Abstract
Notch signalling has an important role in skin homeostasis, promoting keratinocyte differentiation and suppressing tumorigenesis. Here we show that this pathway also has an essential anti-apoptotic function in the keratinocyte UVB response. Notch1 expression and activity are significantly induced, in a p53-dependent manner, by UVB exposure of primary keratinocytes as well as intact epidermis of both mouse and human origin. The apoptotic response to UVB is increased by deletion of the Notch1 gene or down-modulation of Notch signalling by pharmacological inhibition or genetic suppression of ‘canonical' Notch/CSL/MAML1-dependent transcription. Conversely, Notch activation protects keratinocytes against apoptosis through a mechanism that is not linked to Notch-induced cell cycle withdrawal or NF-κB activation. Rather, transcription of FoxO3a, a key pro-apoptotic gene, is under direct negative control of Notch/HERP transcription in keratinocytes, and upregulation of this gene accounts for the increased susceptibility to UVB of cells with suppressed Notch signalling. Thus, the canonical Notch/HERP pathway functions as a protective anti-apoptotic mechanism in keratinocytes through negative control of FoxO3a expression.
Keywords: apoptosis, FoxO3a, Notch, p53, UVB
Introduction
Notch cell surface receptors and their ligands belonging to the Delta and Serrate/Jagged families have a crucial role in cell-fate determination and differentiation, functioning in a cell- and context-specific manner (Bray, 2006). The best-characterized ‘canonical' pathway of Notch activation involves proteolytic cleavage and translocation of the cytoplasmic domain of the receptor to the nucleus, where it associates with the DNA binding protein CSL converting it from a repressor into an activator of transcription (Mumm and Kopan, 2000; Lai, 2002). However, direct binding of Notch to a second ancillary protein, Mastermind-like 1–3, is required for elevated levels of CSL-dependent transcriptional activation through recruitment of further transcriptional co-activators such as p300 (Petcherski and Kimble, 2000; Wu et al, 2000; Oswald et al, 2001). Transcriptional repressors of the HES (Hairy Enhancer of Split)/HERP family are well-characterized direct targets of Notch/CSL activation (Iso et al, 2003).
In mammalian cells, Notch activation is generally thought to maintain stem cell potential and inhibit differentiation, thereby promoting carcinogenesis (Ellisen et al, 1991; Weijzen et al, 2002; Pece et al, 2004; Weng et al, 2004; Balint et al, 2005; Hopfer et al, 2005; Ayyanan et al, 2006). However, in specific cell types such as keratinocytes, increased Notch activity causes exit from the cell cycle and commitment to differentiation, whereas down-modulation or loss of Notch1 function promotes carcinogenesis (Rangarajan et al, 2001; Nicolas et al, 2003; Devgan et al, 2005; Lefort et al, 2007). In mouse keratinocytes, the gene for the cyclin/CDK inhibitor p21WAF1/Cip1 is also induced by the Notch/CSL complex through both a direct and indirect mechanism, with p21WAF1/Cip1 functioning downstream of Notch (Rangarajan et al, 2001; Devgan et al, 2005; Mammucari et al, 2005). In human keratinocytes, Notch activation has more long-term effects, restricting keratinocyte stem cell potential through down-modulation of p63 and Rho/Cdc42 effectors (Nguyen et al, 2006; Lefort et al, 2007). Notch activation also impinges on other pathways important for keratinocyte growth, differentiation and tumour development such as NF-κB (Nguyen et al, 2006; Shin et al, 2006), AP-1 (Talora et al, 2002) and Wnt signalling (Devgan et al, 2005).
Besides its role in growth and differentiation, Notch signalling has also been shown to have a pro- or anti-apoptotic function depending on the context and/or cell type (Weng et al, 2003; Nefedova et al, 2004; Sade et al, 2004; Yang et al, 2004; Zweidler-McKay et al, 2005; Mungamuri et al, 2006; Lewis et al, 2007). In keratinocytes, recent evidence has established Notch1 as a key p53 target gene in tumour suppression, which is induced upon UVB exposure (Lefort et al, 2007; Yugawa et al, 2007). However, the pro- or anti-apoptotic function of the Notch pathway in the keratinocyte UVB response and the underlying mechanisms have not been investigated. This is an important question, considering the impact of UV light on normal skin homeostasis and carcinogenesis.
FoxO3a is a prominent member of the FoxO family, which, similar to other transcription factors with a Fox domain, binds DNA and activates transcription as a monomer (Kaestner et al, 2000). FoxO3a-dependent transcription has been variously linked with control of the cell cycle, apoptosis and differentiation (Accili and Arden, 2004). Notably, the FoxO3a gene is often lost in tumours, consistent with its possible tumour-suppressing function (Galili et al, 1993). FoxO3a activity has been shown to be regulated at the post-transcriptional level, with phosphorylation by the Akt and related SGK kinases keeping this factor in an inactive state in the cytoplasm, whereas its phosphorylation by JNK brings it to the nucleus and activates transcription (Brunet et al, 2001; Quevedo et al, 2007). Additional regulation of FoxO3a function is provided by the tight control of its acetylation state by members of the Sir2 family of protein deacetylases including SIRT1 (Brunet et al, 2004). By de-acetylating FoxO3a, SIRT1 appears to prevent its ability to induce apoptosis (Motta et al, 2004). Surprisingly, little or no information exists on transcriptional control of the FoxO3a gene. We show here that this gene is a negative transcriptional target of Notch/HERP in keratinocytes, with the canonical Notch pathway exerting a protective function in the UVB response of these cells through down-modulation of FoxO3a expression.
Results
UVB exposure induces Notch1 expression and activity in keratinocytes and skin of both mouse and human origin
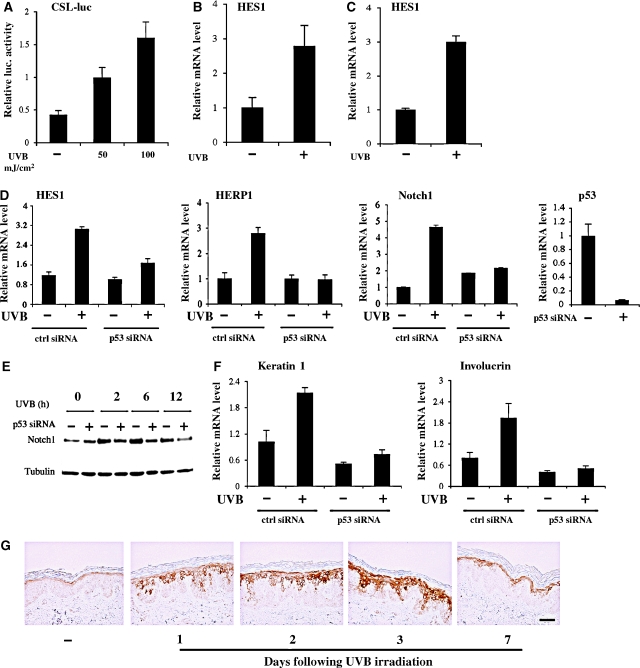
The role of Notch signalling in the UVB response of keratinocytes remains to be established. UVB irradiation of mouse primary keratinocytes caused a dose-dependent activation of Notch signaling, as assessed by the activity of a synthetic Notch-responsive promoter (Figure 1A). Concomitantly, quantitative RT–PCR analysis showed that expression of the endogenous HES1 gene, a ‘canonical' Notch target, is induced by UVB treatment of these cells in vitro (Figure 1B) as well as in vivo, in the intact epidermis (Figure 1C). A similar induction of HES1 and of the related HERP1, as well as of the Notch1 gene itself, was also triggered by UVB treatment of human primary keratinocytes (Figure 1D). Such induction was blocked by siRNA-mediated knock-down of p53 expression, consistent with the recent finding that Notch1 is a direct p53 target gene in keratinocytes (Lefort et al, 2007; Yugawa et al, 2007) (Figure 1D and E). In parallel with increased Notch1 expression and activity, UVB exposure of proliferating keratinocytes also induced differentiation marker expression (keratin 1, involucrin), and induction of these markers, for example induction of keratin 1, was p53-dependent (Figure 1F).
Figure 1.
Increased Notch1 signalling and expression in the keratinocyte UVB response. (A) Primary mouse keratinocytes were transfected with a synthetic Notch-responsive promoter consisting of six CSL binding sites in front of the luciferase gene (CSL-luc), followed, 24 h later, by UVB irradiation (50 or 100 mJ/cm2). Promoter activity was measured 24 h after irradiation by luciferase assays, using a Renilla reporter with a minimal promoter for internal value normalization. (B) Primary mouse keratinocytes were irradiated with UVB (50 mJ/cm2), followed, 2 h later, by measurement of HES1 mRNA levels by real-time RT–PCR. Values are expressed as relative units after internal normalization for GAPDH mRNA levels. (C) Back skin of 3 days old mice was irradiated with UVB (220 mJ/cm2). Eight hours later, the epidermis was separated from the underlying dermis by a brief heat treatment (Nguyen et al, 2006) followed by total RNA preparation and analysis of HES1 mRNA expression by real-time RT–PCR, using GAPDH mRNA for normalization. (D) Primary human keratinocytes transfected with siRNAs for p53 or scrambled siRNA controls for 48 h were irradiated with UVB (50 mJ/cm2) followed, 2 h later, by measurement of HES1, HERP1 and Notch1 expression levels by real-time RT–PCR, with 36B4 mRNA for normalization. Suppression of endogenous p53 was verified by real-time RT–PCR. (E) Primary human keratinocytes were treated as before and Notch1 protein levels were analysed at various times (hours) after irradiation by immunoblotting with the corresponding antibody, using blotting for γ-tubulin as an equal loading control. (F) RNA samples from the same cells as in panel D were analysed for levels of keratin 1 and involucrin differentiation marker expression by real-time RT–PCR with the corresponding specific primers. (G) Mid-back skin of healthy male volunteers, with their informed consent, was irradiated with an FL20S-E Lamp (290–320 nm) (Toshiba, Tokyo). Biopsies taken at the indicated times (days) were processed for immunostaining with antibodies specific for cleaved from of Notch1 (Val1744). Bar, 30 μm.
Consistent with the above findings, immunohistochemistry with an antibody recognizing the cleaved form of Notch1 revealed a significant increase of Notch1 activation also in the intact skin of volunteers at various times of UVB exposure in vivo (Figure 1G).
Notch signalling has a pro-survival function in the UVB and DNA damage response of keratinocytes
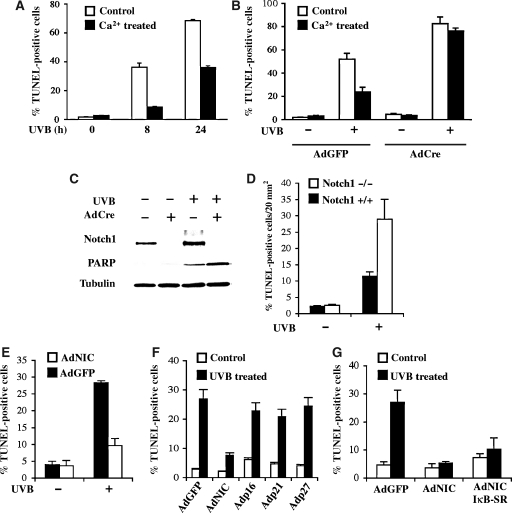
Previous work suggested that differentiating keratinocytes are more resistant to UVB-induced apoptosis than cells of the proliferative compartment (Chaturvedi et al, 2004). To verify this conclusion, mouse keratinocytes under growing conditions and at 24 h after calcium-induced differentiation were subjected to UVB light exposure. TUNEL assays showed a substantially lower apoptotic response of differentiating keratinocytes at both 8 and 24 h of UVB treatment (Figure 2A). To assess whether the higher UVB resistance of differentiating cells is Notch-dependent, primary keratinocytes from mice with the Notch1 gene flanked by loxP sites were infected with a Cre-expressing adenovirus (AdCre) for deletion of this gene. Relative to parallel cultures infected with a control GFP-expressing adenovirus (AdGFP), keratinocytes with deletion of the Notch1 gene exhibited a significantly enhanced apoptotic response to UVB under both growing and differentiating conditions (Figure 2B). Besides TUNEL assays, the increased susceptibility of keratinocytes with Notch1 deletion to UVB-induced apoptosis was confirmed by immunoblot analysis with antibodies against the caspase 3 cleaved form of PARP (Figure 2C). Efficient loss of Notch1 expression upon infection with AdCre was confirmed by immunoblot analysis with an anti-Notch1 antibody (Figure 2C). In vivo, mice with a conditional keratinocyte-specific deletion of the Notch1 gene were exposed to UVB in parallel with corresponding controls. Even in this case, deletion of the Notch1 gene resulted in enhanced sensitivity to UVB-induced apoptosis (Figure 2D).
Figure 2.
Protective function of Notch signalling in the UVB response of mouse keratinocytes and skin. (A) Primary mouse keratinocytes under growing conditions and at 24 h of calcium-induced differentiation (Missero et al, 1996) were treated with UVB (50 mJ/cm2) followed, 8 and 24 h later, by determination of the apoptotic response by TUNEL assays. (B) Primary keratinocytes from mice homozygous for the Notch1 gene flanked by loxP sites (Notch1loxP/loxP) were infected with an AdCre, to induce deletion of the Notch1 gene, or AdGFP control. At 72 h after infection, part of the cells was induced to differentiate by calcium treatment. After 24 h, keratinocytes under either growing or differentiating conditions were treated with UVB (50 mJ/cm2). The apoptotic response was determined 12 h later by TUNEL assays. (C) Primary mouse keratinocytes plus/minus deletion of the Notch1 gene as in the previous panel were analysed, under growing conditions, by immunoblotting with antibodies specific for the caspase 3 cleaved form of PARP in parallel with antibodies against Notch1. (D) Mice with Cre-induced keratinocyte-specific deletion of the Notch1 gene (Notch1loxP/loxP × K5-Cre-PR1) in parallel with their Cre-negative littermates (Notch1loxP/loxP) (Rangarajan et al, 2001) were irradiated with UVB (140 mJ/cm2) on their back skin, at 8 weeks of age. Apoptosis was measured 12 h later by TUNEL assays. (E) Primary mouse keratinocytes were infected with a recombinant adenovirus expressing a constitutive active form of Notch1 (AdNIC) or AdGFP control. After16 h, cells were UVB irradiated (50 mJ/cm2) and the apoptotic response was evaluated 8 h later by TUNEL assays. (F) Primary mouse keratinocytes were infected with AdNIC and AdGFP in parallel with recombinant adenoviruses expressing the CDK inhibitors p16INK4a, p21WAF1/Cip1 and p27Kip1. After 16 h, cells were UVB irradiated (50 mJ/cm2) and the apoptotic response was evaluated 8 h later by TUNEL assays. (G) Primary mouse keratinocytes were infected with AdGFP and with the AdNIC virus individually and in combination with an adenovirus expressing a stabilized super-repressor mutant form of IκBα (IκB-SR) (Wang et al, 1999). Cells were subsequently irradiated and analysed by TUNEL assays as in the previous panels.
To assess whether increased Notch signalling exerts a converse anti-apoptotic function, mouse primary keratinocytes were infected with adenoviruses expressing a constitutive active form of Notch1 (AdNIC) in parallel with AdGFP. As shown in Figure 2E, expression of constitutive active Notch1 rendered keratinocytes more resistant to UVB-induced apoptosis, mirroring the increased sensitivity of cells with Notch1 deletion. These protective effects were not due to cell cycle arrest caused by activated Notch1 expression, as they were not observed after similar adenovirus-mediated expression of CDK inhibitors such as p21WAF1/Cip1, p27Kip1 and p16Ink4a (Figure 2F). As NF-κB activity is induced by Notch activation (Nguyen et al, 2006), an attractive possibility was that the pro-survival effect of Notch activation in keratinocytes depends on NF-κB. However, this does not appear to be the case, as the anti-apoptotic effects of activated Notch1 were counteracted to a very limited extent by NF-κB inhibition as achieved by the concomitant expression of an IκB super-repressor (Wang et al, 1999) (Figure 2G).
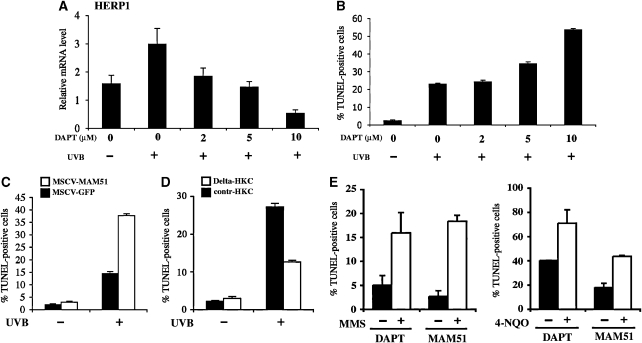
To evaluate whether the Notch pathway has a similar function in human keratinocytes, we relied on two complementary approaches. Treatment with γ-secretase inhibitors, for example DAPT, blocks activation of endogenous Notch receptors (Geling et al, 2002; Morohashi et al, 2006), whereas expression of a 51-amino-acid peptide corresponding to the amino terminus of the MAML1 protein (MAM51) provides an effective method to suppress canonical Notch/CSL/MAML-dependent transcription (Weng et al, 2003). For the first approach, we established a dose–response curve of primary human keratinocytes treated with increasing concentrations of the DAPT plus/minus UVB exposure. Expression levels of the HERP1 gene were used to assess the inhibitory effects on endogenous Notch signalling (Figure 3A). Parallel TUNEL assays showed a good correlation between the extent of inhibition of HERP1 expression by increasing DAPT concentrations and the level of UVB-induced apoptosis (Figure 3B). For the second approach, keratinocytes were infected with a retrovirus expressing the MAM51 dominant-negative peptide fused to GFP, in parallel with a control retrovirus expressing GFP alone. As shown in Figure 3C, cells expressing the MAM51 peptide exhibited a significantly higher apoptotic response to UVB exposure than the controls. Conversely, activation of endogenous Notch signalling by co-culture of human primary keratinocytes with fibroblasts expressing the Notch ligand Delta decreased significantly UVB-induced apoptosis (Figure 3D).
Figure 3.
Protective function of Notch signalling in human keratinocytes against UVB exposure or pharmacologically induced DNA damage. (A) Primary human keratinocytes were treated with DMSO vehicle or increasing concentrations of DAPT for 16 h, followed by UVB irradiation (50 mJ/cm2). After 8 h, cells were analysed for HERP1 mRNA expression levels by real-time RT–PCR analysis. (B) Cultures treated in parallel as in panel A were analysed for their apoptotic response by TUNEL assays. (C) Primary human keratinocytes infected with a retrovirus expressing the MAM51 peptide (MSCV-MAM51) or GFP control (MSCV-GFP) were irradiated with UVB (50 mJ/cm2) and the apoptotic response was measured by TUNEL assays 8 h later. (D) Human primary keratinocytes with stable GFP expression (by retroviral infection) were co-cultured with control mouse NIH3T3 fibroblasts (contr-HKC) or fibroblasts stably expressing full-length Delta 1 (Delta-HKC) for 48 h, followed by UVB irradiation (50 mJ/cm2). Eight hours later, the apoptotic response of the GFP-labelled keratinocytes was measured by TUNEL assays. Values are expressed as a percentage of GFP-labelled cells that were TUNEL positive. (E) Primary human keratinocytes treated with DAPT (+) or DMSO (−) or infected with retroviruses expressing the MAM51 peptide (+) or GFP control (−) were treated with MMS (100 μg/ml) for 4 h or 4-NQO (2 mg/ml) for 1 h. The apoptotic response was measured by TUNEL assays 12 h later.
DNA damage is a main direct consequence of UVB exposure, which triggers apoptosis and which has been recently reported to induce Notch1 expression (Yugawa et al, 2007). As after UVB treatment, human keratinocytes with suppressed Notch signalling, by either DAPT treatment or MAM51 expression, exhibited a similarly increased apoptotic reaction in response to DNA-damaging agents such as MMS (methyl methanesulphonate) or 4-NQO (4-quinoline-1-oxide) (Figure 3E).
Canonical notch signalling protects keratinocytes against UVB-induced apoptosis through negative regulation of FoxO3a expression
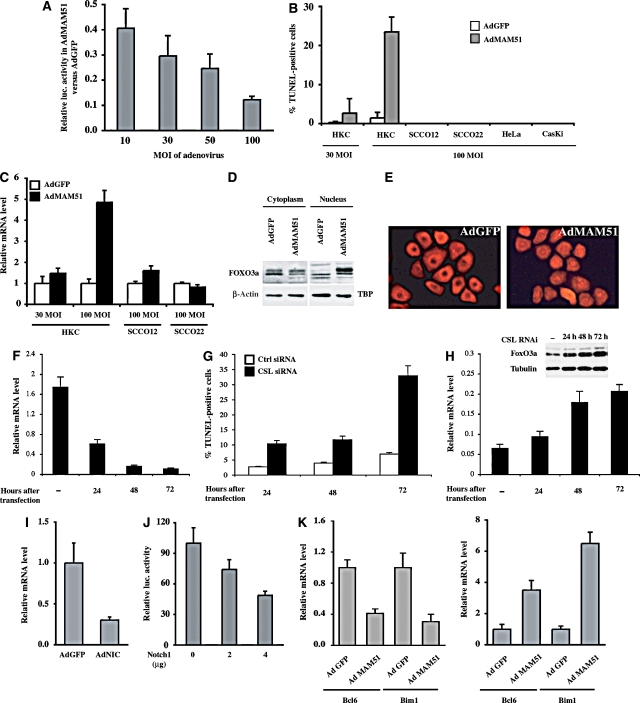
Our finding that the expression of the MAM51 dominant-negative peptide increases the sensitivity of keratinocytes to UVB-induced apoptosis pointed to canonical Notch/CSL/MAML-dependent transcription as the likely underlying mechanism. For further insights, we set up an adenoviral delivery system for expression of the MAM51 peptide in increasing amounts as a function of multiplicity of infection (MOI). Activity of Notch signalling, as assayed by a Notch/CSL promoter reporter assay, was strongly suppressed by infection of primary human keratinocytes with the MAM51-expressing adenovirus (AdMAM51) at high MOI, whereas a progressively lesser suppression was observed after infection with lower viral amounts (Figure 4A). Biologically, greater suppression of Notch signalling by MAM51 expression was already sufficient to induce apoptosis in a large fraction of human primary keratinocytes without any UVB exposure, whereas lower Notch suppression caused a lesser apoptotic response (Figure 4B). The pro-apoptotic effects of elevated MAM51 expression do not reflect aspecific toxicity, as they were not observed in a number of keratinocyte-derived cancer cell lines, including SCCO12, SCCO22, HeLa and CasKi (Figure 4B). Global analysis of gene expression was used to identify molecular targets of Notch/MAML-dependent transcription that may be responsible for its protective anti-apoptotic function. We focused on genes with selectively increased expression in human primary keratinocytes by high versus low levels of MAM51 expression and with opposite modulation by activated Notch1. A restricted number of genes matched these criteria, including FoxO3a, a transcription factor with a key pro-apoptotic function (Essafi et al, 2005; You et al, 2006) (Supplementary Table 1).
Figure 4.
Concomitant induction of apoptosis and FoxO3a expression by dose-dependent suppression of canonical Notch signalling. (A) Primary human keratinocytes were transfected with the Notch-pGA reporter (pGA-Luc; 0.5 μg) together with the phRL-TK Renilla reporter and subsequently infected with an adenovirus expressing the MAM51 peptide or GFP control at the indicated MOI. Promoter activity was measured 24 h later. Results are expressed as a ratio of luciferase activity (after Renilla normalization) in cells infected with the AdMAM51 versus AdGFP viruses at the various MOIs. (B) Human primary keratinocytes, together with keratinocyte-derived cancer cell lines (SCCO12, SCCO22, HeLa and CasKi), were infected with AdGFP and AdMAM51 at the indicated MOI. The fraction of apoptotic cells was assessed 24 h later by TUNEL assays. (C) Human primary keratinocytes and the SCCO12 and SCCO22 cell lines were infected with the AdMAM51 and AdGFP viruses at the indicated MOI and analysed 24 h later for levels of FoxO3a mRNA by real-time RT–PCR. Values are expressed in arbitrary units after normalization for β-actin expression. (D) Human primary keratinocytes were infected with AdMAM51 and AdGFP at an MOI of 100, followed by nuclear and cytoplasmic fractionation and immunoblot analysis for the FoxO3a protein, using the β-actin and TBP proteins as equal loading controls for the cytoplasmic and nuclear fractions, respectively. (E) Human primary keratinocytes were infected with AdMAM51 and AdGFP at an MOI of 100 as before. Cells were fixed 24 h later and processed for immunofluorescence analysis with an antibody against FoxO3a. (F) Human primary keratinocytes were transfected with siRNA specific for CSL in parallel with scrambled siRNA control, followed by assessment of CSL expression at 24, 48 and 72 h after transfection by real-time RT–PCR. (G) Parallel cultures treated as in panel F were analysed by TUNEL assays at various times (hours) after CSL knock-down. (H) Keratinocytes with and without CSL knock-down as in the previous experiments were analysed for levels of FoxO3a expression by real-time RT–PCR and immunoblotting (inset). (I) Primary human keratinocytes were infected with adenoviruses expressing activated Notch1 or GFP control for 24 h and analysed for levels of FoxO3a mRNA by real-time RT–PCR. (J) Primary human keratinocytes were transfected with a FoxO3a-responsive reporter (FHRE-luc) plus increasing amounts of an expression vector for activated Notch1. Promoter activity was determined at 30 h after transfection by luciferase assays, using the phRL-TK Renilla reporter for internal normalization. (K) Primary human keratinocytes infected with adenoviruses expressing activated Notch1 or MAM51 in parallel with GFP controls were analysed by real-time RT–PCR for levels of expression of Bcl6 and Bim1, two well-established FoxO3a targets with pro-apoptotic function.
Real-time RT–PCR analysis confirmed elevated induction of the FoxO3a gene in human primary keratinocytes with high versus low levels of MAM51 expression, with little or no induction in SCCO12 and SCCO22 cancer cell lines (Figure 4C). The induction of FoxO3a gene expression was accompanied by an increased nuclear localization of the protein (Figure 4D and E).
To assess whether other means to suppress Notch signalling, and more specifically Notch/CSL-dependent transcription, elicit the same effects as high doses of MAM51 expression, we transfected human primary keratinocytes with siRNAs specific for the CSL gene in parallel with scrambled siRNA controls. Real-time RT–PCR analysis showed that a progressive suppression of CSL gene expression over time (Figure 4F) was associated with an increasing fraction of cells spontaneously undergoing apoptosis (Figure 4G) and a parallel increase in FoxO3a mRNA and protein expression (Figure 4H).
Mirroring the above findings, expression of activated Notch1 caused down-modulation of FoxO3a gene expression (Figure 4I) in parallel with suppression of FoxO3a activity, as assessed by transient transfection promoter activity assays with a FoxO3a-responsive reporter (Figure 4J) and measurement of the endogenous FoxO3a target genes Bcl6 and Bim1 (Tang et al, 2002; Essafi et al, 2005) (Figure 4K).
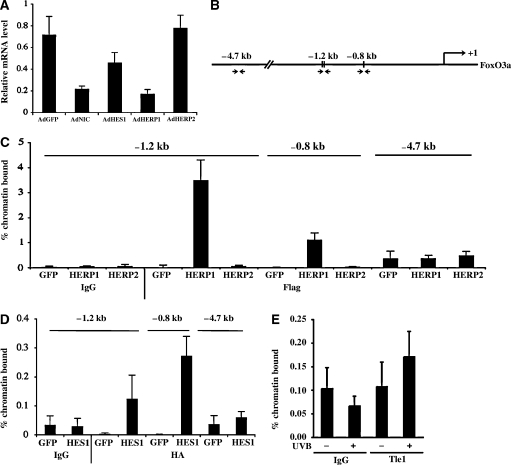
HES/HERP proteins are well-established mediators of the suppressive effects of Notch activation on gene expression. To assess whether increased expression of one or more of these proteins can reproduce the effects of activated Notch1, human keratinocytes were infected with recombinant adenoviruses expressing the HES1, HERP1 and HERP2 proteins. Strong down-modulation of FoxO3a gene expression was caused by increased HERP1 expression, a lesser down-modulation by HES1 and little or no effects by HERP2 (Figure 5A). Sequence analysis of the FoxO3a promoter region revealed the presence of two HES/HERP binding sites around position −1.2 kb (specifically −1.21 and −1.17 kb from the transcriptional start site) and one at position −0.8 kb (Figure 5B). Binding to these regions was assessed by chromatin immunoprecipitation (ChIP) assays with antibodies against the adenovirally expressed epitope-tagged proteins. Paralleling the differential effects on the endogenous gene, we found very effective binding of HERP1 to the −1.2 kb region of the FoxO3a promoter, with lesser binding to the −0.8 kb site and no binding to an upstream region devoid of HES/HERP sites (−4.7 kb) (Figure 5C). By contrast, there was no detectable binding with HERP2, and the HES1 protein bound to the same promoter regions but with lower efficiency (note the difference of scale in the HERP1 versus HES1 graphs) (Figure 5C and D). Antibodies suitable for immunoprecipitation of endogenous HES/HERP proteins are not available. However, HES/HERP proteins bind to a specific transcriptional co-repressor, Tle-1 (Stifani et al, 1992), which can be readily immunoprecipitated with the existing antibodies (Nuthall et al, 2004; Mammucari et al, 2005). ChIP assays with these antibodies showed that UVB exposure of human primary keratinocytes induced association of the Tle-1 protein to the same –1.2 kb region of the FoxO3a promoter bound by the HERP1/HES1 proteins (Figure 5E).
Figure 5.
Differential control of the FoxO3a gene by HES/HERP family members. (A) Primary human keratinocytes were infected with adenoviruses expressing activated Notch1, HES1, HERP1, HERP2 or GFP control for 24 h and analysed for levels of FoxO3a mRNA by real-time RT–PCR. (B) The human FoxO3a promoter region was analysed by the MatInspector (Genomatix) software for analysis of transcription factor binding sites, and three CSL-binding sites at −1.21, −1.17 and −0.8 kb from the transcription initiation site were identified. Arrows refer to the position of the PCR primers utilized for the ChIP analysis of the following panels. (C, D) Primary human keratinocytes were infected with adenoviruses expressing HERP1 (Flag-tagged), HERP2 (Flag-tagged), HES1 (HA-tagged) or GFP. To minimize overexpression of the adenovirally encoded proteins, we used a low MOI for infection of ∼30% of cells. Cells were processed for ChIP with antibodies against the Flag (C) or HA (D) tags and purified nonspecific IgGs as control. Real-time RT–PCR reactions for the −1.2 and −0.8 kb regions of the FoxO3a promoter were performed along with PCR of an upstream region located at −4.7 kb. The primers used in the RT–PCR reactions are listed in Supplementary Table 2. (E) Primary human keratinocytes treated or not with UVB (50 mJ/cm2) were processed 6 h later for ChIP with an antibody against the HES/HERP-associated Tle1 protein (Stifani et al, 1992) or purified rabbit IgGs, followed by real-time PCR analysis of the −1.2 kb region of the FoxO3a promoter.
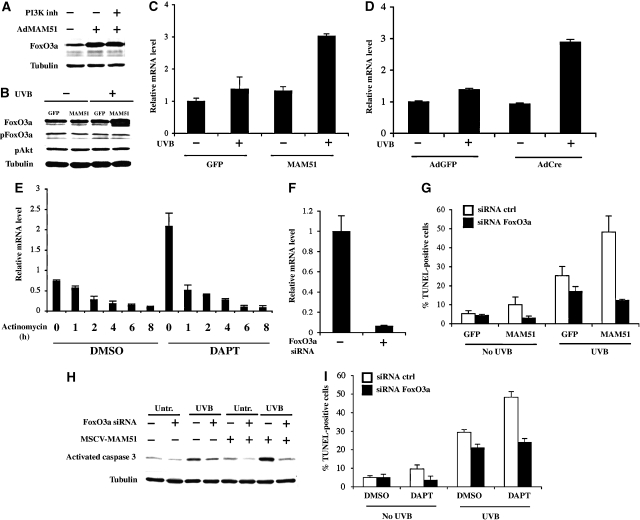
A major form of FoxO3a regulation is Akt-dependent phosphorylation (Brunet et al, 1999). The induction of FoxO3a expression by MAM51 overexpression was unaffected by inhibition of the PI3K/Akt pathway by wortmannin treatment (Figure 6A). Human keratinocytes with lower levels of MAM51 expression (as achieved by retroviral transduction) also exhibited induction of FoxO3a protein expression upon UVB exposure, with no changes in its Akt phosphorylation (Figure 6B). Upregulation of FoxO3a expression at the mRNA level was also observed in these cells (Figure 6C), as well as after UVB irradiation of mouse keratinocytes with Cre-mediated deletion of the Notch1 gene (Figure 6D). FoxO3a mRNA levels were also increased in human keratinocytes concomitantly treated with UVB and DAPT with actinomycin D treatment, showing that the induction of FoxO3a expression by UVB irradiation and Notch suppression is not due to increased message stability (Figure 6E).
Figure 6.
The Notch protective function in the keratinocyte UVB response through down-modulation of FoxO3a expression. (A) Primary human keratinocytes infected with AdMAM51 and GFP control at 100 MOI for 24 h were either untreated or treated with the PI3K inhibitor wortmannin (100 nM) for an additional 8 h, followed by analysis of FoxO3a protein levels by immunoblotting with a corresponding specific antibody. (B) Primary human keratinocytes were stably infected with a MAM51-expressing retrovirus in parallel with a GFP control virus and either untreated or treated with UVB (50 mJ/cm2). Cells were analysed 24 h later for levels of total FoxO3a protein as well as protein phosphorylated at the critical Akt recognition site (Thr32) (pFoxO3a) by immunoblotting with the corresponding specific antibodies. The same extracts were also probed with antibodies against the phosphorylated active form of Akt (pAkt), in parallel with γ-tubulin as an equal loading control. (C) Similarly, retrovirally infected cells plus/minus UVB treatment as in panel B were analysed for FoxO3a mRNA levels by real-time RT–PCR. (D) Primary keratinocytes from Notch1loxP/loxP mice were infected with AdCre, to induce deletion of the Notch1 gene, or AdGFP control, as in Figure 1. Cells were subsequently treated with UVB (50 mJ/cm2) followed, 30 h later, by measurement of FoxO3a mRNA levels by real-time RT–PCR. (E) Human primary keratinocytes were incubated for 16 h with DAPT (10 μM) or DMSO control, UVB-irradiated (50 mJ/cm2) and, 8 h later, either collected or treated with actinomycin D (8 μg/ml) for the indicated times (hours). FoxO3a mRNA levels were assessed by real-time RT–PCR. (F) Human primary keratinocytes were transfected with validated siRNAs for FoxO3a or scrambled controls, followed by measurement of FoxO3a mRNA levels 48 h later by real-time RT–PCR. (G) Human keratinocytes stably infected with the MAM51- and GFP-expressing retroviruses were transfected with FoxO3a siRNAs or scrambled siRNA controls followed, 48 h later, by UVB irradiation (50 mJ/cm2). The apoptotic response was determined 24 h after irradiation by TUNEL assays. (H) Similarly treated cells were analysed for levels of activated caspase 3, by immunoblotting with the corresponding antibodies, with γ-tubulin as an internal control. (I) Human primary keratinocytes were transfected with siRNAs for FoxO3a or scrambled controls and 24 h later treated with DAPT (10 μM) or DMSO control. Cells were UVB irradiated 24 h later and, after another 24 h, were analysed by TUNEL assays.
To assess the functional significance of FoxO3a upregulation in keratinocytes with concomitant UVB treatment and Notch suppression, we employed an siRNA-mediated knock-down approach. Control and MAM51-expressing keratinocytes were transfected with FoxO3a-specific siRNAs in parallel with scrambled siRNA control, followed by real-time RT–PCR at 48 h after transfection to determine the efficiency of gene knock-down (Figure 6F). When challenged with UVB, the increased apoptotic response of the MAM51-expressing keratinocytes, as assessed by both TUNEL assays and immunoblotting for activated caspase 3, was counteracted by the FoxO3a knock-down (Figure 6G and H). Similar results were observed with DAPT-treated cells plus/minus FoxO3a knock-down upon UVB treatment (Figure 6I).
Discussion
UV light is a major aetiological agent of skin ageing and cancer (de Gruijl, 1999; Armstrong and Kricker, 2001). Keratinocytes in the outermost layer of the epidermis are the most exposed to the damaging effects of high-energy UVB but their differentiated state renders them more resistant than cells of the proliferative compartment (Chaturvedi et al, 2004; this communication: Figure 2A). In parallel with its key role in promoting differentiation, we have shown here that Notch signalling has an equally important function in protecting keratinocytes against UVB exposure, both in vitro and in vivo. This protective function is mediated by the ‘canonical' Notch/HERP pathway through a novel mechanism involving transcriptional down-modulation of the FoxO3a gene.
p53 has a key role in the UV/DNA damage response of cells, controlling the decision between growth arrest and apoptosis by a number of alternative mechanisms (Latonen and Laiho, 2005; D'Errico et al, 2007). The recent finding that Notch1 is a p53 target gene in keratinocytes pointed to the possibility that this gene is also implicated in the UVB/DNA damage response of these cells (Lefort et al, 2007; Yugawa et al, 2007). In fact, we have found that the apoptotic response to UVB is increased in the skin of mice with deletion of the Notch1 gene as well as in keratinocytes with down-modulation of Notch signalling, whereas increased Notch signalling protects against this process. Consistent with a protective anti-apoptotic function of Notch in the skin, there is a recent report of spontaneously increased apoptosis in mice with double keratinocyte-specific deletion of the Notch1 and Notch2 genes (Lee et al, 2007).
As for its role in growth/differentiation control, Notch signalling can exert either a pro- or anti-apoptotic function in a manner that is highly cell- and context-dependent. For instance, in neuronal progenitor cells, Notch activation leads to apoptosis through increased nuclear accumulation of p53 and subsequent upregulation of its pro-apoptotic targets (Yang et al, 2004). Similarly, in B-cell leukaemia cells, activation of Notch signalling and induction of its downstream target HES1 lead to growth arrest and cell death (Zweidler-McKay et al, 2005). A variety of mechanisms have been implicated in the Notch pro-survival function. These include, in T cells, the interaction with the orphan receptor protein Nur77 (Jehn et al, 1999); in myeloma cells, the induction of p21WAF/Cip expression; and, in cervical cancer cell lines, the induction of PI3K/Akt/mTOR signalling through as yet undefined mechanisms involving the ‘non-canonical' Notch pathway (Nair et al, 2003; Sade et al, 2004). Other potential cell survival mechanisms in which Notch has been implicated include interference with JNK activation (Kim et al, 2005), upregulation of NF-κB activity (Oswald et al, 1998) and increased expression of proteins with direct anti-apoptotic function, such as Bcl-2 and Mcl-1 (MacKenzie et al, 2004; Oishi et al, 2004). We have shown here that in primary keratinocytes, but not in keratinocyte-derived cancer cell lines, ‘canonical' Notch signalling exerts a pro-survival function that is linked to transcriptional down-modulation of the FoxO3a gene. This occurs without detectable changes in PI3K/Akt-dependent phosphorylation, which is a major form of FoxO3a regulation (Brunet et al, 1999). Rather, the ‘canonical' Notch/HERP pathway is an important negative regulator of FoxO3a expression, through a mechanism involving binding of the HES/HERP/Tle transcription repressor complex to the FoxO3a promoter.
Like Notch, FoxO3a has multiple cellular functions, ranging from cell cycle control, differentiation, resistance to oxidative stress and apoptosis (Burgering and Kops, 2002). Target genes that mediate the pro-apoptotic function of this transcription factor include those for the Bcl-2 family members bNIP3, BCL2L11 and Bim1 (Greer and Brunet, 2005). FoxO3a can also induce apoptosis through more indirect mechanisms, such as induction of the transcriptional repressor Bcl6, which in turn down-modulates expression of the pro-survival Bcl-X(L) gene (Tang et al, 2002). In human keratinocytes, FoxO3a was recently found in a complex with Smad4 to control a subset of genes with cell cycle regulatory function (p21WAF1/Cip1 and p15INK4b), mediators of stress responses (Gadd45α and Gadd45β) and adaptive cell signalling (Jagged1, CDC42EP3 and OVOL1) (Gomis et al, 2006), some of which are also under Notch control in these cells (Nguyen et al, 2006; our unpublished observations). Thus, an intriguing possibility is that FoxO3a participates in the already established cross-talk between Notch and Smad signalling in this cell type (Blokzijl et al, 2003). Irrespective of the detailed mechanism, this Notch1–FoxO3a connection may provide an opportunity for novel drug design approaches to maximize protection against the UVB/DNA damage response of the skin. It will also be interesting to explore whether regulation of FoxO3a by Notch signalling occurs in other cellular and developmental contexts where these important regulators of cell physiology have been implicated.
Materials and methods
Cell culture, viruses and plasmids
Primary mouse keratinocytes were prepared and cultured in minimal essential medium with 4% Chelex-treated fetal calf serum (Hyclone), epidermal growth factor (10 ng/ml; BD Biosciences) and 0.05 mM CaCl2 (low-calcium medium) as described previously (Missero et al, 1996). Primary human keratinocytes from adult skin were isolated and cultured as described previously (Nguyen et al, 2006). NIH fibroblasts expressing full-length Delta or empty vector control (Trifonova et al, 2004) were co-cultured with human primary keratinocytes stably expressing GFP for 48 h as described (Lowell et al, 2000). SCCO12 and SCCO22 cells were provided by Dr J Rocco (Massachusetts General Hospital, Boston, MA), whereas HeLa and CasKi cell lines were from ATCC. The MSCV-MAM51 (Weng et al, 2003), Ad-HES1 (Sriuranpong et al, 2002), Ad-IκB-SR (Wang et al, 1999), Ad-HERP1 (Mammucari et al, 2005), AdNIC, AdCre (Rangarajan et al, 2001), and Adp16, Adp21, Adp27 and AdGFP (Devgan et al, 2005) have been described previously. The recombinant adenovirus expressing truncated form of MAML1 (MAM51) was obtained by removing the insert cDNA (coding for amino acids 13–74 of the MAML1 protein fused to GFP; Weng et al, 2003) from MSCV-MAM51 by ClaI/BglII digestion and inserting it into the HindIII/BglII sites of the pAdTrack-CMV vector, followed by recombination into the adenoviral backbone plasmid pAdEasy-1 in bacteria (He et al, 1998). Recombinant adenoviruses were used at an MOI of 100, unless otherwise specified.
For siRNA experiments, cells were transfected as described previously (Nguyen et al, 2006) with validated Stealth-siRNAs (200 nM) for human p53, CSL (Invitrogen) and FoxO3a (Invitrogen) in parallel with corresponding nonspecific Stealth siRNA negative control Medium GC (Invitrogen).
Methyl methanesulphonate 4-nitroquinoline-1-oxide and wortmannin were from Sigma.
UVB irradiation
Fresh mouse or human keratinocytes were used for in vitro UVB treatment using a custom-made UVB irradiation apparatus with four photochemical lamps (RPR 3000, Southern N.E., Ultraviolet Co., Bradford, CT) as described previously (Wang et al, 2005). The derived UVB dose was measured each time by using a photometer (model IL 1400A, International Light Inc., Newburyport, MA). In vivo treatment of mice (8–9 weeks old female mice or 3 days old newborn as indicated), using the same UVB lamp and photometer as above, was as described (Wang et al, 2005). In vivo treatment of human volunteers, with their informed consent, was performed with an FL20S-E Lamp (290–320 nm) (Toshiba, Tokyo).
Promoter activity assays
The Notch- and FoxO3a-responsive promoters used in these experiments have been described previously (Taniguchi et al, 1998). Transient transfections for promoter activity assays were performed as described previously (Rangarajan et al, 2001) using cotransfection with a TK-Renilla reporter (Promega) for internal normalization. Total quantities of DNA were kept constant by adding appropriate amounts of empty vectors.
Analysis of gene expression and ChIP assays
mRNA expression was quantified by real-time RT–PCR as described previously (Mammucari et al, 2005). Each sample was tested in triplicate and results were normalized by real-time PCR of the same cDNA with GAPDH (for mouse samples) and 36B4 or β-actin (for human samples) primers. cDNA microarray analysis and ChIP assays were carried out as described previously (Devgan et al, 2005; Lefort et al, 2007). The sequence of the specific primers used for these experiments is provided in Supplementary Table 2.
Antibodies
We used the following antibodies: Notch1 (Santa Cruz, C-20), activated Notch1 (Cell Signaling), FoxO3a (Upstate), phospho-FoxO3a (Upstate), Akt (Cell Signaling), phospho-Akt (Cell Signaling), cleaved PARP (Cell Signaling), caspase 3 (Cell Signaling), activated caspase 3 (Cell Signaling), anti-Flag (Sigma), anti-HA (Sigma), γ-tubulin (Sigma), β-actin (Sigma), TATA box binding protein (Abcam), mouse and rabbit serum IgG (Sigma) and anti-Tle1 (Stifani et al, 1992).
Supplementary Material
Supplementary Table 1
Supplementary Table 2
Acknowledgments
We thank Dr Vihren Kolev for careful reading of the manuscript, Dr W Austen (MGH, Boston, MA) for human skin material, Dr Lucy Liaw (Maine Medical Center, Scarborough, Maine) for the HERP1 and HERP2 adenoviruses, Dr F Radtke for the floxed Notch1 mice, Dr M Greenberg for the gift of the FHRE-luc promoter and S Stifani for the gift of anti-Tle1 antibody. This work was supported by NIH grants AR39190, CA16038 and CA73796, the Swiss National Foundation, a grant from the European Union (Epistem, Sixth Framework Program, LSHB-CT-2005-019067) and, in part, by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd Agreement.
References
- Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117: 421–426 [DOI] [PubMed] [Google Scholar]
- Armstrong BK, Kricker A (2001) The epidemiology of UV induced skin cancer. J Photochem Photobiol B 63: 8–18 [DOI] [PubMed] [Google Scholar]
- Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C (2006) Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA 103: 3799–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ (2005) Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest 115: 3166–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibanez CF (2003) Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol 163: 723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ (2006) Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7: 678–689 [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science (New York, NY) 303: 2011–2015 [DOI] [PubMed] [Google Scholar]
- Burgering BM, Kops GJ (2002) Cell cycle and death control: long live Forkheads. Trends Biochem Sci 27: 352–360 [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Qin JZ, Stennett L, Choubey D, Nickoloff BJ (2004) Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J Cell Physiol 198: 100–109 [DOI] [PubMed] [Google Scholar]
- D'Errico M, Lemma T, Calcagnile A, Proietti De Santis L, Dogliotti E (2007) Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat Res 614: 37–47 [DOI] [PubMed] [Google Scholar]
- de Gruijl FR (1999) Skin cancer and solar UV radiation. Eur J Cancer 35: 2003–2009 [DOI] [PubMed] [Google Scholar]
- Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP (2005) p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev 19: 1485–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J (1991) TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66: 649–661 [DOI] [PubMed] [Google Scholar]
- Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW (2005) Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 24: 2317–2329 [DOI] [PubMed] [Google Scholar]
- Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ III, Emanuel BS, Rovera G, Barr FG (1993) Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet 5: 230–235 [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C (2002) A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 3: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis RR, Alarcon C, He W, Wang Q, Seoane J, Lash A, Massague J (2006) A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci USA 103: 12747–12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24: 7410–7425 [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer O, Zwahlen D, Fey MF, Aebi S (2005) The Notch pathway in ovarian carcinomas and adenomas. Br J Cancer 93: 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194: 237–255 [DOI] [PubMed] [Google Scholar]
- Jehn BM, Bielke W, Pear WS, Osborne BA (1999) Cutting edge: protective effects of notch-1 on TCR-induced apoptosis. J Immunol 162: 635–638 [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE (2000) Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev 14: 142–146 [PubMed] [Google Scholar]
- Kim JW, Kim MJ, Kim KJ, Yun HJ, Chae JS, Hwang SG, Chang TS, Park HS, Lee KW, Han PL, Cho SG, Kim TW, Choi EJ (2005) Notch interferes with the scaffold function of JNK-interacting protein 1 to inhibit the JNK signaling pathway. Proc Natl Acad Sci USA 102: 14308–14313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC (2002) Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO Rep 3: 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latonen L, Laiho M (2005) Cellular UV damage responses—functions of tumor suppressor p53. Biochim Biophys Acta 1755: 71–89 [DOI] [PubMed] [Google Scholar]
- Lee J, Basak JM, Demehri S, Kopan R (2007) Bi-compartmental communication contributes to the opposite proliferative behavior of Notch1-deficient hair follicle and epidermal keratinocytes. Development (Cambridge, England) 134: 2795–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort K, Mandinova A, Ostano P, Kolev V, Calpini V, Kolfschoten I, Devgan V, Lieb J, Raffoul W, Hohl D, Neel V, Garlick J, Chiorino G, Dotto GP (2007) Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev 21: 562–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HD, Leveridge M, Strack PR, Haldon CD, O'Neil J, Kim H, Madin A, Hannam JC, Look AT, Kohl N, Draetta G, Harrison T, Kerby JA, Shearman MS, Beher D (2007) Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of notch signaling. Chem Biol 14: 209–219 [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM (2000) Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol 10: 491–500 [DOI] [PubMed] [Google Scholar]
- MacKenzie F, Duriez P, Wong F, Noseda M, Karsan A (2004) Notch4 inhibits endothelial apoptosis via RBP-Jkappa-dependent and -independent pathways. J Biol Chem 279: 11657–11663 [DOI] [PubMed] [Google Scholar]
- Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP (2005) Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell 8: 665–676 [DOI] [PubMed] [Google Scholar]
- Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP (1996) The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev 10: 3065–3075 [DOI] [PubMed] [Google Scholar]
- Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, Tomita T (2006) C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester). J Biol Chem 281: 14670–14676 [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L (2004) Mammalian SIRT1 represses forkhead transcription factors. Cell 116: 551–563 [DOI] [PubMed] [Google Scholar]
- Mumm JS, Kopan R (2000) Notch signaling: from the outside in. Dev Biol 228: 151–165 [DOI] [PubMed] [Google Scholar]
- Mungamuri SK, Yang X, Thor AD, Somasundaram K (2006) Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Cancer Res 66: 4715–4724 [DOI] [PubMed] [Google Scholar]
- Nair P, Somasundaram K, Krishna S (2003) Activated Notch1 inhibits p53-induced apoptosis and sustains transformation by human papillomavirus type 16 E6 and E7 oncogenes through a PI3K–PKB/Akt-dependent pathway. J Virol 77: 7106–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI (2004) Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood 103: 3503–3510 [DOI] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, di Vignano AT, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP (2006) Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev 20: 1028–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, Van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F (2003) Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 33: 416–421 [DOI] [PubMed] [Google Scholar]
- Nuthall HN, Joachim K, Stifani S (2004) Phosphorylation of serine 239 of Groucho/TLE1 by protein kinase CK2 is important for inhibition of neuronal differentiation. Mol Cell Biol 24: 8395–8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Kamakura S, Isazawa Y, Yoshimatsu T, Kuida K, Nakafuku M, Masuyama N, Gotoh Y (2004) Notch promotes survival of neural precursor cells via mechanisms distinct from those regulating neurogenesis. Dev Biol 276: 172–184 [DOI] [PubMed] [Google Scholar]
- Oswald F, Liptay S, Adler G, Schmid RM (1998) NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol 18: 2077–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald F, Tauber B, Dobner T, Bourteele S, Kostezka U, Adler G, Liptay S, Schmid RM (2001) p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol Cell Biol 21: 7761–7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, Zurrida S, Maisonneuve P, Di Fiore PP (2004) Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol 167: 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petcherski AG, Kimble J (2000) Mastermind is a putative activator for Notch. Curr Biol 10: R471–R473 [DOI] [PubMed] [Google Scholar]
- Quevedo C, Kaplan DR, Derry WB (2007) AKT-1 regulates DNA-damage-induced germline apoptosis in C.elegans. Curr Biol 17: 286–292 [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP (2001) Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20: 3427–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade H, Krishna S, Sarin A (2004) The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem 279: 2937–2944 [DOI] [PubMed] [Google Scholar]
- Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA (2006) Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J 25: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM, Nelkin BD, Ball DW (2002) Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol Cell Biol 22: 3129–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifani S, Blaumueller CM, Redhead NJ, Hill RE, Artavanis-Tsakonas S (1992) Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet 2: 343. [DOI] [PubMed] [Google Scholar]
- Talora C, Sgroi DC, Crum CP, Dotto GP (2002) Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev 16: 2252–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA (2002) The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem 277: 14255–14265 [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T (1998) LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol 18: 644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonova R, Small D, Kacer D, Kovalenko D, Kolev V, Mandinova A, Soldi R, Liaw L, Prudovsky I, Maciag T (2004) The non-transmembrane form of Delta1, but not of Jagged1, induces normal migratory behavior accompanied by fibroblast growth factor receptor 1-dependent transformation. J Biol Chem 279: 13285–13288 [DOI] [PubMed] [Google Scholar]
- Wang J, Devgan V, Corrado M, Prabhu NS, El-Deiry WS, Riccardi R, Pandolfi PP, Missero C, Dotto GP (2005) GITR is a p21 Cip1/WAF1 transcriptional target conferring resistance of keratinocytes to UV-induced apoptosis. J Biol Chem 280: 37725–37731 [DOI] [PubMed] [Google Scholar]
- Wang Y, Qin ZH, Nakai M, Chen RW, Chuang DM, Chase TN (1999) Co-stimulation of cyclic-AMP-linked metabotropic glutamate receptors in rat striatum attenuates excitotoxin-induced nuclear factor-kappaB activation and apoptosis. Neuroscience 94: 1153–1162 [DOI] [PubMed] [Google Scholar]
- Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L (2002) Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med 8: 979–986 [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC (2004) Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science (New York, NY) 306: 269–271 [DOI] [PubMed] [Google Scholar]
- Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, Aster JC (2003) Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol 23: 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD (2000) MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet 26: 484–489 [DOI] [PubMed] [Google Scholar]
- Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J (2004) Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol 269: 81–94 [DOI] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW (2006) FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med 203: 1657–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugawa T, Handa K, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T (2007) Regulation of Notch1 gene expression by p53 in epithelial cells. Mol Cell Biol 27: 3732–3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, Aster JC, Allman D, Pear WS (2005) Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood 106: 3898–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Supplementary Table 2