Abstract
The extension and directionality of neurite outgrowth are key to achieving successful target connections during both CNS development and during the re-establishment of connections lost after neural trauma. The degree of axonal elongation depends, in large part, on the spatial arrangement of astrocytic processes rich in growth-promoting proteins. Because astrocytes in culture align their processes on exposure to an electrical field of physiological strength, we sought to determine the extent to which aligned astrocytes affect neurite outgrowth. To this end, dorsal root ganglia cells were seeded onto cultured rat astrocytes that were pre-aligned by exposure to an electric field of physiological strength (500 mV mm−1). Using confocal microscopy and digital image analysis, we found that neurite outgrowth at 24 hours and at 48 hours is enhanced significantly and directed consistently along the aligned astrocyte processes. Moreover, this directed neurite outgrowth is maintained when grown on fixed, aligned astrocytes. Collectively, these results indicate that endogenous electric fields present within the developing CNS might act to align astrocyte processes, which can promote and direct neurite growth. Furthermore, these results demonstrate a simple method to produce an aligned cellular substrate, which might be used to direct regenerating neurites.
Keywords: Astrocytes, neurite, DRG, alignment, electric
INTRODUCTION
During development of the CNS axons must traverse through the complex extracellular matrix of the brain and spinal cord to establish connections with their target. To accomplish this, several chemical and structural guidance cues present within the neuropil facilitate neurite outgrowth and directionality. A key cellular source for such guidance cues is astrocytes, which are present in the extracellular space before neuritic outgrowth. In addition to providing neurotropic and neurotrophic support for growing neurons (Pierret et al., 1998; Gomes et al., 2001), astrocytes provide either a structural or a geometric framework along which neurites can grow (Prochiantz, 1985; Tamamaki, 2002). For example, in the development of many sensory and motor systems, astrocytes extend their processes parallel to the growth axis of a presumptive fiber tract; an orientation that facilitates axon outgrowth in a directional manner (Schmechel and Rakic, 1979; Misson et al., 1988; Voigt, 1989; Cameron and Rakic, 1991). Additionally, astrocytes can express permissive cell-surface molecules, such as N-cadherin and neuronal cell adhesion molecule (NCAM), which further promote neurite extension along their processes (Neugebauer et al., 1988). Although the mechanisms that align astrocyte processes in such an organized manner are unknown, it is interesting to note that this type of process organization can be induced in astrocytes in culture. For example, when astrocytes are exposed to electric fields of physiological strength, they retract and realign their processes in a direction perpendicular to the electric field vector (Borgens et al., 1994).
The presence of endogenous electric fields in many developing biological systems, including the CNS, has been well characterized (reviewed by Colello and Alexander, 2003). For example, ionic currents, ranging from 1–1000 μA cm−2, have been detected in systems as simple as algal eggs and as complex as the vertebrate nervous system (Jaffe, 1981; Nuccitelli, 1992; McCaig and Zhao, 1997). These currents, which are usually focused at either the site of growth or other cellular activity, generate corresponding electrical fields ranging in strength from 1–1000 mV mm−1. Furthermore, disruption of these electric fields induces several developmental abnormalities (Borgens et al., 1979; Jenkins et al., 1996). At the cellular level, endogenous electric fields are responsible for providing the axis of symmetry for cell division and the consequent formation of lamellar structures (Peng and Jaffe, 1976; Zhao et al., 1999). Within the nervous system, endogenous electrical fields have been recorded during development and following injury and affect the orientation, growth rate and branching of neurons and glia in vitro (Jaffe and Poo, 1979; Rajnicek et al., 1992; Borgens et al., 1994; Huang et al., 1997). These studies demonstrate that electric fields directly influence several neural cell types and are likely to have an active role in the development of the CNS. The presence of an inherent pathway in the developing CNS for neurons to follow was coined the ‘blueprint hypothesis’ by Singer et al. (1979). Therefore, one possible function for endogenous electrical fields in the developing CNS is to aid in the alignment of astrocytic processes that might support and direct neurite outgrowth.
Beyond the developmental implications, the generation of an aligned cellular substrate capable of directing neurite outgrowth has important potential applications for the repair and regeneration of CNS injuries. For example, the presence of inhibitory molecules and cellular debris at the site of injury forms major impediments to successful axonal regeneration (Davies and Silver, 1998; Chen et al., 2000). To provide a more permissive substrate for axons regeneration, a number of groups have implanted natural and artificial substrates to bridge the injury site (Giordano et al., 1997; Geller and Fawcett, 2002; Teng et al., 2002; Biran et al., 2003). Though promising, the extent of successful regrowth into these substrates is, to a large part, determined by the geometry and permissiveness of the bridge material (Plant et al., 1997; Geller and Fawcett, 2002). Consequently, the utilization of electrical fields to align astrocytes might yield a substrate with all the key components for successful regeneration. In this study, we aim to determine the extent to which aligned astrocytes influence neurite outgrowth.
OBJECTIVE
We have investigated the ability of electrically stimulated astrocytes to direct neurite growth. To this end, astrocyte cultures have been aligned through exposure to electric fields of physiological strength. Dorsal root ganglia neurons were seeded onto either aligned or unaligned (random) astrocyte cultures and their growth characteristics assessed using confocal microscopy and Fourier image analysis. Finally, we assessed the extent to which fixation of aligned astrocytes influences the outgrowth of neurites.
METHODS
Astrocyte cultures
Primary rat astrocytes were isolated using methods similar to those described previously (McCarthy and de Vellis, 1980). Briefly, cortices from postnatal day 2 (P2) Sprague Dawley rat pups were dissected free then dissociated enzymatically and mechanically into a single-cell suspension. The single-cell suspension was plated at two cortices per 75 cm2 flask in DMEM/F12 medium with 10% fetal bovine serum (FBS) and 10 000 units ml−1 penicillin/streptomycin (PS), and grown to confluency over 14 days. Contaminating cells were removed by shaking the flasks on a rotary shaker at 250 rpm, followed by incubation with 20 μM cytosine arabinoside for 3 days. This method yields cultures of >98% type-I astrocytes, which was confirmed with anti-Vimentin and anti-GFAP immunohistochemistry. Only cultures passaged no more than three times were used in these experiments.
Dorsal root ganglia cultures
Primary rat dorsal root ganglia were isolated using the method described by Sharma and Bigbee (1998). Briefly, spinal cords from embryonic day 16 (E16) Sprague Dawley rat pups were dissected free and dorsal root ganglia collected. Ganglia were dissociated enzymatically in 0.25% trypsin for 45 minutes at 37°C and then triturated, resulting in a single-cell suspension. The cells were centrifuged and resuspended in Eagle’s MEM with 10% FBS and 100 ng ml−1 nerve growth factor. The suspension of dorsal root ganglia cells was seeded immediately into experimental chambers.
Electric field chambers
Electric field chambers were constructed to allow a constant electric current to flow directly over the cell culture chambers. Chambers were constructed in a similar manner to that described by McCaig (1987) and Borgens (1994), but with some modifications to suit our experimental setup. Specifically, superfrost glass slides were cleaned with acid and alcohol washes and rinsed with nanopure water. Following air drying, acetate spacers (average dimensions, 0.12 × 3 × 75 mm) were adhered along the long edge of the glass slide using silicone grease. Slides were then sterilized overnight with UV light and coated with 10 μg bovine fibronectin in 1 ml PBS for 30 minutes. This solution was removed and 1.5 × 105 astrocytes seeded onto the slide in DMEM with 0.5% FCS, PS and G5 supplement (Invitrogen). G5 supplement (Invitrogen) was added to induce stellation of the astrocytes. Following a 2-day incubation, a second glass slide was adhered on top of the first with silicone grease to form a chamber with a cross sectional area of approximately 19 × 0.12 mm.
Culture plates (24 well) were modified to produce an electrical field simultaneously across multiple culture chambers (Fig. 1). Briefly, three culture chambers were connected in series by salt bridges composed of 2% agarose dissolved in DMEM/0.5%FCS/PS. Electric current was passed through the chamber series by placing electrodes into 50 ml flasks filled with PBS, and connecting the cathode to one end of the chamber series and the anode to the other by an additional agarose bridge. Electric current was provided by a constant-current power supply. The electric field strength (E: Mv mm−1) was calculated using the resistivity (ρ: 700 Ωmm, average) of the media, the cross sectional area of the chamber (A: 2.28 mm2, average) and the electric current (I: mA) using the following formula: E = Iρ/A. To produce an electric field strength of either 10 or 500 mV mm−1, a constant electric current of either 0.03 or 1.63 mA (respectively) was applied with a constant-current source and monitored throughout the experiment using an ammeter. During pilot studies, the temperature of the media pools at either end of the chamber, measured with a digital thermometer before, during and following experimentation, was no different than controls (37°C).
Fig. 1. Schematic of the electrical field chamber.
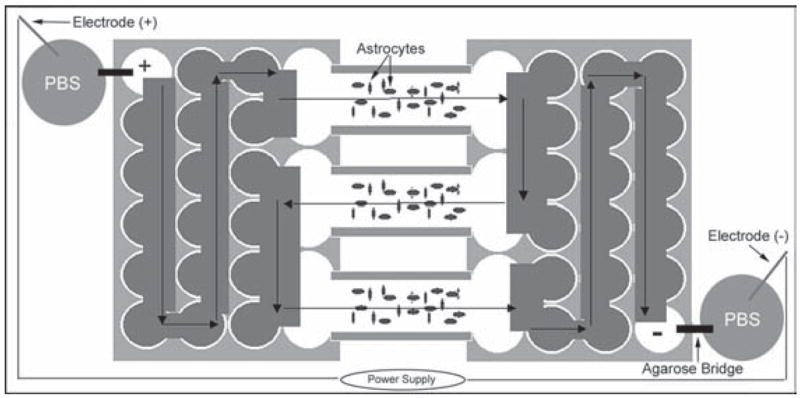
Two 24-well culture plates are modified to expose simultaneously three chambers of astrocytes to an electric field. Current generated by a power supply is passed through the chamber series in the direction indicated by the arrows.
Electric field application and co-cultures
Astrocyte cultures were exposed to 500 mV mm−1 (1.63 mA) for 24 hours to induce alignment. This field strength was chosen because it is within the physiological range recorded from the CNS (Nuccitelli, 1992) and elicits maximal orientation in cultured astrocytes (Borgens et al., 1994). Following electric-field exposure, the overlying glass slide was gently removed from the chamber and the astrocytes either washed with Hank’s Balanced Salt Solution (HBSS) or fixed with 1.5% paraformaldehyde and then washed with HBSS. The fixed astrocytes were washed additionally by five 10-minute rinses with PBS. After washing both live and fixed astrocytes, 5.0 × 104 dorsal root ganglia cells were seeded onto the chambers. The overlying glass slide was then placed gently back on top of the chamber, which was then returned to the incubator. In some instances, an electric field was re-applied following seeding of neurons. In this case, the co-cultures were allowed to settle for 4 hours before application of an electric field of either 10 or 500 mV mm−1 (0.03 and 1.6 mA, respectively).
Immunohistochemistry
Immediately following experimentation cultures were fixed in 4% paraformaldehyde for 15 minutes, washed in PBS, then blocked and permeabilized in PBS containing 5% FBS and 0.3% Triton-X100 (blocking buffer) for 30 minutes. The slides were then incubated with antibodies to GFAP (DAKO, 1:500) and Vimentin (Chemicon, 1:500), which are markers for mature and immature astrocytes, respectively. Concurrently, slides were incubated with an antibody against the neuron marker, TUJ1 (Covance, 1:500), which recognizes beta tubulin III. Antibody incubation was in blocking buffer for 3 hours at room temperature. Following washes in PBS, the cultures were incubated with the appropriate secondary antibodies conjugated with Cy5 (Chemicon, 1:200), Alexafluor 488 (Molecular Probes, 1:200) and Alexafluor 568 (Molecular Probes, 1:200) in PBS containing 5% FBS for 1 hour. Cultures were washed with PBS, the nuclei labeled with Hoechst dye, washed again with water and cover-slipped using Vecta-Shield mounting media (Vector Labs).
Fourier transform analysis of image directionality
Digital image processing methods using the Fast Fourier Transform (FFT) algorithm were used to extract directional information from images of cultured cells (Tonar et al., 2003). This produces an image that is the graphical representation of the frequency content of the original image. To accomplish this transformation, a gray-scale image of either astrocyte or neuron immunostaining was processed with the FFT function of the ImageJ (NIH) image-processing software. The resulting FFT image consists of pixels whose intensity and distribution represents the frequency content of the original image, which is related to the directional content. The pixel intensities of the FFT image, when summed along a straight line from the center to the edge of the image (at angle θ), quantify the relative contribution of objects oriented in that direction. Pixel summing was performed using the ImageJ plug-in ‘Oval Profile’ (authored by Bill O’Connell, http://rsb.info.nih.gov/ij/plugins/oval-profile.html). A graphical representation of the directionality of the original image is obtained by plotting the summed pixel intensities between 0° and 180°. A truly random image would result in a constant pixel intensity independent of direction, which would be plotted as a horizontal line (for example Fig. 2b,c). Conversely, an image preferentially aligned in one direction would have higher pixel intensities along that direction, which would be plotted as having a peak in that direction (for example Fig. 2e,f). It should be noted that the FFT image was first rotated 90° counterclockwise because the results of the FFT yields frequencies orthogonal to those in the original image. In our experiments, this rotation also defines the direction of electric field application along the 0–180° axis (horizontal). The FFT image is symmetrical about the horizontal axis, therefore draws no distinction between objects oriented in opposite directions.
Fig. 2. Electrically-aligned astrocytes guide neurite outgrowth.
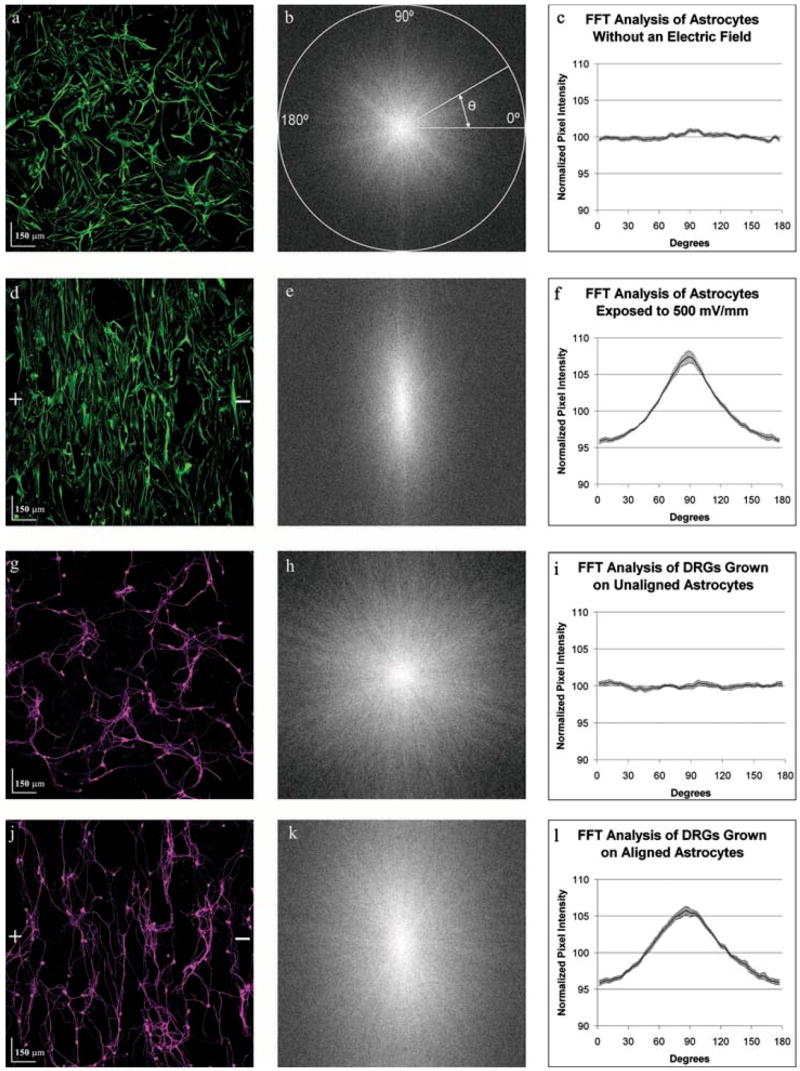
Astrocyte cultures were either exposed to an electric field (500 mV mm−1) for 24 hours or left unexposed as controls. A suspension of dorsal root ganglia cells was then seeded onto the astrocyte cultures and allowed to grow for 48 hours without an electrical field. Images derived from the same field of view show the spatial relationship between control astrocytes (a) and dorsal root ganglion cells (g) and between exposed astrocytes (d) and the dorsal root ganglion cells (j). Plus (+) and minus(−) signs indicate the direction of the electric field vector. (a) Control astrocytes expressing the intermediate filament Vimentin (green), appeared to be randomly oriented. FFT analysis of 12 images provided information about gross image orientation in the form of pixel intensity (b, e, h, k). The pixel intensity of the FFT image is summed along a straight line radiating at the angle, from the center to the edge of the image. (c) Plotting the normalized summed pixel intensity shows equal orientation in every direction. (d) On exposure to an electric field astrocytes display a strong alignment perpendicular to the field. (e,f) FFT analysis of six astrocyte images reveals strong orientation towards 88.8°. (g–i) Similarly, dorsal root ganglia cells seeded on control astrocytes (g) show random orientation that is identical to the astrocyte cultures (h,i). (j–l) Conversely, DRGs seeded on electrically aligned astrocytes (j) preferentially align towards 88.8° (k,l).
Analysis of neurite outgrowth directionality and length
To assess the influence of astrocytic alignment on neurite outgrowth, a total of three chambers of aligned astrocytes and six chambers of unaligned astrocytes were examined. Studies to assess the influence of continued electric field exposure on neurite growth used six chambers of astrocytes aligned at 10 mV mm−1 and 500 mV mm−1. Images of each chamber were acquired with the investigator blinded to the experimental conditions to avoid bias. Dorsal root ganglia often seeded as clumps of cells, which makes imaging difficult. Consequently, representative images of regions with moderate cell density were acquired for analysis. A Leica confocal microscope (TCS-SP2 AOBS) with a 10× objective was used to produce composite images for analysis of astrocyte alignment and the direction and length of neurite outgrowth. Orientation analysis of images was accomplished by performing Fourier transformation. The resulting pixel intensity profile was normalized by dividing each data point in the trace by the trace mean. The cumulative response of similar experiments was computed by a point-by-point average of each trace and graphed with standard error at each data point.
To examine individual neurons, four fields-of-view (FOV) were acquired per chamber using a Leica confocal microscope and a 10× objective. Typically, each image consisted of three neurons that could be traced in their entirety. Neurite direction was measured as the angle from the cell soma to the tip of all the primary neurites. The full length of each neurite was measured by manually tracing the neurite using the ImageJ software.
Statistical analysis
Comparison of orientation curves was done with 2-factor ANOVA and differences tested with Bonferroni corrected post-hoc analysis at each point. Student’s t-test was used to compare all other means. All values are reported as the mean ± S.E.M.
RESULTS
Direction of neurite growth follows electrically aligned astrocytes
To determine the extent to which neurites grow along the processes of electrically aligned glial cells, primary astrocytes were seeded onto fibronectin-coated glass chambers and allowed to adhere for 48 hours before the application of a physiologically relevant electric field of 500 mV mm−1. Control astrocytes were not exposed to an electric field but, otherwise, they were treated identically. Following a 24-hour exposure to the electric field, DRGs were seeded onto the astrocytes and incubated for 48 hours. The mixed cultures were then fixed and immunostained for neuronal (TUJ1) and astrocytic markers (GFAP and Vimentin). Examination of control astrocyte images (Fig. 2a) by FFT analysis (Fig. 2b) showed no orientation preference (n = 12 FOV, Fig. 2c), indicating random orientation. However, following exposure to an electric field (Fig. 2d), FFT image analysis (Fig. 2e) revealed that the astrocytes preferentially align towards the axis perpendicular to the electric field vector, maximally at 88.8 ± 1.30° (n = 6 FOV, Fig. 2f). This alignment is significantly more intense in the electrically stimulated astrocytes than in the control cultures (P<0.001). We then analyzed the same FOVs for directionality of neurite growth using the TUJ1 images. Neurons grown on randomly oriented astrocytes (n = 12 FOV, Fig. 2g) also showed no directional preference (Fig. 2h,i), whereas, neurons grown on electrically aligned astrocytes (Fig. 2j) displayed maximal directionality towards 88.8 ± 2.13° (n = 6 FOV, Fig. 2k,l), which is significantly greater than the respective controls (P<0.001). Based on a survey of the images collected, a typical FOV contained 550 nuclei (Hoechst-positive), of which ~80 cells colocalized with the neuronal marker, TUJ1. This implies that the results obtained above are the cumulative response of approximately 1000 neurons (12 FOV) from the randomly orientated cultures and ~500 neurons (6 FOV) from the electrically aligned cultures. Because the direction of neurite growth in an electric field is substratum-dependent (Rajnicek et al., 1998), we performed a single control experiment to test whether fibronectin-coated slides exposed to an electric field in the absence of any cells induced alignment of DRGs. Following electric-field exposure, either astrocytes or DRGs were seeded onto these slides and incubated for 24 hours. There was no observable orientation of the DRG cultures, indicating that fibronectin does not influence the orientation of eiter neurites or astrocytes (data not shown).
Although the direction of neurite growth was nearly identical to that of astrocyte alignment, it was uncertain whether the neurites make direct contact with astrocyte processes. Examination of the FOV used in the previous analysis indicated that direct contact was made between neurites and astrocyte processes in both randomly oriented cultures (Fig. 3a) and those aligned by electric-field exposure (Fig. 3c). Therefore, we used confocal imaging at higher magnification (63×) to confirm direct interaction between astrocyte processes and neurites. Fig. 3 provides representative images of direct contact between neurites and astrocytes in randomly oriented (Fig. 3b) and electrically aligned cultures (Fig. 3d). Examination of the X–Z sections reveals that neurites are in frequent contact with, and often follow, astrocytic processes. These images indicate that electrically aligned astrocytic processes are permissive to neurite growth and guide the direction of neurite outgrowth.
Fig. 3. Neurites follow the processes of electrically-aligned astrocytes.
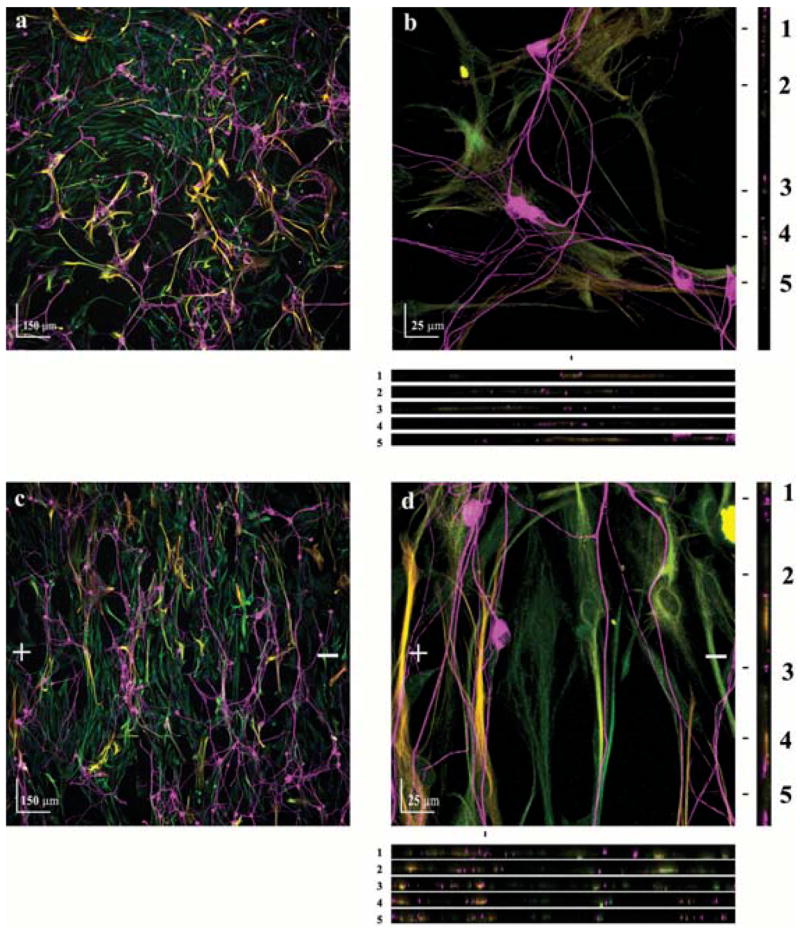
Astrocyte cultures were either exposed to a electric field (500 mV mm−1) for 24 hours or left unexposed as a control. A dorsal root ganglia cell suspension was then seeded onto the astrocyte cultures and allowed to grow for 48 hours. (a,c) Cocultures of neurons (TUJ1+, magenta) and astrocytes (Vimentin+, green and GFAP+, red, which are yellow when colocalized) of control and electrically aligned cultures, respectively. + and − indicate the direction of the electric-field vector. (b,d) Higher magnification indicates that direct contact between neuronal processes and astrocytes is established. Five XZ sections (location marked 1–5) show direct contact between neurites and astrocyte processes in all three dimensions.
Because the image analysis results described above are representative of the cumulative response of many neurons, we substantiated our observations using traditional individual-cell analysis. To accomplish this, it was necessary to identify individual neurons in their entirety, which required a lower seeding density of DRGs on the astrocyte cultures. Therefore, astrocyte cultures were prepared as before and either electrically stimulated (500 mV mm−1) or left unexposed as a control. Dorsal root ganglion cells were then seeded at 10% of the initial density and allowed to grow for 24 hours. The shortened timeframe was to ensure that the leading edge of the neurite did not travel beyond the frame of a single FOV. A total of 37 neurons from three randomly oriented astrocyte chambers (12 FOV) and 39 neurons from three electrically aligned astrocyte chambers (12 FOV) were imaged for this analysis. Superimposing all the cell bodies of neurons grown on control astrocyte substrates showed random neurite outgrowth (Fig. 4a), which is consistent with results obtained from the FFT image analysis as described above. Likewise, preferential alignment towards 90° was observed by superimposing the neurons grown on electrically aligned astrocytes (Fig. 4c), which is also consistent with the FFT analysis. To better illustrate the direction in which the neurites travel, each neurite was binned into a category ranging from 0–180° using 10° increments. In this fashion, 85.3% of the neurites orientated towards 90° ± 30° on electrically aligned astrocytes (Fig. 4d), whereas 34.7% of neurites grown on randomly orientated astrocytes were in the same direction (Fig. 4b). Theoretically, truly random distribution yields 33.3% in this direction, which is similar to neurites grown on randomly aligned astrocytes. For quantification, the direction of each neurite was transformed into an orientation index through the mathematical sine function. An average of this index would produce a value of +1 for neurites oriented at 90° and a value of 0 for neurites oriented at either 0° or 180°. Consequently, the average of this index is 0.5 for a population of neurites that are aligned randomly. The average orientation index of the neurites grown on electrically aligned astrocytes was 0.89 ± 0.02 (n = 95 processes from 35 neurons) (Fig. 4d), whereas those grown on randomly aligned astrocytes (n = 78 processes from 35 neurons) (Fig. 4b) had a significantly lower index of 0.59 ± 0.03 (P<0.001).
Fig. 4. Electrically-aligned astrocytes guide neurite growth and increase neurite length.
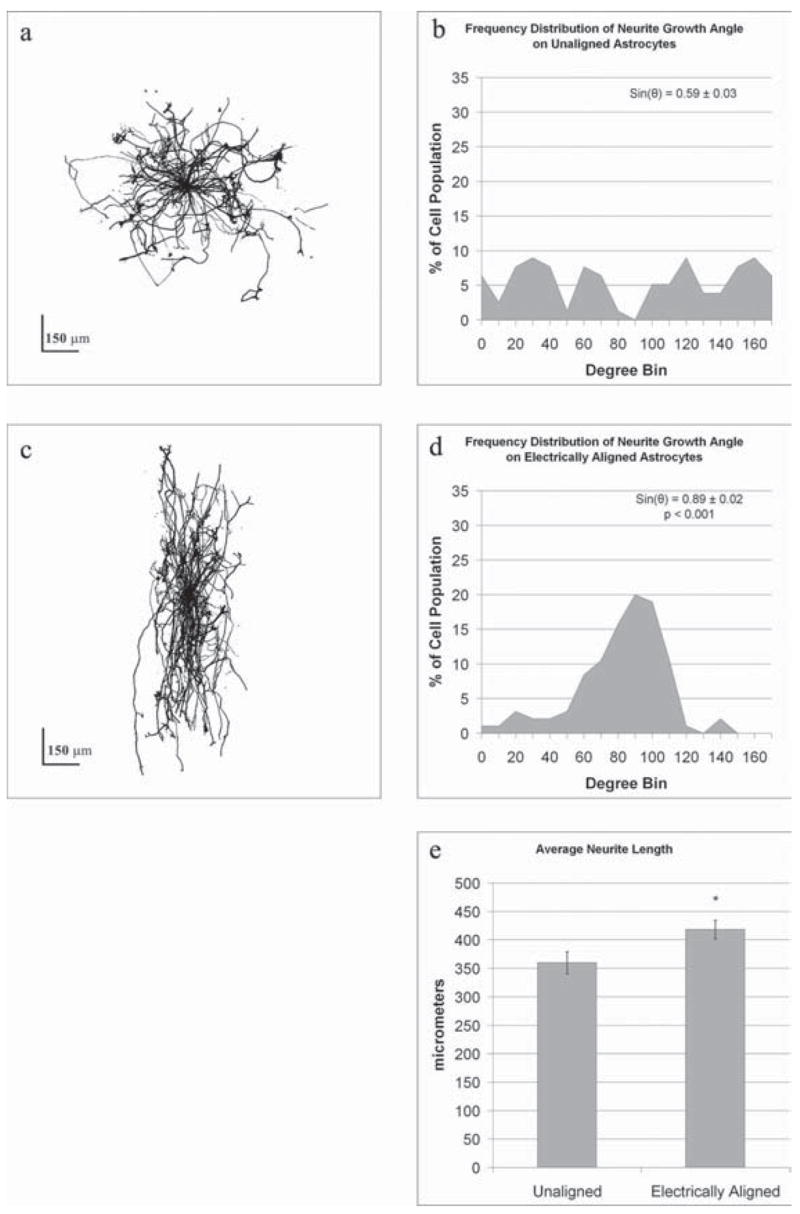
Composite images of multiple neurons grown on either control (a) or electrically aligned (c) astrocytes demonstrate the influence of astrocyte orientation on neurite outgrowth. By superimposing 37 neurons grown on unaligned, control astrocytes it is apparent that neurite outgrowth is random (a). (b) Graphically illustrating the direction of neurite outgrowth using bin sizes of 10° it is apparent that DRGs have no preferred direction of growth on randomly orientated astrocytes. The directionality coefficient for each neurite indicates an average directionality of 0.59 ± 0.03, which is consistent with randomness. (c) Superimposing the outgrowth of 39 neurons grown on electrically aligned astrocytes indicates that neurite outgrowth is aligned. (d) This is illustrated graphically by binning the directionality of neurite outgrowth. The directionality coefficient, 0.89 ± 0.02, is significantly different (P<0.001) to controls, indicating significant orientation perpendicular to the electric field direction. (e) In addition, neurite length on electrically aligned astrocytes is significantly greater (P<0.001, *) than on unaligned astrocytes.
Electrically aligned astrocytes enhance neurite growth
Because DRG neurites preferentially grew in the direction of astrocyte processes, we examined whether aligned astrocytic processes enhance the speed of neurite extension. Therefore, we traced the full length of each neurite branch from its leading edge to the cell soma. Neurites grown on electrically aligned astrocytes over a 24-hour period (n = 95 processes from 39 neurons, 418 ± 16 μm) were significantly longer (P<0.001) than those grown on randomly aligned astrocytes (n = 78 processes from 35 neurons, 360 ± 19 μm) (Fig. 4e). These results indicate that aligned astrocytes are a more permissive substrate for neurite outgrowth than randomly orientated astrocytes.
Effects of continued electrical stimulation neurite growth
Studies by Borgens et al. (1994) indicate that astrocytes slowly lose their alignment following cessation of electric field stimulation. Following electric field-induced astrocyte alignment, we also observed that astrocytes showed some signs of losing their alignment within the 24 hours following exposure. In an effort to maintain astrocyte alignment, which appeared likely to further enhance neurite outgrowth, aligned astrocyte cultures were exposed for an additional 24 hours to an electrical field of either 10 mV mm−1 (weak) or 500 mV mm−1 (strong). Examination of these cultures by FFT image analysis revealed that astrocytes exposed to a weak field maintain their alignment, but below the levels observed in astrocytes exposed to a strong field (Fig. 5a). To determine whether continued astrocytic alignment further enhances of neurite outgrowth, astrocytes were aligned and seeded with DRGs, and then re-exposed to a strong electrical field for an additional 24 hours. Examination of these co-cultures by FFT image analysis demonstrated increased alignment of DRGs (Fig. 5b), which mirrored the improved alignment in the astrocyte cultures. As neurite alignment increases, the peak of the orientation curve tends to become more ‘pointed’. Therefore, the area under the orientation curve peak (specifically within ±10°) of the peak, provides a measurable statistic to quantify the extent of neurite alignment in the direction perpendicular to the electric field vector. We found a significant (P<0.001) increase in neurite alignment (7.4 ± 0.4%) when grown in the presence of a strong electric field compared with those grown on aligned astrocytes without an additional electric field (5.3 ± 0.5%) (Fig. 5c). The length of neurite outgrowth from 41 neurons grown in the presence of a strong electric field was measured, as described above. The length of neurites grown in the presence of a strong electric field (350 ± 16 μm) was significantly shorter (P<0.01) than those grown without continued electric field exposure (418 ± 16 μm). Additionally, there was no difference in neurite length when compared to those grown on randomly oriented astrocyte cultures (360 ± 19 μm) (Fig. 5d).
Fig. 5. Continued exposure to electric fields enhances neurite and astrocyte alignment.
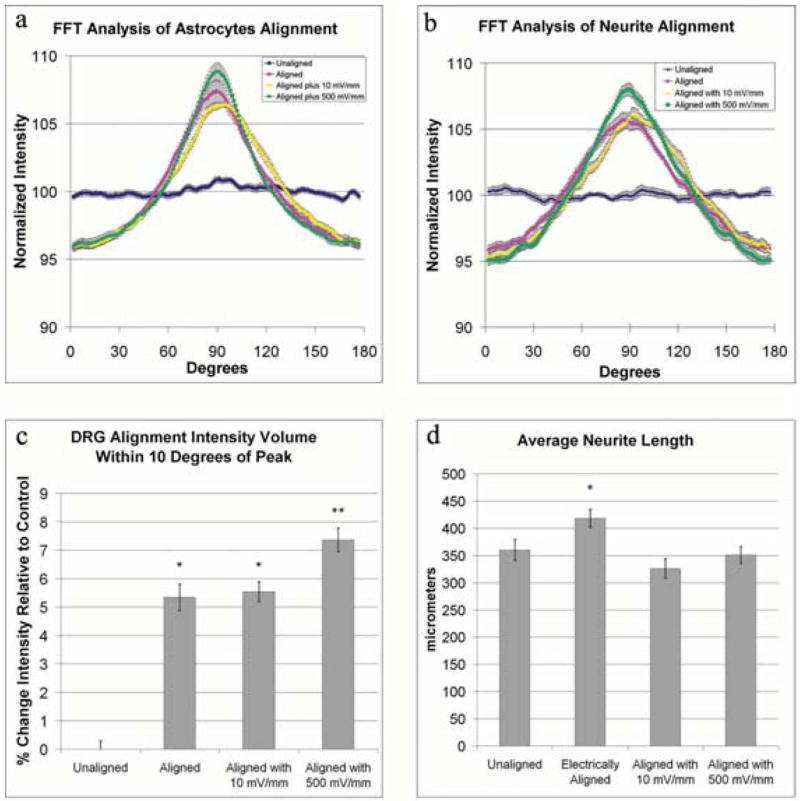
Astrocyte alignment following cessation of electric-field exposure was maintained by exposing aligned astrocytes to an additional weak (10 mV mm−1) or strong (500 mV mm−1) electric field for 24 hours. Cells were stained as described in Fig. 2. (a) FFT analysis of these cultures demonstrates that astrocyte alignment is improved by exposure to a continued electric field of 500 mV mm−1. The influence of continued exposure to an electric field and the enhanced alignment of astrocytes on neurite outgrowth was examined by seeding dorsal root ganglia cells onto aligned astrocytes and exposing the co-culture to 500 mV mm−1 for 24 hours. (b) FFT analysis of neurite orientation shows that continued exposure to electric fields improves alignment. (c) The alignment was quantified by comparing the volume under the curve within ±10° of the peak, which showed a significant increase in alignment of neurites exposed to 500 mV mm−1 (P<0.01, *, P<0.01, **). Examination of neurite length, as in Fig. 3, shows that the total length of neurites is significantly longer when grown on electrically aligned astrocytes compared with unaligned astrocytes (P<0.01, *). (d) With continued exposure to an electric field, there is no longer an increase in neurite length.
After fixation, aligned astrocytes direct neurite growth
A substrate that directs neurite growth has many clinical applications, one of which is as a bridging material to enhance neurite regeneration along transected fiber tracts. We therefore sought to determine if we could fix, and consequently preserve, the aligned orientation of astrocytes exposed to an electric field for use as a substrate on which to direct neurite growth. Therefore, we fixed electrically aligned astrocytes with paraformaldehyde and, following numerous washes, assessed neurite growth on the fixed substrate over 24 hours. Similar to live cultures, neurites exhibited a random orientation on fixed astrocytes that were never aligned to electric fields (Fig. 6a) and a strong alignment when on the fixed, aligned astrocytes (Fig. 6b). By utilizing Fourier image analysis of 12 FOVs from each condition, it we found that neurites directed their growth along the aligned processes of fixed astrocytes (Fig. 6c). Consequently, these results demonstrate that preserving astrocyte alignment with fixation has no deleterious effect on the direction of neurite outgrowth.
Fig. 6. Fixation of aligned astrocytes provides a substrate that induces neurite alignment.
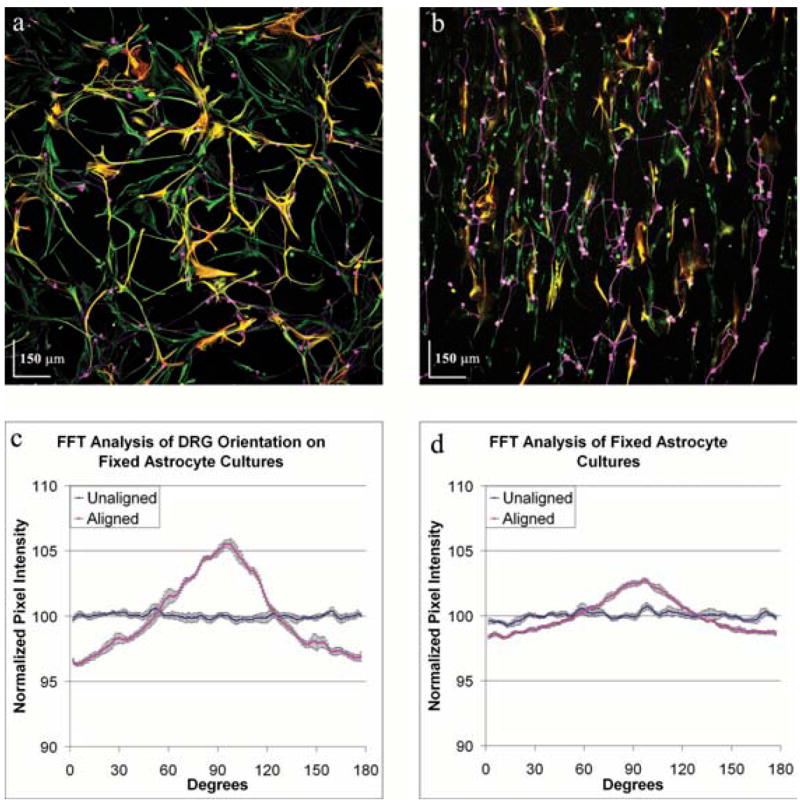
Astrocyte cultures were aligned by exposure to an electric field of 500 mV mm−1 for 24 hours and then fixed immediately in paraformaldehyde. Cells were stained as described in Fig. 2. The astrocyte cultures were washed with PBS and dorsal root ganglia cells were seeded onto the cultures and allowed to grow for 24 hours. FFT analysis of six images of DRGs grown on fixed unaligned astrocytes (a) and six images of DRGs grown on fixed electrically-aligned astrocyte cultures (b) show that the direction of neurite growth (c) is in the direction of astrocyte alignment on the fixed electrically aligned astrocytes (d, red line), whereas orientation of neurites is random on the unaligned astrocytes (d, blue line).
CONCLUSIONS
Astrocytes in culture realign their processes on exposure to electrical fields of physiological strength. Alignment occurs towards the axis perpendicular to the electric-field vector.
Neurons grown on randomly oriented astrocytes showed no directional preference, whereas neurons grown on electrically aligned astrocytes display maximal directionality. Therefore, electrically aligned astrocytic processes are permissive to neurite growth and guide the direction of neurite outgrowth.
After fixation, electrically aligned astrocytes retain the capability to direct neurite growth and guide the direction of neurite outgrowth.
DISCUSSION
The extension and directionality of neurite outgrowth are key elements that are required to achieve successful target connections during both CNS development and during the re-establishment of connections lost after neural trauma. The degree of axonal elongation depends, in large part, on the spatial arrangement of astrocytic processes rich in growth-promoting proteins. Because cultured astrocytes align their processes on exposure to an electrical field of physiological strength, we determined the extent to which aligned astrocytes affect neurite outgrowth. To this end, dorsal root ganglia cells were seeded onto cultured rat astrocytes that were pre-aligned by exposure to an electric field of physiological strength (500 mV mm−1). Using confocal microscopy and digital image analysis, we found that neurite outgrowth after 24 and 48 hours was enhanced significantly, and was consistently directed along the aligned astrocyte processes. Moreover, this directed neurite outgrowth was maintained when grown on fixed, aligned astrocytes. Collectively, these results indicate that endogenous electric fields in the developing CNS might align astrocyte processes, which can promote and direct neurite growth.
One mechanism by which electrically stimulated astrocytes might enhance neurite growth is by expressing cell-surface proteins that stimulate neurons to increase their growth rate. In support of this, we found that neurite length on electrically aligned astrocytes was significantly longer than controls. However, our analysis of neurite length when grown on aligned astrocytes in the presence of an electric field indicates this is not the case. We demonstrate that neurite growth along electrically aligned astrocytes in the presence of an electric field is more representative of growth rates observed when neurites were allowed to grow along randomly oriented astrocytes. If electrical stimulation induced astrocytes to express molecules that enhance neurite growth, neurite growth should be enhanced in the presence of endogenous electric fields. It should be noted that the preferred direction of neurite growth in an electric field is reported to be along the long axis (parallel) of the electric-field vector (Jaffe and Poo, 1979; Rajnicek et al., 1992), which is in contrast to the direction in which astrocyte processes align (perpendicular) (Borgens et al., 1994). Furthermore, it has been shown that neurite growth along the electric-field direction is enhanced. This indicates that the neurites experience competing forces that direct migration if grown on aligned astrocyte substrates with continued exposure to and electric field.
Under conditions in which the substrate does not impart directional information, such as when astrocytes are unaligned, the growth cone of a neurite must constantly sample the microenvironment in an attempt to determine the optimal direction in which to grow. On unaligned substrates, an optimal direction does not exist and, consequently, the neurite moves in random directions while constantly sampling the surrounding region. This continued sampling process requires the constant assembly and disassembly of cytoskeletal proteins, which requires both time and energy. When the process of choosing a direction in which to grow is simplified, as in environments in which a directional cue is present, neurites tend to grow faster along the direction provided by the guidance cue. This is presumably because the more efficient process of determining the direction in which to grow results in quicker assembly of the cytoskeleton. By exposing the growing neurite to two competing forces, we, therefore, produced a situation in which the optimal direction in which to grow was not as easily discernable, which might be reflected in the lack of an enhanced growth rate.
After development, neurite outgrowth also occurs in the CNS following injury to fiber tracts. For example, following spinal cord trauma, transected axons often generate a growth cone at their leading tip and begin traversing the neuropil to re-establish lost connections. This attempt to restore connectivity by the regenerating axon has limited success because several cell types in the lesion express inhibitory factors (Bandtlow and Schwab, 2000; Grimpe and Silver, 2002). Consequently, current investigational therapeutic approaches to repair spinal cord injury employ implantable bridges through which regenerating axons traverse the injury site uninhibited. Several substrates are permissive to neurite growth (Geller and Fawcett, 2002; Giordano et al., 1997; van Dorp et al., 1999). However, without directional guidance cues to aid the axon in traversing the bridge, their use as a conduit for axon growth and, consequently, spinal cord repair is limited (Plant et al., 1997; Geller and Fawcett, 2002). To address this issue, many researchers utilize live cells in the bridge to provide nutritive support and guidance cues. Alternatively, mechanical guidance cues, often in the form of grooves, successfully direct growing axons (Clark et al., 1993; Rajnicek et al., 1997). More recently, however, a hybrid approach that uses physical cues to align live cells in the bridge has been shown to support and direct neurite growth (Deumens et al., 2004; Biran et al., 2003). Astrocytes are often used in such hybrid bridges because they have been demonstrated to enhance and direct neurite outgrowth in vivo and in vitro. Unfortunately, the methods by which alignment of astrocytes within these bridges is accomplished often requires either atypical materials or harsh fabrication processes (Teng et al., 2002; Biran et al., 2003). Studies such as these have demonstrated that it is necessary to impose an aligned cellular substrate for optimal neurite growth across a bridge. Therefore, ideally, astrocyte processes should be aligned in a relatively short period of time by methods that do not require either prefabrication processing or unique material properties.
Our results demonstrate a simple method to induce astrocyte alignment in 24 hours through the application of an electric field. Furthermore, by fixing the electrically aligned astrocyte cultures, we create a substrate that remains permissive to neurite growth and can guide the direction of neurite outgrowth. This approach has the distinct advantage that induction of alignment of astrocyte processes does not depend on fabrication technologies. Consequently, there are fewer limits on the type of substrate on which to align astrocyte processes. This should lead to an improved ability to fabricate bridges for the repair of spinal cord injury.
Acknowledgments
Funded by the National Institute of Neurological Disorders and Stroke (5R01NS039851-03 and 1R21NS048377–01).
References
- Bandtlow CE, Schwab ME. NI-35/250/nogo-a: a neurite growth inhibitor restricting structural plasticity and regeneration of nerve fibers in the adult vertebrate CNS. Glia. 2000;29:175–181. doi: 10.1002/(sici)1098-1136(20000115)29:2<175::aid-glia11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Biran R, Noble MD, Tresco PA. Directed nerve outgrowth is enhanced by engineered glial substrates. Experimental Neurology. 2003;184:141–152. doi: 10.1016/s0014-4886(03)00253-x. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R, Mohr TJ, Jaeger CB. Mammalian cortical astrocytes align themselves in a physiological voltage gradient. Experimental Neurology. 1994;128:41–49. doi: 10.1006/exnr.1994.1111. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Reduction of sodium dependent stump currents disturbs urodele limb regeneration. Journal of Experimental Zoology. 1979;209:377–386. doi: 10.1002/jez.1402090304. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Christ AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Clark P, Britland S, Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. Journal of Cell Science. 1993;105:203–212. doi: 10.1242/jcs.105.1.203. [DOI] [PubMed] [Google Scholar]
- Colello RJ, Alexander J. Electric Fields: Their Nature and Influence on Biological Systems. In: Morkoc H, editor. Advanced Semiconductor and Organic Nanotechniques. Academic Press; 2003. pp. 319–346. [Google Scholar]
- Davies SJ, Silver J. Adult axon regeneration in adult CNS white matter. Trends in Neurosciences. 1998;21:515. doi: 10.1016/s0166-2236(98)01335-6. [DOI] [PubMed] [Google Scholar]
- Deumens R, Koopmans GC, Den Bakker CG, Maquet V, Blacher S, Honig WM, et al. Alignment of glial cells stimulates directional neurite growth of CNS neurons in vitro. Neuroscience. 2004;125:591–604. doi: 10.1016/j.neuroscience.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Fox MA, Alexander JK, Afshari FS, Colello RJ, Fuss B. Phosphodiesterase-I alpha/autotaxin controls cytoskeletal organization and FAK phosphorylation during myelination. Molecular and Cellular Neuroscience. 2004;27:140–150. doi: 10.1016/j.mcn.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Experimental Neurology. 2002;174:125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- Giordano GG, Thomson RC, Ishaug SL, Mikos AG, Cumber S, Garcia CA, et al. Retinal pigment epithelium cells cultured on synthetic biodegradable polymers. Journal of Biomedical Materials Research. 1997;34:87–93. doi: 10.1002/(sici)1097-4636(199701)34:1<87::aid-jbm12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gomes FC, Spohr TC, Martinez R, Moura Neto V. Crosstalk between neurons and glia: highlights on soluble factors. Brazilian Journal of Medical and Biological Research. 2001;34:611–620. doi: 10.1590/s0100-879x2001000500008. [DOI] [PubMed] [Google Scholar]
- Grimpe B, Silver J. The extracellular matrix in axon regeneration. Progress in Brain Research. 2002;137:333–349. doi: 10.1016/s0079-6123(02)37025-0. [DOI] [PubMed] [Google Scholar]
- Haung R, Peng L, Hertz L. Effects of a low-voltage static electric field on energy metabolism in astrocytes. Bioelectromagnetics. 1997;18:77–80. doi: 10.1002/(sici)1521-186x(1997)18:1<77::aid-bem11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. The role of ionic currents in establishing developmental pattern. Philosophical Transactions: Biological Sciences. 1981;295:553–666. doi: 10.1098/rstb.1981.0160. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Poo MM. Neurites grow faster towards the cathode than the anode in a steady field. Journal of Experimental Zoology. 1979;209:115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- Jenkins LS, Duerstock BS, Borgens RB. Reduction of the current of injury leaving the amputation inhibits limb regeneration in the red spotted newt. Developmental Biology. 1996;178:251–262. doi: 10.1006/dbio.1996.0216. [DOI] [PubMed] [Google Scholar]
- McCaig CD. Spinal neurite reabsorption and regrowth in vitro depend on the polarity of an applied electric field. Development. 1987;100:31–41. doi: 10.1242/dev.100.1.31. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. Bioessays. 1997;19:819–826. doi: 10.1002/bies.950190912. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, DeVellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. Journal of Cell Biology. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Identification of radial glial cells within the developing murine central nervous system: studies based upon a new immunohistochemical marker. Developmental Brain Research. 1988;44:95–108. doi: 10.1016/0165-3806(88)90121-6. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. Journal of Cell Biology. 1988;107:1177–1187. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. The EMBO Journal. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R. Endogenous ionic currents and DC electric fields in multicellular animal tissues. Bioelectromagnetics Suppl. 1992;1:147–157. doi: 10.1002/bem.2250130714. [DOI] [PubMed] [Google Scholar]
- Peng HB, Jaffe LF. Polarization of fucoid eggs by steady electrical fields. Developmental Biology. 1976;53:277–284. doi: 10.1016/0012-1606(76)90229-3. [DOI] [PubMed] [Google Scholar]
- Pierret P, Quenneville N, Vandaele S, Abbaszadeh R, Lanctot C, Crine P, et al. Trophic and tropic effects of striatal astrocytes on cografted mesencephalic dopamine neurons and their axons. Journal of Neuroscience Research. 1998;51:23–40. doi: 10.1002/(SICI)1097-4547(19980101)51:1<23::AID-JNR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Plant GW, Woerly S, Harvey AR. Hydrogels containing peptide or aminosugar sequences implanted into the rat brain: influence on cellular migration and axonal growth. Experimental Neurology. 1997;143:287–299. doi: 10.1006/exnr.1997.6407. [DOI] [PubMed] [Google Scholar]
- Prochiantz A. Neuronal growth and shape. Developmental Neuroscience. 1985;7:189–198. doi: 10.1159/000112287. [DOI] [PubMed] [Google Scholar]
- Rajnicek A, Britland S, McCaig C. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. Journal of Cell Science. 1997;110:2905–2913. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- Rajnicek A, Robinson K, McCaig C. The direction of neurite growth in a weak dc electric field depends on the substratum: contributions of adhesivity and net surface charge. Developmental Biology. 1998;203:412–423. doi: 10.1006/dbio.1998.9039. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anatomy and Embryology (Berlin) 1979;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Sharma KV, Bigbee JW. Acetylcholinesterase antibody treatment results in neurite detachment and reduced outgrowth from cultured neurons: further evidence for a cell adhesive role for neuronal acetylcholinesterase. Journal of Neuroscience Research. 1998;53:454–464. doi: 10.1002/(SICI)1097-4547(19980815)53:4<454::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Singer M, Nordlander RH, Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. Journal of Comparative Neurology. 1979;185:1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Tamamaki N. Radial glias and radial fibers: what is the function of radial fibers? Anatomical Science International. 2002;77:2–11. doi: 10.1046/j.0022-7722.2002.00013.x. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Proceedings of the National Academy of Sciences of the USA. Vol. 99. 2002. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells; pp. 3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonar Z, Nemecek S, Holota R, Kocova J, Treska V, Molacek J, et al. Microscopic image analysis of elastin network in samples of normal, atherosclerotic and aneurysmatic abdominal aorta and its biomechanical implications. Journal of Applied Biomedicine. 2003;1:149–159. [Google Scholar]
- van Dorp AG, Verhoeven MC, Koerten HK, van Blitterswijk CA, Ponec M. Bilayered biodegradable poly(ethylene glycol)/poly(butylene terephthalate) copolymer (Polyactive) as substrate for human fibroblasts and keratinocytes. Journal of Biomedical Materials Research. 1999;47:292–300. doi: 10.1002/(sici)1097-4636(19991205)47:3<292::aid-jbm2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. Journal of Comparative Neurology. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Zhao M, Forrester JV, McCaig CD. A small, physiological electric field orients cell division. Proceedings of the National Academy of Sciences of the USA. 1999;96:4942–4946. doi: 10.1073/pnas.96.9.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]


