Abstract
Purpose
To compare the diagnostic accuracy of the Matrix frequency-doubling technology (FDT) 24-2, first-generation FDT N-30 (FDT N-30), and standard automated perimetry (SAP) tests of visual function.
Methods
One eye of each of 85 glaucoma patients and 81 healthy controls from the Diagnostic Innovations in Glaucoma Study was included. Evidence of glaucomatous optic neuropathy on stereophotographs was used to classify the eyes. Matrix FDT 24-2, first-generation FDT N-30, and SAP-SITA 24-2 tests were performed on all participants within 3 months. Receiver operating characteristic (ROC) curves were generated and used to determine sensitivity levels at 80% and 90% specificity for mean deviation (MD), pattern standard deviation (PSD), number of total deviation (TD), and pattern deviation (PD) points triggered at less than 5% and 1%. The tests were compared using the best parameter for each test (that with the highest area under the ROC curve) and with the PSD.
Results
The best parameters were MD for SAP (0.680), PSD for FDT N-30 (0.733), and number of TD less than 5% points for FDT 24-2 (0.774). Using the best parameter, the area under the ROC curve was significantly larger for FDT 24-2 than for SAP (P = 0.01). No statistically significant differences were observed between SAP and FDT N-30 (P = 0.21) and FDT N-30 and FDT 24-2 (P = 0.26). Similar results were obtained when the PSD was used to compare the tests, with the exception that the area under the ROC curve for the FDT N-30 test (0.733) was significantly larger than that of the SAP-SITA (0.641; P = 0.03).
Conclusions
The performance of the Matrix FDT 24-2 was similar to that of the first-generation FDT N-30. The Matrix FDT 24-2 test was consistently better than SAP at discriminating between healthy and glaucomatous eyes. Further studies are needed to evaluate the ability of the Matrix FDT 24-2 to monitor glaucoma progression.
Frequency-doubling technology (FDT) perimetry measures contrast sensitivity. Although participants are not asked to assess the spatial frequency of the FDT stimuli, the test is based on the frequency-doubling illusion first described by Kelly1 and later proposed as a sensitive measure of glaucomatous visual field loss.2,3 This illusion occurs when a sinusoidal grating of low spatial frequency undergoes counterphase flickering at a high temporal frequency. Under these conditions, the sinusoidal grating is perceived to have twice its physical spatial frequency. It was originally believed that the FDT test isolates the spatially nonlinear My cells,2,4 a subset of the magnocellular retinal ganglion cells. However, more recent evidence suggests that the magnocellular pathway is isolated as a whole by the FDT stimulus.5,6 This isolation reduces redundancy within the visual system and is responsible for the greater sensitivity to glaucoma achieved by function-specific tests.7 Several reports suggest that FDT perimetry in both the screening8–11 and the thresholding12 modes is sensitive to glaucomatous visual losses. Medeiros et al.13 showed that FDT is also able to predict future visual loss on standard automated perimetry (SAP). Furthermore, the variability of FDT is independent of visual field loss,14 and its test-retest variability is more uniform than that of SAP over the dynamic range of the instrument.15,16
The proliferation of new tests and instruments to assess vision requires frequent reevaluations, comparing each new test with its precursors and with other tests. Frequency-doubling technology is a useful clinical tool that sensitively detects glaucoma and is robust to blur, pupil size, and refractive errors.17 The FDT instrument is also relatively inexpensive and easy to transport. Although several studies have reported better diagnostic accuracy for FDT than for SAP, most were performed using the first-generation FDT perimeter.18,19 The original FDT perimeter allowed testing of the central 30° of the visual field with screening and thresholding strategies. Targets were relatively large, and a maximum of 19 locations could be tested. Johnson et al.20 showed that the sensitivity of FDT to glaucomatous loss was maintained using smaller targets distributed in a pattern similar to that of the SAP 24-2 test. The Humphrey Matrix was commercially introduced in 2003, offering the following tests: macula, 10-2; N-30-F, 24-2 and 30-2. The Glaucoma Hemifield Test (GHT) is available for the FDT 24-2 and 30-2 tests. The purpose of the present study was to compare the ability of the Matrix FDT 24-2, first-generation FDT N-30, and SAP-SITA 24-2 tests to discriminate between healthy and glaucoma eyes.
METHODS
All participants were selected from the Diagnostic Innovations in Glaucoma Study (DIGS), conducted at the Hamilton Glaucoma Center at the University of California at San Diego (UCSD). DIGS is an ongoing study, prospectively designed to assess structure and function in glaucoma. DIGS participants are selected based on the inclusion/exclusion criteria specified here. The patient participants are referred to the study through the Glaucoma Clinic at the UCSD Department of Ophthalmology and are followed annually. Healthy participants are recruited from the general population through advertisement as well as from the staff and employees of UCSD.
We performed a retrospective cross-sectional analysis of data obtained from one eye of each of 166 participants. Eighty-five participants were glaucoma patients, and 81 were healthy controls. DIGS participants were included in this study if they had at least one reliable SAP-SITA, FDT N-30, and FDT 24-2 test completed within a 3-month period. On average, SAP-SITA tests were performed within 0.32 ± 0.69 months of FDT N-30 tests and within 0.29 ± 0.67 months of FDT 24-2 tests. FDT N-30 and FDT 24-2 tests were performed on average within 0.04 ± 0.22 months of each other. Although visual field reliability in DIGS is defined as less than 33% fixation losses, false-negative responses, and false-positive responses, Table 1 shows that the visual field tests included in this study were highly reliable. The visual fields of two patients showing a false-negative error rate greater than 33% were included in this study because it was evident that this was caused by the severity of the disease. Most participants had prior experience performing visual field tests. Those participants naive to visual field testing were given practice tests on SAP, FDT N-30, and FDT 24-2 as needed. Testing order was randomized across participants, and trained reviewers at the UCSD Visual Field Assessment Center (VisFACT) assessed all visual fields to avoid the inclusion of visual fields with artifacts.
TABLE 1.
Percentages of Fixation Losses, False-Positive, and False-Negative Errors for the SAP, FDT N-30, and FDT 24-2 Tests
| Fixation Losses | False-Positive Errors | False-Negative Errors | |
|---|---|---|---|
| SAP-SITA | 5.77 ± 7.01 | 1.98 ± 2.57 | 0.81 ± 8.36 |
| FDT N-30 | 2.71 ± 7.41 | 1.88 ± 4.89 | 1.81 ± 8.76 |
| FDT 24-2 | 4.76 ± 6.67 | 2.65 ± 5.43 | 2.01 ± 7.29 |
All values are percentages expressed as mean ± SD.
Stereophotographs were taken of most participants within 6 months of the visual field tests, as illustrated in Figure 1. A few participants with glaucomatous optic neuropathy were included in the study even though their stereophotographs were taken more than 6 months before the visual field tests. Stereophotographs graded as normal were all taken within 6 months of the visual field tests to minimize the likelihood that the participant progressed from healthy to glaucomatous during the interval separating the stereophotograph and the visual field tests. Informed consent was obtained from all participants after the nature of the study, and its possible consequences was explained to them. The UCSD Human Research Protections Program approved the DIGS methodology, which adheres to the tenets of the Declaration of Helsinki for research involving human subjects and to the Health Insurance Portability and Accountability Act, or HIPAA.
Figure 1.
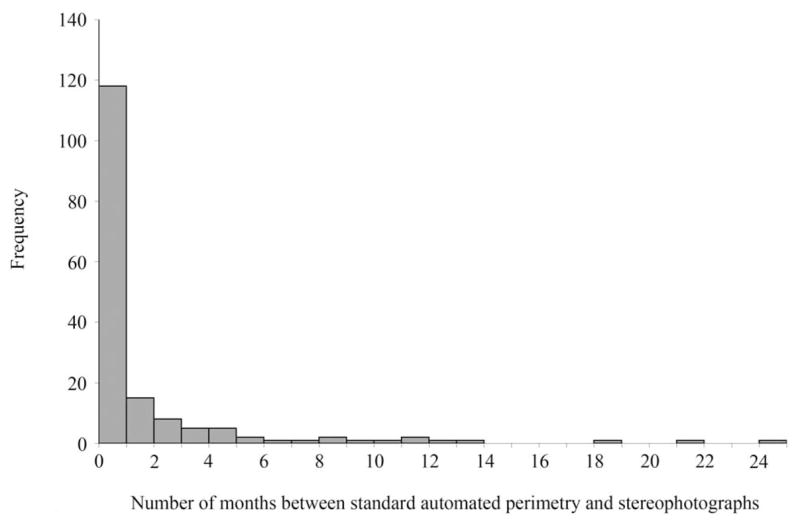
Histogram depicting the distribution of months between standard automated perimetry (SAP) and stereophotographs.
Inclusion Criteria for DIGS
Participants underwent complete ophthalmologic examinations, including slit lamp biomicroscopy, intraocular pressure measurement, and dilated stereoscopic fundus examination. Simultaneous stereoscopic photographs with good clarity and stereopsis were obtained for all participants. At study entry, all participants had open angles, best-corrected acuity of 20/40 or better, spherical refraction within ±5.0 diopters (D), and cylinder correction within ±3.0 D. A family history of glaucoma was allowed.
Exclusion Criteria for DIGS
Participants were excluded if they had a history of intraocular surgery except for uncomplicated cataract or glaucoma surgery. We also excluded all participants with secondary causes of elevated IOP (e.g., iridocyclitis, trauma), other intraocular eye disease, other diseases affecting the visual field (e.g., pituitary lesions, demyelinating diseases, HIV or AIDS, or diabetic retinopathy), those taking medications known to affect visual field sensitivity, and those with problems other than glaucoma that affected color vision.
Study Groups
Participants were classified based on the presence of structural damage to the optic disc as assessed by simultaneous stereophotographs.21 Visual field results were not used to classify participants into the study groups. The descriptive statistics for each study group are presented in Table 2.
TABLE 2.
Descriptive Measures for Each Study Group
| Controls (n = 81) | Glaucoma (n = 85) | P | |
|---|---|---|---|
| Age, mean ± SD (years) | 59.0 ± 10.7 | 61.2 ± 12.2 | 0.22 |
| Eye (% right eye) | 54.3 | 51.8 | 0.74 |
| Sex (% male) | 33.3 | 52.9 | 0.03 |
| Cataract extraction before study (%) | 3 (3.7) | 15 (17.7) | 0.003 |
| SAP MD, mean ± SD (dB) | −0.96 ± 1.51 | −3.82 ± 6.12 | <0.001 |
| SAP MD, range (dB) | −5.98–1.50 | −30.63–1.19 | — |
| SAP PSD, mean ± SD (dB) | 1.84 ± 0.82 | 3.82 ± 3.75 | <0.001 |
| SAP PSD, range (dB) | 1.05–6.93 | 1.02–16.11 | — |
Age, mean deviation (MD), and pattern standard deviation (PSD) for standard automated perimetry (SAP) were analyzed with t-tests, whereas eye, sex, and number of cataract extractions in the study eye before the study were analyzed with chi-square tests.
Healthy Controls
Participants included in this group had normal appearance of the optic disc on stereophotographs. They also had normal dilated fundus examination results, no history of ocular hypertension defined as IOP ≥23 mm Hg, and no history of use of glaucoma medication.
Glaucoma Patients
Participants included in this group had abnormal appearance of the optic disc on simultaneous stereophotographs. Abnormal appearance of the optic disc was defined as having more than a 0.2 cup-to-disc ratio asymmetry between the two eyes, evidence of excavation, neuroretinal rim thinning, notching, or nerve fiber layer defects.
Simultaneous Stereophotographs
The appearance of the optic disc of each participant was assessed with simultaneous stereophotographs (TRC-SS; Topcon, Paramus, NJ). Stereoscopic sets of slides were examined using a stereoscopic viewer (Asahi; Pentax, Golden, CO) by two trained graders who determined whether the optic disc appeared to be normal or glaucomatous. The graders were masked to the identity of the participants and to the assessment of the other grader. When the two graders disagreed, a third experienced grader served as an adjudicator.
Measures of Visual Function
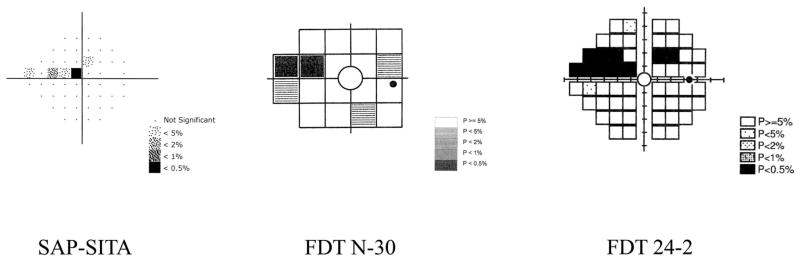
Visual function was assessed with standard automated perimetry and two different frequency-doubling technology perimetry tests. Figure 2 shows the locations tested and the abnormal areas for each test for one of the glaucoma patients included in this study. The two locations above and below the blind spot were not included in the analysis for SAP but were included for the FDT tests. Participants were required to fixate centrally for the duration of each test. Adequate refraction was provided for each device, and the pupils had a diameter of at least 3 mm. The pupils were dilated when this requirement was not met.
Figure 2.
Examples of the pattern deviation plots are presented for SAP-SITA, FDT N-30, and FDT 24-2 in a patient with glaucomatous optic neuropathy. Each plot shows the locations tested and the results expressed as a grayscale pattern (denser patterns indicate deeper defects). Probabilities are shown in the corresponding keys.
Standard Automated Perimetry
Each participant underwent standard automated perimetry using the 24-2 program on the Humphrey Field Analyzer II, using the Swedish Interactive Thresholding Algorithm (SITA)22 (Carl Zeiss Meditec, Dublin, CA). The targets consist of Goldmann size III (0.43°) white lights, presented on a dimly illuminated white background (31.5 apostilbs). The participant was asked to respond when a flash of light was detected.
FDT N-30
FDT N-30 was performed with the Frequency Doubling Visual Field Instrument (Carl Zeiss Meditec) using Welch-Allyn technology (Welch-Allyn, Skaneateles, NY). This test uses a modified binary search23 staircase threshold procedure with stimuli presented for a maximum of 720 ms. The targets consist of a square patch containing a 0.25 cyc/deg sinusoidal grating that undergoes 25-Hz counterphase flicker. Each target subtends 10° of visual angle and is presented at each of 16 locations within the central 20° of the visual field and at two additional locations in the nasal hemifield. A circular target is also presented centrally. The participant was asked to respond when any contrast or flicker was detected.
FDT 24-2
The FDT 24-2 test was performed with the Humphrey Matrix (Carl Zeiss Meditec) using Welch-Allyn technology. The Matrix FDT uses the Zippy Estimation by Sequential Testing (ZEST)23 thresholding algorithm with a flat previous probability density function and fixed termination criterion. Each target subtends five degrees of visual angle, has a spatial frequency of 0.5 cyc/deg and a temporal frequency of 18 Hz. The participant was asked to respond when any contrast or flicker was detected.
Data and Statistical Analyses
Descriptive statistics were performed using JMP version 5.1.2 (SAS Institute Inc., Cary, NC). For each test, receiver operating characteristic (ROC) curves were generated for the mean deviation (MD), pattern standard deviation (PSD), and the number of total deviation (TD) and pattern deviation (PD) points triggered at less than 5% and 1%. The area under the ROC curves was calculated for each parameter for each test. The best parameter for each test was defined as the one with the highest area under the ROC curve. The area under the ROC curves for the best parameter of each test and for the PSD was compared statistically with the method of DeLong et al.24 using Matlab (The Math-Works Inc., Natick, MA), and the 95% confidence intervals for the difference between each area under the ROC curve comparison were calculated. The ROC curves were used to estimate the sensitivity of each parameter for each test at set specificities of 80% and 90%. The alpha level for statistical significance was set at P < 0.05. The level of agreement between the tests was assessed using the kappa (κ) statistic. Kappa values range from zero to one, with values between 0.00 and 0.20 indicating slight agreement, 0.21 and 0.40 indicating fair agreement, 0.41 and 0.60 indicating moderate agreement, 0.61 and 0.80 indicating substantial agreement, and 0.81 and 1.00 indicating almost perfect agreement.25 The agreement between the results of the SAP-SITA and FDT 24-2 Glaucoma Hemifield Test (GHT) and the participant classification based on the stereophotographs was also assessed with the kappa statistic. For this analysis “borderline” and “general reduction of sensitivity” GHT results were considered within normal limits.
RESULTS
Table 3 shows the area under the ROC curves for each parameter of each test, the SE associated with the area under the ROC curves, the sensitivities at set specificities of approximately 80% and 90%, and the criteria that yielded these values. The statistical power to detect a difference of 0.1 between the areas under the ROC curves was 0.80. Within each test, no statistically significant differences were observed between any of the parameters.
TABLE 3.
Area under the Receiver Operating Characteristic Curve and Associated SE Are Presented for Each Parameter for Each Test (N = 166)
| AUC | SE | Sensitivity/Specificity (%) | Criteria for 80% Specificity | Sensitivity/Specificity (%) | Criteria for 90% Specificity | |
|---|---|---|---|---|---|---|
| SAP-SITA | ||||||
| MD | 0.680 | 0.041 | 46/80 | −1.98 | 38/90 | −2.49 |
| PSD | 0.641 | 0.043 | 45/80 | 2.10 | 30/90 | 2.28 |
| TD <5% | 0.668 | 0.042 | 46/81 | 13 | 27/91 | 20 |
| TD <1% | 0.640 | 0.043 | 49/83 | 3 | 33/90 | 6 |
| PD <5% | 0.645 | 0.043 | 51/79 | 9 | 31/90 | 13 |
| PD <1% | 0.622 | 0.011 | 45/83 | 3 | 33/89 | 4 |
| FDT N-30 | ||||||
| MD | 0.660 | 0.042 | 44/80 | −3.75 | 33/90 | −4.74 |
| PSD | 0.733 | 0.038 | 56/80 | 4.91 | 40/90 | 5.72 |
| TD <5% | 0.662 | 0.042 | 44/79 | 9 | 24/91 | 13 |
| TD <1% | 0.689 | 0.041 | 54/78 | 2 | 40/90 | 4 |
| PD <5% | 0.728 | 0.039 | 59/79 | 5 | 47/89 | 7 |
| PD <1% | 0.690 | 0.041 | 56/73 | 1 | 42/90 | 2 |
| FDT 24-2 | ||||||
| MD | 0.763 | 0.037 | 55/80 | −3.69 | 45/89 | −5.09 |
| PSD | 0.755 | 0.037 | 56/80 | 3.39 | 38/90 | 4.07 |
| TD <5% | 0.774 | 0.036 | 60/78 | 9 | 44/90 | 19 |
| TD <1% | 0.761 | 0.037 | 64/78 | 2 | 46/90 | 6 |
| PD <5% | 0.731 | 0.038 | 52/80 | 8 | 44/90 | 12 |
| PD <1% | 0.756 | 0.037 | 62/75 | 3 | 47/90 | 5 |
Criteria for each parameter that yielded approximately 80% and 90% specificity are also presented, as are sensitivity at 80% and 90% specificity for each parameter of each test. Boldface text within the table indicates the best parameter for each test (MD for SAP, PSD for N-30, and TD < 5% for FDT 24-2). MD, mean deviation; PSD, pattern standard deviation; TD, total deviation; PD, pattern deviation.
The best parameter (that with the highest area under the ROC curve) was MD for SAP (area under the ROC curve, 0.680), PSD for FDT N-30 (area under the ROC curve, 0.733), and the number of TD points triggered at less than 5% for FDT 24-2 (area under the ROC curve, 0.774). The ROC curves for the best parameter of each test are presented in Figure 3. A statistical comparison of the areas under the ROC curves for the best parameter of each test was performed and shows a significant difference between SAP and FDT 24-2 (P = 0.01; 95% CI, 0.02 to 0.17). No significant differences were observed between the areas under the ROC curves for the best parameters of SAP and FDT N-30 (P = 0.21; 95% CI, −0.03 to 0.14) and between FDT N-30 and FDT 24-2 (P = 0.26; 95% CI, −0.03 to 0.11). At a set specificity of 80%, the sensitivity associated with the best parameter of each test was 46% for SAP, 56% for FDT N-30, and 60% for FDT 24-2. At a set specificity of 90%, sensitivity was 38% for SAP, 40% for FDT N-30, and 44% for FDT 24-2.
Figure 3.
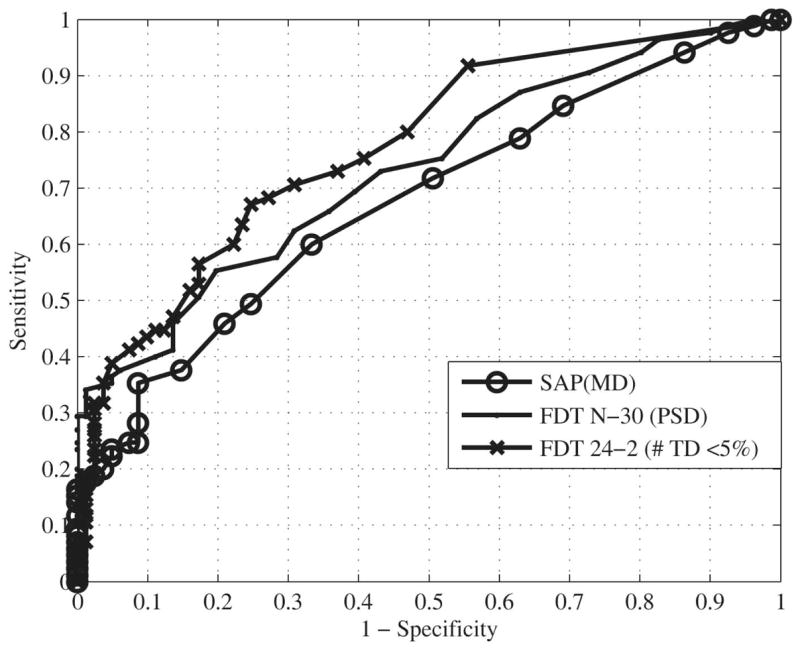
Receiver operating characteristic (ROC) curves for the best parameter of SAP-SITA (area under the ROC curve, 0.680), FDT N-30 (area under the ROC curve, 0.733), and FDT 24-2 (area under the ROC curve, 0.774) are presented.
Using the PSD to compare all tests, we obtained areas under the ROC curve of 0.641 for SAP, 0.733 for FDT N-30, and 0.755 for the FDT 24-2 test. Significant differences were observed between the areas under the ROC curves of SAP and FDT 24-2 (P = 0.002; 95% CI, −0.19 to −0.04) and between SAP and FDT N-30 (P = 0.03; 95% CI, −0.17 to −0.01). The difference between FDT N-30 and FDT 24-2 (P = 0.51; 95% CI, −0.09 to 0.04) was not significant.
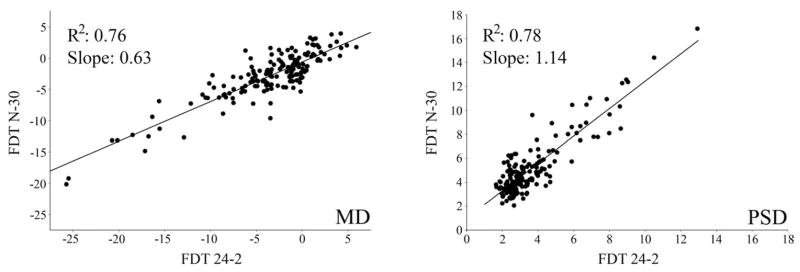
Figure 4 shows the relationship between the mean deviation (R2 = 0.76; slope = 0.63) and pattern standard deviation (R2 = 0.78; slope = 1.14) of the FDT N-30 and FDT 24-2. Scatter plots, similar to those reported by Brusini et al.,26 showing the relationship between the MD and PSD values for the glaucoma patients (n = 85) for SAP-SITA, FDT N-30, and FDT 24-2 are presented in Figure 5. The agreement between the three tests in classifying the glaucoma eyes (n = 85) is presented in the Venn diagram shown in Figure 6. This figure is based on the criteria for the best parameter of each test at 80% specificity. Table 4 presents the kappa statistic and the strength of agreement between each test combination using the best parameter and the PSD for the glaucoma group (n = 85). Fair agreement was found between the participant classification based on the stereophotographs and the Glaucoma Hemifield Test (GHT) for both the FDT 24-2 (kappa = 0.364) and SAP (kappa = 0.237) (n = 166).
Figure 4.
This graph depicts the relationship between the mean deviation (MD) of the FDT N-30 and FDT and between the pattern standard deviation (PSD) of these two tests.
Figure 5.
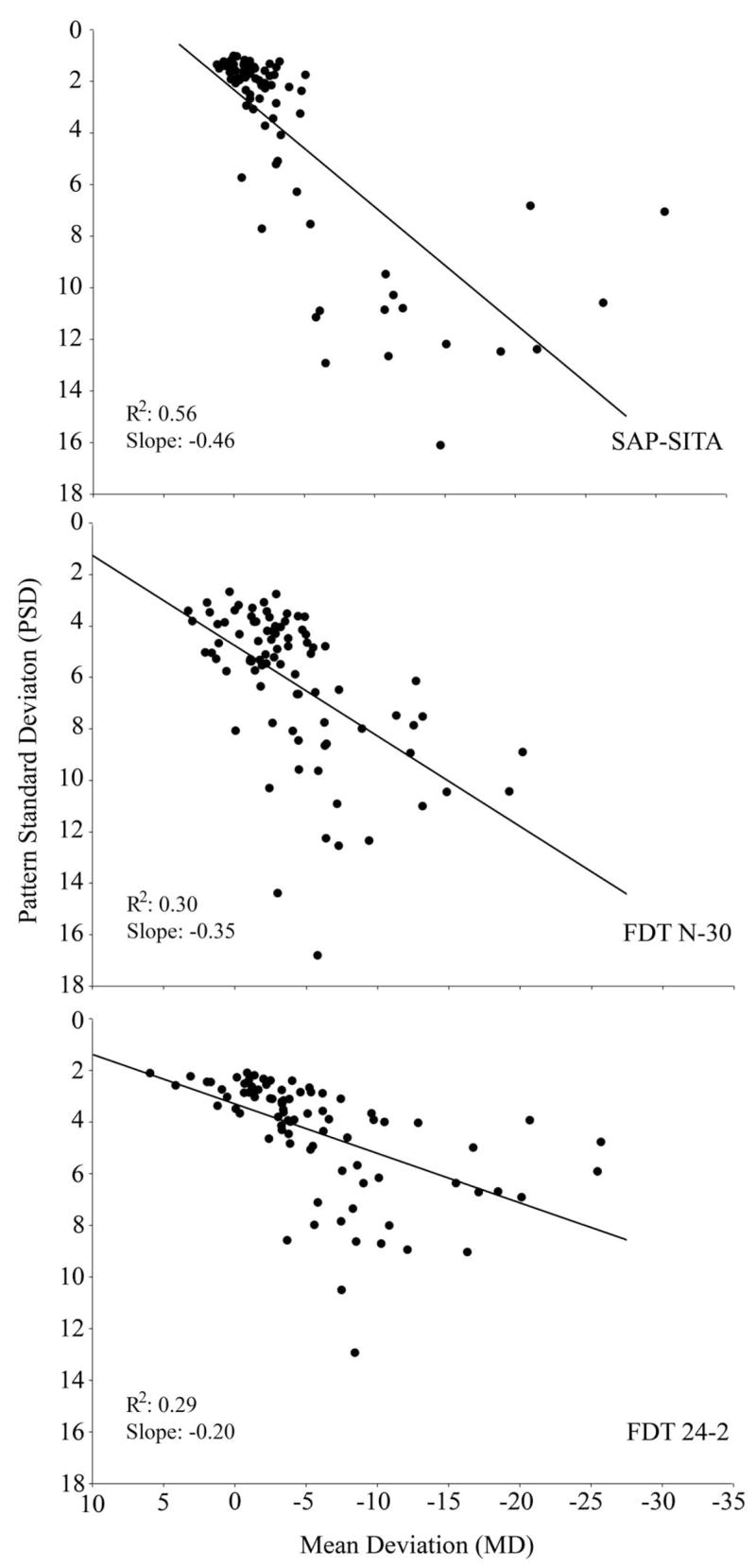
Scatter plots showing the relationship between the mean deviation (MD) and pattern standard deviation (PSD) for the glaucoma patients (n = 85) for SAP-SITA, FDT N-30, and FDT 24-2.
Figure 6.
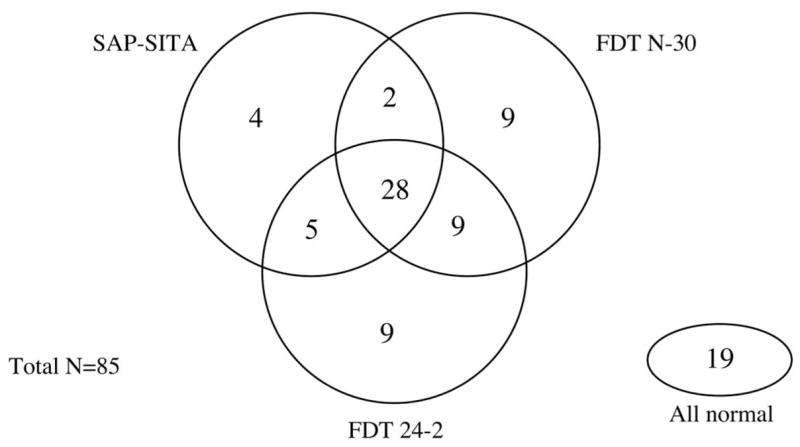
Venn diagram showing the overall agreement between SAP-SITA, FDT N-30, and FDT 24-2 in classifying the glaucoma eyes (n = 85). Abnormality is based on the criteria for the best parameter of each test that yielded approximately 80% specificity.
TABLE 4.
Agreement between Each Test Combination (Kappa Statistic and Strength of Agreement) for the Glaucoma Patients (n = 85) Using the Best Parameter for Each Test and the PSD Criteria
| Kappa Statistic | Strength of Agreement | ||
|---|---|---|---|
| Best parameter | SAP and FDT 24-2 | 0.444 | Moderate |
| SAP and FDT N-30 | 0.371 | Fair | |
| FDT 24-2 and FDT N-30 | 0.396 | Fair | |
| PSD | SAP and FDT 24-2 | 0.628 | Substantial |
| SAP and FDT N-30 | 0.489 | Moderate | |
| FDT 24-2 and FDT N-30 | 0.617 | Substantial |
PSD, pattern standard deviation.
A histogram depicting the distribution of test durations for each test is presented in Figure 7. An analysis of variance (ANOVA) and Tukey post hoc test show a significant difference in test duration between the three tests (P < 0.001), with the FDT N-30 (272 ± 21 seconds) taking on average less time to perform than both SAP-SITA (318 ± 54 seconds) and FDT 24-2 (315 ± 14 seconds). No difference in mean test duration was observed between SAP-SITA and FDT 24-2.
Figure 7.
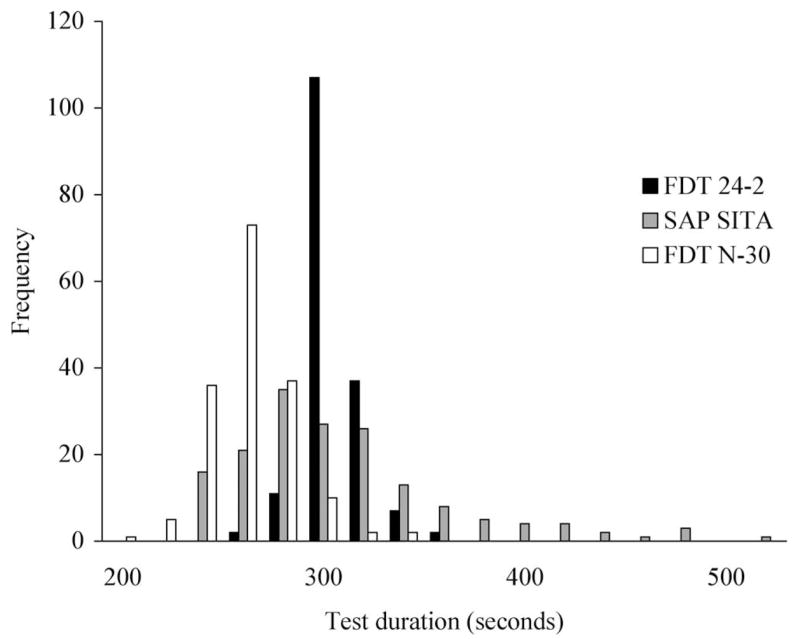
Histogram depicting the distribution of test durations for FDT 24-2, SAP-SITA, and FDT N-30.
DISCUSSION
With only a few reports available in the literature, there is no consensus on whether the Matrix FDT 24-2 test is more sensitive to glaucoma than SAP-SITA. Our results suggest that the FDT 24-2 is better able to discriminate between healthy and glaucomatous eyes than SAP-SITA, a finding consistent with the results reported by Brusini et al.,27 Leeprechanon et al.,28 and Medeiros et al.29 Two other studies, however, have reported similar performance between the FDT 24-2 and SAP.30,31 A direct comparison between our results and those reported by Spry et al.30 is complicated by their inclusion of patients with several types of glaucoma (pseudoexfoliation, primary open-angle glaucoma, normal tension), patients with ocular hypertension, and glaucoma suspects and by their use of the SITA-Fast strategy for SAP. Selection bias is likely responsible for the finding reported by Patel et al.31 that the FDT 24-2 failed to detect 36% of abnormal SAP-SITA test results in their sample because only patients with abnormal results on SAP-SITA were included.
The best parameter for each test in this study was selected because it produced the highest area under the ROC curve. From a clinical perspective, however, the usefulness of the pattern standard deviation for early detection of glaucomatous visual field loss is solidly established.32,33 Given that no significant differences were found between the best parameter for each test and all other parameters, we also compared the tests using the PSD. Similar to when the best parameter was used, the FDT 24-2 performed significantly better than SAP, and no difference was observed between the FDT 24-2 and FDT N-30 tests. The comparison between the SAP and FDT N-30 tests, however, differed depending on which parameter was used to compare the tests. When the best parameter for each test was used, no difference was observed between the two tests. Consistent with previous reports,19,33 however, significantly better performance was observed for the FDT N-30 test compared to SAP-SITA when the PSD was used to compare the tests. Better agreement between the tests was also observed when the PSD was used to compare the tests.
Differences were observed in the relationship between the mean deviation and pattern standard deviation within each test for patients with glaucoma (Fig. 5). A much tighter distribution was observed for SAP compared with the FDT N-30 and FDT 24-2 tests. SAP tended to cluster patients with glaucomatous optic neuropathy within the normal range of MD and PSD values, and this may explain the poorer ability of SAP to discriminate between healthy and glaucomatous eyes. Patients were more evenly distributed along the severity continuum for both FDT tests, resulting in better diagnostic accuracy.
We did not find any difference between the performance of the FDT 24-2 and FDT N-30 tests in discriminating between healthy and glaucomatous eyes. When plotting the mean deviations for the FDT N-30 and FDT 24-2 tests against each other, however, the slope (0.63) was not equal to 1, as might have been expected. A 1-unit reduction in the MD of the FDT N-30 test resulted in a greater decrease in the MD of the FDT 24-2 MD test. This is likely because of the eccentricity and the smaller size of the stimulus used in the FDT 24-2 test, consistent with reports showing a reduction in sensitivity with increasing eccentricity when smaller stimuli are used.20,34
The level of agreement observed between the tests could be better interpreted if we knew the level of agreement achieved when the same test is performed twice. Although no study has directly compared the intertest and intratest agreement within the same cohort, the Ocular Hypertension Treatment Study has shown that normal visual field test results can occur in patients who previously had two and even three abnormal visual field results.35 This highlights the considerable variability associated with current perimetric techniques. The Venn diagram presented in Figure 6 shows that though 28 patients were classified with an abnormality by all three tests, 19 were classified with normal results by all three tests. It is possible that these 19 patients experienced detectable structural changes before any evidence of visual loss showed. It is also possible that some participants were misclassified by the criteria used in this study (i.e., evidence of glaucomatous optic neuropathy on stereophotographs). Ideally, we would have classified our participants based on a more definitive diagnosis, such as evidence of progressive glaucomatous optic neuropathy.36 Unfortunately, this information was not available for all the participants included in this study.
Important strengths of the present study include the comparison of the three visual field tests in the same sample and the use of a classification criterion (glaucomatous optic neuropathy on stereophotographs) independent of visual function. A fair comparison of the three visual function tests, which use different stimuli and assess different visual pathways, was achieved by setting their respective specificity at equal levels with the use of the ROC analysis. In conclusion, the results of the present study suggest that the Matrix FDT 24-2 and FDT N-30 tests are better able than SAP-SITA to discriminate between healthy and glaucomatous eyes. No differences were observed between the Matrix FDT 24-2 and the FDT N-30 tests. Future studies are needed to determine whether the Matrix FDT 24-2 will be useful to monitor glaucoma progression.
Acknowledgments
The authors thank Madhusudhanan Balasubramanian for assistance with graphics and Chris A. Johnson for helpful comments during the revision process.
Supported by National Eye Institute Grants EY 08208 (PAS) and EY 11008 (LMZ). Participant retention incentive grants in the form of glaucoma medication at no cost: Alcon Laboratories Inc, Allergan, Pfizer Inc, and Santen Inc.
Footnotes
Disclosure: L. Racette, None; F.A. Medeiros, Carl Zeiss Meditec (F, R), Heidelberg Engineering (R); L.M. Zangwill, Carl Zeiss Meditec (F), Heidelberg Engineering (F, R); D. Ng, None; R.N. Weinreb, Carl Zeiss Meditec (F, R), Heidelberg Engineering (F, R); P.A. Sample, Carl Zeiss Meditec (F), Haag-Streit (F), Heidelberg Engineering (F), Welch-Allyn (F)
References
- 1.Kelly DH. Frequency doubling in visual responses. J Opt Soc Am. 1966;56:1628–1633. [Google Scholar]
- 2.Maddess T. Performance of nonlinear visual units in ocular hypertension and glaucoma. Clin Vision Sci. 1992;7:371–383. [Google Scholar]
- 3.Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997;38:413–425. [PubMed] [Google Scholar]
- 4.Maddess T, Hemmi JM, James AC. Evidence for spatial aliasing effects in the Y-like cells of the magnocellular visual pathway. Vision Res. 1998;38:1843–1859. doi: 10.1016/s0042-6989(97)00344-1. [DOI] [PubMed] [Google Scholar]
- 5.Anderson AJ, Johnson CA. Mechanisms isolated by frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2002;43:398–401. [PubMed] [Google Scholar]
- 6.White AJ, Sun H, Swanson WH, Lee BB. An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci. 2002;43:3590–3599. [PubMed] [Google Scholar]
- 7.Johnson CA. Selective versus nonselective losses in glaucoma. J Glaucoma. 1994;3(suppl):S32–S44. [PubMed] [Google Scholar]
- 8.Thomas R, Bhat S, Muliyil JP, Parikh R, George R. Frequency doubling perimetry in glaucoma. J Glaucoma. 2002;11:46–50. doi: 10.1097/00061198-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Yamada N, Chen PP, Mills RP, et al. Screening for glaucoma with frequency-doubling technology and Damato campimetry. Arch Ophthalmol. 1999;117:1479–1484. doi: 10.1001/archopht.117.11.1479. [DOI] [PubMed] [Google Scholar]
- 10.Casson R, James B, Rubinstein A, Ali H. Clinical comparison of frequency doubling technology perimetry and Humphrey perimetry. Br J Ophthalmol. 2001;85:360–362. doi: 10.1136/bjo.85.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paczka JA, Friedman DS, Quigley HA, Barron Y, Vitale S. Diagnostic capabilities of frequency-doubling technology, scanning laser polarimetry, and nerve fiber layer photographs to distinguish glaucomatous damage. Am J Ophthalmol. 2001;131:188–197. doi: 10.1016/s0002-9394(00)00644-9. [DOI] [PubMed] [Google Scholar]
- 12.Trible JR, Schultz RO, Robinson JC, Rothe TL. Accuracy of glaucoma detection with frequency-doubling perimetry. Am J Ophthalmol. 2000;129:740–745. doi: 10.1016/s0002-9394(00)00354-8. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol. 2004;137:863–871. doi: 10.1016/j.ajo.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Spry PG, Johnson CA, McKendrick AM, Turpin A. Variability components of standard automated perimetry and frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2001;42:1404–1410. [PubMed] [Google Scholar]
- 15.Chauhan BC, Johnson CA. Test-retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Invest Ophthalmol Vis Sci. 1999;40:648–656. [PubMed] [Google Scholar]
- 16.Artes PH, Hutchison DM, Nicolela MT, LeBlanc RP, Chauhan BC. Threshold and variability properties of matrix frequency-doubling technology and standard automated perimetry in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:2451–2457. doi: 10.1167/iovs.05-0135. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CA, Demirel S. The role of spatial and temporal factors in frequency-doubling perimetry. In: Wall M, Heijl A, editors. Perimetry Update 1996/9: Proceedings of the XIIth International Perimetric Society Meeting. Amsterdam: Kugler; 1996. pp. 13–19. [Google Scholar]
- 18.Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmol Clin North Am. 2003;16:213–225. doi: 10.1016/s0896-1549(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 19.Sample PA, Bosworth CF, Blumenthal EZ, Girkin C, Weinreb RN. Visual function-specific perimetry for indirect comparison of different ganglion cell populations in glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1783–1790. [PubMed] [Google Scholar]
- 20.Johnson CA, Cioffi GA, Van Buskirk EM. Frequency doubling technology perimetry using a 24-2 stimulus presentation pattern. Optom Vis Sci. 1999;76:571–581. doi: 10.1097/00006324-199908000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Johnson CA, Sample PA, Zangwill LM, et al. Structure and function evaluation (SAFE), II: comparison of optic disk and visual field characteristics. Am J Ophthalmol. 2003;135:148–154. doi: 10.1016/s0002-9394(02)01930-x. [DOI] [PubMed] [Google Scholar]
- 22.Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol Scand. 1997;75:368–375. doi: 10.1111/j.1600-0420.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 23.Turpin A, McKendrick AM, Johnson CA, Vingrys AJ. Performance of efficient test procedures for frequency-doubling technology perimetry in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43:709–715. [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 26.Brusini P, Filacorda S. Enhanced Glaucoma Staging System (GSS 2) for classifying functional damage in glaucoma. J Glaucoma. 2006;15:40–46. doi: 10.1097/01.ijg.0000195932.48288.97. [DOI] [PubMed] [Google Scholar]
- 27.Brusini P, Salvetat ML, Zeppieri M, Parisi L. Frequency doubling technology perimetry with the Humphrey Matrix 30–2 test. J Glaucoma. 2006;15:77–83. doi: 10.1097/00061198-200604000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Leeprechanon N, Giangiacomo A, Fontana H, Hoffman D, Caprioli J. Frequency-doubling perimetry: comparison with standard automated perimetry to detect glaucoma. Am J Ophthalmol. 2007;143:263–271. doi: 10.1016/j.ajo.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Medeiros FA, Sample PA, Zangwill LM, Liebmann JM, Girkin CA, Weinreb RN. A statistical approach to the evaluation of covariate effects on the receiver operating characteristic curves of diagnostic tests in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2520–2527. doi: 10.1167/iovs.05-1441. [DOI] [PubMed] [Google Scholar]
- 30.Spry PG, Hussin HM, Sparrow JM. Clinical evaluation of frequency doubling technology perimetry using the Humphrey Matrix 24-2 threshold strategy. Br J Ophthalmol. 2005;89:1031–1035. doi: 10.1136/bjo.2004.057778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel A, Wollstein G, Ishikawa H, Schuman JS. Comparison of visual field defects using matrix perimetry and standard achromatic perimetry. Ophthalmology. 2007;114:480–487. doi: 10.1016/j.ophtha.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson CA, Sample PA, Cioffi GA, Liebmann JR, Weinreb RN. Structure and function evaluation (SAFE), I: criteria for glaucomatous visual field loss using standard automated perimetry (SAP) and short wavelength automated perimetry (SWAP) Am J Ophthalmol. 2002;134:177–185. doi: 10.1016/s0002-9394(02)01577-5. [DOI] [PubMed] [Google Scholar]
- 33.Sample PA, Medeiros FA, Racette L, et al. Identifying glaucomatous vision loss with visual-function-specific perimetry in the diagnostic innovations in glaucoma study. Invest Ophthalmol Vis Sci. 2006;47:3381–3389. doi: 10.1167/iovs.05-1546. [DOI] [PubMed] [Google Scholar]
- 34.Anderson AJ, Johnson CA, Fingeret M, et al. Characteristics of the normative database for the Humphrey matrix perimeter. Invest Ophthalmol Vis Sci. 2005;46:1540–1548. doi: 10.1167/iovs.04-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keltner JL, Johnson CA, Levine RA, et al. Normal visual field test results following glaucomatous visual field end points in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123:1201–1206. doi: 10.1001/archopht.123.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Use of progressive glaucomatous optic disk change as the reference standard for evaluation of diagnostic tests in glaucoma. Am J Ophthalmol. 2005;139:1010–1018. doi: 10.1016/j.ajo.2005.01.003. [DOI] [PubMed] [Google Scholar]