Abstract
Degenerative disc disease (DDD) is still a poorly understood phenomenon because of the lack of availability of precise definition of healthy, ageing and degenerated discs. Decreased nutrition is the final common pathway for DDD and the status of the endplate (EP) plays a crucial role in controlling the extent of diffusion, which is the only source of nutrition. The vascular channels in the subchondral plate have muscarinic receptors but the possibility of enhancing diffusion pharmacologically by dilation of these vessels has not been probed. Although it is well accepted that EP damage will affect diffusion and thereby nutrition, there is no described method to quantify the extent of EP damage. Precise definitions with an objective method of differentiating healthy, ageing and degenerated discs on the basis of anatomical integrity of the disc and physiological basis of altered nutrition will be useful. This information is an urgent necessity for better understanding of DDD and also strategizing prevention and treatment.
Seven hundred and thirty endplates of 365 lumbar discs from 73 individuals (26 healthy volunteers and 47 patients) with age ranging from 10–64 years were evaluated by pre-contrast and 10 min, 2, 4, 6 and 12 h post contrast MRI after IV injection of 0.3 mmol/kg of Gadodiamide. End plates were classified according to the extent of damage into six grades and an incremental score was given for each category. A total endplate score (TEPS) was derived by adding the EP score of the two endplates for each concerned disc. The base line value (SIbase) and the signal intensity at particular time periods were used to derive the enhancement percentage for each time period (Enhancement (%) = SItp – SIbase/SIbase × 100). The enhancement percentage for each time period, the time for peak enhancement (T-max) and the time intensity curve (TIC) over 12 h were used to study and compare the diffusion characteristics. The differences in pattern of diffusion were obvious visually at 4 h which was categorized into five patterns—Pattern A representing normal diffusion to Pattern E representing a total abnormality in diffusion. Degeneration was classified according to Pfirrmann’s grading and this was correlated to the TEPS and the alterations in diffusion patterns. The relationship of TEPS on the increase in DDD was evaluated by a logistic curve and the cut point for severe DDD was found by ROC curve. The influence of the variables of age, level, Modic changes, instability, annulus fibrosis defect (DEBIT), TEPS and diffusion patterns on DDD was analyzed by multiple and stepwise regression analysis.
Oral nimodipine study: Additional forty lumbar end-plates from four young healthy volunteers were studied to document the effect of oral nimodipine. Pre-drug diffusion levels were studied by pre and post contrast MRI (0.3 mmol/kg of gadodiamide) at 10 min, 2, 4, 6, 12 and 24 h. Oral nimodipine was administered (30 mg QID) for 5 days and post-contrast MRI studies were performed similarly. Enhancement was calculated at vertebral body-VB; subchondral bone-SCB; Endplate Zone-EPZ and at superior and inferior peripheral nucleus pulposus-PNP and central nucleus pulposus-CNP, using appropriate cursors by a blinded investigator. Paired sample t test and area under curve (AUC) measurements were done.
The incidence of disc degeneration had a significant correlation with increasing TEPS (Trend Chi-square, P < 0.01). Only one out of 83 (1.2%) disc had either Pfirrmann Grade IV or V when the score was 4 or below when compared to 34/190 (17.9%) for scores 5–7; 41 of 72 (56.9%) for scores 8–10 and 18 of 20 (90%) for scores 11 and 12 (P < 0.001 for all groups). Pearson’s correlation between TEPS and DDD was statistically significant, irrespective of the level of disc or different age groups (r value was above 0.6 and P < 0.01 for all age groups). Logistic curve fit analysis and ROC curve analysis showed that the incidence of DDD increased abruptly when the TEPS crossed six. With a progressive increase of end plate damage, five different patterns of diffusion were visualized. Pattern D and E represented totally altered diffusion pattern questioning the application of biological method of treatment in such situations. Four types of time intensity curves (TIC) were noted which helped to differentiate between healthy, aged and degenerated discs. Multiple and stepwise regression analysis indicated that pattern of disc diffusion and TEPS to be the most significant factors influencing DDD, irrespective of age.
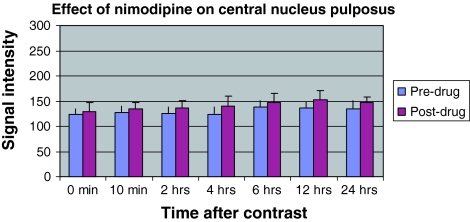
Nimodipine increased the average signal intensity for all regions—by 7.6% for VB, 8% for SCB and EPZ and 11% for CNP at all time intervals (P < 0.01 for all cases). Although the increase was high at all time intervals, the maximum increase was at 2 h for VB, SCB and EPZ; 4 h for PNP and 12 h for CNP. It was also interesting that post-nimodipine, the peak signal intensity was attained early, was higher and maintained longer compared to pre-nimodipine values.
Our study has helped to establish that EP damage as a crucial event leading to structural failure thereby precipitating DDD. An EP damage score has been devised which had a good correlation to DDD and discs with a score of six and above can be considered ‘at risk’ for severe DDD. New data on disc diffusion patterns were obtained which may help to differentiate healthy, ageing and degenerated discs in in-vivo conditions. This is also the first study to document an increase in diffusion of human lumbar discs by oral nimodipine and poses interesting possibility of pharmacological enhancement of lumbar disc nutrition.
Keywords: Disc degeneration, End plate, Post-contrast diffusion, Ageing, Nimodipine
Introduction
Degenerative disc disease (DDD) has been approached from various angles of epidemiological studies [34, 38], genetic inheritance [4, 36, 65, 72], molecular biology [5, 11, 17], mechanical analysis [1, 25, 26, 27, 30, 40, 41, 43, 69, 81] and FEM studies [18, 37, 42, 49, 50] but a complete understanding still remains elusive [12, 21, 44, 48]. Many fundamental issues on the initiating factor, the extent of natural healing, adaptive remodeling and secondary changes are not still clear [2, 12, 21, 44, 48]. Methods to confidently differentiate healthy, ageing and degenerated discs are not clearly known [2]. When biological treatment and regenerative medicines [3, 35, 39–41, 53, 56, 67, 73, 79, 83, 84] are on the horizon, it is important that an ageing disc be differentiated from a degenerative disc, as the treatment for both may not be the same [2].
Although multifactorial, a stable mechanical environment with normal hydrostatic pressure and intact nutritional pathway are considered to be important for preventing DDD [2]. The endplate plays a crucial role in both [16, 62, 71, 77]. Unfortunately, the EP is highly susceptible to mechanical failure and forms a ‘weak link’ in the structural integrity of the functional motion segment [2]. Cracks in the EP as the first step of failure have been predicted by FEM studies [50] and confirmed by microscopic studies [77], autopsy studies [47, 71] and diffusion MRI studies [59]. The healing ability of damaged EPs is still unknown and endplate damage can precipitate disc degeneration in a variety of ways including decreased nutrition [59], loss of viscoelastic properties of the nucleus pulposus [23], loss of protein macromolecules, altered matrix synthesis, secondary annular damage [5, 11, 17, 64, 71, 78] and vascularisation of the nucleus pulposus with autoimmune changes [55, 68]. There is growing belief that the fundamental basis of disc degeneration is structural failure [2, 5, 47, 59, 71, 77] and that EP damage may play a crucial role in initiation and progress of disc degeneration.
There is no described method in literature to quantify the severity of end plate damage (EPD) in vivo and document the effect of such damage on the physiology of nutrition and the resultant risk for DDD. The human disc has little capacity for healing and regeneration [2, 12, 21, 44, 48] and it may have a ‘point of no return’ after which reversal by natural healing or adaptive remodeling is impossible. Diffusion patterns in vivo may also indicate the nutritional status of the matrix and the receptiveness of the ‘soil’, a concern when biological methods for reversing DDD are on the pipeline. Stem cell therapy, genetic manipulation, anabolic stimuli and growth factors, which are all identified as possible treatments of future, will fail if the mechanical environment and nutritional pathways are blocked [2]. There is an urgent need to evolve a method to quantify the EP damage in vivo and to assess the alterations in diffusion and resultant degeneration.
Previous studies on EP physiology and diffusion have all been under laboratory conditions, cadaveric studies [41, 71], by radio-active isotopes [70, 76] and animal studies [28, 29, 51, 52]. Our study on 730 EPs in 365 discs of 73 patients is the largest human study and the first to classify EP damage on an incremental grade and document the in vivo effect on diffusion and to calculate the probability of DDD. The study has also evolved an EP damage score which allows quantification of the damage and its correlation with the extent of DDD. New data on disc diffusion patterns have been obtained which have helped to differentiate healthy, ageing and degenerative discs in-vivo on basis of anatomy and functional status.
Methods to improve disc nutrition has commanded the interest of spine surgeons for long, albeit with little success [63, 70, 75, 80]. The capillaries in end plate region have muscarinic receptors that regulate blood flow in response to external stimulus [10, 80]. Histological studies have documented that calcium channel antagonist Nimodipine increases endplate vascularity in rats [74]. There is no corresponding data for humans and whether endplate hypervascularity leads to increase in diffusion is not known. This prospective study in human volunteers reports for the first time an increase in diffusion following Nimodipine by serial post contrast MRI study.
Materials and methods
The study was approved by the Ethical Committee of the Institution and all healthy volunteers and patients were counseled and informed consent obtained.
Study population
Seven hundred and thirty endplates of 365 discs from 73 patients (26 healthy human volunteers and 47 patients) with age ranging from 10–64 years (male 52; female 21) were evaluated by pre-contrast MRI with T1, T2 sequences for changes in the endplate and disc degeneration. The distribution of the discs as per the age, level of the discs, degeneration, status of annulus and presence of Modic changes is given in Table 1.
Table 1.
Details of the 365 discs analysed in this study
| Level of disc | Age(years) | Pfirrmann’s grading | Modic changesa | AFD gradingb | Instability | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 0 | 1 | 2 | 3 | ||||
| L1-2 | <20 | 11 | 3 | 0 | 1 | 1 | 13 | 2 | 1 | 16 | 0 | 0 | 0 | 0 | 16 |
| 21–40 | 12 | 23 | 1 | 1 | 2 | 32 | 3 | 4 | 39 | 0 | 0 | 0 | 0 | 39 | |
| >40 | 3 | 9 | 3 | 1 | 2 | 14 | 3 | 1 | 18 | 0 | 0 | 0 | 0 | 18 | |
| L2-3 | <20 | 7 | 5 | 0 | 3 | 1 | 11 | 1 | 4 | 16 | 0 | 0 | 0 | 0 | 16 |
| 21–40 | 7 | 26 | 0 | 3 | 3 | 32 | 2 | 5 | 37 | 1 | 0 | 1 | 0 | 39 | |
| >40 | 0 | 8 | 8 | 2 | 0 | 12 | 6 | 0 | 17 | 1 | 0 | 0 | 0 | 18 | |
| L3-4 | <20 | 6 | 4 | 4 | 2 | 0 | 11 | 2 | 3 | 16 | 0 | 0 | 0 | 0 | 16 |
| 21–40 | 4 | 26 | 2 | 7 | 0 | 32 | 6 | 1 | 35 | 4 | 0 | 0 | 0 | 39 | |
| >40 | 0 | 7 | 3 | 5 | 3 | 14 | 2 | 2 | 15 | 3 | 0 | 0 | 1 | 18 | |
| L4-5 | <20 | 6 | 5 | 2 | 3 | 0 | 12 | 2 | 2 | 14 | 1 | 1 | 0 | 0 | 16 |
| 21–40 | 4 | 12 | 6 | 15 | 2 | 33 | 4 | 2 | 16 | 17 | 6 | 0 | 2 | 39 | |
| >40 | 0 | 4 | 1 | 2 | 11 | 9 | 4 | 5 | 4 | 7 | 6 | 1 | 4 | 18 | |
| L5-S1 | <20 | 4 | 6 | 4 | 2 | 0 | 12 | 3 | 1 | 11 | 5 | 0 | 0 | 0 | 16 |
| 21–40 | 4 | 17 | 3 | 11 | 4 | 30 | 4 | 5 | 18 | 10 | 11 | 0 | 3 | 39 | |
| >40 | 0 | 6 | 5 | 3 | 4 | 11 | 4 | 3 | 8 | 8 | 2 | 0 | 2 | 18 | |
| Total | 68 | 161 | 42 | 61 | 33 | 278 | 48 | 39 | 282 | 55 | 26 | 2 | 12 | 365 | |
a 0 no modic changes, 1 modic change in one end plate, 2 modic change in both end plates
b 0 intact annulus, 1 bulge in annulus, 2 protrusion, 3 extrusion/sequestration
Plain MRI evaluation
TSE T1 and T2 weighted images (4 mm sagittal sections) were obtained by using a 1.5 T MR Imager [Siemens, Symphony, Germany (T1: TR = 600, TE = 15.0; T2:TR = 4,000, TE = 95.0)]. Disc degeneration was assessed by Pfirrmann’s grading [57] and discs were considered to be healthy if they were Grade I, II or III (n = 271) and degenerated if they were Grade IV or V (n = 94). Twelve discs were found to be frankly unstable—three had lysis, one had retrolisthesis and eight had spondylolisthesis. The nature of the sub-chondral bone changes were classified as Type I, II or III as per the description of Modic et al. [45] and the extent of involvement was classified as absent (n = 278) or occurring in one single end plate (n = 48) or occurring in both end plates of the respective discs (n = 39).
The endplates were assessed in T1 weighted images and were classified into six types according to the severity of damage (Fig. 1). Type I EP had no damage and was seen as a uniformly symmetrical concave hypo-intense band without EP breaks or associated Modic changes. Type II EP had focal thinning either at the centre or periphery but without any EP break or Modic changes. Type III EP demonstrated focal disc marrow contact regions without alteration in the contour of the endplate. Modic changes were absent. Type IV EP damage resemble a typical schmorl’s node occupying upto 25% of the endplate area with typical depression. Sub-chondral changes were usually present. Type V EP involve more extensive damage occupying upto 50% of the EP area and were always associated with sub-chondral changes. Type VI EP represented complete EP damage with gross irregularity or sclerosis. Depending on the severity of EPD, an EP score was given to denote the extent of damage of each EP (Fig. 1) and a ‘Total End Plate damage Score (TEPS)’ was derived for each disc by adding up the score of both endplates.
Fig. 1.
This figure shows six varieties of end plates according to severity of damage. Type I is a normal end plate and is seen as a uninterrupted hypo-intense band and symmetrically concave. Type II is an end plate with areas of thinning either in the centre or periphery; no obvious break is visualized. Type III is an end plate which show focal defects with established disc marrow contact. The contour of the end plate is maintained and there is no sub-chondral bone change. Type IV end plates have breaks less than 25% with sub-chondral depression. Modic changes are usually present around the node. Type V are larger end plate defects occupying up to 50% of the end plate with associated modic changes. Type VI end plates have extensive damage involving almost the entire end plate
Diffusion studies
Diffusion characteristics of the disc were assessed by serial post contrast T1 images of MRI by injecting 0.3 mmol/kg Gadodiamide and performing serial post contrast studies at 10 min, 2, 4, 6 and 12 h [59]. The diffusion patterns (Fig. 2) and time intensity curves (TIC) depicting the temporal pattern of diffusion over 12 h (Fig. 3) and the diffusion patterns were studied.
Fig. 2.
Five visually distinguishable diffusion patterns were observed in serial post contrast MRI. Pattern A represented normal diffusion pattern observed in healthy discs with intact end plates. The diffusion bands were uninterrupted and parallel to the end plates and slowly progressed to the central nucleus pulposus. No abnormal disc marrow contacts were seen. Pattern B was seen in discs with focal end plate defects with patent disc marrow contacts which became very prominent in 2 h post contrast pictures. The typical diffusion bands were seen in the rest of the areas and there was no abnormal pooling of the dye. Pattern C was seen in defects with involvement of the sub-chondral bone and large disc marrow contacts. Here there was pooling of the dye at the region of the defect but the diffusion bands were maintained in the other areas. There was no leakage of the dye to the centre of the nucleus pulposus. Pattern D was associated with pooling of the dye in the peripheral nucleus pulposus by 2 h extending into small areas of the central nucleus pulposus subsequently. Pattern E indicated a total disruption of the disc and the entire disc space was filled with the dye even by 10 min
Fig. 3.
a shows the MRI appearance and the corresponding TIC curves of variations in diffusion. TIC curve 1 shows well delineated bands parallel to the end plate and slowly moving towards the centre. The corresponding TIC shows a increasing enhancement after an initial lag to reach peak enhancement at 6 h. This pattern obviously represents ‘healthy’ disc. Figure 3b shows an MRI pattern in a typically ageing disc. The disc appears black but without structural failure. The TIC curve shows decreased diffusion and a delayed achievement of peak enhancement. Fig. 3c shows a double peak pattern which is often seen in degenerating disc. The MRI picture shows patchy diffusion. The TIC shows an initial peak which probably corresponds to the vascular phase and the second peak represent diffusion from the partially intact end plates. This appearance is often seen in patients with partial disc degeneration. Figure 3d shows the appearance of a completely degenerated disc with extensive pooling of the dye in the entire disc space. The TIC shows a very early peak enhancement which is sustained over 12 h
Diffusion patterns
Five distinct diffusion patterns were identified (Fig. 2; Table 2). The diffusion pattern was normal (Pattern A) if uninterrupted smooth diffusion bands parallel to the endplate were noticed at 2 h and slowly progressing to the central nucleus pulposus (CNP). Measurement of signal intensity at various zones also confirmed the presence of ‘diffusion march’ phenomenon and ‘endplate delay’ [59]. Pattern B diffusion had diffusion spikes across the EP enhancing the disc marrow contact zones. The peripehral nucleus pulposus (PNP) at the site of the break showed enhancement even at 10 min, but diffusion band over the other areas was intact and there was no pooling inside the matrix (Fig. 4). Pattern C diffusion was associated with defects with subchondral bone depression in the endplate and had pooling restricted to the area of defect. The diffusion bands were maintained at other areas and there was no pooling in the central nucleus pulposus. Pattern D diffusion had more extensive defects where the pooling of the dye extended from the sub-chondral defect to the peripheral nucleus pulposus and areas of the central nucleus pulposus. Patchy areas of normal diffusion pattern were still seen. Pattern E diffusion had a complete loss of normal diffusion and there was complete pooling occupying the entire central nucleus pulposus.
Table 2.
Various Patterns of Diffusion in post contrast MRI
| Patterns | Diffusion characteristics |
|---|---|
| A | Uninterrupted uniform band of diffusion |
| End plate delay and diffusion march present | |
| No enhancement spikes or pooling | |
| B | Areas of disc marrow contact |
| Normal diffusion band maintained in other areas | |
| No gross changes in the diffusion pattern | |
| C | Focal pooling in PNP* at site of defect |
| Diffusion band is affected at defect level only | |
| No pooling inside CNP+ | |
| D | Focal pooling within the defect and extending into the CNP |
| End plate delay and diffusion march is altered | |
| E | Totally abnormal diffusion pattern with gross pooling in CNP |
| No diffusion bands visualized |
PNP peripheral nucleus pulposus
CNP central nucleus pulposus
Fig. 4.
At the zones of disc marrow contacts, cursors were placed at the SCB, EP, the area of nucleus pulposus immediately adjacent to the endplate (PNP), and the CNP (a). Enhancement of the region of disc adjacent to the zone of endplate breakage resembled that of the EP rather than the other regions of nucleus pulposus (b). Endplate delay was absent, and SImax was achieved at 10 min (c). The enhancement percentage of the EP break and the disc adjacent was isointense during most of the time–intensity curve (b). The regions of the disc without EPZ break showed little enhancement at 10 min and achieved SImax only at 6 h [59, 60] (SCB sub-chondral bone, PNP peripheral nucleus pulposus, CNP central nucleus pulposus)
Time intensity curve (TIC)
The baseline value obtained from the pre-contrast film [signal intensity (SI)base] and the signal intensity at a particular time period (SItp) were used to derive the enhancement percentage for each time period (enhancement percentage = SItp – SIbase/SIbase × 100). The peak enhancement rise time (Trise) was the time between SIbase and SImax (the maximum signal intensity in 12 h). The enhancement percentage of each time period, the Trise for each segment, and the time–intensity curve of each ROI over 12 h (derived by plotting enhancement percentage against time) were used to study and compare the diffusion characteristics [59] (Fig. 3).
The TEPS and diffusion patterns were independently assessed by three different observers in 50 discs and the intra-observer agreement rate was calculated by intra-class correlation method [20] and was found to be good [0.815 (95% CI—0.563, 0.945) and 0.837 (95% CI—0.598, 0.951), respectively].
Distribution of the discs with various degrees of DDD for each TEPS was tabulated (Table 3).The relationship between TEPS and DDD was found by calculating Pearson’s correlation coefficient for all the discs considered together and also for sub-groups of age less than 20, 21–40 and 41 and above. The influence of increasing TEPS over the entire range of scores on DDD was analysed by constructing a logistic curve [13].
Table 3.
Incidence of various grades of DDD according to TEPS
| DDD | Total end plate score (TEPS) | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | 4 | 10 | 16 | 10 | 20 | 5 | 2 | 1 | 0 | 0 | 0 | 68 |
| 2 | 4 | 7 | 39 | 34 | 46 | 14 | 12 | 4 | 0 | 0 | 1 | 161 |
| 3 | 0 | 0 | 2 | 4 | 18 | 5 | 4 | 6 | 2 | 1 | 0 | 42 |
| 4 | 0 | 0 | 1 | 8 | 15 | 6 | 12 | 9 | 8 | 2 | 0 | 61 |
| 5 | 0 | 0 | 0 | 0 | 2 | 3 | 1 | 6 | 5 | 0 | 16 | 33 |
| Total | 8 | 17 | 58 | 56 | 101 | 33 | 31 | 26 | 15 | 3 | 17 | 365 |
DDD degenerative disc disease, TEPS total end plate score
An ROC curve [85] was constructed between TEPS and DDD to calculate the cut point of TEPS beyond which the incidence of DDD was high. The correlation of diffusion and DDD was tabulated and the correlation was tested by trend chi-square. The correlation of the TEPS to the diffusion pattern was also tested. Step-wise regression analysis was performed with DDD as the dependant variable and age, level of lesion, sex, modic changes, instability, diffusion pattern and TEPS as independent variables to identify the factors which influenced DDD most.
Oral nimodipine study
Forty lumbar endplates from four young (age range 28–31 years; mean age 29.5) male healthy volunteers formed the study group for the second part of the study.
In the step 1 base line (pre drug, pre contrast) T1 weighted sagittal MR images of lumbar spine were obtained from all the four volunteers. In step 2, 0.3 mmol/kg body weight of Gadodiamide was administered intravenously and post contrast MR images were obtained at 10 min, 2, 4, 6, 12 and 24 h. The data obtained from the steps 1 and 2 were used to calculate the pre-drug diffusion characteristics. After a Gadodiamide wash out period of 10 days, a plain MR examination was performed to ensure return of signal intensity values to the base line (step 3). Then all volunteers received oral nimodipine (30 mg QID) for 5 days (step 4) following which post-drug pre-contrast MR images (step 5) and post-drug post-contrast images (step 6) at 10 min, 2, 4, 6, 12, 24 h were obtained. The data obtained from the steps 5 and 6 were used to calculate the post-drug diffusion characteristics.
Venous blood samples were obtained from all the volunteers pre-drug and 1 h after consumption of each tablet on days 1, 3 and 5 to estimate the serum nimodipine concentration. Nimodipine plasma concentration were analyzed by high performance liquid chromatography following the method of the Quin and Gallo [22, 58] with two pumps with Diode array detector and programme class LC10. (Stationary phase: C8 250 × 4.6 mm, particle size 5 μm (nucleosyl/100, C8), volume of injection: 100 ml (automatic). Mobile phase: acetonetril: water pH 2.5 with phosphoric acid (65; 35), Flow rate; 2.0 ml/min. Ambient temperature of 25°C, UV detector to 241 nm. Detection limit: 0.69 ng/ml.)
Results
Of the 365 discs, 68 were of Pfirrmann’s grade I, 161 were Pfirrmann’s grade II, 42 were Pfirrmann’s grade III, 61 were Pfirrmann’s grade IV and 33 were Pfirrmann’s grade V. 282 discs had no annulus defect whereas 55 had bulge, 26 had protrusion and 2 had extrusion of disc. Of the 12 discs with instability, 3 had lysis, 1 had gross retrolisthesis and 8 had spondylolisthesis. The distribution of the discs according to the age, level, presence of Modic changes, annulus disc bulge graded based on DEBIT [32] and instability is provided in Table 1.
The relationship of TEPS and disc degeneration was analyzed (Table 3). The incidence of DDD was found to significantly increase with increase in TEPS. Only 1 of 83 (1.2%) discs had either Pfirrmann Grade IV or V degeneration when the score was 4 or below; compared to 34 of 190 (17.9%) for the score of 5–7; 41 of 72(56.9%) for the score of 8 to 10 and 18 of 20 (90%) for the score of 11 or 12 (Trend chi-square, P < 0.001 for all groups). The correlation between TEPS and DDD was found to be good and statistically significant irrespective of the age and levels of disc (Table 4).
Table 4.
Correlation Co-efficient between TEPS and DDD
| No of discs | Correlation (r) | P value | |
|---|---|---|---|
| Correlationa of TEPS with DDD for levels of discs | |||
| L1-2 | 73 | 0.657 | <0.001 |
| L2-3 | 73 | 0.639 | <0.001 |
| L3-4 | 73 | 0.535 | <0.001 |
| L4-5 | 73 | 0.629 | <0.001 |
| L5-S1 | 73 | 0.572 | <0.001 |
| Correlationa of TEPS with DDD for various age groups | |||
| <20 years | 75 | 0.617 | <0.001 |
| 21–40 years | 179 | 0.732 | <0.001 |
| >40 years | 65 | 0.696 | <0.001 |
| All discs | 365 | 0.650 | <0.001 |
The TEPS had a significant Correlation with DDD in all levels of disc and also for all age groups
aPearson’s correlation co-efficient
The influence of TEPS on the increase in DDD over the entire spectrum of TEPS was estimated by deriving a logistic curve [13] which showed a good curve fit with a correlation of R = 0.9. A logistic curve formula [y = a/(1+be−cx) coefficient data: a = 5.1875717; b = 189.39131; c = 0.85979856] was also derived which allowed estimation of the DDD for any TEPS score (Fig. 5). The goodness of the curve was tested by using the formula in 50 new discs. There was no significant difference between the observed and estimated value (chi square value = 0.05). The response of increase in DDD to per unit increase in TEPS was found to be ‘slow’ till a TEPS value of six after which the response was ‘rapid’ with a steep increase in the curve. The curve indicated a critical TEPS score of 6 after which the risk for degeneration was increased many fold. A receiver operating curve (ROC) was constructed between TEPS and DDD which showed a good correlation, the area under the curve being 0.838 (SE = 0.022, 95% CI—0.079, 0.876) (Fig. 6). The curve confirmed a cut point of 6 in TEPS beyond which the incidence of DDD was very high.
Fig. 5.
Logistic curve was constructed to infer the influence of TEPS on DDD over the various values of TEPS. The curve had a good fit with correlation coefficient of R = 0.9 and the curve formula showing the association was y = a/(1+b*exp(−cx)); Coefficient data: (a = 5.187; b = 189.391; c = 0.859). It is seen that the incidence of DDD was negligible in patients with a TEPS score of less than four and then slowly rising till six. The curve showed a steep response till the score of nine after which there was a asymptote indicating that most discs were degenerated by the time the score was nine. The curve indicated that the critical TEPS score was six beyond which the incidence of DDD was very high
Fig. 6.
An ROC curve constructed for TEPS and DDD showed a good correlation with an area under the curve (AUC) of 0.838. The curve indicated a cut point of six beyond which the incidence of DDD is very high
Five visually distinguishable diffusion patterns were observed (Table 2; Fig. 2).
Pattern A represented normal diffusion and was found in 170 discs.
Pattern B diffusion was found in EP with disc marrow contact and was seen in 100 discs.
Pattern C having focal leakage in the subchondral bone and PNP without extension to CNP was found in 63 discs.
Pattern D pattern showing more extensive leak with extension to CNP was seen in 12 discs.
Pattern E showing extensive pooling occupying the entire disc space was seen in 20 discs.
Alterations in diffusion showed a good correlation with DDD (Table 5). Only 12 of 170 (7%) with Pattern A had DDD compared to 19 of 20 (95%) for discs with pattern E (Trend chi-square, P < 0.001). The relationship between the TEPS and pattern of diffusion was analysed (Table 6). As the TEPS increased, the diffusion pattern also was found to increase significantly towards pattern E (trend chi-square, P < 0.001).
Table 5.
Distribution of Grades of Degeneration in various Diffusion patterns
| Pfirrmann’s grading | Diffusion patterns | Total | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| 1 | 46 | 19 | 3 | 0 | 0 | 68 |
| 2 | 99 | 46 | 14 | 2 | 0 | 161 |
| 3 | 13 | 18 | 8 | 2 | 1 | 42 |
| 4 | 12 | 15 | 32 | 2 | 0 | 61 |
| 5 | 0 | 2 | 6 | 6 | 19 | 33 |
| Total | 170 | 100 | 63 | 12 | 20 | 365 |
Trend chi square: P < 0.001
Table 6.
Distribution of diffusion patterns in various TEPS
| Diffusion | TEPS | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| A | 7 | 15 | 49 | 37 | 45 | 9 | 7 | 1 | 0 | 0 | 0 | 170 |
| B | 0 | 2 | 8 | 15 | 44 | 15 | 11 | 4 | 1 | 0 | 0 | 100 |
| C | 1 | 0 | 1 | 3 | 11 | 7 | 11 | 15 | 11 | 2 | 1 | 63 |
| D | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 3 | 2 | 0 | 2 | 12 |
| E | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 1 | 14 | 20 |
| Total | 8 | 17 | 58 | 56 | 101 | 33 | 31 | 26 | 15 | 3 | 17 | 365 |
With increasing TEPS, increasingly abnormal diffusion patterns were noted
TEPS total end plate score
Analysis of the TIC showed many individual variations, but four dominant types were seen (Fig. 3). Type I TIC was characterized by an initial lag period followed by an increase of signal intensity reaching peak enhancement at 6 h and the presence of the phenomenon of ‘Diffusion March’ and ‘end plate delay’. Type II TIC was similar but the peak enhancement was substantially lower and the time to achieve peak enhancement was also delayed. Type III TIC had a double peak curve with the first peak occurring before 2 h and the second peak occurring at 6 h. Type IV TIC was seen in patients with complete dye leakage. High signal intensity was achieved even at 2 h and the enhancement was maintained considerably over a long period.
Stepwise multiple regression analysis was performed with the grades of degeneration as the dependant variable and the factors of age, sex, level of discs, presence of Modic changes, instability, diffusion patterns and TEPS and AFD as independent variables. It showed that diffusion pattern was the most significant variable influencing DDD and accounting for 43% (t = 16.69, SE 0.043; P < 0.001) followed by age and TEPS.
Oral nimodipine study
The details of the signal intensities of various regions of interest before and subsequent to the administration of nimodipine is furnished in Table 7. Nimodipine was found to increase the signal intensity in all ROI significantly, both pre-contrast and also at all time intervals (Figs. 7, 8). The time at which the maximum difference was achieved however differed being at 2 h in the vertebral body, subchondral bone and End plate zone; 4 h in the PNP and 12 h in the CNP. Although the post nimodipine studies showed an increase in signal intensity at all time periods , the maximum difference achieved in the vertebral body was 18 at 2 h; at subchondral bone was 17.9 at 2 h; at EP was 9.2 at 2 h; at PNP was 17.6 at 4 h and at CNP was 16.15 at 12 h. It was interesting to note that following nimodipine, the maximum signal intensity was achieved earlier, was greater and was sustained over a longer period of time.
Table 7.
Increase in Signal Intensity following Nimodipine in various regions of Interest at various time intervals
| Time after contrast | Body | Subchondral bone | Endplate | PNP | CNP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Increase in SI | Increase in percentage (%) | p value | Average Increase in SI | Increase in percentage (%) | P value | Average Increase in SI | Increase in percentage (%) | p value | Average Increase in SI | Increase in percentage (%) | p value | Average Increase in SI | Increase in percentage (%) | P value | |
| 0 min | 11.29 | 7 | 0 | 12.68 | 8.94 | 0 | 5.15 | 7.19 | 0 | 6.63 | 5.29 | 0 | 5.5 | 4.42 | 0 |
| 10 min | 15.84 | 7.61 | 0.01 | 15.08 | 7.98 | 0 | 1.93 | 2.23 | 0 | 9.18 | 7.14 | 0 | 6.2 | 4.85 | 0 |
| 2 h | 18 | 10.24 | 0 | 17.98 | 11.35 | 0 | 9.2 | 10.96 | 0 | 16.9 | 13.02 | 0 | 10.85 | 8.69 | 0.03 |
| 4 h | 13.77 | 8.31 | 0 | 14.07 | 9.67 | 0 | 3.7 | 4.61 | 0 | 17.63 | 13.59 | 0 | 15.67 | 12.62 | 0 |
| 6 h | 7.96 | 4.57 | 0.01 | 7.65 | 4.83 | 0 | 5.7 | 6.86 | 0 | 11.93 | 8.48 | 0 | 9.7 | 7.04 | 0 |
| 12 h | 7.21 | 4.27 | 0.03 | 7.88 | 5.11 | 0 | 6.78 | 8.7 | 0 | 14.4 | 10.38 | 0.01 | 16.15 | 11.85 | 0.05 |
| 24 h | 6.58 | 3.88 | 0.02 | 8.42 | 5.51 | 0 | 4.3 | 5.66 | 0 | 10.83 | 7.94 | 0 | 11.4 | 8.43 | 0 |
Fig. 7.
The graphical representation of the signal intensities in end-plate zone. It is noted that post-drug signal intensity is high both pre-contrast and post-contrast and at all time periods
Fig. 8.
The graphical representation of the signal intensities in CNP. It is noted that post-drug signal intensity is high both pre-contrast and post-contrast and at all time periods
Discussion
Precise understanding of when a healthy disc starts ageing or degenerating and the exact difference between both is an urgent need [2, 12, 46]. It will help epidemiologists to identify risk factors, medico legal experts to differentiate disease from normal changes and help clinicians strategize treatment [2].
Unfortunately, universally acceptable definitions for both are still not available. Regardless of the definition used, DDD is known to increase with age [2, 12, 21, 44, 48, 77], is most common in the lower lumbar spine [12, 21, 44, 48] and is influenced by a myriad of factors such as genetic inheritance [4, 65, 72], ageing [2, 34, 38, 65], decreased nutrition [12, 21, 28, 29, 44, 47, 51, 52, 59, 62, 70, 76, 77], mechanical overloading [1, 25, 26, 27, 30, 40, 41, 81] and miscellaneous factors like smoking [24]. Although multifactorial, decreased nutrition and structural failure affecting the mechanical environment of the NP are considered to be the two important pathways for disc degeneration. The EP has attracted attention as its integrity and function is crucial to the maintenance of mechanical environment and also proper nutrition to the avascular disc [5, 11, 12, 21, 23, 44, 47, 48, 50, 59, 64, 71, 77, 78]. However, it is a weak structure exhibiting micro cracks and damage even from infancy and progressing with age [9, 59, 60]. While minor breaks may heal, macro breaks can lead to structural failure and damage the discs by a variety of methods [5, 11, 12, 17, 21, 23, 44, 47, 48, 50, 59, 64, 71, 77, 78].
There is a volume of literature on the role of EP in DDD [5, 11, 12, 17, 21, 44, 48, 50, 59, 64, 71, 77, 78]. They are mostly based on laboratory studies [70, 76], animal studies [28, 29, 51, 62] and cadaveric studies [9]. There is still no method described to quantify the end plate damage and to study the effects in vivo. MRI is the most commonly used investigation to assess the status of discs and a MRI based radiological score of EPD will be most useful. While the other two components of the disc—the annulus and the nucleus pulposus are clearly defined in the MRI, a clear MRI description of the EP is not available in literature. Modic et al. [45] have described three types of changes in the endplate region which are now popularly termed as ‘EP changes’. However, these are changes in the vertebral marrow and subchondral bone and do not depict the status of the EP. Uniform hypo-intense bands that separate the nucleus pulposus and the subchondral bone and clearly seen in T1 and T2 weighted images have been reported to functionally represent the endplate in the MRI [59, 60] (Fig. 9). The present study also noted that EP damages of varying severity can be identified as breaks in the hypointense band (Fig. 1).
Fig. 9.
The existing phrase “endplate changes,” which has already found wide usage and acceptance in the literature causes confusion of the exact representation of EP in literature. The MRI frequently shows signal intensity changes of the subchondral bone (gray arrows), which have been termed “endplate changes.” The three types of changes that have been described actually represent the MRI appearance of the changes in the vertebral bone marrow. b–d The changes frequently extend into the body and are not those of the endplate. In the normal T1 MRI picture, there is a hypointense zone between the subchondral bone and the nucleus pulposus (white arrows), which probably represents the endplate rather than the hyperintense zone of the bone margins (a). The zone is anatomically distinct and functionally represents the endplate, as it is sharply demarcated from the bone and the nucleus pulposus by being hypointense by at least 60–100 U. The time–intensity curve of this zone is also distinct from the neighboring bone and nucleus pulposus. Anatomic alterations of this zone seen in MRI were seen to effect substantial changes in the diffusion pattern of the disc [59, 60]
Type I EP represented a healthy EP with normal diffusion. An uninterrupted band of diffusion parallel to EPs and progressively moving to the centre was seen. Peak enhancement was achieved sequentially in the vertebral bone at 10 min, end plate at 2 h and peripheral and central nucleus pulposus at 6 h (diffusion march) [59, 60]. An intact but thinned out EP was classified separately as Type II. Although the diffusion pattern resembled the normal, a separate classification was made for thinning as focal thinning of the EP has been noted to increase the probability for future structural damage [6, 46]. Type III EP had minor breaks with disc marrow contact zones demonstrated by enhancement. There was early enhancement of the PNP at the site of the break, but normal diffusion pattern was still present in the remaining areas. There was also no change in the matrix nor reduction of disc height. Whether these focal breaks represent failure of EP at areas of vascular channels and whether they would heal or progress to a larger structural failure is unknown [6–8, 33, 46, 54]. However, the alteration in diffusion pattern was restricted and the incidence of degeneration was low. Type IV, Type V and Type VI end plates showed breaks with depression of subchondral bone of incremental severity (Fig. 1) and would fit the traditional description of Schmorl’s nodes. Large disc marrow contact channels were established with pooling of dye to variable degree. An incremental end plate score from 1 to 6 was allotted to represent the increased damage from Type I to Type IV.
The calculated TEPS for 365 discs was found to have a good correlation to the incidence of DDD. Only one of the 83 discs (1.2%) with TEPS below 4 had degeneration and the incidence increased to 14% when score was 5, 16.8% in 6, 27.2% in 7, 41.9% in 8, 57.6% in 9, 86.7% in 10 and 90% in 11 and 12 (Trend Chi square, P < 0.001). TEPS had an over-riding influence on degeneration with significant correlation at 1% level irrespective of the level of the disc or the age of the patient (Table 4). Irrespective of age, discs were healthy when the EPs were intact and were degenerated when EPs were damaged. This explains the frequent observation of elderly patients having ‘young discs’ and vice versa.
The relationship of TEPS on DDD was analysed by constructing a logistic curve [13]. The influence of TEPS on DDD was found to be ‘slow’ till a score of six was achieved after which there was a rapid response with severe increase in DDD for each unit increase in TEPS. Beyond a score of nine, the response was asymptote as majority of the discs had undergone maximum degeneration changes. The curve analysis indicated a score of six to be a critical score beyond which the incidence of DDD increased many fold. That a score of six was critical was also confirmed by an ROC curve which showed a good correlation between TEPS and DDD.
What does a critical TEPS of six mean in clinical practice? The risk of DDD is low in the presence of a normal EP (Type I), thinning of EP (Type II) or only focal disc marrow contacts without breaks (Type III). Histological studies have shown that significant temporo-spatial variations of histological and macroscopic levels of DDD can occur regionally without affecting the entire disc [9]. Our study also showed that a disc marrow contact without a break in end plate was found to produce only patchy changes adjacent to the marrow contact zones with no major alteration in the matrix or diffusion pattern of the disc. The incidence of such small breaks increases with age [9, 59] and these may heal with inflammation and sclerosis and be the cause for a decrease in the density of pores with increasing age [77]. In contrast, the incidence of DDD is high with a TEPS score of six or above. This score is usually achieved only when there is a macro break with large areas of pooling in the subchondral bone extending into the peripheral and central nucleus pulposus. The incidence of DDD was only 10.8% (26 of 240) when the EP was intact Type I, II and III) compared to 54.5% (68 of 125) when the EPs were damaged even minimally (Type IV, V and VI) (P < 0.001). This indicated that end plate damage reaching a TEPS of six was associated with a high incidence of DDD.
While most of the discs with a score of 6 or above were degenerated, 49 were still in Pfirrmann grade II or III and did not show complete degeneration. Injuries affecting the inner annulus and EPs have been shown to be incapable of healing and always followed by degeneration [1]. However, degeneration is a slow process [2] and it is possible that these discs are still in the process of slow evolution of degeneration. Perhaps these discs should be considered as ‘degenerating’ or ‘discs at risk’ for severe degeneration.
Alterations in diffusion
Discs depend entirely on diffusion for transport of small molecules and on bulk fluid flow for larger molecules [63, 75, 76]. Impairment of diffusion is critical as chronic lack of oxygen makes disc cells quiescent and lack of glucose kills them [31]. Our previous work has shown that diffusion can be assessed accurately by post-contrast serial MRI studies [59, 60]. In the present study, we identified differences in visual patterns and also TIC by measurement which correlated well with EP damage. When the EP was intact (Type I and II), the normal diffusion pattern A was found. Small disc marrow contacts without macro breaks or subchondral bone involvement did not change the overall diffusion pattern and hence the risk for DDD was low. The diffusion pattern was completely altered once there were macro breaks, the abnormality increasing progressively from Type IV to Type VI EP. The large disc marrow contact channels in these types of EPs resulted in pooling of the dye which was seen only in the periphery in Type IV but to occupy the entire disc space in Type VI. Pooling must not be mistaken for increased diffusion and nutrition as it represents vascularisation of the disc space and extra cellular accumulation of the dye, both indicating matrix degradation. Increasing abnormalities in diffusion was found to correlate with increasing degeneration irrespective of age and level of disc.
Analysis of the individual TIC Curves showed many different patterns but generally four distinct patterns were obvious (Fig. 3). Type I TIC represented the normal pattern where there was an intact end plate (Fig. 3a). The average SIrise at peak enhancement was 40.5 ± 20.7. This pattern represented disc with intact end plates and no abnormality of the matrix.
In Type II TIC, the pattern was similar but the SIrise at peak enhancement was less than 20 and the Tmax was also achieved only by 12 h (Fig. 3b). This was seen in 27 discs which had the following in common:
There was no structural failure either by end plate or annulus fibrosis defects.
The peak enhancement was considerably reduced and was also achieved after a delay, usually only by12 h.
The disc height was maintained and there was no pooling or abnormal diffusion patterns.
This normal diffusion pattern but at a reduced rate without any abnormal patterns, suggests that these discs are in the process of ageing rather than degeneration.
Type III TIC showed a double peak which has been noted in degenerating discs [59, 60]. These discs had areas of disc marrow contacts and the initial peak probably represented abnormal early enhancement due to patchy vascularisation at these regions. The second peak probably indicated normal diffusion occurring in the areas of normal matrix from the adjacent normal end plates.
Type IV TIC was seen in discs with extensive pooling of the dye. There was abnormal early enhancement even by 10 min over the entire region of the disc. This enhancement was maintained for prolonged periods and often over the entire 12 h period. This pattern represented a disc in end stages of degeneration.
Abnormalities in diffusion reflect indirectly the nutritional status of the disc. Type I TIC indicates a disc with intact nutritional pathways. Type II TIC represents a structurally intact disc with reduced nutrition probably due to reduced porosity and sclerosed end plates. Type III and IV TICs represent discs with structural failure and severe damage to nutritional pathways. This raised the question whether biological methods of treatment would be successful in discs exhibiting Type III or Type IV TICs.
Health, ageing and degeneration
Observation of three distinct groups of discs, those with normal structure and normal diffusion; those with normal structure and reduced diffusion and those with abnormal structure and altered diffusion brings up the question if they represent the three different entities namely health, ageing and degeneration. Oxford Dictionary defines ageing as ‘length of time that a person or thing has existed’ whereas degeneration has its origin from the latin word ‘degeneratus’ which means ‘no longer of its kind’. Ageing is a normal process but with reduced function whereas degeneration is a condition with altered function. Suggestions have been made that segregation of ageing and degeneration in the disc must be on anatomical and physiological basis [2, 6]. Our study also supports this concept and we propose the following definitions:
‘Healthy discs’ are those which are structurally intact and functionally normal irrespective of age of patient. They can be identified in vivo by having Type I end plate, pattern A diffusion and Type I TIC curve. They are Pfirrmann Grade I and have normal disc height and structurally intact.
‘Ageing discs’ are those which are structurally intact but with reduced diffusion, irrespective of age of patient. They can be identified in vivo by absence of EP damage, pattern A diffusion and Type II TIC curve. These discs can present with different hues of ‘black discs’ and can be Pfirrmann II, III, or IV. There is no structural failure and disc height is maintained. The reduced diffusion is probably secondary to poor permeability of the EP secondary to progressive mineralization and sclerosis that have been noted with age.
‘Degenerated discs’ are those that have structural failure and abnormal diffusion irrespective of age of patient. They can be identified in vivo by the presence of EP damage (Type IV, V and VI EP), abnormal patterns of diffusion with various grades of pooling of the dye (pattern C, D or E) and a TIC Type III or IV. Type III TIC has a double peak pattern which represents probably a ‘degenerating disc’ and Type IV TIC indicates a completely degenerated disc which has no evidence of normal matrix.
Pooling of the dye in the nucleus pulposus is due to leak through large disc marrow contact channels and vascularisation of the disc matrix. Vascularisation of the disc has been found to be associated with autoimmune reactions, accumulation of immunoglobulins and destruction of nucleus pulposus and persistent marrow disc channels are a source of loss of protein complexes [55, 68]. The end result would be a nucleus pulposus which is ‘degeneratus’ or ‘no longer of its kind’. Thus pooling of the dye in nucleus pulposus strongly indicates destroyed matrix and can be considered as a critical radiological sign to differentiate degeneration and ageing. The source of leakage of the dye is usually the EP break but also can be from the posterior or anterior annulus fibrosus in the presence of disc bulge or instability (Fig. 10).
Fig. 10.
Structural failure leading to disc degeneration can be identified by post-contrast diffusion studies. Here, the leakage of the dye is from the end plate (a); from the tear in the posterior annulus (b) and in the anterior annulus(c)
Post-contrast MRI allows the identification of healthy, ageing, degenerating and completely degenerated discs in patients on the basis of alterations in structure and function (Table 8). It has also identified that ageing and degeneration are dependant on the status of the end plate. An ageing and degenerating disc may present as black disc but could be differentiated on the basis of the diffusion patterns and TIC (Fig. 11). However, some difficulty will exist since discs starts degenerating from a very early age and are also known to have patchy changes of degeneration. So, in some discs both may overlap and may be difficult to distinguish.
Table 8.
Characteristics of healthy, ageing and degenerative discs
| Healthy | Ageing | Degeneration | |
|---|---|---|---|
| Structural failure | Absent | Absent | Present |
| End plate Type | Type I or II | Type I or II or III | Type IV or V or VI |
| Diffusion pattern | Normal | Reduced | Abnormal |
| TIC | Type I | Type II | Type III , IV |
| Pfirrmann grading | Grade I | Grade II , III and IV | Grade IV and V |
Fig. 11.
Ageing versus degeneration. Disc showing intermediary changes such as black disc often create confusion between ageing and degeneration. Diffusion pattern can help to identify the status of the disc. Here, there are two ‘black discs’ which appear similar in pre-contrast MRI but show two different diffusion patterns. The disc in (a), although black, shows typical diffusion bands without any abnormal leak. This signifies reduced physiology and indicates ageing. In comparison, the disc in (b) is a black disc with EP damage and shows a abnormal leak pattern indicating altered diffusion and could be termed as ‘degenerating disc’
Value of post-contrast studies in deciding biological treatment
There are multiple reports attempting to halt or even reverse disc degeneration by biological treatment methods such as the use of recombinant proteins, cytokines and growth factor [15, 19, 61, 82], molecular therapy [3, 35, 39, 73, 83], gene transfer techniques [53, 56, 79] and stem cell therapy [14, 66, 67, 84]. Before these methods are ready for clinical usage, it is important to establish firm guidelines for indications. Recipients of this treatment must be carefully selected. Biological therapies may succeed in ageing discs with normal endplate and normal diffusion pattern since this will ensure adequate nutrition for the implanted cells. On the contrary these therapies will fail when employed in a degenerated disc which have altered mechanical and nutritional environment. Our study indicates that a TEPS of six and above and discs showing gross pooling of the dye in the nucleus pulposus may not be suitable for biological and regenerative therapies (Fig. 12).
Fig. 12.
The spectrum of treatment available for symptomatic DDD. Biological treatment and regenerative medicine therapies would be suitable for discs with a TEPS score less than six and with a diffusion pattern of a and b which indicate good nutritional status. Motion preservation techniques with distraction and unloading the disc have also showed good results. The above therapy would probably fail in discs with established degeneration or in discs with a TEPS of seven and more or a diffusion pattern of c, d or e. They would probably require arthroplasty or fusion
Oral nimodipine study
Pharmacological enhancement of diffusion offers exciting possibilities of halting or reversing DDD. The capillaries in the end plate region have muscuranic receptors that regulate blood flow in response to external stimulus [10, 80]. And histological studies by Turgut et al. [74] have proved that treatment with nimodipine increase the vascular channel counts in rats. They postulated that increased proportion of vascular contacts at the end-plate might be beneficial to the disc by increasing nutrition of the disc and suggested for further studies in humans.
In our study, nimodipine was found to increase the signal intensity for all ROI significantly at all time intervals including the base values (pre-contrast post-nimodipine) (P < 0.01). In the endplate zone (EPZ) which represents the anatomical cartilaginous endplate, there was a significant increase in the mean peak signal intensity by 8% documenting the beneficial effects of nimodipine on endplate vascularity in human endplates. More importantly, this led to an increase in endplate intensity by an average of 14.7 accounting for 11% increase. The results of our study is the first documentation in literature on the favorable effects of nimodipine on endplate vascularity with resultant increase in disc diffusion in humans in in vivo conditions. The exact mechanism of the increase however remains to be established.
The fact that nimodipine can increase the diffusion across end plate opens up the avenue for medical management of DDD in future. However detailed studies in this context are required to derive firm conclusions. The exact mechanism of action of nimodipine, the duration of treatment, whether nimodipine will act on degenerating or degenerated discs is all the key issues of concern. All these need to be answered before embarking on the clinical trials of pharmacological enhancement.
Limitations of the study
The study is purely a radiological assessment of degeneration and the clinical symptoms have not been considered. Another limitation is that disc degeneration being an ongoing phenomenon needs a serial study over a period of time, which was not done in this study. It would have also been very useful to have histological supportive data to explain the changes in the endplate and nucleus pulposus but this is not obviously possible in an in vivo human study.
Conclusion
Our study confirms that damage to the endplate may be the initiating factor for disc degeneration by both altering the mechanical environment and also affecting the nutritional pathways. An incremental score to quantify the endplate damage by plain MRI studies has been devised which has been shown to have a good correlation with disc degeneration irrespective of the level or age of the patient. A logistic curve and ROC for TEPS and DDD indicated a critical score of ‘6’, beyond which there is a high incidence of degeneration. Five different diffusion patterns have been documented and pooling of the dye in the disc matrix has been identified as an important feature of DDD. Based on the diffusion pattern and TIC, definition for healthy, ageing and degenerated discs has been proposed. A TEPS of more than six and ‘pooling of dye’ in CNP represent structural failure and altered nutrition and may indicate an environment unsuitable for biological treatments and regenerative therapy. Our study also is the first in literature to document the feasibility of pharmacological modulation of endplate vascularity and disc diffusion.
Abbreviations
- MRI
Magnetic resonance imaging
- EP
End plate
- EPZ
End plate zone
- NP
Nucleus pulposus
- SCB
Sub-chondral bone
- VB
Vertebral body
- PNP
Peripheral nucleus pulposus
- CNP
Central nucleus pulposus
- DDD
Degenerative disc disease
- AFD
Annulus fibrosus defect
- TEPS
Total end plate score
- EPD
End plate defect
- Tmax
Time taken to achieve peak enhancement percentage
- SI
Signal intensity
- SIbase
Baseline signal intensity in pre-contrast MRI
- SImax
Maximum signal intensity within 12 h
- SItp
Signal intensity at a particular time period
References
- 1.Adams MA, Freeman BJC, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 4.Annunen S, Paassilta P, Lohiniva J, et al. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou J, Goudsouzian NM, Heathfield TF, et al. The human lumbar endplate: Evidence of changes in biosynthesis and denaturation of the extra-cellular matrix with growth, maturation, ageing, and degeneration. Spine. 1996;21:1153–1161. doi: 10.1097/00007632-199605150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Ariga K, Miyamoto S, Nakase T, et al. The relationship between apoptosis of endplate chondrocytes and ageing and degeneration of the intervertebral disc. Spine. 2001;26:2414–2420. doi: 10.1097/00007632-200111150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Benoist M. Natural history of the ageing spine. Eur Spine J. 2003;12(Suppl 2):S86–S89. doi: 10.1007/s00586-003-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernick S, Caillet R. Vertebral end-plate changes with ageing of human vertebrae. Spine. 1982;7:97–102. doi: 10.1097/00007632-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Brown MF, Hukkanen MVJ, McCarthy ID, Redfern DRM, Batten JJ, Crock HV, Hughes SPF, Polak JM. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79-B:147–153. doi: 10.1302/0301-620X.79B1.6814. [DOI] [PubMed] [Google Scholar]
- 11.Buckwalter JA. Fine structural studies of the human intervertebral disc. In: White AA, Gorden SL, editors. Idiopathic low back pain. St. Louis: CV Mosby; 1982. pp. 108–43. [Google Scholar]
- 12.Buckwalter JA. Spine update: ageing and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Cerny LC, Stasiw DM, Zuk W. The logistic curve for the fitting of sigmoidal data. Physiol Chem Phys. 1981;13(3):221–230. [PubMed] [Google Scholar]
- 14.Cheung KM, Ho G, Leung VY, et al. The effect of severity of disc degeneration on mesenchymal stem cells’ ability to regenerate the intervertebral disc: a rabbit model. Eur Cell Mater. 2005;10(Suppl 3):45. [Google Scholar]
- 15.Crean JK, Roberts S, Jaffray DC et al. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine 199, 722:2877–2884 [DOI] [PubMed]
- 16.Edwards WT, Zheng Y, Ferrara LA, Yuan HA. Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine. 2001;26:218–225. doi: 10.1097/00007632-200101150-00019. [DOI] [PubMed] [Google Scholar]
- 17.Eyre DR. Collagens of the disc. In: Ghosh P, editor. The biology of the intervertebral disc, vol I. Boca Raton: CRC Press, Inc; 1988. pp. 171–188. [Google Scholar]
- 18.Goel VK, Monroe BT, Gilbertson LG, et al. Interlaminar shear stresses and laminae separation in a disc. Spine. 1995;20:689–698. [PubMed] [Google Scholar]
- 19.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:612–626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 20.Greenfield ML, Kuhn JE, Wojtys EM. A statistics primer: validity and reliability. Am J Sports Med. 1998;26:483–485. doi: 10.1177/03635465980260032401. [DOI] [PubMed] [Google Scholar]
- 21.Gruber HE, Hanley EN., Jr Recent advances in disc cell biology. Spine. 2003;28:186–193. doi: 10.1097/00007632-200301150-00017. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Hernández R, Coll T, Rachitzky P, Armas-Hernández MJ, Armas-Padilla MC, Velasco M, Rizzo A. Comparison of two nimodipine formulations in healthy volunteers. J Hum Hypertens. 2002;16(S1):S142–S144. doi: 10.1038/sj.jhh.1001361. [DOI] [PubMed] [Google Scholar]
- 23.Holm S, Holm AK, Ekstrom L, et al. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004;17:64–71. doi: 10.1097/00024720-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93:91–99. doi: 10.1517/03009734000000042. [DOI] [PubMed] [Google Scholar]
- 25.Hutton WC, Elmer WA, Boden SD, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine. 1999;24:1507–1515. doi: 10.1097/00007632-199908010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hutton WC, Toribatake Y, Elmer WA, et al. The effect of compressive force applied to the intervertebral disc in vivo: a study of proteoglycans and collagen. Spine. 1998;23:2524–2537. doi: 10.1097/00007632-199812010-00007. [DOI] [PubMed] [Google Scholar]
- 27.Hutton WC, Yoon ST, Elmer WA, et al. Effect of tail suspension (or simulated weightlessness) on the lumbar intervertebral disc: study of proteoglycans and collagen. Spine. 2002;27:1286–1290. doi: 10.1097/00007632-200206150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim MA, Haughton VM, Hyde JS. Effect of disk maturation on diffusion of low molecular weight gadolinium complexes:An experimental study in rabbits. Am J Neuroradiol. 1995;16:1307–1311. [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim MA., Jesmanowicz A, Hyde JS, et al. Contrast enhancement of normal intervertebral disks: time and dose dependance. Am J Neuroradiol. 1994;15:419–423. [PMC free article] [PubMed] [Google Scholar]
- 30.Ishihara H, McNally DS, Urban JPG, et al. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80:839–846. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- 31.Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MC, Kelly AP, Brant-Zawadzki MN. MRI of degenerative disease of the lumbar spine. Magn Reson Q. 1994;10:173–190. [PubMed] [Google Scholar]
- 33.Kang JD, Stefanovic-Racic M, McIntyre LA, et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2 and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 34.Kauppila LI. Prevalence of stenotic changes in arteries supplying the lumbar spine. A postmortem angiographic study on 140 subjects. Ann Rheum Dis. 1997;56:591–595. doi: 10.1136/ard.56.10.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami M, Matsumoto T, Hashizume H, et al. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine. 2005;30:1933–1939. doi: 10.1097/01.brs.0000176319.78887.64. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi Y, Osada R, Kanamori M, et al. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y. Prediction of peripheral tears in the annulus of the intervertebral disc. Spine. 2000;25:1771–1774. doi: 10.1097/00007632-200007150-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kurunlahti M, Tervonen O, Vanharanta H, et al. Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine. 1999;24:2080–2084. doi: 10.1097/00007632-199910150-00003. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Leo BM, Beck G, et al. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine. 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 40.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1482. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 41.Lotz JC, Hsieh AH, Walsh AL, et al. Mechanobiology of the intervertebral disc. Biochem Soc Trans. 2002;30:853–858. doi: 10.1042/BST0300853. [DOI] [PubMed] [Google Scholar]
- 42.Lu YM, Hutton WC, Gharpuray VM. Do bending, twisting, and diurnal fluid changes in the disc affect the propensity to prolapse? A viscoelastic finite element model. Spine. 1996;21:2570–2579. doi: 10.1097/00007632-199611150-00006. [DOI] [PubMed] [Google Scholar]
- 43.MacLean JJ, Lee CR, Grad S, et al. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–981. doi: 10.1097/00007632-200305150-00004. [DOI] [PubMed] [Google Scholar]
- 44.Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus. 2002;13:1–6. doi: 10.3171/foc.2002.13.2.2. [DOI] [PubMed] [Google Scholar]
- 45.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 46.Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J. 2006;15(Suppl. 3):S333–S337. doi: 10.1007/s00586-006-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore RJ, Vernon-Roberts B, Fraser RD, et al. The origin and fate of herniated lumbar intervertebral disc tissue. Spine. 1996;21:2149–2155. doi: 10.1097/00007632-199609150-00018. [DOI] [PubMed] [Google Scholar]
- 48.Nachemson AL. Newest knowledge of low back pain: a critical look. Clin Orthop. 1992;279:8–20. [PubMed] [Google Scholar]
- 49.Natarajan RN, Andersson GBJ. The influence of lumbar disc height and cross-sectional area on the mechanical response of the disc to physiologic loading. Spine. 1999;24:1873–1881. doi: 10.1097/00007632-199909150-00003. [DOI] [PubMed] [Google Scholar]
- 50.Natarajan RN, Ke JH, Andersson GBJ. A model to study the disc degeneration process. Spine. 1994;19:259–265. doi: 10.1097/00007632-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen CM, Haughton VM, Papke RA, An H, Censky SC. Measuring diffusion of solutes into intervertebral disks with MR imaging and paramagnetic contrast medium. Am J Neuroradiol. 1998;19:1781–1784. [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen CM, Riley L, Ho KC, Xu R, An H, Haughton VM. Effect of degeneration of the intervertebral discs on the process of diffusion. Am J Neuroradiol. 1997;18:435–442. [PMC free article] [PubMed] [Google Scholar]
- 53.Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 54.Oda J, Tanaka H, Tsuzuki N. Intervertebral disc changes with ageing of human cervical vertebra from the neonate to the eighties. Spine. 1988;13:1205–1211. doi: 10.1097/00007632-198811000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Palmgren T, Gronblad M, Virri J, et al. An immunohistochemical study of nerve structures in the annulus fibrosus of human normal lumbar intervertebral discs. Spine. 1999;24:2075–2079. doi: 10.1097/00007632-199910150-00002. [DOI] [PubMed] [Google Scholar]
- 56.Paul R, Haydon RC, Cheng H, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755–763. doi: 10.1097/00007632-200304150-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfirrmann CWA, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 58.Qian M, Gallo JM. High-performance liquid chromatographic determination of the calcium channel blocker nimodipine in monkey plasma. J Chromatogr. 1992;578(2):316–320. doi: 10.1016/0378-4347(92)80432-P. [DOI] [PubMed] [Google Scholar]
- 59.Rajasekaran S, Naresh Babu J, Arun R, et al. ISSLS prize winner. A study of diffusion in human lumbar discs. Spine. 2004;29:2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 60.Rajasekaran S, Naresh-Babu J, Murugan S. Review of postcontrast MRI studies on diffusion of human lumbar discs. J Magn Reson Imaging. 2007;25(2):410–8. doi: 10.1002/jmri.20853. [DOI] [PubMed] [Google Scholar]
- 61.Roberts S, Caterson B, Menage J, et al. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 62.Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14:166–174. doi: 10.1097/00007632-198902000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Roberts S, Urban JPG, Evans H, et al. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 64.Roberts S, Caterson B, Evans H, et al. Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalisation study of animal and human tissues. Histochem J. 1994;26:402–411. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- 65.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in ageing, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–874. doi: 10.1042/BST0300869. [DOI] [PubMed] [Google Scholar]
- 66.Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/S0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 67.Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 68.Satoh K, Konno S, Nishiyama, et al. Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine. 1999;24:1980–1984. doi: 10.1097/00007632-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 69.Sauerland K, Raiss RX, Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis Cartil. 2003;11:343–350. doi: 10.1016/S1063-4584(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 70.Stairman JW, Holm S, Urban JPG. Factors influencing oxygen concentration gradients in the intervertebral disk: a theoretical analysis. Spine. 1991;16:444–449. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka M, Nakahara S, Inoue H. A pathologic study of discs in the elderly. Separation between the cartilaginous endplate and the vertebral body. Spine. 1993;18:1456–1462. doi: 10.1097/00007632-199310001-00034. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi M, Haro H, Wakabayashi Y, et al. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491–495. doi: 10.1302/0301-620X.83B4.11617. [DOI] [PubMed] [Google Scholar]
- 73.Takegami K, An HS, Kumano F, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–238. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Turgut M, Uysal A, Uslu S, Tavus N, Yurtseven ME. The effects of calcium channel antagonist nimodipine on end-plate vascularity of the degenerated intervertebral disc in rats. J Clin Neurosci. 2003;10(2):219–223. doi: 10.1016/S0967-5868(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 75.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology. 1978;15:203–221. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 76.Urban JPG, Maroudas A. Measurement of fixed charge density and partition coefficients in the intervertebral disc. Biochim Biophys Acta. 1979;586:166–178. [Google Scholar]
- 77.Vernon-Roberts B. Age-related and degenerative pathology of intervertebral discs and apophyseal joints. In: Jayson MIV, editor. The lumbar spine and back pain, 4th edn. Edinburgh: Churchill Livingstone; 1992. pp. 17–41. [Google Scholar]
- 78.Videman T, Sarna S, Battie MC, et al. The long-term effect of physical loading and exercise lifestyles on back-related symptoms, disability and spine pathology among men. Spine. 1995;20:699–709. doi: 10.1097/00007632-199503150-00011. [DOI] [PubMed] [Google Scholar]
- 79.Wallach CJ, Sobajima S, Watanabe Y, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 80.Wallace AL, Wyatt BC, McCarthy ID, Hughes SP. Humoral regulation of blood flow in the vertebral endplate. Spine. 1994;19(12):1324–1328. doi: 10.1097/00007632-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–337. doi: 10.1016/S0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- 82.Weiler C, Nerlich AG, Zipperer J, et al. SSE award competition in basic sciences: expression of major matrix metalloproteinases is associated with intervertebral disc degeneration and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29:2603–2611. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- 84.Zhang YG, Guo X, Xu P, et al. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop. 2005;430:219–226. doi: 10.1097/01.blo.0000146534.31120.cf. [DOI] [PubMed] [Google Scholar]
- 85.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]