Abstract
Cell migration requires integration of signals arising from both the extracellular matrix and messengers acting through G protein-coupled receptors (GPCRs). We find that increased levels of G protein-coupled receptor kinase 2 (GRK2), a key player in GPCR regulation, potentiate migration of epithelial cells towards fibronectin, whereas such process is decreased in embryonic fibroblasts from hemizygous GRK2 mice or upon knockdown of GRK2 expression. Interestingly, the GRK2 effect on fibronectin-mediated cell migration involves the paracrine/autocrine activation of a sphingosine-1-phosphate (S1P) Gi-coupled GPCR. GRK2 positively modulates the activity of the Rac/PAK/MEK/ERK pathway in response to adhesion and S1P by a mechanism involving the phosphorylation-dependent, dynamic interaction of GRK2 with GIT1, a key scaffolding protein in cell migration processes. Furthermore, decreased GRK2 levels in hemizygous mice result in delayed wound healing rate in vivo, consistent with a physiological role of GRK2 as a regulator of coordinated integrin and GPCR-directed epithelial cell migration.
Keywords: GIT1, GRK2, integrins, MAPK, migration
Introduction
Signal-directed migration requires a spatiotemporal integration of information from mechanical cues and from diffusible molecules such as chemokines, bioactive lipids and growth factors. Failures in this process might result in aberrant migration, leading to chronic inflammatory disorders, tumour metastasis, impaired wound healing or vascular diseases (Ridley et al, 2003).
Cellular motility demands dynamic regulation of cell anchorage. Adhesion structures are nucleated by integrins, membrane receptors that orchestrate multi-protein complexes, namely focal adhesions and focal contacts that modulate cell growth, survival and cytoskeleton remodelling, linking the actin cellular cytoskeleton to components of the extracellular matrix (Brunton et al, 2004). Focal adhesion turnover is under the control of multiple signalling inputs. Activation of members of the FAK and Src families of tyrosine kinases decreases adhesive interactions and disorganizes the associated cytoskeleton by means of phosphorylation of numerous proteins (Brunton et al, 2004). In addition, extracellular signal-regulated protein kinase/mitogen-activated protein kinase (ERK/MAPK) activity can also promote focal adhesion assembly/disassembly by modifying paxillin, calpain or MLCK activities (Carragher and Frame, 2004; Webb et al, 2004), thereby enhancing cell migration.
For migration to progress efficiently, pro-migratory signals from a variety of G protein-coupled receptors (GPCRs) must be precisely coordinated with those arising from integrins (Li et al, 2003; Luttrell and Luttrell, 2004; DeFea, 2007). Most chemokines and lipid messengers, such as sphingosine-1-phosphate (S1P), emit signals to the actin cytoskeleton and to adhesion structures by binding to plasma membrane receptors of the GPCR family (Spiegel et al, 2002; DeFea, 2007). Concomitantly, GPCR occupancy activates desensitization mechanisms that are also required for proper migration. Agonist-occupied receptors are phosphorylated by a family of serine/threonine kinases known as G protein-coupled receptor kinases (GRKs), an event that triggers binding of arrestins, uncoupling from G proteins and receptor internalization (Penela et al, 2003). The GRK family of kinases comprises seven isoforms, of which G protein-coupled receptor kinase 2 (GRK2) is the most ubiquitously expressed (Penela et al, 2003). Consistent with its role in GPCR desensitization, GRK2 has been shown to attenuate chemokine-induced migration in T cells and monocytes (revised by Vroon et al, 2006), thus emerging as a relevant modulator of inflammatory responses. However, pro-migratory effects of β-arrestins and of a member of a distinct GRK subfamily, GRK6, have also been reported in response to different GPCRs in several cell types (Vroon et al, 2006; DeFea, 2007), thereby suggesting other unknown functional roles different from those related to GPCR desensitization. Interestingly, GRK2 has been shown to interact with a variety of proteins involved in migration (MEK, Akt, ezrin, PI3Kγ or GIT; reviewed by Ribas et al, 2007). However, the functional role of such interactions in cell migration and whether GRK2 influences motility in cellular types other than immune cells and towards different chemotactic stimuli have not been investigated.
In this report, we have studied the impact of altering GRK2 protein levels on integrin-dependent chemotaxis. We show for the first time that GRK2 positively regulates epithelial cell migration by mechanisms involving coordinated fibronectin- and S1P-mediated signalling and the modulation of the Rac/PAK/MEK/ERK1/2 pathway in response to S1P and adhesion, based on the dynamic regulation of the interaction of GRK2 with GIT1, a key scaffolding protein in cell migration processes.
Results
Increased GRK2 expression promotes profound changes in epithelial cell morphology and enhances motility towards fibronectin
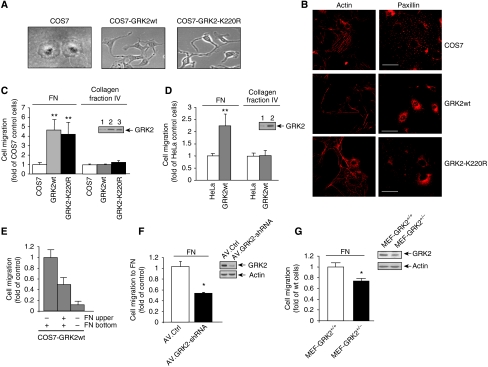
To explore the potential impact of GRK2 on cell migration, wild-type (wt) GRK2 was stably overexpressed in two different model epithelial-derived cell lines, COS7 and HEK-293 cells. Increased kinase expression resulted in marked morphological changes characteristic of motile cells (Figure 1A and Supplementary Figure S1A). Increased GRK2 levels trigger cortical actin rearrangements at the cell periphery, accompanied by a reduction in stress fibres. Interestingly, expression of a kinase-dead mutant of GRK2 (GRK2-K220R) at comparable levels promoted changes similar to those observed with wt GRK2 (Figure 1B). In both cases, increased GRK2 expression promoted a diffuse cytosolic localization of paxillin, in marked contrast to its dot-like distribution at peripheral and central focal adhesions observed in control COS7 cells (Figure 1B). Such paxillin redistribution suggested a role for GRK2 in focal adhesion turnover independent of kinase activity.
Figure 1.
GRK2 expression levels influence cell morphology and migration to fibronectin. (A, B) COS7 cells stably overexpressing wt GRK2 or the inactive GRK2-K220R mutant were plated on coverslips coated with 10 μg/ml fibronectin (FN) in the presence of 10% serum and their morphology was compared to that of parental cells by phase-contrast microscopy (objective × 20) (A) or by confocal fluorescence microscopy as described in Materials and methods. Scale bars, 50 μm (B). (C–E) GRK2 expression enhances fibronectin-directed cell migration. Serum-starved COS7 (C) or HeLa cells (D) expressing or not increased wt GRK2 or GRK2-K220R levels (3- to 10-fold over endogenous protein levels as determined by immunoblot analysis; see insets) were seeded on Transwell filters precoated with 20 μg/ml FN or 10 μg/ml collagen fraction IV and cell migration was assessed as detailed in Materials and methods. Data are the mean±s.e.m. of 4–6 independent experiments performed in duplicate. **P<0.01, compared to control cells. Number of control HeLa cells migrating to FN and collagen was 32±10 and 22±9 cells/field, respectively, whereas that of COS7 cells migrating to FN was 73±19 cells/field (means±s.d.). In (E), directed migration of COS7 cells expressing wt GRK2 was analysed upon addition of FN (20 μg/ml) to either the bottom chamber alone (chemotactic gradient) or to both upper and bottom chambers (uniform concentration). (F, G) Cellular migration to FN was significantly decreased upon reduction of GRK2 expression (see inset blots) in HeLa cells infected with an adenoviral-GRK2 shRNA construct (F) and in MEFs (G) derived from hemizygous GRK2+/− mice as compared to parental HeLa cells and MEFs from wt GRK2 mice, respectively. Data are the mean±s.e.m. of 3–4 independent experiments performed in duplicate. *P<0.05, **P<0.01, compared to control cells. Control values for HeLa and wt MEFs migrating to FN were 37±7 and 201±33 cells/field (means±s.d.), respectively.
To address whether such GRK2-induced changes result in altered cell motility, we analysed migration towards fibronectin in different epithelial cell lines stably expressing GRK2 or the GRK2-K220R mutant. Increased GRK2 expression markedly enhanced migration in COS7, HeLa (Figure 1C and D) or HEK-293 cells (Supplementary Figure S1B), in a kinase activity-independent manner. Such response did not depend on the interaction of GRK2 with Gαq subunits (Supplementary Figure S1C). Expression of a C-terminal deletion mutant no longer capable of binding to Gβγ subunits did not significantly enhance cell migration, whereas a membrane-targeted GRK2 mutant strongly enhanced cell motility, suggesting the involvement of a scaffolding function for GRK2 when recruited to specific membrane locations. This pro-migratory effect was clearly reduced when the fibronectin gradient was abolished, indicating that GRK2 impacts preferentially the cell chemotactic responses (Figure 1E). Furthermore, the effect of GRK2 is specific and does not affect the overall cell motility, as migration of COS7 or HeLa cells towards collagen fraction IV was unaltered by overexpression of wt GRK2 or GRK2-K220R (Figure 1C and D).
Interestingly, migration towards fibronectin was significantly reduced upon downregulation of endogenous GRK2 expression in HeLa cells using either a specific GRK2-siRNA construct (not shown) or adenoviral-mediated shRNA delivery (Figure 1F), and in mouse embryonic fibroblasts (MEFs) derived from hemizygous GRK2+/− mice, which display a 40–50% reduction in kinase levels compared to wt animals (Figure 1G). These results indicate that GRK2 expression levels positively correlate with cell migration to specific matrix proteins in primary cells and in different epithelial-derived cell lines.
GRK2-dependent increased migration to fibronectin is mediated by a PTX-sensitive GPCR
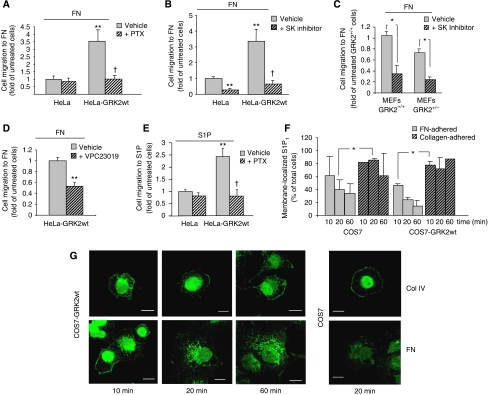
Regarding the mechanisms underlying the effect of GRK2 on cell migration, initial experiments excluded the participation of a Rho-dependent pathway (Supplementary Figure S2A). Given the key role of GRK2 in GPCR modulation and signalling, we hypothesized that a GPCR activity might be involved. Addition of pertussis toxin (PTX), an inhibitor of Gi-coupled receptor signalling, completely suppressed the increase in GRK2-dependent migration (Figure 2A). As our assays were performed in the absence of soluble stimuli and using cells deprived of serum, we hypothesized that an autocrine signal acting through GPCRs might be implicated. In this regard, S1P is endogenously generated by the activity of sphingosine kinase (SK) and is able to stimulate S1P1 (Gi-coupled) and S1P3 GPCRs, present in fibroblasts and epithelial cell lines, in a paracrine/autocrine manner (Spiegel et al, 2002; El-Shewy et al, 2006; Hait et al, 2006).
Figure 2.
The effect of GRK2 on cell migration to FN involves a PTX-sensitive S1P GPCR. Migration to FN of HeLa cells overexpressing or not wt GRK2 (A, B, D) or MEFs derived from wt or hemizygous GRK2 mice (C) was determined as in Figure 1D in the presence of vehicle or upon cell treatment with pertussis toxin (PTX) (D), SKI (B, C) or VPC23019 (D) as detailed in Materials and methods. In control conditions, the number of HeLa cells migrating to FN was 15±4 (A), 26±4 (B) and 44±2 (D), whereas that of wt MEFs was 123±22 (C) cells/field (means±s.d.). (E) Increased GRK2 expression enhances the migratory response of HeLa cells upon direct challenge of Gi-coupled S1P receptors. Migration of cells treated or not with PTX was assessed towards 1 μM S1P as above. In all panels, data are the mean±s.e.m. of four independent experiments performed in duplicate. *P<0.05, **P<0.01, compared to untreated control cells; †P<0.05, comparison between vehicle and inhibitor-treated HeLa cells stably expressing wt GRK2. (F, G) COS7 cells with or without extra GRK2 were kept in suspension for 1 h in the presence of serum and allowed to adhere on FN-coated (10 μg/ml) or collagen IV-coated (10 μg/ml) coverslips for the indicated times. Adhered cells were fixed, permeabilized and stained with an anti-S1P1 polyclonal antibody to analyse receptor subcellular localization by confocal fluorescence microscopy (G). Despite showing an unspecific nuclear staining, anti-S1P1 antibody clearly labels plasma membrane and intracellular vesicles. Cells positive for membrane receptor presence were quantified as detailed in Supplementary data (F). Data are the mean±s.e.m. of three independent experiments. *P<0.05, compared to collagen-adhered cells. Scale bars, 10 μm.
Interestingly, pharmacological inhibition of SK abolished the effect of increased GRK2 expression on migration to fibronectin in HeLa cells (Figure 2B) and COS7 cells (Supplementary Figure S3) as well as impaired motility in wt and GRK2+/− MEFs (Figure 2C). The presence of a selective S1P1/S1P3 receptor antagonist decreased the migration rate of HeLa cells stably expressing GRK2 (Figure 2D), thus ruling out receptor-independent actions of S1P. Treatment with PTX decreased migration in both GRK2-expressing and control cells, indicating that specificity of receptor coupling is not altered by GRK2 overexpression (Figure 2E). Moreover, exogenous S1P reversed the effects of the SK inhibitor SKI on cell migration towards fibronectin in HeLa and COS7 cells with or without extra GRK2 (Supplementary Figure S3A and B), further supporting the paracrine/autocrine actions of S1P. We next used endogenous S1P1 receptor internalization as a read-out of physiologically relevant S1P production leading to S1P receptor transactivation. We found that in the presence of fibronectin (but not collagen), a clear S1P1 receptor internalization takes place in wt or GRK2-overexpressing HeLa cells, being slightly increased in the latter (Figure 2F and G). Direct determination of S1P levels indicated increased autocrine production of this messenger in cells stably expressing GRK2 (0.96±0.13 μM in conditioned medium and 9.61±2.2 μM in the cytosolic fraction) compared to wt HeLa cells (0.56±0.21 and 5.46±1.3 μM, respectively). Overall, these results suggested that the pro-migratory effects of GRK2 in epithelial cells involved SK activation and paracrine/autocrine actions of S1P signalling through Gi-coupled receptors.
Confirming such functional interplay, cells stably expressing GRK2 or the GRK2-K220R mutant displayed higher migration rates in response to exogenously added S1P than control cells (Figure 2E and Supplementary Figure S3C), whereas S1P-induced migration was significantly reduced upon knockdown of endogenous GRK2 in HeLa cells (Supplementary Figure S3D) or in MEFs from GRK2+/− mice compared to wt cells (data not shown). Overall, our data suggested that fibronectin triggers an autocrine loop involving S1P receptors to potentiate epithelial cell migration, and that this component is positively modulated by GRK2 protein levels.
GRK2 facilitates activation of the ERK pathway upon direct or integrin-mediated S1P receptor stimulation
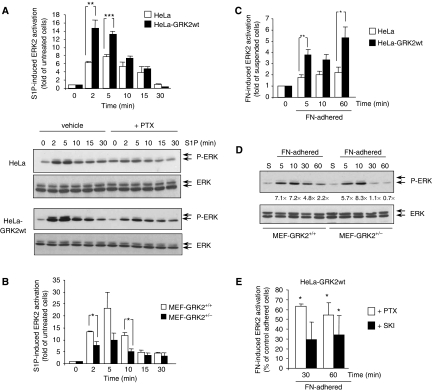
To define the role of GRK2 downstream S1P receptor signalling, we analysed the impact of altering GRK2 levels on S1P-dependent ERK1/2 activation. Increased GRK2 expression in HeLa cells promoted an enhanced (≈12-fold versus ≈8-fold) ERK1/2 peak stimulation, followed by a similar decay to baseline levels (Figure 3A). A similar effect was noted in COS7 cells with extra GRK2 or GRK2-K220R levels (Supplementary Figure S4). ERK1/2 stimulation involved Gi-coupled S1P receptors (Figure 3A, bottom panel) as in S1P-mediated migration. Consistent with the notion that GRK2 levels positively modulate S1P receptor-dependent ERK1/2 activation, decreased stimulation of this pathway by S1P was observed in MEFs from GRK2+/− mice compared to wt animals (Figure 3B).
Figure 3.
GRK2 expression levels modulate S1P-triggered or adhesion-dependent stimulation of ERK1/2. (A) Increased GRK2 expression promotes a more rapid and potent activation of ERK in response to S1P. The indicated cells were serum-starved for 6 h and challenged with 1 μM S1P for the indicated times. ERK1/2 activation was determined in cell lysates by using an anti-phospho-ERK1/2 antibody (P-ERK). The immunoblot was then stripped and the total cellular ERK1/2 was detected with specific antibodies. Phospho-ERK2 band densities were normalized to cognate total ERK2 densities. Preincubation with PTX markedly inhibits ERK activation. A representative blot is shown. (B) ERK1/2 activation is reduced in cells displaying lower levels of GRK2 protein. MEFs derived from GRK2+/+ or GRK2+/− mice were starved for 12 h in 0.1% serum, stimulated with 1 μM S1P for the indicated times and ERK1/2 activation was assessed as in (A). (C–E) GRK2 levels modulate ERK1/2 activity upon adhesion in a Gi protein-dependent and S1P-mediated manner. HeLa cells stably overexpressing or not wt GRK2 (C, E) or MEFs from GRK2+/+ or GRK2+/− mice (D) with or without PTX or SKI (E) were serum-starved and kept in suspension (S) for 2 h before adhesion on 10 μg/ml FN-coated plates for different periods of time. ERK stimulation was assessed as in (A). In all panels, data are the mean±s.e.m. of 3–4 independent experiments. *P<0.05, **P<0.01, ***P<0.001, compared to control cells or to vehicle-treated cells at each time point of adhesion (E). In (D), the normalized fold stimulation of ERK2 activity versus basal (suspension) conditions is indicated in the representative blot.
Similarly, increased GRK2 expression results in a stronger stimulation of ERK1/2 upon integrin-mediated adhesion (Figure 3C), whereas a more rapid attenuation of ERK stimulation was detected in GRK2+/− compared to wt MEFs in these conditions (Figure 3D). Consistently, knockdown of endogenous GRK2 in HeLa cells also leads to decreased fibronectin- or S1P-mediated ERK stimulation (data not shown). Moreover, the effect of GRK2 on ERK1/2 activation during adhesion was attenuated in the presence of either PTX or an SK1 inhibitor (Figure 3E), thus indicating cooperation between S1P and integrin receptors in this signalling pathway.
We next explored whether β-arrestins are involved in the positive effects of GRK2 on ERK1/2 activation and cell migration by using MEFs lacking β-arrestin-1 and -2 expression (βarr1/2-KO MEFs). S1P promoted a robust increase in ERK signalling in these cells, and migration to fibronectin was markedly increased (2.5-fold) in knockout MEFs compared to controls (Supplementary Figure S4A and B). Interestingly, we had previously reported that GRK2 levels are higher in βarr1/2-KO MEFs compared to control MEFs (Salcedo et al, 2006), which could promote enhanced migration and S1P/ERK signalling. Moreover, stably increasing GRK2 levels in β-arrestin KO MEFs further enhanced migration towards fibronectin (Supplementary Figure S4C), thus stressing a β-arrestin-independent effect of GRK2 on cell motility. In keeping with this, GRK2-K220R, a mutant that does not trigger β-arrestin recruitment to the receptor complex, potentiates S1P-induced ERK1/2 stimulation as efficiently as wt GRK2 (Supplementary Figure S4D).
Phosphorylation of GRK2 on tyrosine and serine residues regulates ERK signalling and cell motility in response to S1P and fibronectin
Interestingly, the effect of GRK2 on fibronectin-directed epithelial cell migration was clearly decreased by pharmacological inhibition of the MEK/ERK pathway or the activity of c-Src or PI3K, but did not involve Akt activation (Supplementary Figures S2 and S5). We have previously reported that GRK2 itself can be phosphorylated by c-Src upon GPCR activation (Ribas et al, 2007), which modulates its activity and interaction with other molecules (Mariggio et al, 2006), whereas phosphorylation of serine 670 by ERK1/2 impairs Gβγ-dependent catalytic activity (Penela et al, 2003). Interestingly, cell adhesion and S1P challenge were able to promote such phosphorylation events (Supplementary Figure S6). Remarkably, GRK2 phosphorylation at S670 was preceded by an increase in GRK2 phosphotyrosine levels and by maximal ERK1/2 pathway activity (see Figure 3) either upon S1P stimulation or cellular adhesion, consistent with a functional link between these processes.
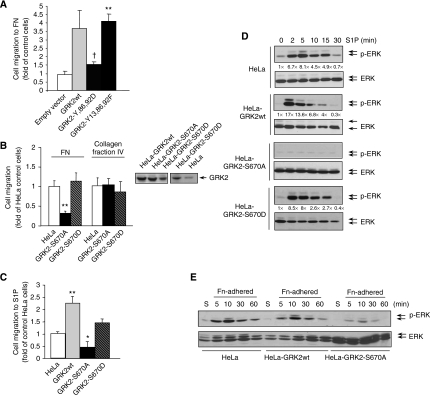
Next, we addressed whether an adequate timing of tyrosine and serine phosphorylation of GRK2 might influence cellular responses to either S1P or fibronectin, by using specific GRK2 phosphorylation mutants. Mimicking ‘permanent' tyrosine phosphorylation of GRK2 (GRK2-Y2D mutant) did not cause an increase in cell migration observed with the transient expression of either wt GRK2 or a mutant with decreased phosphorylation by c-Src (Figure 4A). On the other hand, HeLa cells stably expressing a GRK2 mutant unable to be phosphorylated at S670 (GRK2-S670A) displayed a markedly decreased migration towards fibronectin (Figure 4B). Interestingly, the presence of a GRK2 mutant that would mimic permanent phosphorylation at this site, GRK2-S670D, was unable to enhance motility of HeLa cells as effectively as wt GRK2. A similar trend was observed in the migratory responses to S1P challenge (Figure 4C). The effect of GRK2 mutants on motility was not caused by an unspecific impairment in cellular locomotion, as cell migration towards collagen was unaltered in the presence of such mutants (Figure 4B). Moreover, blockade of migration by expression of GRK2-S670A does not result from an altered cell attachment (Supplementary Figure S7).
Figure 4.
Phosphorylation of GRK2 at defined tyrosine or serine residues regulates migration and ERK1/2 signalling in response to S1P challenge and FN-mediated adhesion. (A–C) HeLa cells transiently transfected with either wt GRK2 or the tyrosine phoshorylation mutants GRK2-Y13,86,92F or GRK2-Y86,92D (A) or stably overexpressing similar levels (B, inset blot) of wt GRK2 or the GRK2-S670A or GRK2-S670D mutants (B, C) were subjected to migration assays towards 20 μg/ml FN (A, B), 10 μg/ml collagen fraction IV (B) or 1 μM S1P (C), as detailed in Materials and methods. Data are the mean±s.e.m. of 2–4 independent experiments performed in duplicate. *P<0.05, **P<0.01, compared to control HeLa cells; †P<0.05, compared to cells transfected with GRK2-Y13,86,92F. Control cells migrating towards fibronectin were 21±1 (A), 33±10 (B) or 23±1 (C) cells/field (mean±s.d.), and towards collagen 21±4 (B). (D, E) Expression of GRK2-S670A completely abrogates ERK1/2 activation in response to S1P and upon adhesion to fibronectin. HeLa cells or cells stably expressing wt GRK2, GRK2-S670A or GRK2-S670D were serum-starved and challenged with 1 μM S1P (D) or kept in suspension (S) before adhesion to 10 μg/ml FN (E) for the indicated times. ERK activation was determined as in Figure 3. Representative blots from four independent experiments are shown, and fold stimulation of ERK2 activity normalized by total ERK2 is included in (D).
These results indicate that timely and dynamic regulation of serine and tyrosine phosphorylation of GRK2 was strictly required, in a step following integrin engagement, for proper migration in response to S1P and fibronectin. As the extent and duration of ERK1/2 activation is instrumental in cell motility (Brahmbhatt and Klemke, 2003), we explored whether GRK2 phosphorylation status could modulate agonist-mediated stimulation of the ERK1/2 pathway. Expression of GRK2-S670A completely abrogated ERK1/2 activation in response to S1P where that of GRK2-S670D led to a pattern of stimulation similar to that observed in control HeLa cells expressing endogenous levels of GRK2 (Figure 4D, see quantified blots for comparison), in line with the fact that these cells migrate similar to HeLa parental cells in response to S1P and fibronectin. Activation of ERK1/2 during adhesion to fibronectin was also severely inhibited in the presence of GRK2-S670A (Figure 4E), thus stressing the notion that GRK2 modulates cell motility by means of regulating ERK activation.
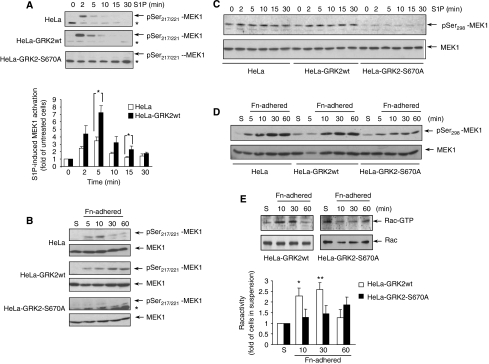
Signalling integration between adhesion and the ERK pathway involves integrin-induced phosphorylation of MEK1 at S298 by PAK1, which enhances both phosphorylation of MEK1 by Raf and MEK1–ERK interaction, thus leading to efficient ERK activation (Slack-Davis et al, 2003; Edin and Juliano, 2005). Interestingly, S1P triggered a more potent increase of Raf-dependent phosphorylation of MEK1 in cells overexpressing wt GRK2 (or GRK2-S670D) as compared to control cells (Figure 5A), which correlates with the higher activation of ERK observed in HeLa-GRK2 wt cells (Figure 3). In contrast, the presence of GRK2-S670A completely abrogated MEK1 activation by S1P (Figure 5A). A similar trend in MEK1 activation was observed upon adhesion to fibronectin in cells expressing wt GRK2 or the S670A mutant (Figure 5B). Moreover, PAK-mediated MEK1 phosphorylation at S298 was markedly impaired in the presence of such GRK2 mutant in S1P-stimulated adherent cells (Figure 5C) and upon adhesion to fibronectin (Figure 5D).
Figure 5.
Phosphorylation of GRK2 at S670 is required for triggering Rac/PAK1/MEK1 activation in response to S1P or fibronectin. HeLa cells with or without extra wt GRK2 or GRK2-S670A were serum-starved and stimulated with 1 μM S1P (A, C) or kept in suspension for 2 h (S) and then adhered to FN-coated plates (B, D, E) for the indicated times. The phosphorylation status of MEK1 at relevant activation sites promoted by PAK1 and Raf1 kinases was analysed by using specific anti-phospho-S298 and anti-phospho-T217/221 antibodies, respectively. Total MEK1 expression is also shown. Data are representative and/or mean±s.e.m. of 4–5 independent experiments. *P<0.05, compared to control HeLa cells. In (A), the asterisk denotes a cross-reacting unspecific band. (E) Rac1 activation pattern upon adhesion to FN is altered in cells expressing GRK2-S670A. HeLa cells stably expressing wt GRK2 or GRK2-S670A were lysed at the indicated times of adhesion and Rac activity was determined as detailed in Supplementary data. Data are the mean±s.e.m. of 4–5 independent experiments. *P<0.05, **P<0.01, compared to cells kept in suspension (S). A representative gel is shown.
These data suggested that timely serine phosphorylation of GRK2 at S670 might be required for MEK1 activation by facilitating MEK modulation by PAK or PAK activation itself. In this regard, we observed that PAK activation in response to S1P was compromised in the presence of the GRK2-S670A mutant (Supplementary Figure S8). Moreover, adhesion to fibronectin stimulated a rapid and transient activation of Rac in cells expressing wt GRK2, whereas such pattern was not observed in cells expressing GRK2-S670A (Figure 5E). Overall, these data indicated that GRK2 levels, by a mechanism involving its dynamic phosphorylation at S670, would regulate the extent and duration of Rac activation by integrins and, therefore, the ability of Rac to stimulate PAK (Bokoch, 2003).
GRK2 affects S1P-induced ERK signalling by modulating the scaffolding function of GIT1
It has been reported that GRK2 interacts with GIT1, a scaffolding protein that recruits active Rac in close proximity to its downstream effector PAK through a βPIX-mediated recruitment of both molecules, thus leading to PAK activation both at focal adhesions and at the cell leading edge (Hoefen and Berk, 2006). GIT1 also acts as a scaffold for MEK/ERK1/2 activation at focal adhesions (Yin et al, 2004, 2005), which promotes their disassembly and causes increased motility (Zhao et al, 2000). To address whether this scaffold protein could be involved in the GRK2-dependent modulation of MEK/ERK signalling, we expressed a GIT1 mutant lacking the SHD domain (aa 258–346), which is required for MEK recruitment and ERK activation upon different stimuli (Hoefen and Berk, 2006) but dispensable for GRK2 binding. The presence of GIT1-ΔSHD markedly decreased S1P-induced ERK stimulation in both wt and GRK2-overexpressing cells (Supplementary Figure S9), strongly suggesting that the scaffold role of GIT1 is involved in mediating the effects of GRK2.
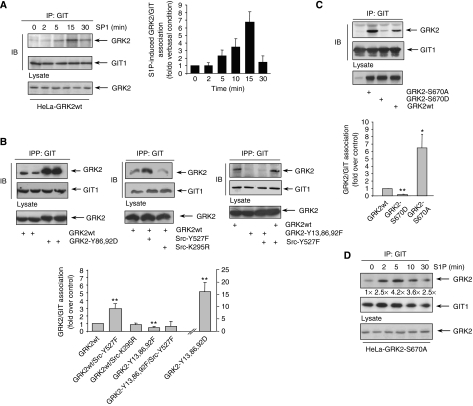
Interestingly, in cells stably expressing wt GRK2 and endogenous GIT1, S1P or cell adhesion stimulated a transient co-immunoprecipitation of both proteins (Figure 6A and data not shown). Next, we investigated whether such transient association of GRK2 with GIT1 might be dictated by GRK2 phosphorylation status. We observed that an enhanced GRK2/GIT1 association is detected in the presence of active Src (Figure 6B), and that tyrosine phosphorylation of GRK2 is critical for such effect, as an increased co-immunoprecipitation of these proteins is not observed in the presence of active Src when key GRK2 tyrosine sites are mutated, whereas mimicking phosphorylation at Y86/92 suffices to enhance such association (Figure 6B). On the other hand, the GRK2-S670A mutant displayed a markedly increased association (six- to seven-fold) to GIT1 when compared to wt kinase, whereas binding of the GRK2-S670D mutant was decreased (Figure 6C).
Figure 6.
Dynamic GRK2 phosphorylation at tyrosine and serine residues modulates its association to GIT1. (A) S1P challenge promotes transient association of GRK2 with GIT1. HeLa- GRK2 wt cells were treated with 1 μM S1P for the indicated times and processed for GIT1 immunoprecipitation (IP) as described in Materials and methods. The presence of GRK2 and GIT1 in the immune complexes was assessed by immunoblot analysis (IB) with specific antibodies. Data (mean±s.e.m. of five independent experiments) indicate the amount of co-precipitated GRK2 protein, normalized by immunoprecipitated GIT1 and referred to association in basal (i.e., non-stimulated) conditions. (B, C) HEK-293 cells were transiently co-transfected with GIT1 and wt GRK2 or the indicated phosphorylation mutants in the presence or absence of active (Y527F) or kinase-dead (K295R) c-Src constructs as indicated. GIT1 immunoprecipitates were analysed as above. Data are the mean±s.e.m. of 3–5 experiments. *P<0.05, **P<0.01, compared to wt GRK2/GIT1 association. (D) GIT1 interaction with GRK2-S670A in response to S1P occurs earlier and is more persistent. HeLa cells stably expressing GRK2-S670A were challenged with S1P, and GIT1 immunoprecipitates were analysed as above. Fold stimulation of GRK2 co-immunoprecipitation normalized by total GIT1 is shown. Results are representative of three independent experiments. Representative co-immunoprecipitation and total cell lysate expression blots are shown in all panels.
Overall, our results demonstrated that phosphorylation of GRK2 tightly regulates its interaction with GIT1. In response to S1P or during adhesion, the sequential or spatial stimulation of signalling pathways (c-Src and ERK1/2) with opposing effects on GRK2/GIT binding would lead to a dynamic association of both proteins. Consistent with this notion, S1P promotes an earlier and more sustained association between GIT1 and GRK2-S670A (Figure 6D) compared to wt GRK2 (Figure 6A).
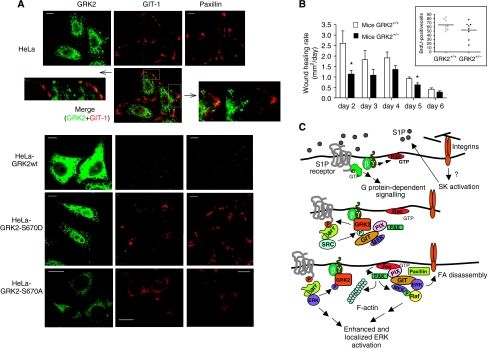
Interestingly, GRK2 association to GIT1 is favoured by membrane targeting, as the GRK2-gg mutant strongly interacts with GIT1 (Supplementary Figure S10A), which is paralleled by increased cell motility (Supplementary Figure S1C). However, the GRK2-Δct mutant, which also strongly interacts with GIT1 (Supplementary Figure S10A), fails to promote migration (Supplementary Figure S1C), thus suggesting that the GRK2/GIT1 association must occur at specific cellular locations to be functional. The scaffolding functions of GIT1 require an appropriate spatial localization to render functional signalling complexes. In control HeLa cells, GIT1 was mainly confined to focal adhesion-like structures and showed a distribution pattern similar to that of paxillin (Figure 7A). Endogenous GRK2 and GIT1 displayed a largely distinct subcellular distribution, although a partial colocalization was appreciated in some peripheral adhesion structures, consistent with their transient and dynamic interaction. An increase in wt GRK2 levels led to a dramatic change of this pattern, as shown by the loss of GIT1 (and paxillin) signal at adhesion-like structures and the appearance of a diffuse cytoplasmic staining. As total protein levels of both proteins were not decreased (Supplementary Figure S10B), reduction in their staining at focal adhesions is consistent with the occurrence of focal adhesion disassembly, similar to that observed in COS7 cells overexpressing GRK2 (see Figure 1). Interestingly, expression of GRK2-S670D (which shows reduced binding to GIT1 compared to wt) was unable to promote this effect (Figure 7A), whereas expression of GRK2-S670A (which displays a marked increase in steady-state GIT1 association) was able to promote loss of focal adhesion staining for both GIT1 and paxillin.
Figure 7.
(A) Effect of GRK2 on the subcellular localization of GIT1 and paxillin. Control HeLa cells or cells stably expressing wt GRK2, GRK2-S670A or GRK2-S760D were plated on coverslips coated with 10 μg/ml FN in the presence of 10% serum, fixed and stained with specific antibodies to detect GRK2, GIT1 and paxillin, and analysed by fluorescence confocal microscopy. Zoomed images correspond to the indicated areas in the low-magnification merged image to show clearly partial colocalization of endogenous GIT1 and GRK2. (B) Wound healing rate is significantly decreased in GRK2+/− compared to wt mice. Animals were wounded with a 4 mm biopsy punch and healing rate was calculated as described in Materials and methods. Data of wound healing rates (mean±s.e.m.) from days 2–6 post-wounding for wt (white bar; n=8–9) and GRK2+/− mice (black bar; n=8–9) are graphically summarized. *P<0.05, wt versus GRK2+/−. Wound sections from BrdU-labelled mice were stained with a specific anti-BrdU antibody and positive cells in the hyperproliferative epithelium were quantified (inset), indicating that keratinocyte proliferation was not significantly altered. (C) A model for the role of GRK2 in fibronectin/S1P-mediated ERK signalling and cell migration. Integrins would promote SK stimulation leading to paracrine/autocrine activation of S1P receptors (upper part of the scheme), which would recruit GRK2 to the vicinity of the plasma membrane. The dynamic association of GRK2 to the GIT1 scaffold, enhanced upon tyrosine phosphorylation of GRK2 (P, light blue, middle part) and decreased upon phosphorylation by ERK at S670 (P, purple, lower part), would facilitate the localized activation of the Rac/PAK/MEK/ERK pathway, leading to increased focal adhesion (FA) turnover and cell migration. See text for additional discussion. SK, sphingosine kinase; ?, unknown mechanism; β-arr, β-arrestin, SRC, Src-like tyrosine kinase.
Wound healing rates are altered in GRK2 hemizyogous mice
An in vitro ‘wound healing' assay indicated that knockdown of endogenous GRK2 expression in COS7 cells impairs the closure process (Supplementary Figure S11). Re-epithelialization of wounded skin represents a well-established process involving epithelial cell migration in response to specific mechanical (integrin-mediated) and chemotactic (S1P) cues (Jacinto et al, 2001; Grose et al, 2002; Vogler et al, 2003), thus recapitulating several of the processes affected by altering GRK2 expression in cell culture. Analysis of wound healing rates from day 2 until 6 in GRK2+/− mice (Figure 7b) revealed a significant decrease compared to wt littermates (F(1,16)=12.988; P<0.01). Moreover, analysis of data obtained on separate days revealed a significant decrease in wound healing rates at days 2 and 5 (P<0.05). Proliferation of epithelial cells was not significantly different in these mice groups (Figure 7B, inset, and Supplementary Figure S11B), thus further supporting that cell migration is the key process altered in the delayed re-epithelialization in GRK2+/− mice. In summary, our data strongly indicate that GRK2 positively regulates wound healing in vivo.
Discussion
The concerted action of integrin receptors and GPCRs has been shown to be important in the modulation of cell migration and adhesion (Brunton et al, 2004; Carragher and Frame, 2004; Hait et al, 2006; DeFea, 2007). In this report, we demonstrate that GRK2, a well-known GPCR regulatory protein, positively modulates epithelial cell migration by regulating the spatiotemporal interplay of signals arising from integrin engagement and S1P challenge into the ERK1/2 pathway.
Consistent with the observation that increased GRK2 levels promote cytoskeletal structural changes that favour cell motility, we found that migration rates of fibroblasts and different epithelial cell lines towards fibronectin, but not collagen IV, positively correlate with the expression of this kinase, in a GRK2 activity-independent manner. Interestingly, activation of Gi-coupled S1P receptors is required for the effect of GRK2 on fibronectin-directed migration, as PTX treatment, inhibition of endogenous S1P with an SK inhibitor or S1P1/S1P3 receptor blockade by means of a selective antagonist strongly impaired such response. Enhanced fibronectin-induced S1P1 receptor internalization and direct determination of S1P levels further support that the pro-migratory effects of GRK2 in epithelial cells involve SK activation and paracrine/autocrine actions of S1P signalling. Remarkably, enhanced GRK2 expression enables more efficient activation of the ERK1/2 pathway in response to either S1P receptor activation or integrin engagement. The positive effects of GRK2 on cell motility and signalling are independent of β-arrestin function.
Although previous reports have described the association of GRK2 to the S1P1 receptor complex at the plasma membrane (Alderton et al, 2001) and GRK2-mediated phosphorylation of S1P1 or S1P3 receptors (Watterson et al, 2002; Rutherford et al, 2005), our results describe for the first time a functional consequence of GRK2 in S1P receptor signalling in an endogenous system. Importantly, in contrast to what has been reported for chemokine receptors in immune cell types (Vroon et al, 2006), GRK2 appears to function as a positive regulator of S1P receptors signalling to the ERK cascade and the migration machinery. In lymphocytes, increased GRK2 expression reduces chemokine-mediated ERK stimulation, whereas MEK activation is not altered (Jimenez-Sainz et al, 2006). However, in response to S1P receptor activation in epithelial cells, the expression of GRK2 positively correlates with both ERK and MEK stimulation, thus revealing a new mode of action. Our data support the notion that GRK2 has positive signalling functional roles different from those related to GPCR desensitization, which represents a new paradigm in GPCR transduction, and are in line with recent observations in the Smoothened receptor system (Meloni et al, 2006; Molnar et al, 2007). Our results further indicate that changes in GRK2 expression levels may lead to different outcomes regarding cell migration, depending on the cell type, the specific stimuli acting through GPCRs, RTK or integrin receptors or the signalling context, and involve its dynamic interaction with a variety of cellular proteins, leading to differential networks of interaction of GRK2 with cell migration-related signalosomes.
Interestingly, the functional interplay between integrins and S1P receptors at the level of ERK1/2 signalling is influenced by the expression level of GRK2. Signal cross-talk between integrins and structurally diverse receptors such as growth factor tyrosine kinase receptors (Tzima et al, 2002; Slack-Davis et al, 2003) and heptahelical chemokine receptors (Campbell and Butcher, 2000) has been described. Regarding S1P receptors, integrin activity seems to be required for endothelial cell migration in response to S1P (Paik et al, 2001). Our data extend these previous observations demonstrating that S1P receptors cooperate with integrins to modulate cellular adhesion and migration in epithelial cells, pointing to GRK2 as a novel co-actor in this signalling integration.
S1P receptor transactivation seems to represent a general strategy for endowing non-GPCRs with the ability to trigger G protein-dependent signalling cascades by which cell migration can be modulated, such as the MEK1/ERK1/2 pathway (Spiegel et al, 2002; El-Shewy et al, 2006). Our data are compatible with integrin activation promoting cellular production of the S1P receptor ligand S1P by means of SK activation, as has been described for several growth factors and cytokines through SK1 stimulation (Milstien and Spiegel, 2006), although the detailed molecular mechanisms involved remain to be investigated. Alternatively, S1P receptors might activate inside-out mechanisms leading to integrin modulation (Milstien and Spiegel, 2006) as has been reported for other GPCRs.
Our data indicate that the dynamic phosphorylation of GRK2 at tyrosine and serine residues markedly affects ERK signalling and cell motility in response to S1P and fibronectin by means of altering its interaction with GIT1, a key scaffolding protein involved in cell motility and cellular adhesion. Interestingly, S1P receptors have been shown to modulate GIT1 complexes and promote its translocation to focal adhesions (Shikata et al, 2003).
It has been reported that GIT1 serves as a scaffold for MEK1 and ERK1/2 activation in focal adhesions (Yin et al, 2004, 2005) and has a critical role in delivering signalling molecules to the cell leading edge (Hoefen and Berk, 2006). Our results are consistent with a model in which integrin/S1P stimulation would cause Gβγ-dependent translocation of GRK2 to the plasma membrane and GRK2-mediated recruitment of GIT1, in a phosphotyrosine-dependent manner, to sites wherein chemotactic activation is taking place (see scheme in Figure 7C). Such events would facilitate Rac1-dependent PAK1 activation and localized ERK1/2 stimulation. Active ERK at the cell leading edge would promote turnover of integrin-mediated adhesive structures, required for directional movement, by phosphorylating paxillin, FAK or calpain, among other molecules. Adequate migration would also require the disassembly of GRK2/GIT1 complexes by means of localized phosphorylation of GRK2 by ERK at S670. Alterations in such dynamic scenario as a result of either modified GRK2 protein levels or expression of phosphorylation-impaired GRK2 mutants would compromise ERK1/2 activation in response to both S1P and fibronectin as well as cellular migration to either stimulus. GRK2-S670A would act as a dominant-negative mutant by keeping away GIT1 from endogenous GRK2. In contrast, GRK2-S670D-expressing cells do not mimic the effect of wt GRK2 overexpression on ERK activation or cell migration, as this mutant associates poorly to the GIT1 scaffold and would not prevent endogenous GRK2 from dynamically interacting with GIT1.
The mechanism(s) by which dynamic GRK2 association facilitates GIT1-dependent ERK1/2 activation and focal adhesion turnover remains to be established. Interestingly, GRK2 interacts with the central region of GIT1 that targets the protein to cytoplasmic complexes (Manabe et al, 2002). GRK2-mediated regulation of GIT1 trafficking between cytoplasmic and adhesion structures might contribute to GRK2-induced cell migration. Alternatively, GRK2 might modulate either the function of GIT1 as a scaffold in the Rac/PAK/ERK1/2 cascade or the activity of such GIT1-recruited molecules. As GRK2-S670A not only prevents PAK1-mediated MEK1 phosphorylation and PAK1 activation but also hampers Rac1 activity, improper GRK2/GIT1 interaction might interfere either at the GIT1/Rac1 or Rac1/PAK1 interfaces. Whether modulation of additional signalling cascades also participates in GRK2-facilitated migration deserves future investigation.
The notion that GRK2 function and expression levels might be relevant for migratory events involving either S1P or fibronectin cues in epithelial cells in vivo is confirmed by our results showing that hemizygous GRK2 mice exhibit decreased wound healing rates. Interestingly, some of the mechanisms operating in adult epithelial cell repair are recapitulated during embryonic morphogenetic movements. We have reported that GRK2 is highly expressed in areas of the mouse embryo undergoing active migration and differentiation processes (Sefton et al, 2000). On the other hand, aberrant epithelial cell motility has a key role in cancer progression wherein S1P and integrin signalling actively participate (Guo and Giancotti, 2004; Milstien and Spiegel, 2006). Increased S1P receptor functionality and SK1 upregulation is common in breast and other solid tumours correlating with metastasis and chemoresistance (Milstien and Spiegel, 2006; Spiegel and Milstien, 2007), whereas overexpressed β1 and α6β4 integrins promote carcinoma invasion (Guo and Giancotti, 2004; Brockbank et al, 2005). In this regard, we have recently reported an increase in GRK2 protein levels in malignant breast epithelial cells depending on the activity of the AKT/Mdm2 pathway (Salcedo et al, 2006). The possibility that in some contexts increased GRK2 expression levels might contribute to enhanced migration and invasion of tumour cells emerges as an attractive hypothesis that is currently being addressed in our laboratory.
Materials and methods
Cell culture
COS7, HEK-293 or HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA) at 37°C in a humidified 5% CO2 atmosphere. GRK2+/− and wt MEFs were derived from embryonic day 12 embryos and propagated in DMEM plus 10% FBS until pass 5. Immortalized βarr1/2-KO MEFs were cultured as reported (Salcedo et al, 2006). The cells were serum-starved for 12 h (migration assays) or 4–6 h (S1P signalling assays) and stimulated with S1P (1 μM) at 37°C in serum-free DMEM media for the desired time periods. Details on cell transfection are provided in Supplementary data.
Cell migration assays
Cells serum-starved in 0.1% FBS (MEFs) or DMEM (HeLa, HEK-293 and COS7 cells) were seeded (30 000 cells/well) onto uncoated or fibronectin-coated (10–20 μg/ml) or collagen type IV-coated (10 μg/ml) 6.5-mm Transwell filters with 8-μm pores (Costar, Cambridge, MA, USA) in the presence of S1P (1 μM) or serum-free medium as indicated. Cell treatment with the SK inhibitor SKI (10 μM), which depletes endogenous cellular S1P, the Src inhibitor PP2 (5 μM), the MEK kinase inhibitor PD98059 (50 μM), VPC23019 (10 μM), a selective antagonist for the S1P receptors 1 and 3 or the ROCK inhibitor Y27632 (30 μM) was initiated 30 min before the migration assay and maintained thereafter by addition to the upper cell chamber. For inactivation of Gi proteins, cells were pretreated with PTX (100 ng/ml) 12 h before the assay. After approximately 16 h (HEK-293 and COS7) or 5 h (COS7, HeLa and MEFs) of migration, cells were fixed and stained with 5 μg/ml DAPI. Five random fields of each filter were counted.
Adhesion assays
Cells were detached and kept in suspension on 150-mm Petri dishes precoated with 1% BSA (lipid-free) for 2 h in serum-free medium. Cells were then either immediately lysed (cells in suspension (S)) or allowed to adhere for the indicated time periods to culture dishes coated with 10 μg/ml fibronectin, followed by lysis in RIPA buffer. Cellular lysates were resolved by 8–10% SDS–PAGE and subjected to immunoblot analysis.
Wound healing assay
Hemizygous GRK2+/− mice backcrossed to C57bl/6 mice for 10 generations and wt littermates were used in this study. Mice were housed in the animal facility of Utrecht University under standard conditions. The animal committee of the University Medical Center Utrecht approved the experiments. Two symmetrical, full-thickness wounds were created on the dorsum below the inferior angle of the scapula of ketamine- and xylazine-anaesthetized mice using a sterile 4 mm biopsy punch (Stiefel Laboratorium, Germany). Wounds were photographed daily using a Sony digital camera. The digital image of each wound was analysed using ImageJ software (NIH, USA). To prevent magnification and angulation errors, the wound area was determined as a ratio to a 4 mm circular standard. Wound healing rate was calculated as the difference in wound area that day compared to the day before. All wound measurements were performed by the same individual, who was blinded to the experimental groups.
See Supplementary data for further details on materials and experimental methods regarding GRK2 knockdown, immunoprecipitation and western blot analysis, determination of ERK1/2, MEK, PAK and Rac activities or S1P levels, immunofluorescence, cell proliferation assay and statistics.
Supplementary Material
Supplementary data
Acknowledgments
We thank Richard Premont for experimental tools, Veronica Rivas and Ilana den Hartog for expert technical help and Gloria Escribano for secretarial assistance. This work was funded by grants from Ministerio de Educación y Ciencia (SAF2005-03053), Fundación Ramón Areces, Fundacion Mutua Madrileña, The Cardiovascular Network (RECAVA) of Ministerio Sanidad y Consumo-Instituto Carlos III (RD06-0014/0037), Comunidad de Madrid (S-SAL-0159-2006) and the MAIN European Network (LSHG-CT-2003-502935). PP and CR are recipients of ‘Ramón y Cajal' contracts and IA is a ‘Juan de la Cierva' postdoctoral fellow.
References
- Alderton F, Rakhit S, Kong KC, Palmer T, Sambi B, Pyne S, Pyne NJ (2001) Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J Biol Chem 276: 28578–28585 [DOI] [PubMed] [Google Scholar]
- Bokoch GM (2003) Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781 [DOI] [PubMed] [Google Scholar]
- Brahmbhatt AA, Klemke RL (2003) ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J Biol Chem 278: 13016–13025 [DOI] [PubMed] [Google Scholar]
- Brockbank EC, Bridges J, Marshall CJ, Sahai E (2005) Integrin beta1 is required for the invasive behaviour but not proliferation of squamous cell carcinoma cells in vivo. Br J Cancer 92: 102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC (2004) Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta 1692: 121–144 [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Butcher EC (2000) Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol 12: 336–341 [DOI] [PubMed] [Google Scholar]
- Carragher NO, Frame MC (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol 14: 241–249 [DOI] [PubMed] [Google Scholar]
- DeFea KA (2007) Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol 69: 535–560 [DOI] [PubMed] [Google Scholar]
- Edin ML, Juliano RL (2005) Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol Cell Biol 25: 4466–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shewy HM, Johnson KR, Lee MH, Jaffa AA, Obeid LM, Luttrell LM (2006) Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. J Biol Chem 281: 31399–31407 [DOI] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S (2002) A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315 [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG (2004) Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 5: 816–826 [DOI] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S (2006) Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026 [DOI] [PubMed] [Google Scholar]
- Hoefen RJ, Berk BC (2006) The multifunctional GIT family of proteins. J Cell Sci 119: 1469–1475 [DOI] [PubMed] [Google Scholar]
- Jacinto A, Martinez-Arias A, Martin P (2001) Mechanisms of epithelial fusion and repair. Nat Cell Biol 3: E117–E123 [DOI] [PubMed] [Google Scholar]
- Jimenez-Sainz MC, Murga C, Kavelaars A, Jurado-Pueyo M, Krakstad BF, Heijnen CJ, Mayor F Jr, Aragay AM (2006) G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Mol Biol Cell 17: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D (2003) Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114: 215–227 [DOI] [PubMed] [Google Scholar]
- Luttrell DK, Luttrell LM (2004) Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene 23: 7969–7978 [DOI] [PubMed] [Google Scholar]
- Manabe R, Kovalenko M, Webb DJ, Horwitz AR (2002) GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci 115: 1497–1510 [DOI] [PubMed] [Google Scholar]
- Mariggio S, Garcia-Hoz C, Sarnago S, De Blasi A, Mayor F Jr, Ribas C (2006) Tyrosine phosphorylation of G-protein-coupled-receptor kinase 2 (GRK2) by c-Src modulates its interaction with Galphaq. Cell Signal 18: 2004–2012 [DOI] [PubMed] [Google Scholar]
- Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler-Reya RJ, Lefkowitz RJ, Caron MG (2006) Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol 26: 7550–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstien S, Spiegel S (2006) Targeting sphingosine-1-phosphate: a novel avenue for cancer therapeutics. Cancer Cell 9: 148–150 [DOI] [PubMed] [Google Scholar]
- Molnar C, Holguin H, Mayor F Jr, Ruiz-Gomez A, de Celis JF (2007) The G protein-coupled receptor regulatory kinase GPRK2 participates in Hedgehog signaling in Drosophila. Proc Natl Acad Sci USA 104: 7963–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Chae S, Lee MJ, Thangada S, Hla T (2001) Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem 276: 11830–11837 [DOI] [PubMed] [Google Scholar]
- Penela P, Ribas C, Mayor F Jr (2003) Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal 15: 973–981 [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F Jr (2007) The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta 1768: 913–922 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302: 1704–1709 [DOI] [PubMed] [Google Scholar]
- Rutherford C, Ord-Shrimpton FU, Sands WA, Pediani JD, Benovic JL, McGrath JC, Palmer TM (2005) Phosphorylation-independent internalisation and desensitisation of the human sphingosine-1-phosphate receptor S1P3. Cell Signal 17: 997–1009 [DOI] [PubMed] [Google Scholar]
- Salcedo A, Mayor F Jr, Penela P (2006) Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J 25: 4752–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton M, Blanco MJ, Penela P, Mayor F, Nieto MA (2000) Expression of the G protein-coupled receptor kinase 2 during early mouse embryogenesis. Mech Dev 98: 127–131 [DOI] [PubMed] [Google Scholar]
- Shikata Y, Birukov KG, Garcia JG (2003) S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol 94: 1193–1203 [DOI] [PubMed] [Google Scholar]
- Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, Catling AD (2003) PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J Cell Biol 162: 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, English D, Milstien S (2002) Sphingosine 1-phosphate signaling: providing cells with a sense of direction. Trends Cell Biol 12: 236–242 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S (2007) Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem 282: 2125–2129 [DOI] [PubMed] [Google Scholar]
- Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA (2002) Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 21: 6791–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler R, Sauer B, Kim DS, Schafer-Korting M, Kleuser B (2003) Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J Invest Dermatol 120: 693–700 [DOI] [PubMed] [Google Scholar]
- Vroon A, Heijnen CJ, Kavelaars A (2006) GRKs and arrestins: regulators of migration and inflammation. J Leukoc Biol 80: 1214–1221 [DOI] [PubMed] [Google Scholar]
- Watterson KR, Johnston E, Chalmers C, Pronin A, Cook SJ, Benovic JL, Palmer TM (2002) Dual regulation of EDG1/S1P(1) receptor phosphorylation and internalization by protein kinase C and G-protein-coupled receptor kinase 2. J Biol Chem 277: 5767–5777 [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF (2004) FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6: 154–161 [DOI] [PubMed] [Google Scholar]
- Yin G, Haendeler J, Yan C, Berk BC (2004) GIT1 functions as a scaffold for MEK1–extracellular signal-regulated kinase 1 and 2 activation by angiotensin II and epidermal growth factor. Mol Cell Biol 24: 875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Zheng Q, Yan C, Berk BC (2005) GIT1 is a scaffold for ERK1/2 activation in focal adhesions. J Biol Chem 280: 27705–27712 [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L (2000) Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol 20: 6354–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data