, P=0.03) and sessions (F(4, 76)=29.19, P<0.01), and a significant two-way treatment×session interaction (F(8, 76)=3.26, P<0.01). Tukey HSD post hoc comparisons of the treatment main effect revealed differences between the 3.0 nmol group and the vehicle group, but no difference between the 0.3 nmol group and vehicle.
Fig. 3.
Effects of infusions the D1 antagonist SCH-23390 in the BLA on instrumental learning, performance and nosepoking. Rats received infusions of SCH-23390 or vehicle prior to sessions 1–5, and prior to session 11, but not prior to sessions 6–10 or session 12. (A) Mean number of LP per session per group for sucrose pellets (±S.E.M.). * P<0.05 different from vehicle (Tukey HSD). (B) Mean number of NP per session per group (±S.E.M.). * P<0.05 different from vehicle.
During sessions 6–10, the number of correct LP increased for all three groups; a main effect of session was found (F(4, 76)=14.70, P<0.01) but the effects of prior treatment and interaction were not statistically reliable (F’s=.71 and 0.88, respectively). ANOVA on LP in session 11 and 12 were not statistically differentiated.
Fig. 3B shows the average number of NP per session across session. ANOVA on NP during the first five sessions revealed significant main effects of treatment (F(2, 19)=9.67, P<0.01) and sessions (F(4, 76)=17.70 P<0.01), but no significant treatment×session interaction (F=1.25, P=0.28). Tukey HSD on the main effect of treatment revealed that both drug-treated groups were lower than the vehicle-treated group. ANOVA on NP during session 6–10 revealed no main effect of prior treatment (F(2, 16)=1.91, P=0.18), no main effect of sessions (F(4, 64)=2.06, P=0.10), or treatment×session interaction (F(8, 64)=0.56, P=0.81), ANOVA on NP during sessions 11 and 12 revealed no effect of treatment, sessions, or interaction.
Microstructural analyses
The results of two of the microstructural behavior analyses are presented in Fig. 4. Panels A and B show the probability of a NP given a sucrose pellet delivery (Pr(NP Reinf), termed a “Reinf” although it includes pellets delivered by the RT-30” which, technically, are not Reinf) when SCH-23390 was infused into the CeA and BLA, respectively. In both drug-treated and vehicle-treated rats, in both amygdala subnuclei, the Pr(NP Reinf) started near 0.70 and slowly decreased to about 0.50. This measure may repre sent learning about the events surrounding feeder operation (e.g. the magazine light, the sound of the feeder) and the sucrose pellets (a stimulus-outcome, or S-O relation). In other words, Fig. 4A and B shows that when a Reinf was delivered, there was a high likelihood that the next measured event for all rats was a NP, regardless of treatment. The decrease in subsequent sessions may have been due to the fact that during later sessions the contingencies changed within the session (from FR-1 after 50 LP to an RR-2); it became less advantageous to NP after every LP, and more advantageous to lever-press with increasing rapidly due to the positive feedback function of the RR-2 schedule (more presses=more Reinf). Although there appeared to be no differences in the Pr(NP Reinf), because this is a relative measure the time between the pellet delivery and NP could have been affected by drug infusions. Panels C and D show that the latency between pellet delivery and a NP was not impaired by drug infusions in either site, especially during sessions 1 and 2 when each individual rat experienced about 30 pellet deliveries (due to the conjoint RT-30” schedule). The lack of error bars during some of the sessions, and the lack of a point for other sessions, merely reflect the fact that only one rat made more than five LP during that session (no error bars) or that no rat in the group made more than five LP in a session (no point), and did not meet the requirement for inclusion in the data analysis. The exclusion of these data was necessary so as to not skew the data due to a small sample.
Fig. 4.
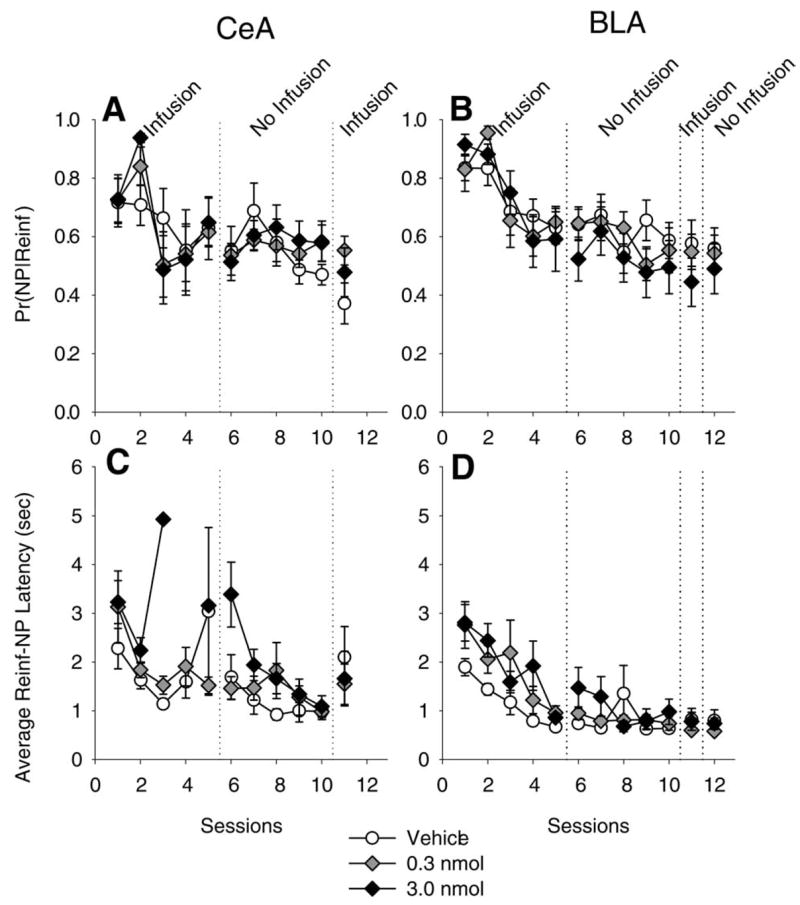
Microstructural behavioral analysis of D1 antagonism in the CeA and the basolateral amygdala (BLA). Panels A and B show the conditional probability of a NP given a Reinf was the last recorded event (Pr(NP Reinf)) in the CeA and BLA. Panels C and D shows the average latency of a NP following a Reinf. Note that during session 3, in the CeA, only one 3.0 nmol-treated rat (the dark diamond) earned enough Reinf (i.e. more than five) to meet the criteria for computing a mean latency (see method for description of computations).
Locomotion and feeding
As can be seen in Table 1, neither SCH-23390 infusions into the CeA, nor in the BLA, produced any statistical differences in the measures of spontaneous locomotor behavior or feeding.
Table 1.
SCH-23390 infused into the CeA or BLA: effects on spontaneous locomotor and feeding behavior
| Site Treatment | Total intake (g sucrose) | Latency to start eating (s) | Feeding duration (s) | Feeding bouts (number) | Avg feeding bout length (s) | Avg drinking duration (s) | Locomotion (center crossings) | Rears (paws Above Horizontal line) |
|---|---|---|---|---|---|---|---|---|
| CeA | ||||||||
| Vehicle | 7.0±0.76 | 20.91±4.9 | 590.0±41.9 | 19.5±2.4 | 35.97±7.0 | 11.8±9.0 | 26.0±2.6 | 27.0±4.9 |
| 0.3 nmol SCH-23390 | 6.8±0.87 | 23.53±4.0 | 570.0±39.7 | 19.8±2.6 | 32.93±5.4 | 20.7±14.2 | 22.0±2.5 | 23.0±3.6 |
| 3.0 nmol SCH-23390 | 5.9±0.74 | 23.14±6.6 | 549.6±27.2 | 20.3±1.9 | 29.28±3.7 | 19.1±11.5 | 24.1±0.7 | 19.9±3.1 |
| F (P) | 1.67 (0.22) | 0.06 (0.95) | 0.68 (0.52) | 0.02 (0.97) | 0.41 (0.67) | 0.22 (0.80) | 0.91 (0.43) | 0.86 (0.44) |
| BLA | ||||||||
| Vehicle | 6.0±0.77 | 14.75±2.6 | 502.0±60.1 | 12.8±1.4 | 41.1±5.4 | 3.9±3.2 | 24.5±3.4 | 25.8±3.3 |
| 0.3 nmol SCH-23390 | 6.9±0.68 | 11.92±2.2 | 630.0±41.4 | 11.9±1.1 | 59.1±9.7 | 0.3±0.1 | 17.8±2.4 | 18.6±3.1 |
| 3.0 nmol SCH-23390 | 5.6±0.59 | 14.47±2.8 | 515.9±55.4 | 13.3±0.6 | 38.2±3.5 | 0.9±0.3 | 25.1±2.0 | 26.6±4.6 |
| F (P) | 0.82 (0.46) | 0.32 (0.73) | 1.46 (0.26) | 0.33 (0.72) | 2.02 (0.16) | 1.07 (0.37) | 2.36 (0.13) | 2.14 (0.15) |
DISCUSSION
The present findings suggest a novel role for DA D1 activation in the amygdala in instrumental learning and are consistent with theories postulating a key role for D1 activation in neural plasticity. They are the first to suggest that plasticity in the amygdala is required for instrumental learning through which “organisms learn the consequences of their own behavior” (Catania, 1998). Control experiments show that D1 activation in the amygdala serves a selective and specific role in the cellular neuromodulatory mechanisms governing early learning (e.g. acquisition of new behavior), as opposed to a role in performance of the behavior. These results are consistent with previous experimentation demonstrating a crucial role for D1 activation in the amygdala in learning, such as second-order fear conditioning (Nader and LeDoux, 1999), and in drug-related processes, like cocaine-seeking in the cocaine-reinstatment paradigm (Ciccocioppo et al., 2001; Di Ciano and Everitt, 2004).
D1 receptor activation in the amygdala is required for instrumental learning but not performance
In the present experiments, infusions of SCH-23390 into the CeA or BLA dose-dependently impaired the acquisition of a novel instrumental behavior (Figs. 2A and 3A). After acquisition, however, D1 activation was not required for performance of the behavior, and control experiments revealed no effects on food-directed or general motor behavior. Thus, the nature of the impairment was neither motoric nor motivational, but likely the result of blocking cellular mechanisms involved in instrumental learning.
D1-receptor antagonism in the CeA and BLA appeared to produce similar behavioral effects, however several notable differences were observed. High-dose infusions of SCH-23390 in the CeA completely abolished learning, but by session 5, BLA-treated rats were responding at a substantial rate. Albeit regarding a different form of learning, a prominent theory of fear conditioning holds that information proceeds serially from the BLA to the CeA; neurons in CeA project directly to the brain stem producing adaptive behavior (Pare et al., 2004). If instrumental learning involves serial processing from the BLA to the CeA, then manipulations of the CeA may disrupt output from both the BLA and CeA, whereas BLA manipulations would not affect CeA processing, although a major input would be affected.
Secondly, there was a statistical difference in nosepoking between drug-treated and the vehicle-treated groups in the BLA, but not in the CeA. However, the ability of the rats to retrieve a Reinf was unaffected by drug treatment, either in terms of the probability that the next behavior was a NP (Pr(NP Reinf, Fig. 4A and B) or the latency to retrieve the pellet (Reinf-NP Latency, Fig. 4C and D). These results, taken together with the results of the control experiments, support the contention that motivational processes were unaffected; when sucrose pellets were delivered, drug-treated rats were unimpaired in their ability to retrieve them. In addition, the between-group difference observed in nosepoking in the BLA experiment is most likely a result of an unusual pattern and high rates emitted by the vehicle-infused group, rather than a drug effect. In other words, the CeA-vehicle-treated rats in the Fig. 2B showed the more typical pattern of high rates during the first two sessions, with a precipitous drop-off during session 3. In contrast, the group receiving vehicle infusions in the BLA displayed a more gradual decrement in nose-poking across sessions 1–5. Previous experimentation indicates that the pattern emitted by the CeA-vehicle-treated rats is the more typical pattern.
However, due to the close proximity of the two subnuclei, it is possible that the drug diffused from one subnuclei (e.g. CeA into BLA) into the other or that tissue integrity has been compromised. In other words, it may be that the CeA is not involved in instrumental learning at all because infusions diffused into the BLA, a structure important in instrumental learning (or vice versa). We have shown in previous reports, however, differential effects of drug infusions on performance of instrumental lever-pressing in these two structures, thereby attenuating some of these concerns. For example, lever-pressing was reduced by an infusion of the NMDA receptor antagonist AP-5 into the CeA prior to session 11 (Andrzejewski et al., 2004), but not by an AP-5 infusion into the BLA (Baldwin et al., 2000). These results suggest that regional specificity has been produced under conditions and methods that are identical to the present experiments. In addition, target coordinates for the two sites were different in all three directions. BLA infusions were targeted to be more posterior (−2.8 vs. −2.0), more lateral (±4.8 vs. ±4.0) and more ventral (−8.3 vs. −8.2). Lastly, the rate of diffusion of SCH-23390 in the CeA, as measured by quantitative autoradiography, suggests that it takes about 20 min for the drug to begin to diffuse outside of the CeA (Caine et al., 1995). Although larger injections volumes were used in the present studies (0.5 μl vs. 0.33 μl) than in the aforementioned study, sessions were only 15 min in length. Taken together, these three factors, experience, targets, and diffusion rate, suggest that if diffusion were producing some of the impairment in learning, the effects were sufficiently minimized.
Nonetheless, it is important to consider drug effects on nosepoking, and its relation to other events, because it may provide a glimpse of the possible role of Pavlovian influences on instrumental behavior. That is, food cup-responding (nosepoking) is often considered a measure of the associative strength (stimulus–outcome) between the pellets and the operation of the feeder (e.g. sound, illumination of the food cup, etc.), and is thought to reflect the motivational significance of the cues signaling reward (Gallagher and Holland, 1994). Because no effects of SCH-23390 infusions were found on food cup-responding, it appears that while some Pavlovian processes were intact, instrumental learning, which is dependent on response-outcome contingencies, was impaired by D1 inactivation. In contrast, Dickinson et al. (2000) demonstrated that systemic injections of the DA D2 receptor antagonist pimozide and the mixed DA D1/D2 receptor antagonist α-flupenthixol attenuated the facilitation of instrumental performance by Pavlovian conditional stimuli (presumably mediated by S-O associations), but had no effect on instrumental incentive learning (presumed to be mediated by R-O associations). However, while Dickinson et al. (2000) dissociated important dopaminergic modulation of distinct learning processes, our results are not directly contradicted because their pharmacological manipulations were systemic and engaged D2 receptors. Further experimentation is needed to differentiate D1 and D2 receptor function on these important learning processes.
An alternate, and arguably more parsimonious, interpretation regarding the effect of CeA and BLA manipulations on nose-poking is that this behavior is less sensitive to CeA manipulations than BLA manipulations, regardless of its associative nature (e.g. Pavlovian vs. instrumental). Indeed, the present experiments provided little insight into the nature of the associative structures that may have been influenced by D1 antagonism because they were not designed to assess those relations. However, from the experimenter’s point of view, the contingencies (e.g. R-O relations) between the rat’s behavior and other events were manipulated in some of the present experiments allowing for, at the very least, some exploratory data analyses and speculation regarding associative mechanisms, in light of contemporary theories of learning. Certainly, additional data from multiple methodologies and paradigms are needed to more fully understand the neurobiology of instrumental learning.
Discrepancies between pharmacological and lesion studies
While recent lesion experiments suggest dissociable roles for both the CeA and BLA in some forms of learning, the clear impairment of instrumental learning in the present study stands in marked contrast to these investigations. For example, lesions of the CeA or BLA appear to leave initial instrumental learning and first-order Pavlovian conditioning (food-cup responding) intact (Balleine et al., 2003; Cador et al., 1989; Hall et al., 2001; Harmer et al., 1997; Weiss et al., 2000; Whitelaw et al., 1996). However, after first-order Pavlovian conditioning, BLA lesions block “CS-potentiated feeding,” the enhancement of food consumption by food-related stimuli, but do not block Pavlovian-to-instrumental transfer (“PIT”), whereas CeA lesions block PIT but not CS-potentiated feeding (Holland and Gallagher, 2003; Holland et al., 2002). While these data suggest important roles for the CeA and BLA in important aspects of learning, the finding that amygdala lesions do not affect initial acquisition of lever-pressing is discrepant with our current findings.
Although further experimentation may be required to resolve this discrepancy, we propose that compensatory neuroadaptations following lesion-induced damage may obscure the amygdala’s fundamental role in instrumental learning. Similar discrepancies have been noted in pigeons, where N-methyl-D-aspartate receptor blockade with AP-5 in the prefrontal cortex produced deficits in extinction learning, but lesions did not (Lissek and Gunturkun, 2003). Abundant evidence suggests that neuroadaptations in multiple cellular functions occur following lesions, including presynaptic increases in synthesis, metabolism, and fractional release of DA in the remaining terminals (Abercrombie et al., 1990; Castaneda et al., 1990; Robinson and Whishaw, 1988; Touchet and Bennett, 1989; Zhang et al., 1988), and post-synaptic DA-receptor supersensitivity (Creese et al., 1977; Mishra et al., 1974; Neve et al., 1982). Therefore, while lesion-induced deficits in certain paradigms are well-documented, the lack of deficits in some lesion studies makes them difficult to interpret, given the likely recovery of function. The fact that selective pharmacological blockade of NMDA receptors (Andrzejewski et al., 2004; Baldwin et al., 2000), muscarinic receptors (See et al., 2003) and now D1 receptors, within the amygdala, disrupts aspects of appetitive instrumental learning indeed demonstrates a role for the amygdala in the cellular plasticity that underlies this learning.
DA in the amygdala mediates the acquisition of significance of environmental stimuli
Several lines of evidence support the present data suggesting a role for amygdala DA in instrumental learning. Firstly, in vivo microdialysis studies show that DA levels increase in distinct subnuclei of the amygdala during Pavlovian approach conditioning, during discriminated instrumental training, during drug challenge, and during drug-cue exposure in rats (Harmer et al., 1997; Harmer and Phillips, 1999; Hori et al., 1993; Weiss et al., 2000). Amygdalar DA also increases in humans during reading or performance of a working memory task (Fried et al., 2001). Secondly, immunohistochemical data corroborate the finding that DA activity is elevated in the amygdala during Pavlovian conditioning and during instrumental conditioning (Phillips et al., 2003). Thirdly, in fear-related paradigms, SCH-23390 infusions into the CeA or BLA block acquisition and performance of fear-potentiated startle (Greba and Kokkinidis, 2000; Lamont and Kokkinidis, 1998), impair second-order fear conditioning (Nader and LeDoux, 1999), and modulate responses to stress (Stevenson and Gratton, 2004). Lastly, addiction-related studies show that amygdalar D1 activation is involved in drug conditioning and self-administration (Caine et al., 1995; Di Ciano and Everitt, 2004; Zarrindast et al., 2003). Thus, it is clear from Pavlovian and instrumental conditioning paradigms, trained with a variety of unconditional stimuli and Reinf, that D1 function in the amygdala modulates important aspects of learning, most likely the attribution of significance to environmental stimuli.
Synaptic plasticity in the amygdala and the role of DA
There is abundant cellular evidence for synaptic plasticity within the amygdala. For example, in a number of models, both in vitro and in vivo, long-term potentiation (LTP), as well as other forms of synaptic plasticity, has been demonstrated in the amygdala (Blair et al., 2001; Chapman et al., 1990; Huang and Kandel, 1998; McKernan and Shinnick-Gallagher, 1997; Pare and Collins, 2000; Rogan and LeDoux, 1995; Watanabe et al., 1995). DA has been shown to modulate sensory, thalamic and prefrontal inputs to the BLA (Rosenkranz and Grace, 2001, 2002); ultra-structural analysis reveals that dopaminergic afferents to both BLA and CeA are in a position to modulate these extrinsic inputs (Asan, 1997). Thus, synaptic plasticity in the amygdala is hypothesized to represent a major mechanism governing learning, and DA is likely to participate in the process for both fear- and reward-related learning.
Function of amygdala DA within a cortico-striatal network: learning the value of actions
In recent years there has been substantial interest in the integration of knowledge concerning the relative roles of specific brain structures within the overall functioning of a neural systems network (Cardinal et al., 2002; Holland and Gallagher, 2004; Schoenbaum et al., 2003). Instrumental learning requires a process in which the value of actions (initially generated randomly) is somehow represented when followed by a positive, unexpected event. Given (i) the proposed role for striatal and particularly prefrontal-striatal circuits and their outputs in selection of motor actions (Wise et al., 1996), and (ii) the assumption that the amygdala participates in assigning value to stimuli in the environment (Baxter and Murray, 2002), and (iii) the notion that dopaminergic neurons within the midbrain signal unpredicted, surprising, or salient events in the environment and release DA in terminal regions (Schultz and Dickinson, 2000), we propose that D1 receptor activation within the amygdala participates in the assigning of value to motor actions. As D1 receptors appear to be required only in the acquisition process (at least for instrumental learning), it is possible that amygdala DA release during early learning enables the network to associate unexpected positive events (food reward) with the immediately preceding action. Further experiments will be required to test this hypothesis. In sum, the present findings provide behavioral evidence for DA D1-mediated plasticity within both the BLA and CeA in the acquisition of novel instrumental behavior.
Acknowledgments
This research was supported by National Institutes of Health grants DA016465-01 (M.E.A.) and DA 004788–18 (A.E.K.).
Abbreviations
- BLA
basolateral portion of the amygdala
- CeA
central nucleus of the amygdala
- DA
dopamine
- FR-1
Fixed Ratio-1
- HSD
honestly significant difference
- LP
lever press
- NP
nose poke
- PIT
Pavlovian-to-instrumental transfer
- Reinf
reinforcer
- RR-2
Random Ratio-2
- RT-30”
random time 30”
- SCH-23390
R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-[a-zA-Z][0-9]-3-benzazepine hydrochloride
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525(1):36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Sadeghian K, Kelley A. Central amygdalar and dorsal striatal NMDA-receptor involvement in instrumental learning and spontaneous behavior. Behav Neurosci. 2004;118(4):715–729. doi: 10.1037/0735-7044.118.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288(3):449–469. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci. 2000;114(1):84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23(2):666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Gerdjikov T. The role of signaling molecules in reward-related incentive learning. Neurotox Res. 2004;6(1):91–104. doi: 10.1007/BF03033301. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev. 1998;22(2):335–345. doi: 10.1016/s0149-7634(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8(5):229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Boyson S, McGonigle P, Molinoff P. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30(1):77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692(1–2):47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Castaneda E, Whishaw IQ, Robinson TE. Changes in striatal dopamine neurotransmission assessed with microdialysis following recovery from a bilateral 6-OHDA lesion: variation as a function of lesion size. J Neurosci. 1990;10(6):1847–1854. doi: 10.1523/JNEUROSCI.10-06-01847.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania AC. Learning. Upper Saddle River, NJ: Prentice-Hall; 1998. [Google Scholar]
- Chapman PF, Kairiss EW, Keenan CL, Brown TH. Long-term synaptic potentiation in the amygdala. Synapse. 1990;6(3):271–278. doi: 10.1002/syn.890060306. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98(4):1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197(4303):596–598. doi: 10.1126/science.877576. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Gehlert DR, McCabe RT, Barnett A, Wamsley JK. D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci. 1986;6(8):2352–2365. doi: 10.1523/JNEUROSCI.06-08-02352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24(32):7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114(3):468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Amygdala-ventral striatal interactions and reward-related processes. In: Aggleton JP, editor. Amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 401–429. [Google Scholar]
- Fried I, Wilson CL, Morrow JW, Cameron KA, Behnke ED, Ackerson LC, et al. Increased dopamine release in the human amygdala during performance of cognitive tasks. Nat Neurosci. 2001;4(2):201–206. doi: 10.1038/84041. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. Understanding the function of the central nucleus: Is simple conditioning enough? In: Aggleton JP, editor. Amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 307–321. [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci U S A. 1994;91(25):11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greba Q, Kokkinidis L. Peripheral and intraamygdalar administration of the dopamine D1 receptor antagonist SCH 23390 blocks fear-potentiated startle but not shock reactivity or the shock sensitization of acoustic startle. Behav Neurosci. 2000;114(2):262–272. doi: 10.1037//0735-7044.114.2.262. [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13(10):1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hitchcott PK, Morutto SL, Phillips GD. Repeated d-amphetamine enhances stimulated mesoamygdaloid dopamine transmission. Psychopharmacology (Berl) 1997;132(3):247–254. doi: 10.1007/s002130050342. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience. 1999;90(1):119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Phillips GD. Effects of intra-amygdala R(+) 7-OH-DPAT on intra-accumbens d-amphetamine-associated learning. I. Pavlovian conditioning. Psychopharmacology (Berl) 1998a;140(3):300–309. doi: 10.1007/s002130050771. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Phillips GD. Effects of intra-amygdala R(+) 7-OH-DPAT on intra-accumbens d-amphetamine-associated learning. II.Instrumental conditioning. Psychopharmacology (Berl) 1998b;140(3):310–318. doi: 10.1007/s002130050772. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17(8):1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14(2):148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76(1):117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Hori K, Tanaka J, Nomura M. Effects of discrimination learning on the rat amygdala dopamine release: a microdialysis study. Brain Res. 1993;621(2):296–300. doi: 10.1016/0006-8993(93)90119-8. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21(1):169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795(1–2):128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Lissek S, Gunturkun O. Dissociation of extinction and behavioral disinhibition: the role of NMDA receptors in the pigeon associative forebrain during extinction. J Neurosci. 2003;23(22):8119–8124. doi: 10.1523/JNEUROSCI.23-22-08119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Gardner EL, Katzman R, Makman MH. Enhancement of dopamine-stimulated adenylate cyclase activity in rat caudate after lesions in substantia nigra: evidence for denervation supersensitivity. Proc Natl Acad Sci U S A. 1974;71(10):3883–3887. doi: 10.1073/pnas.71.10.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113(5):891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Neve KA, Kozlowski MR, Marshall JF. Plasticity of neostriatal dopamine receptors after nigrostriatal injury: relationship to recovery of sensorimotor functions and behavioral supersensitivity. Brain Res. 1982;244(1):33–44. doi: 10.1016/0006-8993(82)90901-5. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17(3):429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20(7):2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92(1):1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego, CA: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Setzu E, Vugler A, Hitchcott PK. Immunohistochemical assessment of mesotelencephalic dopamine activity during the acquisition and expression of Pavlovian versus instrumental behaviours. Neuroscience. 2003;117(3):755–767. doi: 10.1016/s0306-4522(02)00799-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450(1–2):209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15(1):127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Romo R, Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol. 1990;63(3):592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21(11):4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417(6886):282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res. 2003;146(1–2):19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J Neurophysiol. 1990;63(3):607–624. doi: 10.1152/jn.1990.63.3.607. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154(3):301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117(2):477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Gratton A. Role of basolateral amygdala dopamine in modulating prepulse inhibition and latent inhibition in the rat. Psychopharmacology Berl. 2004:139–145. doi: 10.1007/s00213-004-1879-6. [DOI] [PubMed] [Google Scholar]
- Touchet N, Bennett JP., Jr The metabolism of systemically-administered L-dihydroxyphenylalanine, by intact and dopamine-denervated striata, as revealed by brain microdialysis. Neuropharmacology. 1989;28(11):1217–1222. doi: 10.1016/0028-3908(89)90214-1. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Saito H, Abe K. Nitric oxide is involved in long-term potentiation in the medial but not lateral amygdala neuron synapses in vitro. Brain Res. 1995;688(1–2):233–236. doi: 10.1016/0006-8993(95)00563-6. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97(8):4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127(3):213–224. [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13(6):685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10(3–4):317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Young AMJ, Rees KR. Dopamine release in the amygdaloid complex of the rat, studied by brain microdialysis. Neurosci Lett. 1998;249(1):49–52. doi: 10.1016/s0304-3940(98)00390-5. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Rezayof A, Sahraei H, Haeri-Rohani A, Rassouli Y. Involvement of dopamine D1 receptors of the central amygdala on the acquisition and expression of morphine-induced place preference in rat. Brain Res. 2003;965(1–2):212–221. doi: 10.1016/s0006-8993(02)04201-4. [DOI] [PubMed] [Google Scholar]
- Zhang WQ, Tilson HA, Nanry KP, Hudson PM, Hong JS, Stachowiak MK. Increased dopamine release from striata of rats after unilateral nigrostriatal bundle damage. Brain Res. 1988;461(2):335–342. doi: 10.1016/0006-8993(88)90264-8. [DOI] [PubMed] [Google Scholar]