Abstract
Chickpea is an important food legume crop of Turkey and is largely grown for human consumption on low moisture or salt-affected soils. The objective of the study was to find the effects of NaCl stress at electrical conductivities of 4.5, 8.6, 12.7 and 16.3 dS/m and seed sizes (7, 8 and 9 mm) on germination and early seedling growth of three popular chickpea cultivars (AKN-97, Gokce and Uzunlu-99). Mean frequency of germination, germination time, germination index, root length, shoot length and seedling fresh weight showed seed size-dependent responses of cultivars to salt stress. In general, small seeds germinated and grew more rapidly compared to medium and large seeds of the same cultivars against all levels of salt stress, with the best results in cultivar Uzunlu-99. No effect of NaCl treatments was observed on frequency of germination; however, a drastic decrease in early seedling growth was recorded at increased NaCl concentrations. Regression analysis results showed a significantly positive relationship (P<0.01) between seed size and mean germination time, whereas a significantly negative relationship was recorded between seed size and germination index, root length, shoot length. Moreover, linear regression values apparently confirmed that increased seed size in each cultivar affected decreased germination index, root and shoot lengths with enhanced mean germination time. Thus, it was concluded that the use of small seeds could considerably reduce the production costs of chickpea in salt-affected soils.
Keywords: Chickpea (Cicer arietinum L.), NaCl stress, Cultivar, Seed size, Germination
INTRODUCTION
Chickpea (Cicer arietinum L.) is an annual grain legume that is used extensively for human consumption in Turkey. Chickpea is classified into ‘desi’ and ‘kabuli’ types based primarily on seed color. Desi chickpea has a pigmented (tan to black) seed coat and small seed size (>100 seeds/28.47 g). Kabuli chickpea has white to cream-colored seed coat and ranges in size from small to large (>100 to <50 seeds/28.47 g) (Miller et al., 2002).
Chickpea is largely grown on low moisture soils of Anatolia where evaporation exceeds precipitation, resulting in salt accumulation on the soil surface (Meiri, 1984; Pessarakli, 1999). There are conflicting reports on the effects of salinity on seed germination and growth in chickpea and many other species and cultivars (Bliss et al., 1986; Hampson and Simpson, 1990). However, it could not be determined clearly that differences between cultivars against salt tolerance are mainly due to genetic or morphologic factors. Soltani et al.(2002) proposed that large seeds had an advantage in producing more vigorous chickpea seedlings under saline or non-saline conditions. Gan et al.(2003) indicated that the use of small seeds and shallow planting reduced the production costs without reduction in seed yield.
Although chickpea, like other legumes, is largely salt sensitive (Mass and Hoffman, 1977; Lauter and Munns, 1986; Ashraf and Waheed, 1993; Özdemir and Engin, 1994; Welfare et al., 2002; Ozcan et al., 2000), there are significant differences among chickpea cultivars against salt tolerance during germination. However, Kheradnam and Ghorashy (1973) recorded relatively small differences among four Iranian chickpea cultivars in their germination response to NaCl and tolerance to salinity. While Goel and Varshney (1987) and Yadav et al.(1989) agreed that germination of chickpea is relatively less affected by salinity than subsequent early seedling growth, Kumar (1985) emphasized that later growth stages of chickpea are more sensitive to salt stress compared to earlier growth stages.
The plants flower profusely with indeterminate growth habit, and continue to flower and set pods as long as climatic conditions are favorable. This results in seeds of different sizes even in the same variety as they are composed of seed mixtures extracted from early, middle or late pollinating flowers with wide range of variation in chickpea seed size (Upadhyaya, 2003). Variation in seed size is associated with geographical effects on genetic factors, irregular flowering, number of seeds per pod, and number of pods per plant (Pedersen et al., 1961; Smithson et al., 1985; Miller et al., 2002). The seeds of chickpea cultivars differ in several respects including size, color and shape. Two loci B and Fr are responsible for changes in seed size and shape. However, their effects are modified by combination of factors including rainfall, temperature and number of seeds per pod (Smithson et al., 1985).
The aim of the study was to investigate the influence of salt stress on factors affecting germination of seeds with different sizes from three Turkish chickpea cultivars.
MATERIALS AND METHODS
The seeds of Uzunlu-99, Gokce and AKN-97 (kabuli type) were obtained from Central Field Crops Research Institute, Ankara, Turkey, with mean protein percentages of 19.91%, 25.30% and 23.12%, respectively. They were separated into small (7 mm), medium (8 mm) and large (9 mm) sized seeds by passing through a series of sieves. The 100-seed weights of three cultivars are shown in Table 1.
Table 1.
One-hundred-seed weights (g) of cultivars in relation to seed size
| Seed sizes (mm) | 100-seed weight (g) |
||
| AKN-97 | Gokce | Uzunlu-99 | |
| 7 | 31.3±0.31 | 30.9±0.76 | 32.6±0.16 |
| 8 | 42.6±0.10 | 40.3±0.18 | 42.7±0.18 |
| 9 | 50.5±1.45 | 48.3±0.65 | 48.7±1.68 |
Data represent mean±SD of four replicates; LSD=1.42 (P<0.05)
NaCl concentrations at electrical conductivities of 4.5, 8.6, 12.7 and 16.3 dS/m were adjusted before the start of the experiment. Distilled water served as a control (0.0 dS/m).
Four replicates of 50 seeds for each cultivar (50×4=200 seeds) were germinated between four rolled filter papers with 60 ml of respective test solutions. The papers were replaced every 2 d to prevent accumulation of salts (Rehman et al., 1996). Each rolled paper was placed in a sealed polythene bag to prevent evaporation. Seeds were allowed to germinate at (20±1) °C in the dark for 10 d. A seed was considered to be germinated when the emerging radicle elongated to 2 mm. Germination percentage was recorded every 24 h for 10 d. Mean germination time (MGT) was calculated for the rate of germination (Ellis and Roberts, 1980) with the following formula:
| MGT=∑(fx)/∑f, |
where f is the number of newly germinated seeds on each day and x is days of counting.
Germination index (GI) was calculated by the method of Wang et al.(2004) with the formula as following:
| GI=∑(Gi/Tt), |
where Gi is the germination percentage at the ith day, and T t is days of germination test.
The higher GI results show the higher seed quality and better performance (Wang et al., 2004). Root and shoot lengths and seedling fresh weight were measured on the 10th day. Ten grams of seeds respectively from each seed size and cultivar were placed in Petri dishes containing distilled water to determine water uptake of seeds necessary for germination. The water uptake was measured as fresh weight percentage increase in seed weight.
The experiment was designed with 3 factors and arranged at random. The first factor was cultivars (Uzunlu-99, Gokce and AKN-97), the second was seed sizes (7, 8 and 9 mm), and the third was different NaCl solution levels (0.0, 4.5, 8.6, 12.7 and 16.3 dS/m). Data given in percentages were subjected to arcsine transformation before statistical analysis. Analysis of variance (ANOVA) for all investigated parameters and linear regression analysis to investigate the effect of seed size on investigated parameters were performed by using the MSTAT-C computer software, version 2.10 (Michigan State University, USA). Significant differences among means were compared by LSD test (P<0.05).
RESULTS
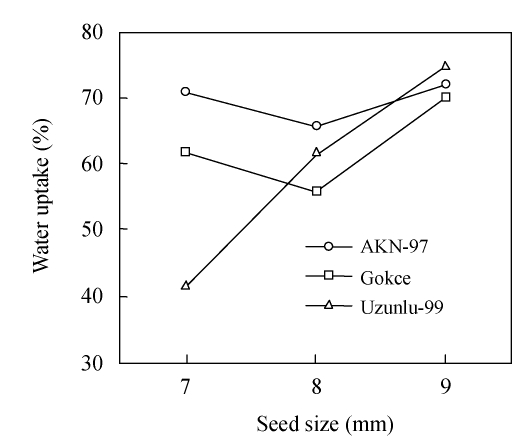
Water uptake was significantly (P<0.05) affected by the interaction between cultivars and seed size, with variable behavior in the three cultivars (Fig.1). The small, medium and large seeds of Uzunlu-99 uptook 41.5%, 61.5% and 74.9% water before germination, respectively. This showed that increase in seed size of Uzunlu-99 was accompanied by corresponding increase in the water uptake to germinate seeds. Contrarily, the small, medium and large seeds of Gokce utilized 61.7%, 55.8% and 69.99% water, respectively, and the small, medium and large seeds of AKN-97 used 70.8%, 65.6% and 71.9% water, respectively. Importantly, the medium seeds of both Gokce and AKN-97 needed less water compared to small and large seeds to germinate. Moreover, the amount of water needed to germinate small and large seeds of AKN-97 was similar, whereas the amount of water needed to germinate small and large seeds of Gokce varied significantly.
Fig. 1.
Water uptake of three chickpea cultivars (AKN-97, Gokce and Uzunlu-99) in relation to seed size, expressed as fresh weight percentage increase in 10 g-seed weight
LSD=7.03 (P<0.05)
NaCl solutions had no effect on frequency of germination, and 100% germination was observed under all NaCl treatments (data not shown). A significant 3-way interaction (cultivar×seed size×NaCl level) was observed for all other investigated characters (P<0.05, df=90). MGT increased with each increase in NaCl stress and seed size in all cultivars. The small seeds (7 mm) of AKN-97, Gokce and Uzunlu-99 took the minimum MGT to germinate under all salt stress conditions. Contrarily, the large seeds (9 mm) of three cultivars took the maximum MGT to germinate under all salt stress conditions in general (Table 2).
Table 2.
Interactive effects of NaCl levels and seed sizes on mean germination time (MGT) (d) of chickpea cultivars
| Cultivars | Seed sizes (mm) |
MGT (d) |
||||
| 0.0 dS/m | 4.5 dS/m | 8.6 dS/m | 12.7 dS/m | 16.3 dS/m | ||
| AKN-97 | 7 | 2.88±0.19 | 2.90±0.10 | 3.30±0.10 | 3.80±0.09 | 3.87±0.13 |
| 8 | 3.22±0.23 | 3.48±0.08 | 3.37±0.03 | 3.90±0.25 | 4.12±0.24 | |
| 9 |
3.53±0.40 |
3.62±0.20 |
3.72±0.15 |
4.62±0.50 |
4.62±0.50 |
|
| Gokce | 7 | 2.32±0.12 | 2.64±0.13 | 2.83±0.13 | 2.98±0.18 | 3.72±0.13 |
| 8 | 2.93±0.20 | 3.12±0.06 | 3.52±0.25 | 3.52±0.31 | 4.07±0.20 | |
| 9 |
3.13±0.33 |
3.45±0.22 |
4.30±0.44 |
3.98±0.32 |
4.88±0.15 |
|
| Uzunlu-99 | 7 | 2.75±0.17 | 2.57±0.13 | 3.16±0.24 | 2.82±0.13 | 3.83±0.08 |
| 8 | 2.98±0.08 | 2.98±0.13 | 3.80±0.01 | 3.10±0.09 | 4.20±0.05 | |
| 9 | 3.33±0.34 | 3.50±0.22 | 3.50±0.05 | 3.88±0.12 | 4.45±0.36 | |
Data represent mean±SD of four replicates; LSD=0.34 (P<0.05, df=90)
GI was significantly affected by NaCl and seed size. Small seeds of all cultivars gave the highest GI values at all NaCl concentrations, while increasing NaCl concentration reduced GI variably (Table 3). Small seeds of Uzunlu-99 gave higher GI at both 4.5 and 8.6 dS/m compared to the control (0.0 dS/m).
Table 3.
Interactive effects of NaCl levels and seed sizes on germination index (GI) of chickpea cultivars after 10 d incubation
| Cultivars | Seed sizes (mm) |
GI |
||||
| 0.0 dS/m | 4.5 dS/m | 8.6 dS/m | 12.7 dS/m | 16.3 dS/m | ||
| AKN-97 | 7 | 25.0±0.01 | 25.0±0.00 | 18.9±1.96 | 18.1±1.96 | 17.8±1.90 |
| 8 | 23.3±2.42 | 23.3±2.42 | 20.0±0.01 | 18.1±1.96 | 16.6±0.01 | |
| 9 |
20.0±0.01 |
20.0±0.00 |
18.9±1.90 |
13.9±1.04 |
15.3±0.01 |
|
| Gokce | 7 | 33.3±0.01 | 26.1±1.96 | 20.0±0.01 | 25.0±0.01 | 20.0±0.01 |
| 8 | 25.0±0.01 | 21.7±1.91 | 18.9±1.96 | 25.0±0.01 | 16.7±0.01 | |
| 9 |
23.3±2.42 |
20.6±2.42 |
17.8±1.90 |
17.0±1.96 |
13.7±1.04 |
|
| Uzunlu-99 | 7 | 23.3±2.42 | 25.0±0.01 | 27.7±2.42 | 23.3±2.42 | 15.3±1.04 |
| 8 | 23.3±2.82 | 25.0±0.01 | 20.0±0.01 | 20.0±0.01 | 17.8±1.90 | |
| 9 | 20.0±0.01 | 20.6±2.42 | 18.1±1.96 | 18.1±1.91 | 12.1±1.04 | |
Data represent mean±SD of four replicates; LSD=3.82 (P<0.05, df=90)
Increased NaCl concentration decreased root length of all cultivars; however, this decrease was more prominent with large seeds (9 mm) of respective cultivars. Small seeds (7 mm) gave the longest roots at control (Table 4). Increased seed size and salt concentration caused corresponding decreases in root length in all cultivars. The longest roots were obtained from small seeds in each cultivar under all salt stress conditions.
Table 4.
Interactive effects of NaCl levels and seed sizes on root length (cm) of chickpea cultivars after 10 d incubation
| Cultivars | Seed sizes (mm) | Root length (cm) |
||||
| 0.0 dS/m | 4.5 dS/m | 8.6 dS/m | 12.7 dS/m | 16.3 dS/m | ||
| AKN-97 | 7 | 11.27±0.90 | 9.57±0.06 | 7.57±0.32 | 5.83±0.72 | 3.80±0.35 |
| 8 | 9.77±0.32 | 7.37±0.21 | 7.17±0.61 | 4.83±0.20 | 3.47±0.35 | |
| 9 |
9.93±0.68 |
7.70±0.91 |
4.83±0.61 |
3.77±0.58 |
2.57±0.37 |
|
| Gokce | 7 | 11.77±0.76 | 9.17±1.11 | 7.33±0.06 | 5.27±0.32 | 3.63±0.40 |
| 8 | 10.03±0.95 | 8.60±0.57 | 7.60±0.70 | 4.03±0.25 | 2.57±0.31 | |
| 9 |
6.73±1.42 |
10.13±0.70 |
6.07±0.66 |
4.13±0.15 |
2.43±0.21 |
|
| Uzunlu-99 | 7 | 11.37±0.83 | 11.27±0.06 | 8.37±0.81 | 5.83±0.69 | 3.13±0.43 |
| 8 | 10.27±0.40 | 9.70±0.72 | 5.33±0.81 | 4.57±0.46 | 2.73±0.05 | |
| 9 | 9.97±0.63 | 8.43±0.55 | 6.80±0.26 | 4.43±0.38 | 2.97±0.47 | |
Data represent mean±SD of four replicates; LSD=1.29 (P<0.05, df=90)
The longest shoots were obtained from Uzunlu-99 on small seeds (7 mm) under all NaCl concentrations. Shoot length was severely influenced by salt stress with complete inhibition and no shoots at NaCl stress of 16.3 dS/m in all cultivars (Table 5). Medium and large seeds of Gokce and large seeds of AKN-97 also failed to develop shoots at NaCl stress of 12.7 dS/m. The results further show that small seeds were superior to medium and large ones at NaCl stresses of 4.5 to 12.7 dS/m.
Table 5.
Interactive effects of NaCl levels and seed sizes on shoot length (cm) of chickpea cultivars after 10 d incubation
| Cultivars | Seed sizes (mm) | Shoot length (cm) |
||||
| 0.0 dS/m | 4.5 dS/m | 8.6 dS/m | 12.7 dS/m | 16.3 dS/m | ||
| AKN-97 | 7 | 5.23±0.80 | 3.70±0.30 | 2.60±0.26 | 1.33±0.15 | − |
| 8 | 4.60±0.35 | 2.93±0.25 | 1.97±0.20 | 1.17±0.17 | − | |
| 9 |
4.30±0.62 |
2.40±0.20 |
1.40±0.01 |
− |
− |
|
| Gokce | 7 | 4.37±0.67 | 2.83±0.04 | 2.33±0.05 | 1.40±0.10 | − |
| 8 | 4.57±0.15 | 2.97±0.25 | 1.40±0.04 | − | − | |
| 9 |
2.43±0.30 |
2.30±0.26 |
1.10±0.20 |
− |
− |
|
| Uzunlu-99 | 7 | 6.37±0.47 | 5.63±0.30 | 3.30±0.31 | 1.93±0.16 | − |
| 8 | 5.30±0.44 | 4.37±0.75 | 1.23±0.19 | 1.03±0.10 | − | |
| 9 | 4.33±0.55 | 2.20±0.10 | 1.27±0.15 | 1.00±0.10 | − | |
Data represent mean±SD of four replicates; −: Data could not be taken due to no shoot growth; LSD=0.78 (P<0.05, df=90)
As no shoot growth was recorded in the medium and large seeds of Gokce and large seeds of AKN-97 at NaCl stress of 12.7 dS/m and all seeds of all cultivars at NaCl stress of 16.3 dS/m, therefore their root growth was evaluated as seedling fresh weight (Table 6). Compared to control, each increase in NaCl concentration and seed size resulted in remarkable decrease in seedling fresh weight for all cultivars. Although seed sizes had different responses to each NaCl concentration, the highest seedling fresh weight (71.2 mg/plant) was observed from medium seeds of Uzunlu-99 (control).
Table 6.
Interactive effects of NaCl levels and seed sizes on seedling fresh weight (mg/plant) of chickpea cultivars after 10 d incubation
| Cultivars | Seed sizes (mm) | Seedling fresh weight (mg/plant) |
||||
| 0.0 dS/m | 4.5 dS/m | 8.6 dS/m | 12.7 dS/m | 16.3 dS/m | ||
| AKN-97 | 7 | 60.3±5.16 | 38.3±3.33 | 32.9±1.07 | 24.7±1.58 | 15.9±1.54 |
| 8 | 60.5±2.85 | 35.9±2.72 | 30.5±3.06 | 24.3±1.08 | 19.4±1.86 | |
| 9 |
54.7±3.25 |
38.9±3.09 |
23.1±1.57 |
16.8±1.82 |
12.5±1.03 |
|
| Gokce | 7 | 61.2±4.31 | 44.2±1.97 | 30.1±0.87 | 22.1±2.25 | 8.8±0.56 |
| 8 | 62.3±2.52 | 44.1±3.82 | 23.4±2.36 | 20.6±2.24 | 10.0±1.01 | |
| 9 |
65.3±2.24 |
34.0±3.45 |
25.0±2.84 |
20.7±1.73 |
10.7±0.98 |
|
| Uzunlu-99 | 7 | 52.1±7.04 | 37.9±2.95 | 27.7±1.85 | 23.8±1.93 | 15.7±1.65 |
| 8 | 71.2±3.78 | 44.8±3.49 | 32.2±3.01 | 17.0±0.92 | 11.4±1.15 | |
| 9 | 48.1±4.38 | 45.8±2.81 | 27.6±2.09 | 16.7±1.31 | 11.4±0.79 | |
Data represent mean±SD of four replicates; LSD=6.42 (P<0.05, df=90)
Linear regression analysis results showed non-significant relationship between seed size and germination percentage or seedling fresh weight (data not given). However, a significantly positive relationship (P<0.01) was recorded between seed size and mean germination time, whereas a significantly negative relationship was recorded between seed size and GI, root and shoot lengths (Table 7). Linear regression values confirmed that increased seed size apparently resulted in decreased GI, root and shoot lengths with enhanced mean germination time.
Table 7.
Linear regression equations for predicting the investigated parameters (y) from seed size (x, mm) of chickpea cultivars after 10 d of NaCl treatment
| Characters | R2 | Regression equations |
| Mean germination time | 0.292 | y=0.252+0.404x |
| Germination index | 0.201 | y=40.469−2.481x |
| Root length | 0.052 | y=13.229−0.809x |
| Shoot length | 0.072 | y=6.971−0.603x |
Relationship between seed size and mean germination time, germination index, root and shoot lengths was significant (P<0.01)
DISCUSSION
The primary action of osmotic inhibition is retardation of water uptake, which is crucial for germination (Kahn, 1960). Seed size affected the water uptake and consequently growth parameters of the investigated cultivars. The effect of salinity stress was more prominent on AKN-97 and Gokce compared to Uzunlu-99.
NaCl adversely affected the germination time and early seedling growth of chickpea, but any inhibitory effects of NaCl on germination percentage were not determined. The results of this study are in agreement with the observations of Murillo-Amador et al.(2002) in cowpea and Okcu et al.(2005) in pea. They observed that NaCl did not adversely affect germination percentage but delayed MGT. The lower MGT in control (0.0 dS/m) and reduced MGT in small seeds compared to medium and large seeds of all cultivars under all levels of NaCl stress may be due to more rapid water uptake; different concentration of NaCl may have created an osmotic barrier resulting in inhibition of corresponding water uptake, which is in agreement with Nieman (1965), Wignarajah et al. (1975) and Yasseen et al.(1987), who emphasized that NaCl inhibits growth by reducing both cell division and cell enlargement. GI of small seeds of each cultivar was higher compared to medium and large seeds under no stress (0.0 dS/m) or all levels of NaCl stress. Wang et al.(2004) indicated that the GI gave the best results to predict field emergence of Sudan grass.
Root length was severely influenced by NaCl stress; however, small seeds produced the longest roots. It is assumed that small seeds absorbed water more rapidly compared to medium and large seeds, which resulted in fast root growth. Contrarily, Soltani et al.(2002) observed that root length was diminished by increasing NaCl concentration. They also noted that large seeds gave longer roots compared to medium and small seeds. It is presumed that the differences could be due to different genotypes used in this study.
Shoot growth was more adversely affected by hypocotyl injury due to different levels of NaCl than the root especially at 16.3 dS/m in all cultivars. The results are in agreement with Esechie et al.(2002), who proposed that hypocotyl injury was more severe under saline soils compared to non-saline soils. On the other hand, small seeds exhibited better performance than large seeds. Soltani et al.(2002) found that increasing seed size resulted in decreasing shoot length, while no significant differences were determined for the investigated cultivars in the current study.
As a result of decreasing root and shoot lengths, seedling fresh weight decreased under increased NaCl concentrations in all cultivars of chickpea. This study further shows that NaCl had greater inhibitory effects on early seedling growth rather than on germination, in agreement with similar observation in alfalfa (Redmann, 1974). It is assumed that reducing cell division and plant growth metabolism induced by accumulation of Na+ ion caused changes in ion balances and the imbalance of mineral nutrients resulted in a reduction or an inhibition of plant growth (Mer et al., 2000). The linear positive relationship between seed size and mean germination time showed that each increase in salt stress resulted in corresponding increase in the germination time of small, medium and large seeds of three cultivars. The significantly negative relationship between seed size and GI, root and shoot lengths suggested that each increase in NaCl concentration resulted in these inhibitory or toxic effects of NaCl, which was observed only after the start of elongation of radicle. Furthermore, the beneficial effects of small seeds under both saline and non-saline conditions were clearly observed in this study. Small seeds (7 mm) germinated and grew more rapidly under NaCl stress, showing that they could be preferred on salt-infected soils to achieve higher levels of crop yield. It is thus concluded that NaCl had no toxic effect on seed germination; however, it affected early seedling growth negatively.
Acknowledgments
The authors acknowledge the help from Prof. Dr. Sebahattin Ozcan, Department of Field Crops, University of Ankara, Turkey for practical guidance and Mrs. Aysegul Gurbuz, Central Agricultural Research Institute, Ankara, Turkey for providing seeds and protein analyses.
References
- 1.Ashraf M, Waheed A. Response of some genetically diverse lines of chickpea (Cicer arietinum L.) to salt. Plant and Soil. 1993;154(2):257–266. doi: 10.1007/BF00012531. [DOI] [Google Scholar]
- 2.Bliss RD, Platt-Aloia KA, Thomson WW. Osmotic sensitivity in relation to salt sensitivity in germinating barley seeds. Plant Cell Environ. 1986;9(9):721–725. doi: 10.1111/j.1365-3040.1986.tb02104.x. [DOI] [Google Scholar]
- 3.Ellis RH, Roberts EH. Towards a Rational Basis for Testing Seed Quality. In: Hebblethwaite PD, editor. Seed Production. London: Butterworths; 1980. pp. 605–635. [Google Scholar]
- 4.Esechie HA, Al-Saidi A, Al-Khanjari S. Effect of sodium chloride salinity on seedling emergence in chickpea. Journal of Agronomy and Crop Science. 2002;188(3):155–160. doi: 10.1046/j.1439-037X.2002.00554.x. [DOI] [Google Scholar]
- 5.Gan YT, Miller PR, McDonald CL. Response of kabuli chickpea to seed size and planting depth. Can J Plant Sci. 2003;83(1):39–46. [Google Scholar]
- 6.Goel N, Varshney KA. Note on seed germination and early seedling growth of two chickpea varieties under saline conditions. Legume Res. 1987;10(1):34–36. [Google Scholar]
- 7.Hampson CR, Simpson GM. Effects of temperature, salt and osmotic potential on early growth of wheat (Triticum aestivum). I. Germination. Can J Bot. 1990;68(3):524–528. doi: 10.1139/b90-072. [DOI] [Google Scholar]
- 8.Kahn A. An analysis “dark osmotic inhibition” of germination of lettuce seeds. Plant Physiol. 1960;35(1):1–7. doi: 10.1104/pp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kheradnam M, Ghorashy SR. Salt tolerance of chickpea varieties during germination. Agron J. 1973;65(2):329. [Google Scholar]
- 10.Kumar D. Emergence, establishment and seed yield of chickpea as affected by sodicity. Annals of Arid Zone. 1985;24(4):334–340. [Google Scholar]
- 11.Lauter DJ, Munns DN. Salt resistance of chickpea genotype in solutions salinized with NaCl. Annu Rev Plant Physiol. 1986;84(2):455–461. [Google Scholar]
- 12.Mass EV, Hoffman GJ. Crop salt tolerance—current assessment. J Irrig Drainage Div ASCE. 1977;103(2):115–134. [Google Scholar]
- 13.Meiri A. Plant Response to Salinity: Experimental Methodology and Application to the Field. In: Shainberg I, Shalhevet J, editors. Soil Salinity under Irrigation Processes and Management. Berlin: Springer-Verlag; 1984. [Google Scholar]
- 14.Mer RK, Prajith PK, Pandya DH, Pandey AN. Effect of salts on germination of seeds and growth of young plants of Hordeum vulgare, Triticum aestivum, Cicer arietinum and Brassica juncea . J Agron Crop Sci. 2000;185(4):209–217. doi: 10.1046/j.1439-037x.2000.00423.x. [DOI] [Google Scholar]
- 15.Miller P, McKay K, Jenks B, et al. Montguide: Montan State University, Extension Service; 2002. Growing Chickpea in the Northern Great Plains. MT200204 AG 3/2002,(Available from: http://www.montana.edu/wwwpb/pubs/mt200204.pdf) [Google Scholar]
- 16.Murillo-Amador B, Lopez-Aguilar R, Kaya C, Larrinaga-Mayoral J, Flores-Hernandez A. Comparative effects of NaCl and polyethylene glycol on germination, emergence and seedling growth of cowpea. Journal of Agronomy and Crop Science. 2002;188(4):235–247. doi: 10.1046/j.1439-037X.2002.00563.x. [DOI] [Google Scholar]
- 17.Nieman RH. Expansion of bean leaves and its suppression by salinity. Plant Physiol. 1965;40(1):156–161. doi: 10.1104/pp.40.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okcu G, Kaya MD, Atak M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.) Turk J Agric For. 2005;29(4):237–242. [Google Scholar]
- 19.Ozcan H, Turan MA, Koc O, Cikili Y, Taban S. Growth and variations in proline, sodium, chloride, phosphorus and potassium concentrations of chickpea (Cicer arietinum L. cvs.) varieties under salinity stress. Turk J Agric For. 2000;24(6):649–654. [Google Scholar]
- 20.Özdemir S, Engin M. Effect of NaCl concentrations on germination and seedling growth in chickpea (Cicer arietinum L.) Turk J Agric For. 1994;18(3):323–328. [Google Scholar]
- 21.Pedersen MW, Jones LG, Rogers TH. Producing Seeds of the Legumes. In: Stefferud A, editor. Seeds. US Department of Agriculture; 1961. pp. 171–181. Yearbook. [Google Scholar]
- 22.Pessarakli M. Handbook of Plant and Crop Stress. New York: Marcel Decker Inc; 1999. [Google Scholar]
- 23.Redmann RE. Osmotic and specific ion effect on the germination of alfalfa. Can J Bot. 1974;52(4):803–808. [Google Scholar]
- 24.Rehman S, Harris PJC, Bourne WF, Wilkin J. The effect of sodium chloride on germination and the potassium and calcium content of Acacis seeds. Seed Sci Technol. 1996;25(1):45–57. [Google Scholar]
- 25.Smithson JB, Thompson JA, Summerfield RJ. Chickpea (Cicer arietinum L.) In: Summerfield RJ, Roberts EH, editors. Grain Legume Crops. London: Collins Publications; 1985. pp. 312–390. [Google Scholar]
- 26.Soltani A, Galeshi S, Zeinali E, Latifi N. Germination, seed reserve utilization and seedling growth of chickpea as affected by salinity and seed size. Seed Sci Technol. 2002;30(1):51–60. [Google Scholar]
- 27.Upadhyaya HD. Geographical patterns of variation for morphological and agronomic characteristics in the chickpea germplasm collection. Euphytica. 2003;132(3):343–352. doi: 10.1023/A:1025078703640. [DOI] [Google Scholar]
- 28.Wang YR, Yu L, Nan ZB, Liu YL. Vigor tests used to rank seed lot quality and predict field emergence in four forage species. Crop Sci. 2004;44(2):535–541. [Google Scholar]
- 29.Welfare W, Yeo AR, Flowers TJ. Effects of salinity and ozone, individually and in combination, on the growth and ion contents of two chickpea (Cicer arietinum L.) varieties. Environ Pollut. 2002;120(2):397–403. doi: 10.1016/S0269-7491(02)00109-4. [DOI] [PubMed] [Google Scholar]
- 30.Wignarajah K, Jennings DH, Handley JF. Effect of salinity on growth of Phaseolus vulgaris L. II. The effect of internal solute concentration. Ann Bot. 1975;39(5):1039–1055. [Google Scholar]
- 31.Yadav HD, Yadav OP, Dhankar OP, Oswal MC. Effect of chloride salinity and boron on germination, growth and mineral composition of chickpea (Cicer arientinum L.) Annals of Arid Zone. 1989;28(1):63–67. [Google Scholar]
- 32.Yasseen BT, Jurjee JA, Sofaly SA. Changes in some growth processes induced by NaCl in individual leaves of two barley cultivars. Indian J Plant Physiol. 1987;30(1):1–6. [Google Scholar]