Abstract
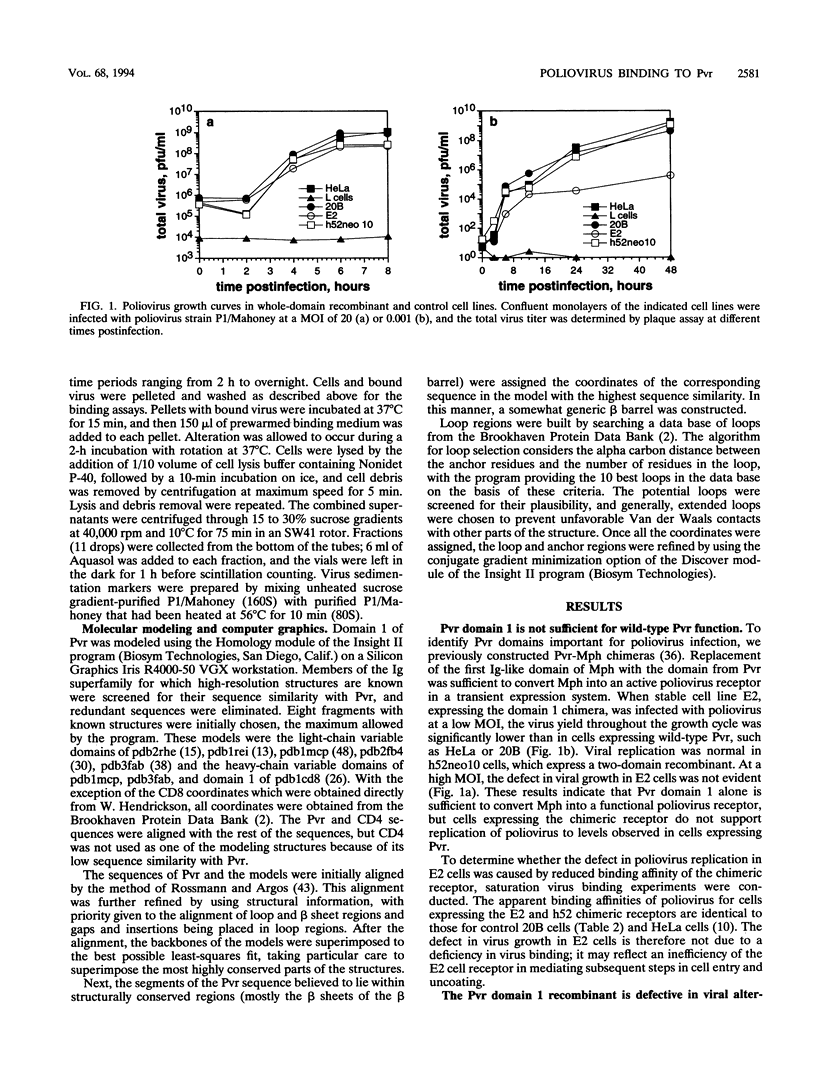
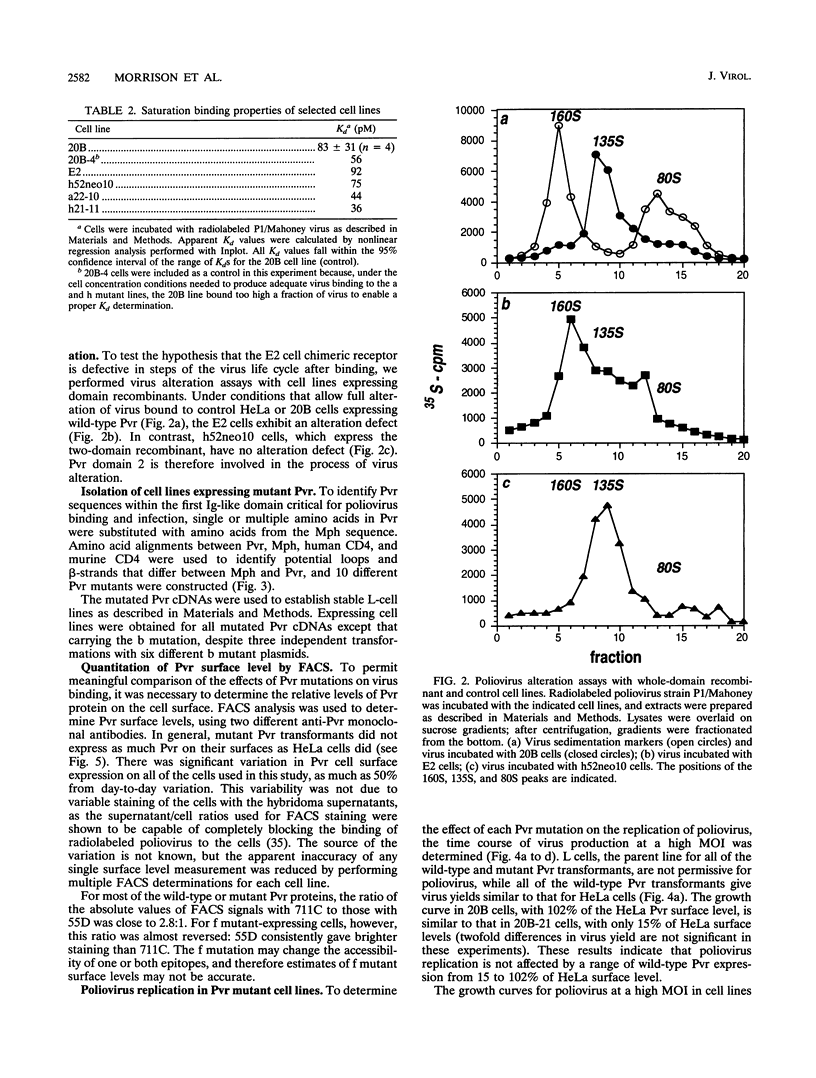
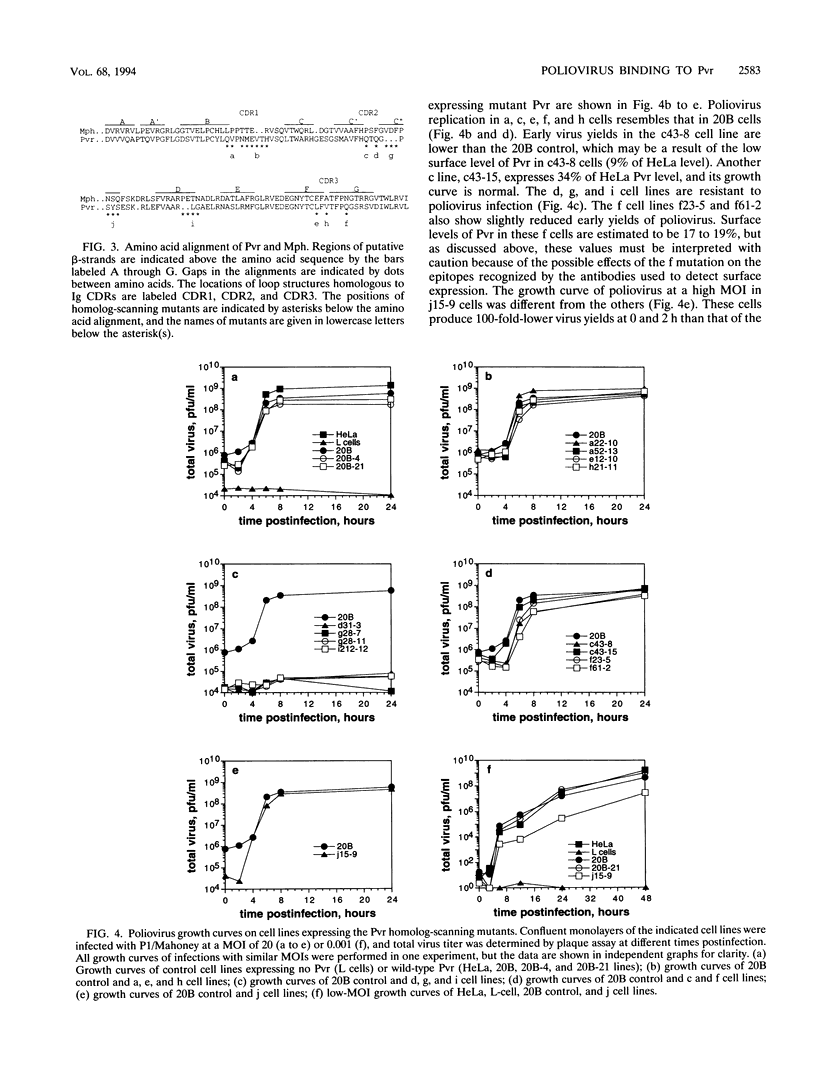
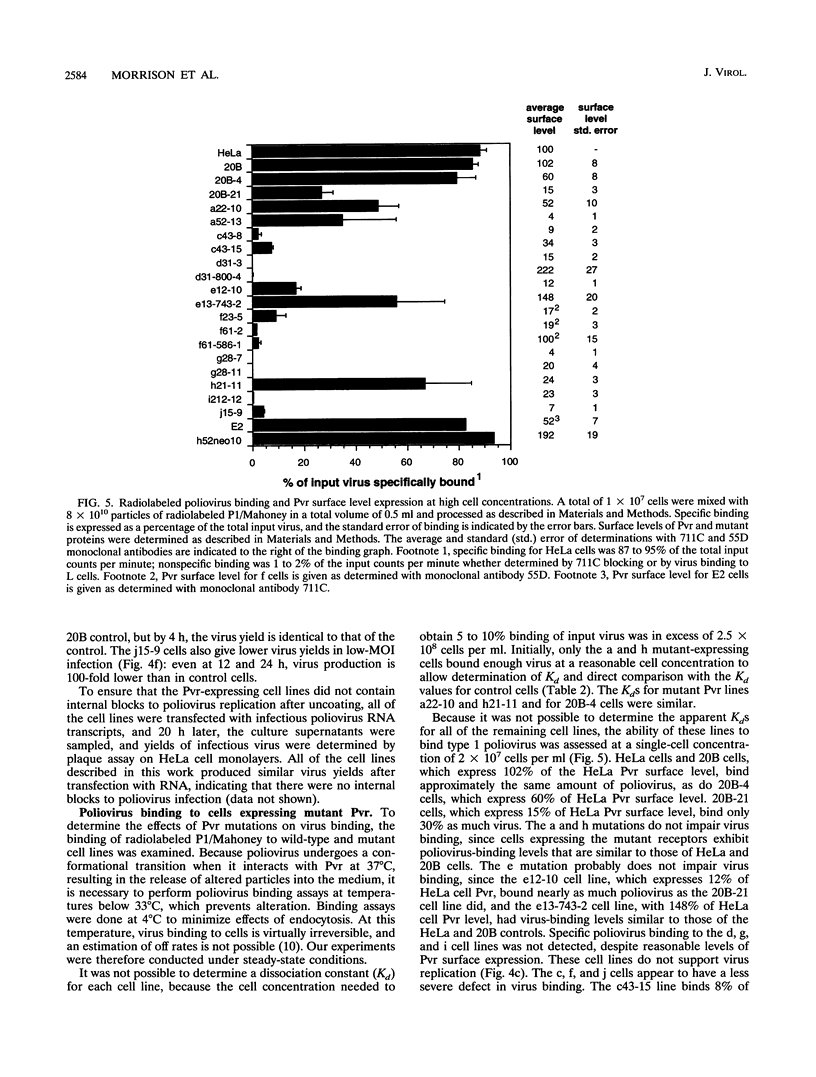
Poliovirus initiates infection of primate cells by binding to the poliovirus receptor, Pvr. Mouse cells do not bind poliovirus but express a Pvr homolog, Mph, that does not function as a poliovirus receptor. Previous work has shown that the first immunoglobulin-like domain of the Pvr protein contains the virus binding site. To further identify sequences of Pvr important for its interaction with poliovirus, stable cell lines expressing mutated Pvr molecules were examined for their abilities to bind virus and support virus replication. Substitution of the amino-terminal domain of Mph with that of Pvr yields a molecule that can function as a poliovirus receptor. Cells expressing this chimeric receptor have normal binding affinity for poliovirus, yet the kinetics of virus replication are delayed. Results of virus alteration assays indicate that this chimeric receptor is defective in converting native virus to 135S altered particles. This defect is not observed with cells expressing receptor recombinants that include Pvr domains 1 and 2. Because altered particles are believed to be an intermediate in poliovirus entry, these findings suggest that Pvr domains 2 and 3 participate in early stages of infection. Additional mutants were made by substituting variant Mph residues for the corresponding residues in Pvr. The results were interpreted by using a model of Pvr predicted from the known structures of other immunoglobulin-like V-type domains. Analysis of stable cell lines expressing the mutant proteins revealed that virus binding is influenced by mutations in the predicted C'-C" loop, the C" beta-strand, the C"-D loop, and the D-E loop. Mutations in homologous regions of the immunoglobulin-like CD4 molecule alter its interaction with gp120 of human immunodeficiency virus type 1. Cells expressing Pvr mutations on the predicted C" edge do not develop cytopathic effect during poliovirus infection, suggesting that poliovirus-induced cytopathic effect may be induced by the virus-receptor interaction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bowman M. R., MacFerrin K. D., Schreiber S. L., Burakoff S. J. Identification and structural analysis of residues in the V1 region of CD4 involved in interaction with human immunodeficiency virus envelope glycoprotein gp120 and class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9052–9056. doi: 10.1073/pnas.87.22.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore L., Rose J. K. Blockade of human immunodeficiency virus type 1 production in CD4+ T cells by an intracellular CD4 expressed under control of the viral long terminal repeat. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2695–2699. doi: 10.1073/pnas.90.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore L., Rose J. K. Prevention of HIV-1 glycoprotein transport by soluble CD4 retained in the endoplasmic reticulum. Nature. 1990 Jun 14;345(6276):625–628. doi: 10.1038/345625a0. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Olson D., Shapiro R., Winkler G., Rosa J. J., Thomas D. W., Williams C., Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J Immunol. 1992 Sep 1;149(5):1779–1787. [PubMed] [Google Scholar]
- Clayson E. T., Brando L. V., Compans R. W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989 May;63(5):2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Hussey R. E., Steinbrich R., Ramachandran H., Husain Y., Reinherz E. L. Substitution of murine for human CD4 residues identifies amino acids critical for HIV-gp120 binding. Nature. 1988 Sep 22;335(6188):363–366. doi: 10.1038/335363a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Herzenberg L. A. Selection of hybridomas and hybridoma variants using the fluorescence activated cell sorter. J Immunol Methods. 1982;52(1):1–14. doi: 10.1016/0022-1759(82)90344-1. [DOI] [PubMed] [Google Scholar]
- Epp O., Lattman E. E., Schiffer M., Huber R., Palm W. The molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI refined at 2.0-A resolution. Biochemistry. 1975 Nov 4;14(22):4943–4952. doi: 10.1021/bi00693a025. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey W., Jr, Wang B. C., Yoo C. S., Sax M. Structure of a novel Bence-Jones protein (Rhe) fragment at 1.6 A resolution. J Mol Biol. 1983 Jul 5;167(3):661–692. doi: 10.1016/s0022-2836(83)80104-1. [DOI] [PubMed] [Google Scholar]
- Giranda V. L., Chapman M. S., Rossmann M. G. Modeling of the human intercellular adhesion molecule-1, the human rhinovirus major group receptor. Proteins. 1990;7(3):227–233. doi: 10.1002/prot.340070304. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Freistadt M. S., Racaniello V. R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990 Oct;64(10):4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Racaniello V. R. Down regulation of poliovirus receptor RNA in HeLa cells resistant to poliovirus infection. J Virol. 1991 Apr;65(4):1829–1835. doi: 10.1128/jvi.65.4.1829-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koike S., Ise I., Nomoto A. Functional domains of the poliovirus receptor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4104–4108. doi: 10.1073/pnas.88.10.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouteiller P. P., Mishal Z., Lemonnier F. A., Kourilsky F. M. Quantification by flow cytofluorimetry of HLA class I molecules at the surface of murine cells transformed by cloned HLA genes. J Immunol Methods. 1983 Jul 29;61(3):301–315. doi: 10.1016/0022-1759(83)90224-7. [DOI] [PubMed] [Google Scholar]
- Leahy D. J., Axel R., Hendrickson W. A. Crystal structure of a soluble form of the human T cell coreceptor CD8 at 2.6 A resolution. Cell. 1992 Mar 20;68(6):1145–1162. doi: 10.1016/0092-8674(92)90085-q. [DOI] [PubMed] [Google Scholar]
- Lloyd R. E., Bovee M. Persistent infection of human erythroblastoid cells by poliovirus. Virology. 1993 May;194(1):200–209. doi: 10.1006/viro.1993.1250. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- McClelland A., deBear J., Yost S. C., Meyer A. M., Marlor C. W., Greve J. M. Identification of monoclonal antibody epitopes and critical residues for rhinovirus binding in domain 1 of intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7993–7997. doi: 10.1073/pnas.88.18.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Moebius U., Clayton L. K., Abraham S., Harrison S. C., Reinherz E. L. The human immunodeficiency virus gp120 binding site on CD4: delineation by quantitative equilibrium and kinetic binding studies of mutants in conjunction with a high-resolution CD4 atomic structure. J Exp Med. 1992 Aug 1;176(2):507–517. doi: 10.1084/jem.176.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Sattentau Q. J., Klasse P. J., Burkly L. C. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992 Aug;66(8):4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. E., Racaniello V. R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992 May;66(5):2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell M. K., Haughn L. J., Maroun C. R., Julius M. H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990 Sep 20;347(6290):286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Chen B. L., Phizackerley R. P., Saul F. The three-dimensional structure of the fab' fragment of a human myeloma immunoglobulin at 2.0-angstrom resolution. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3440–3444. doi: 10.1073/pnas.71.9.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet P., Carayon P. Cytofluorometric quantification of cell-surface antigens by indirect immunofluorescence using monoclonal antibodies. J Immunol Methods. 1985 Dec 17;85(1):65–74. doi: 10.1016/0022-1759(85)90274-1. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Register R. B., Uncapher C. R., Naylor A. M., Lineberger D. W., Colonno R. J. Human-murine chimeras of ICAM-1 identify amino acid residues critical for rhinovirus and antibody binding. J Virol. 1991 Dec;65(12):6589–6596. doi: 10.1128/jvi.65.12.6589-6596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. A comparison of the heme binding pocket in globins and cytochrome b5. J Biol Chem. 1975 Sep 25;250(18):7525–7532. [PubMed] [Google Scholar]
- Ryu S. E., Kwong P. D., Truneh A., Porter T. G., Arthos J., Rosenberg M., Dai X. P., Xuong N. H., Axel R., Sweet R. W. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 1990 Nov 29;348(6300):419–426. doi: 10.1038/348419a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N., van Hille B., Geuskens M., Rommelaere J. Partial reversion of conditional transformation correlates with a decrease in the sensitivity of rat cells to killing by the parvovirus minute virus of mice but not in their capacity for virus production: effect of a temperature-sensitive v-src oncogene. J Virol. 1989 Nov;63(11):4797–4807. doi: 10.1128/jvi.63.11.4797-4807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Schockmel G. A., Somoza C., Davis S. J., Williams A. F., Healey D. Construction of a binding site for human immunodeficiency virus type 1 gp120 in rat CD4. J Exp Med. 1992 Jan 1;175(1):301–304. doi: 10.1084/jem.175.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinka H. C., Zibert A., Wimmer E. A chimeric poliovirus/CD4 receptor confers susceptibility to poliovirus on mouse cells. J Virol. 1992 Apr;66(4):2523–2526. doi: 10.1128/jvi.66.4.2523-2526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinka H. C., Zibert A., Wimmer E. Poliovirus can enter and infect mammalian cells by way of an intercellular adhesion molecule 1 pathway. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3598–3602. doi: 10.1073/pnas.88.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Gaur A., Chan P. Y., Springer T. A. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J Immunol. 1992 May 15;148(10):3271–3274. [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Tomassini J. E., Graham D., DeWitt C. M., Lineberger D. W., Rodkey J. A., Colonno R. J. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4907–4911. doi: 10.1073/pnas.86.13.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]



