Abstract
The contents of total phenolics and extractable condensed tannins in the leaves, twigs and stem bark of Canarium album were determined. The structural heterogeneity of condensed tannins from stem bark was characterized by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and nuclear magnetic resonance (NMR) analyses. The results show the predominance of signals representative of procyanidins and prodelphinidins. In addition, epicatechin and epigallocatechin polymers with galloylated procyanidin or prodelphinidin were also observed. The tannins were screened for their potential antioxidant activities using 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ferric reducing/antioxidant power (FRAP) model systems. Tannins extracted from leaves, twigs and stem bark all showed a very good DPPH radical scavenging activity and ferric reducing power.
Keywords: Canarium album, Tannins, Antioxidant capacity, Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)
INTRODUCTION
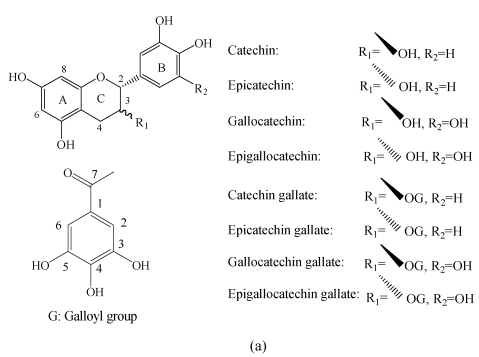
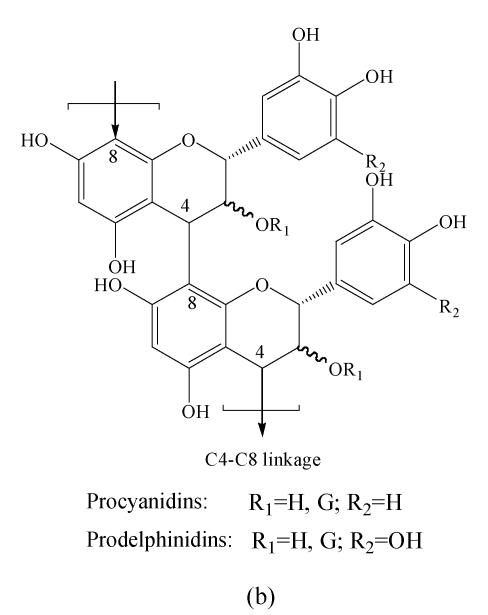
Tannins known as the group of phenolic compounds are the significant plant secondary metabolites. Tannins in vascular plants occur as two types, the condensed and the hydrolysable (Hernes et al., 2001). Condensed tannins are also known as proanthocyanidins (PAs), the oligomeric and polymeric flavan-3-ols, which are linked through C4-C8 or C4-C6 linkages. The diversity of condensed tannins is given by the structural variability of the monomer units (different hydroxylation patterns of the aromatic rings A and B, and different configurations at the chiral centers C2 and C3) (Fig.1). The size of PA molecules can be described by their degrees of polymerization (DPs). The molecules are water-soluble and can form complexes with proteins and polysaccharides (Haslam, 1998). PAs are of great interest in nutrition and medicine because of their potent antioxidant capacity and possible protective effects on human health (Santos-Buelga and Scalbert, 2000). They have antioxidant properties related to their radical scavenging capacity (Ricarda Da Silva et al., 1991), and these properties have been used against heart disease through reducing lipid oxidation. It was hypothesized that the free radical scavenging properties of PAs may reduce the risk of cardiovascular diseases, cancer (Bagchi et al., 2000) and blood clotting, and certain types of trimeric PAs may protect against urinary tract infections (Santos-Buelga and Scalbert, 2000). However, tannins are diverse compounds with great variation in structure and concentration within and among plant species. Therefore, biomedical researches on the health benefits and risks of increased tannins consumption are severely limited by lack of methods for rapid characterization and standardization.
Fig. 1.
Chemical structures of flavan-3-ol (a) monomers and (b) polymers
Canarium album or Chinese olive (Burseraceae), is the native species in the southeast of China. It is a good fruit species because of its tolerance to poor soils (e.g., rocky hillsides, saline or alkaline soils) (Wei et al., 1999). The dried fruits are a traditional medicine material that has some pharmacological functions such as anti-bacterium, anti-virus, anti-inflammation and detoxification (Ding, 1999). The fresh fruits are widely used in food industry, while the leaves and stem bark of the plants are discarded as waste products.
Polyphenols make a major contribution to free radical scavenging capacities of Mediterranean olives species (Visioli et al., 1998). Previous studies indicated that there was a direct relationship between antioxidant activity and total phenolics content in selected herbs, vegetables and fruits (Oszmianski et al., 2007; Wu, 2007). In this study, contents of total phenolics and extractable condensed tannins of leaves, twigs and stem bark of C. album were determined, and the structural heterogeneity of condensed tannins from stem bark was characterized by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and nuclear magnetic resonance (NMR) analyses. Meanwhile, the free radical scavenging capacities and ferric reducing power of condensed tannins from leaves, twigs and stem bark were also discussed.
MATERIALS AND METHODS
Samples
Leaves, twigs and stem bark samples of C. album were collected in Fuzhou in the southeast of China. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-S-triazine (TPTZ), ascorbic acid (AA), butylated hydroxyanisole (BHA), gallic acid and ellagic acid were purchased from Sigma (USA). Sephadex LH-20 was purchased from Amersham (USA). All chemicals were of analytical reagent (AR) purity grade.
Methods
1. Solvent extraction
Ten grams of fresh materials were weighed and extracted with 100 ml acetone-water solution (70:30, v/v) twice at room temperature. The extract was filtered and pooled, and the solvent was removed under reduced pressure by using a rotary vacuum-evaporator at 40 °C. The remaining aqueous fraction was extracted thrice with hexane in order to remove chlorophyll and lipophilic compounds. The remaining crude tannins fraction was chromatographed on an Sephadex LH-20 column (3 cm×22 cm) which was first eluted with methanol:water (50:50, v/v) to remove oligomeric tannins and then followed by acetone:water (70:30, v/v). The last fraction, containing the polymeric tannins, was freeze-dried and stored at −20 °C until required.
2. Determination of total phenolics and condensed tannins (PAs)
Established procedures (Lin et al., 2006) were used to determine total phenolics and condensed tannins. Total phenolics were measured with the Prussian blue method (Graham, 1992), and condensed tannins were assayed by the butanol-HCl method (Terrill et al., 1992), using purified tannins from C. album and tannic acid as the standards.
3. NMR analysis
13C NMR spectra were recorded in CD3COCD3-D2O mixture with a Varian Metcury-600 spectrometer at 150 MHz (proton decoupling mode for carbon).
4. MALDI-TOF MS analysis
The MALDI-TOF MS spectra were recorded on a Bruker Reflex III. The irradiation source was a pulsed nitrogen laser with a wavelength of 337 nm, and the duration of the laser pulse was 3 ns. In the positive reflectron mode, an accelerating voltage of 20.0 kV and a reflectron voltage of 23.0 kV were used. The spectra of condensed tannins were obtained from a sum of 100~150 shots and calibrated using Angiotensin II (1046.5 MW), Bombesin (1619.8 MW), ACTHclip1839 (2465.2 MW), and Somatostatin28 (3147.47 MW) as external standards. 2,5-Dihydroxy benzoic acid (DHB, 10 mg/ml aqueous solution) was used as the matrix. The sample solutions (7.5 mg/ml aqueous) were mixed with the matrix solution at a volumetric ratio of 1:3. The mixture (1 μl) was applied to the steel target. Amberlite IRP-64 cation-exchange resin (Sigma-Aldrich, USA), equilibrated in deionized water, was used to deionize the analyte-matrix solution thrice. Cesium trifluoroacetate (1 mg/ml) was mixed with the analyte-matrix solution (1:3, v/v) to promote the formation of a single type of ion adduct ([M+Cs]+) (Xiang et al., 2006).
5. Hydrolysis of the tannins
The tannins from leaves and twigs (0.25 g) were hydrolyzed with 2 ml of 2 mol/L HCl in a boiling water bath for 1 h. After cooling, 2 ml of 2 mol/L NaOH and then 6 ml methanol were added to the vial. The slurry was sonicated for 20 min with occasional shaking. Further, the slurry was centrifuged at 19 000×g and the supernatant was used for HPLC analysis. The HPLC apparatus consisted of an Aglient 1100 diode array detector and a quaternary pump.
The samples were previously dissolved in mobile phase and then filtrated through membrane filter with an aperture size of 0.45 µm. Ten milliliters of the clear supernatant after centrifugation at 19 000×g for 5 min was injected. Separation was performed on a Hypersil ODS column (4.6 mm×250 mm, 5 μm) thermostatted at 30 °C. The mobile phase was composed of solvent A (0.1% (v/v) trifluoroacetic acid (TFA) in water) and solvent B (0.1% (v/v) TFA in acetonitrile). The gradient condition was: 0~2nd minutes 100% A, 2nd~6th minutes 100%~95% A, 6th~10th minutes 95% A, 10th~15th minutes 95%~80% A, 15th~20th minutes 80% A, and 20th~25th minutes 70% A. Other chromatographic conditions were as follows: flow rate at 1 ml/min, detection at 280 nm, and scanning performed between 200 and 600 nm.
6. Free-radical scavenging activity
The free-radical scavenging activity was measured according to the method of Braca et al.(2001). A 100 µl of sample at different concentration (15~250 µg/ml) was added to 3 ml of DPPH solution (0.004% (w/w) methanolic solution). Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. The IC 50 value, defined as the amount of antioxidant necessary to decrease the initial DPPH concentration by 50%, was calculated from the results and used for comparison. The capability to scavenge the DPPH radical was calculated by using the following equation:
| DPPH scavenging effect (%)=[(A1−A2)/A1]×100, |
where A 1 is the absorbance of the control reaction, and A 2 is the absorbance in the presence of the sample. BHA and ascorbic acid were used as the controls.
7. Ferric reducing/antioxidant power (FRAP) assay
FRAP assay is a simple and reliable colorimetric method commonly used for measuring the total antioxidant capacity (Benzie and Strain, 1996). Briefly, 3 ml of FRAP reagent prepared freshly was mixed with 100 µl of test sample or methanol (for the reagent blank). The FRAP reagent was prepared from 300 mmol/L acetate buffer (pH 3.6), 20 mmol/L ferric chloride and 10 mmol/L TPTZ made up in 40 mmol/L hydrochloric acid. All the above three solutions were mixed together in the ratio of 25:2.5:2.5 (v/v/v). The absorbance of reaction mixture at 593 nm was measured spectrophotometrically after incubation at 25 °C for 10 min. The FRAP values, expressed in mmol ascorbic acid equivalents (AAE)/g dried tannins, were derived from a standard curve.
8. Statistical analysis
All measurements were replicated three times. One-way analysis of variance (ANOVA) was used, and the differences were considered to be significant at P<0.05. All statistical analyses were performed with SPSS 11.0.
RESULTS AND DISCUSSION
Content of total phenolics and extractable condensed tannins
Total phenolic content of the leaves was the highest, followed by twigs, and then stem bark for C. album (Table 1). The leaves of C. album had higher extractable condensed tannin content than twigs and stem bark.
Table 1.
Contents of total phenolics and extractable condensed tannins in leaves, twigs and stem bark of C. album
| Samples | Total phenolics (mg/g)a | Extractable condensed tannins (mg/g)b |
| Leaves | 164.39±14.67 | 117.70±9.39 |
| Twigs | 161.99±13.07 | 48.99±2.40 |
| Stem bark | 54.96±3.12 | 39.98±3.05 |
Using tannic acid as the standard;
Using purified leaf tannins as the standard
NMR analysis
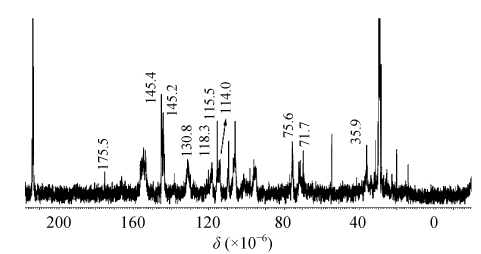
The purified condensed tannins from stem bark were analyzed by 13C NMR spectroscopy. The 13C NMR spectra showed distinct signals at 145.2×10−6 and 145.4×10−6, which are assignable to C3′ and C4′ in procyanidin units (catechin/epicatechin) (Fig.2). The dominance of the procyanidin unit of the polymeric sample was further corroborated by the presence of strong peaks at 114.0×10−6~115.5×10−6 consistent with the C2′ and C5′ chemical shifts of the catechol B-ring, and at 118.1×10−6~120.2×10−6 consistent with the C6′ chemical shift of the catechol B-ring. In addition, a strong signal at 145×10−6~146×10−6 was also obtained. Prodelphinidin units (gallocatechin/epigallocatechin) generally showed a typical resonance at 146×10−6 (Behrens et al., 2003). The presence of a clear signal with such a chemical shift in the spectra of the condensed tannins from stem bark revealed that they are mostly composed of procyanidin and prodelphinidin units.
Fig. 2.
13C NMR spectrum of condensed tannins from stem bark of C. album
The region between 70×10−6 and 90×10−6 is sensitive to the stereochemistry of the C-ring. The determination of the ratio of the 2,3-cis to 2,3-trans stereochemistries could thus be achieved through the distinct differences in their respective C2 chemical shifts (Es-Safi et al., 2006). Whereas C3 of both cis and trans isomers occurs at 73×10−6 (in the presence of galloyl groups), C2 gives a resonance at 76×10−6 for the cis form and at 84×10−6 for the trans form. The absence of the latter signal peak in the spectrum of the condensed tannins fraction indicates the only presence of epicatechin and epigallocatechin subunits. The presence of a signal at 35.9×10−6 was consistent with a C4 being shifted upfield by the presence of a 3-O-gallate unit. This was further confirmed by the observation of signals for ester carbonyl carbons at 175.5×10−6 (Gal-C7) and galloyl ring carbons at 114.0×10−6 (Gal-C2, Gal-C6), 130.8×10−6 (Gal-C1) and 143×10−6~145×10−6 (Gal-C4). These results thus show that the polymeric PA fraction of the stem bark is predominantly constituted of procyanidin and prodelphinidin with (−)-epicatechin and (−)-epigallo-catechin as main constitutive monomers, some with galloyl groups attached (Spencer et al., 2007).
MALDI-TOF MS analysis
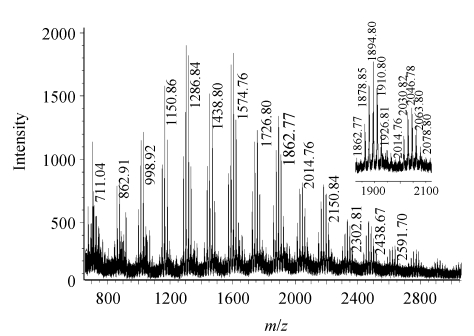
Individual oligomers of vegetable tannins are well resolved in MALDI-TOF MS spectra with their molecular weights determinated in the analysis of condensed tannins (Ohnishi-Kameyama et al., 1997; Yang and Chien, 2000; Ishida et al., 2005). When MALDI-TOF MS is used to characterize tannins, the mass spectra tend to favor an association with naturally abundant Na+ or K+ over the formation of a protonated molecular ion [M+H]+ (Ohnishi-Kameyama et al., 1997; Krueger et al., 2003). In order to promote the formation of single tannin-ion adducts, K+, Na+, Ag+ or Cs+ is usually added to the analyte/matrix and the adducts are detected in the positive ion mode. However, MALDI-TOF mass spectra of condensed tannins are notably affected by various added and naturally abundant ions, except for the over-evaluation of hydroxyl substitution in flavan-3-ol oligomers due to the formation of both [M+Na]+ and [M+K]+ from one species (Krueger et al., 2003). Using MALDI-TOF with deionization and selection of Cs+ as the cationization reagent rather than selection of Na+, condensed tannin polymers of higher DP were observed. Meanwhile, the polymer with the highest intensity ion peak changed with the ion adducts used (Xiang et al., 2007).
Fig.3 shows the MALDI-TOF mass spectrum of the polymeric mixture, recorded as Cs+ adducts in the positive ion reflectron mode and showing a series of repeating procyanidin polymers. The polymeric character is reflected by the periodic peak series representing different chain lengths. The results indicate that condensed tannins from stem bark are characterized by mass spectra with a series of peaks with distances of 288 Da corresponding to a mass difference of one catechin/epicatechin between each polymer. Therefore, prolongation of condensed tannins is due to the addition of catechin/epicatechin monomers. The spectrum showed a series polyflavan-3-ols extending from the dimer (m/z 711) to the octamer (m/z 2439) that did not contain ions with 2 Da lower than that predicted in positive ion reflectron mode (Table 2).
Fig. 3.
MALDI-TOF positive ion reflectron mode mass spectrum of the tannins from C. album stem bark and the enlarged spectrum of the polyflavan-3-ol hexamer
Table 2.
Observed and calculated massesa of heteropolyflavan-3-ols by MALDI-TOF MS
| Polymer | n1 | n2 | n3 | C1 | C2 |
| Dimer | 2 | 0 | 0 | 711 | 711.04 |
| 2 | 0 | 1 | 863 | 862.91 | |
| 1 | 1 | 0 | 727 | 727.09 | |
| 1 | 1 | 1 | 879 | 878.88 | |
| 0 | 2 | 0 | 743 | 742.88 | |
| 0 |
2 |
1 |
895 |
894.93 |
|
| Trimer | 3 | 0 | 0 | 999 | 998.92 |
| 3 | 0 | 1 | 1151 | 1150.86 | |
| 2 | 1 | 0 | 1015 | 1014.85 | |
| 2 | 1 | 1 | 1167 | 1166.82 | |
| 1 | 2 | 0 | 1031 | 1030.84 | |
| 1 | 2 | 1 | 1183 | 1182.80 | |
| 0 | 3 | 0 | 1047 | 1046.75 | |
| 0 |
3 |
1 |
1199 |
1198.69 |
|
| Tetramer | 4 | 0 | 0 | 1287 | 1286.84 |
| 4 | 0 | 1 | 1439 | 1438.80 | |
| 3 | 1 | 0 | 1303 | 1302.79 | |
| 3 | 1 | 1 | 1455 | 1454.79 | |
| 2 | 2 | 0 | 1319 | 1318.80 | |
| 2 | 2 | 1 | 1471 | 1470.77 | |
| 1 | 3 | 0 | 1335 | 1334.78 | |
| 1 |
3 |
1 |
1487 |
1486.77 |
|
| Pentamer | 5 | 0 | 0 | 1575 | 1574.76 |
| 5 | 0 | 1 | 1727 | 1726.80 | |
| 4 | 1 | 0 | 1591 | 1590.79 | |
| 4 | 1 | 1 | 1743 | 1742.78 | |
| 3 | 2 | 0 | 1607 | 1606.78 | |
| 3 | 2 | 1 | 1759 | 1758.76 | |
| 2 | 3 | 0 | 1623 | 1622.73 | |
| 2 |
3 |
1 |
1775 |
1775.64 |
|
| Hexamer | 6 | 0 | 0 | 1863 | 1862.77 |
| 6 | 0 | 1 | 2015 | 2014.76 | |
| 5 | 1 | 0 | 1879 | 1878.85 | |
| 5 | 1 | 1 | 2031 | 2030.82 | |
| 4 | 2 | 0 | 1895 | 1894.80 | |
| 4 | 2 | 1 | 2047 | 2046.78 | |
| 3 | 3 | 0 | 1911 | 1910.80 | |
| 3 | 3 | 1 | 2063 | 2063.80 | |
| 2 | 4 | 0 | 1927 | 1926.81 | |
| 2 |
4 |
1 |
2079 |
2078.80 |
|
| Heptamer | 7 | 0 | 0 | 2151 | 2150.84 |
| 7 | 0 | 1 | 2303 | 2302.81 | |
| 6 | 1 | 0 | 2167 | 2167.78 | |
| 6 | 1 | 1 | 2319 | 2319.82 | |
| 5 | 2 | 0 | 2183 | 2182.83 | |
| 5 | 2 | 1 | 2335 | 2335.69 | |
| 4 | 3 | 0 | 2199 | 2198.84 | |
| 4 |
3 |
1 |
2351 |
2350.76 |
|
| Octamer | 8 | 0 | 0 | 2439 | 2438.67 |
| 8 | 0 | 1 | 2591 | 2591.70 | |
| 7 | 1 | 0 | 2455 | 2455.74 | |
| 7 | 1 | 1 | 2607 | 2606.81 | |
| 6 | 2 | 0 | 2471 | 2471.74 | |
| 6 | 2 | 1 | 2623 | 2623.77 | |
| 5 | 3 | 0 | 2487 | 2487.81 | |
| 5 | 3 | 1 | 2639 | 2639.78 |
n 1: Number of catechin unit; n 2: Number of gallocatechin unit; n 3: Number of galloylated esters; C 1: Calculated [M+Cs]+ mass; C 2: Observed [M+Cs]+ mass.
| M=290+288a+304b+152c+133, |
In addition to the predicted homopolyflavan-3-ol mass series mentioned above, each DP had a subset of masses 16, 32 and 64 Da higher (Fig.3 and Table 2). These masses can be explained by heteropolymers of repeating flavan-3-ol units containing an additional hydroxyl group (∆16 Da) at the position 5 of the B-ring. Given the absolute masses corresponding to each peak, it was further suggested that they contain procyanidin and prodelphinidin, as have already been indicated in the 13C NMR spectrum.
On the basis of the structures described by Krueger et al.(2003), an equation was formulated to predict heteropolyflavan-3-ols of a higher DP (Table 2). The equation is M=290+288a+304b+152c+133, where M is the calculated mass; 290, 288, 304 and 152 are the molecular weights of the terminal epicatechin unit, the epicatechin extending unit, the epigallocatechin extending unit and the galloyl ester, respectively; 133 is the atomic weight of cesium (Cs); a and b are the DPs contributed by the epicatechin extending unit and the epigallocatechin extending unit, respectively; c is the number of galloyl esters. Application of this equation to the experimentally obtained data revealed the presence of a series of condensed tannins consisting of well-resolved oligomers. The broad peaks in these spectra indicate that there is a large structural heterogeneity within DP.
Each peak of the condensed tannins was always followed by mass signals at a distance of 152 Da corresponding to the addition of one galloyl group at the heterocyclic C-ring. Thus, peak signals corresponding to monogalloylated derivatives of various procyanidin oligomers were easily attributed. No procyanidins with more than one galloyl group were detected. Therefore, MALDI-TOF MS indicated the simultaneous occurrence of a mixture of procyanidin polymers and monogalloylated derivatives of procyanidin polymers, showing that there was a mixture of galloylated procyanidin and procyanidin occurring in procyanidin oligomers of stem bark.
The series of compounds with 2 Da multiples lower than those described in the predictive equation for heteropolyflavan-3-ols were not detected, so A-type interflavan ether linkage does not occur between adjacent flavan-3-ol subunits.
Hydrolysis of the tannins
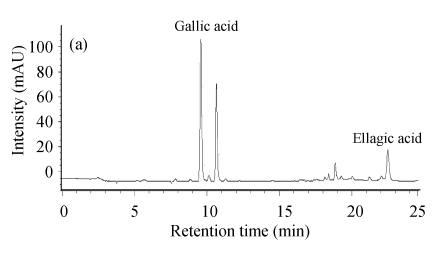
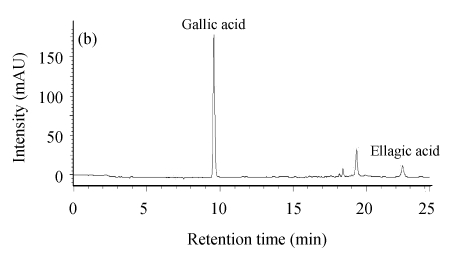
Gallic acid, as a constituent of gallotannins, was observed at high concentration in leaves ((56.02±0.51) mg/g) and twigs ((85.68±0.48) mg/g) (Fig.4).
Fig. 4.
HPLC chromatogram (280 nm) of the tannins from C. album leaves (a) and twigs (b) after hydrolysis
Ellagic acid (ellagotannins constituent) was also found in leaves ((16.48±0.79) mg/g) and twigs ((9.13±0.30) mg/g), but lower than gallic acid. It was indicated that C. album had antimicrobial activity on some common bacteria, mildews and microzymes, and gallic acids might be an important composition for this function (Yuan et al., 2001).
Radical-scavenging activities on DPPH
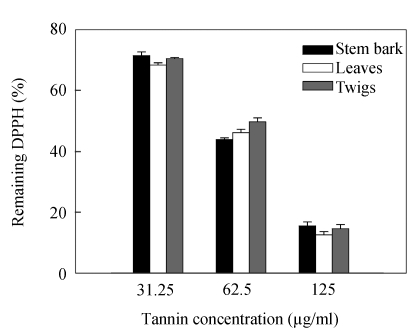
Relatively stable organic radical DPPH has been widely used in the determination of antioxidant activity of single compounds as well as the different plant extracts (Brand-Williams et al., 1995). The free radical-scavenging activities of tannins from leaves, twigs and stem bark along with the reference standards of ascorbic acid and BHA were determined by the DPPH assay. Because activities are expressed as the tannins concentration required to achieve a 50% decrease in absorbance at 517 nm (IC 50), the smaller tannins concentration indicates the higher DPPH radical-scavenging activity. Tannins from leaves, twigs and stem bark all showed the significantly higher inhibition percent of DPPH radical compared to reference ascorbic acid. Great decreases in a concentration-dependent manner of remaining DPPH indicated that C. album possesses potent free radical-scavenging activity (Table 3 and Fig.5).
Table 3.
Antioxidant activities of the tannins from C. album using the DPPH free radical-scavenging assay and ferric reducing antioxidant assay
| Sample | IC50 (µg/ml)* | FRAP** |
| Twigs | 62.31±2.05b | 3.74±0.07c |
| Stem bark | 54.80±0.50c | 4.49±0.11ab |
| Leaves | 56.86±1.56c | 4.28±0.02b |
| BHA | 57.46±0.97c | 4.66±0.10a |
| AA | 78.25±1.41a | − |
The antioxidant activity was evaluated as the concentration of the test sample required to decrease the absorbance at 517 nm by 50% in comparison to the control;
FRAP values are expressed in mmol ascorbic acid equivalent (AAE)/g sample in dry weight. BHA: Butylated hydroxyanisole; AA: Ascorbic acid. Data are presented as mean±SD; Means with different letters in the same column are significantly different at P<0.05 level
Fig. 5.
Remaining DPPH after addition of the tannins from C. album for 30 min
Ferric reducing antioxidant power
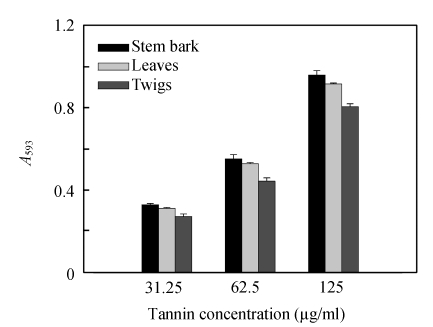
The reduction capacity of a compound may serve as a significant indicator of its potential antioxidant activity (Meir et al., 1995). Antioxidant potential of tannins from C. album was estimated from their ability to reduce TPTZ-Fe(III) complex to TPTZ-Fe(II) complex (Table 3 and Fig.6).
Fig. 6.
Ferric reducing power after addition of the tannins from C. album
A 593: Absorbance at 593 nm
In accordance with findings from the DPPH assay, the FRAP values ranged from 3.74 to 4.49 mmol AAE/g dried tannins. A higher absorbance corresponds to a higher ferric reducing power. All tannins showed increased ferric reducing power with the increasing concentration. At 125 µg/ml, the reducing power of tannins from stem bark (A 593 (absorbance at 593 nm)=0.958) was superior to those of leaves (A 593=0.915) and twigs (A 593=0.804).
In brief, the reducing power of the tannins followed the order: stem bark (4.49 mmol AAE/g dried tannins)≈leaves (4.28 mmol AAE/g dried tannins)>twigs (3.74 mmol AAE/g dried tannins). But the reducing power of the tannins was less than that of BHA (4.66 mmol AAE/g dried sample). High FRAP and DPPH values of tannins from leaves, twigs and stem bark corresponded to structural heterogeneity of tannins. The polymeric procyanidins from stem bark showed significant radical-scavenging and antioxidant activities.
CONCLUSION
C. album leaves had a relatively high level of total phenolics and extractable condensed tannins. Structure of condensed tannins from stem bark characterized by NMR and MALDI-TOF MS analyses showed that the condensed tannins consisted of predominantly procyanidins and prodelphinidins with 2,3-cis stereochemistry. The mean DP and the average molecular weight were 5.3 and 1578.25 Da, respectively. Tannins extracted from leaves, twigs and stem bark all showed very good DPPH radical scavenging activity (IC 50 of 56.86, 62.31 and 54.80 µg/ml) and ferric reducing power (4.28, 3.74 and 4.49 mmol AAE/g dried tannins).
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30671646), the Program for New Century Excellent Talents in University, China (No. NCET-07-0725), and the Program for Innovative Research Team in Science and Technology in Fujian Province University, China
References
- 1.Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148(2-3):187–197. doi: 10.1016/S0300-483X(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 2.Behrens A, Maie N, Knicker H, Kögel-Knabner I. MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry. 2003;62(7):1159–1170. doi: 10.1016/S0031-9422(02)00660-X. [DOI] [PubMed] [Google Scholar]
- 3.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 4.Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis . Journal of Natural Products. 2001;64(7):892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 5.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 6.Ding BP. Pharmacology of Qingguo pills on relieving cough. China Traditional Patent Medicine. 1999;21:27–28. (in Chinese) [Google Scholar]
- 7.Es-Safi NE, Guyot S, Ducrot PH. NMR, ESI/MS, and MALDI-TOF/MS analysis of pear juice polymeric proanthocyanidins with potent free radical scavenging activity. Journal of Agricultural and Food Chemistry. 2006;54(19):6969–6977. doi: 10.1021/jf061090f. [DOI] [PubMed] [Google Scholar]
- 8.Graham HD. Stabilization of the Prussian blue color in the determination of polyphenols. Journal of Agricultural and Food Chemistry. 1992;40(5):801–805. doi: 10.1021/jf00017a018. [DOI] [Google Scholar]
- 9.Haslam E. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action. Cambridge: Cambridge University Press; 1998. p. 422. [Google Scholar]
- 10.Hernes PJ, Benner R, Cowie GL, Goni MA, Bergamaschi BA, Hedges JI. Tannin diagenesis in mangrove leaves from a tropical estuary: a novel molecular approach. Geochimica et Cosmochimica Acta. 2001;65(18):3109–3122. doi: 10.1016/S0016-7037(01)00641-X. [DOI] [Google Scholar]
- 11.Ishida Y, Kitagawa K, Goto K, Ohtani H. Solid sampling technique for direct detection of condensed tannins in bark by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2005;19(5):706–710. doi: 10.1002/rcm.1845. [DOI] [PubMed] [Google Scholar]
- 12.Krueger CG, Vestling MM, Reed JD. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of heteropolyflavan-3-ols and glucosylated heteropolyflavans in sorghum [Sorghum bicolor (L.) Moench] Journal of Agricultural and Food Chemistry. 2003;51(3):538–543. doi: 10.1021/jf020746b. [DOI] [PubMed] [Google Scholar]
- 13.Lin YM, Liu JW, Xiang P, Lin P, Ye GF, Sternberg L da SL. Tannin dynamics of propagules and leaves of Kandelia candel and Bruguiera gymnorrhiza in the Jiulong River Estuary, Fujian, China. Biogeochemistry. 2006;78(3):343–359. doi: 10.1007/s10533-005-4427-5. [DOI] [Google Scholar]
- 14.Meir S, Kanner J, Akiri B, Hadas SP. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. Journal of Agricultural and Food Chemistry. 1995;43(7):1813–1815. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- 15.Ohnishi-Kameyama M, Yanagida A, Kanda T, Nagata T. Identification of catechin oligomers from apple (Malus pumila cv. Fuji) in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and fast-atom bombardment mass spectrometry. Rapid Communications in Mass Spectrometry. 1997;11(1):31–36. doi: 10.1002/(SICI)1097-0231(19970115)11:1<31::AID-RCM784>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Oszmianski J, Wojdylo A, Lamer-Zarawska E, Swiader K. Antioxidant tannins from Rosaceae plant roots. Food Chemistry. 2007;100(2):579–583. doi: 10.1016/j.foodchem.2005.09.086. [DOI] [Google Scholar]
- 17.Ricarda Da Silva JM, Darmon N, Fernandez Y. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. Journal of Agricultural and Food Chemistry. 1991;39(9):1549–1552. doi: 10.1021/jf00009a002. [DOI] [Google Scholar]
- 18.Santos-Buelga C, Scalbert A. Proantocyanidins and tannin-like compounds: nature, occurrence dietary intake and effects on nutrition and health. Journal of the Science of Food and Agriculture. 2000;80(7):1094–1117. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1094::AID-JSFA569>3.0.CO;2-1. [DOI] [Google Scholar]
- 19.Spencer P, Sivakumaran A, Fraser K, Foo LY, Lane GA, Edwards PJB, Meagher LP. Isolation and characterization of procyanidins from Rumex obtusifolius . Phytochemical Analysis. 2007;18(3):193–203. doi: 10.1002/pca.967. [DOI] [PubMed] [Google Scholar]
- 20.Terrill TH, Rowan AM, Douglas GB, Barry TN. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. Journal of the Science of Food and Agriculture. 1992;58(3):321–329. doi: 10.1002/jsfa.2740580306. [DOI] [Google Scholar]
- 21.Visioli F, Bellomo G, Galli C. Free radical-scavenging properties of olive oil polyphenols. Biochemical and Biophysical Research Communications. 1998;247(1):60–64. doi: 10.1006/bbrc.1998.8735. [DOI] [PubMed] [Google Scholar]
- 22.Wei H, Peng W, Mao YM. Chemical constituents of fruit from Canarium album . Journal of Chinese Material Medicine. 1999;24(7):421–423. (in Chinese) [PubMed] [Google Scholar]
- 23.Wu L. Effect of chlorogenic acid on antioxidant activity of Flos lonicerae extracts. Journal of Zhejiang University SCIENCE B. 2007;8(9):673–679. doi: 10.1631/jzus.2007.B0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang P, Lin YM, Lin P, Xiang C. Effects of adduct ions on matrix-assisted laser desorption/ionization time of flight mass spectrometry of condensed tannins: a prerequisite knowledge. Chinese Journal of Analytical Chemistry. 2006;34(7):1019–1022. doi: 10.1016/S1872-2040(06)60047-9. [DOI] [Google Scholar]
- 25.Xiang P, Lin YM, Lin P, Xiang C, Yang ZW, Lu ZM. Effect of cationization reagents on the matrix-assisted laser desorption/ionization time-of-flight mass spectrum of Chinese gallotannins. Journal of Applied Polymer Science. 2007;105(2):859–864. doi: 10.1002/app.26373. [DOI] [Google Scholar]
- 26.Yang Y, Chien M. Characterization of grape procyanidins using high-performance liquid chromatography/mass spectrometry and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Journal of Agricultural and Food Chemistry. 2000;48(9):3990–3996. doi: 10.1021/jf000316q. [DOI] [PubMed] [Google Scholar]
- 27.Yuan JG, Liu X, Tang ZQ. Preliminarily study on the antimicrobial activity of olive and its functional compositions. Chinese Journal of Food Science. 2001;22(3):82–84. (in Chinese) [Google Scholar]