Abstract
The aim of this cross-sectional case-control study is the comparison of the weight and height between a group of children with Scheuermann’s disease (SD) and a comparable group of healthy ones and also the correlation of them with the degree and the morphology of the kyphotic curve. Following a school-screening program of 10,057 school students, aged between 11 and 17 years old, 175 adolescents with Scheuermann’s disease were diagnosed. The mean height and weight of 175 adolescents diagnosed to have SD compared with this of a group of normal children taken randomly from the group of 9,882 healthy children screened. The control group was comparable with the study group concerning age (p = 0.605) and sex. The weight, height and body mass index (BMI) were significantly lower in the healthy (control) group (p < 0.001). However, there was no correlation between weight (r = –0.019, p = 0.804), height (r = 0.053, p = 0.484) and BMI (r = –0.177, p = 0.019) with the magnitude of kyphotic curve. There was also no correlation between weight (r = –0.27, p = 0.722), height (r = –0.025, p = 0.744) and BMI (r = –0.038, p = 0.619) with Voutsinas index as well. Scheuermann’s disease is probably a multifactorial skeletal deformity. Weight and height do not seem to affect the magnitude and morphology of the main kyphotic curve in SD. It seems probably that this observation is not part of the pathogenetic mechanism of SD but a result of its cascade. The increased weight and height of these patients may be the secondary result of other disturbances (i.e. hormonal), which may play more crucial role in Scheuermann’s disease pathogenesis.
Keywords: Scheuermann’s disease, Deformity, School screening, Scheuermann’s pathogenesis, Thoracic kyphosis
Introduction
Scheuermann’s disease is a deformity of the spine that develops prior to puberty and becomes most prominent during the adolescent growth spurt. It is a common cause of structural kyphosis of thoracic or thoracolumbar-spine associated with wedged vertebral bodies and disturbances of the vertebral end plates [22]. The typical patient has age between 12 and 15 years with a round-shouldered appearance [22]. In most cases, the progression is slow and ceases as axial skeletal maturity is achieved [9].
The cause of Scheuermann’s disease is still unknown. Several theories have been suggested, concerning the increased release of growth hormone, defective formation of collagen fibrils with subsequent weakening of the vertebral end plates, juvenile osteoporosis, trauma, vitamin A deficiency, poliomyelitis and epiphysitis [1, 10, 13, 23]. A dominant autosomal inheritance pattern with high penetrance and variable expressivity has also been described for Scheuermann’s disease, suggesting that in some patients a genetic predisposition may be present [2, 4, 12].
Mechanical factors are also suspected to be responsible for the pathogenesis of Scheuermann’s disease [10, 11, 18, 20]. It was reported that children with Scheuermann’s disease are heavier and taller in comparison with healthy ones [11, 18–20]. In addition, it is widely supported that mechanical stress may play a role in the severity of kyphotic curve and symptoms [20]. However, it is not clear whether this observation is true and if it is related with SD pathogenesis. The aim of this cross-sectional case-control study was the comparison of the weight and height between a group of children with SD and a comparable group of healthy ones and also the correlation of the weight and height of patients with the degree and the morphology of the kyphotic curve.
Patients and methods
This study was approved by the Committee of Education and it was conducted in accordance with the World Medical Association Declaration of Helsinki, 1975, as revised in 1983. The parents of all students were informed about their children’s participation in the study and informed consent was obtained from them all.
This is a cross-sectional, case-control study concerning comparison of the weight and height of a group of 175 adolescents with Scheuermann’s disease and an age-matched group of healthy ones. The weight and height of adolescents with Scheuermann’s disease was correlated with the degree and deformity of the kyphotic curve.
Following a school-screening program of 10,057 school students, aged between 11 and 17 years, 175 adolescents with Scheuermann’s disease were diagnosed. The diagnosis of the disease was based on clinical and radiographic criteria. Scheuermann’s disease of the thoracic spine creates a rigid kyphosis. The flexible curve in postural kyphosis corrects with the forward bending test performed with the neck extended, whereas with Scheuermann’s disease the kyphosis accentuated. A compensatory cervical and lumbar hyperlordosis is also usually found in these patients. The children that presented, according to the examiners, kyphosis with the Adam’s test, hyperlordosis of lumbar spine, reported chronic pain in the thoracic, in thoracolumbar junction or in the lumbar region or children whose clinical examination was ambiguous were selected for radiological control.
Children whose lateral radiograph of the thoracolumbar-spine presenting one or more vertebrae with anterior wedging of their body ≥5°, vertebral end-plate irregularities combined with global kyphosis (upper surface of T3–under surface of T12) as measured by the Cobb angle >40° were considered as suffering from Scheuermann’s disease.
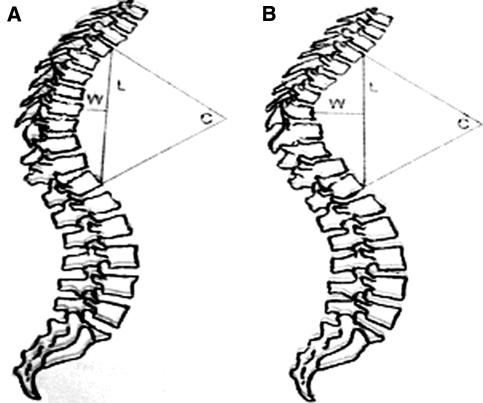
The age, the height in upright position with a tape measure and the weight of all children were recorded. Their body mass index was also estimated. The magnitude of the kyphotic curve was estimated from the upper surface of T3 to lower surface of T12, with Cobb’s method. Voutsinas index of the kyphosis was estimated, as well. Voutsinas index is defined as the quotient of W/L × 100, where L is the length of the curve while W is the maximum inside distance of the curve from the L line (Fig. 1).
Fig. 1.
Voutsinas index is defined as the quotient of W/L × 100, where L is the length of the curve while W is the maximum inside distance of the curve from the L line. When we are using Cobb’s method to measure the deformities, the degrees of curvature are identical (C)
All measurements were taken by one doctor. The mean height and weight of 175 adolescents were diagnosed to have SD, compared with this of a same size group of normal children taken randomly from the group of 9,882 healthy children screened. The control group was comparable with the study group concerning age and sex (Table 1).
Table 1.
Baseline characteristics of the study and control group
| Children with Scheuermann’s disease | Normal children | p | |
|---|---|---|---|
| N | 175 | 175 | |
| Maleb | 147 (84) | 147 (84) | |
| Femaleb | 28 (16) | 28 (16) | |
| Age (years)a | 13.07 (0.87) | 13.14 (0.84) | 0.266c |
| Height (cm)a | 166.8 (7.59) | 164.4 (6.3) | 0.002c |
| Weight (kg)a | 66.12 (4.04) | 61.2 (4.68) | 0.000c |
| BMI (kg/m2)a | 23.88 (2.38) | 22.68 (1.69) | 0.000c |
aThe values are given as the mean with the standard deviation in parentheses
bThe values are given as raw numbers with the percentages (%) in parentheses
cTests performed using Mann–Whitney test
The height and weight of adolescents with SD was correlated with the magnitude and severity of the kyphotic curve. These measurements were repeated for many other comparable groups concerning size and age taken randomly from the initial group of healthy children.
Statistical analysis
Standard statistical methods have been used for descriptive statistics. Normality of different groups’ data distribution was tested according to Kolmogorov–Smirnov test. All statistical tests were two-tailed. The alpha level for all analyses was set at 0.05. Analyses were performed with SPSS statistical software (SPSS, Chicago, IL, USA)
The Mann–Whitney test was used to calculate the statistical difference between the mean age, height and weight of children with Scheuermann's disease and normal children. The same test was used to calculate the statistical difference of the mean BMI between these two groups of children.
Spearman correlation coefficient was used to determine the correlation between the weight, the height and the BMI with the magnitude of kyphotic curve. The same test was used to correlate patients’ weight, height and BMI with Voutsinas index.
Partial correlation was used to explore these relationships again when controlling for age.
Results
Baseline characteristics of two groups are apparent in Table 1. Both groups consisted of exactly the same number of boys and girls and were comparable according to age (p = 0.266) (Table 1).
However, the mean height of healthy children was statistically significant, lower in comparison with the mean height of children with Scheuermann’s disease (p < 0.001). In addition, children diagnosed to have SD were significantly heavier in comparison with healthy ones (p = 0.002) (Table 1). Statistically significant difference was found between two groups, as far as the body mass index (BMI) of patients was concerned. The mean BMI of children with Scheuermann’s disease was greater in relation with this of control group (p < 0.001) (Table 1).
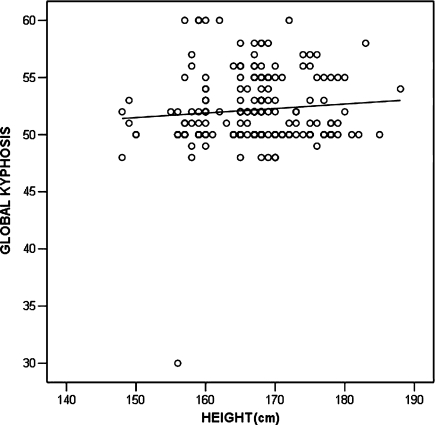
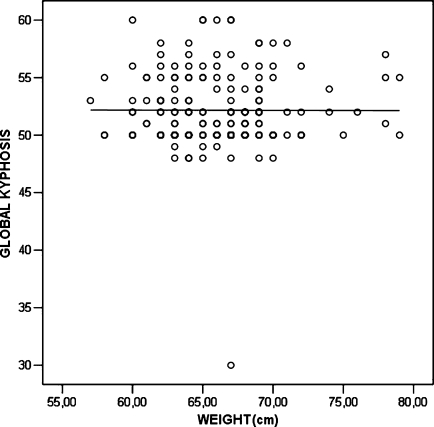
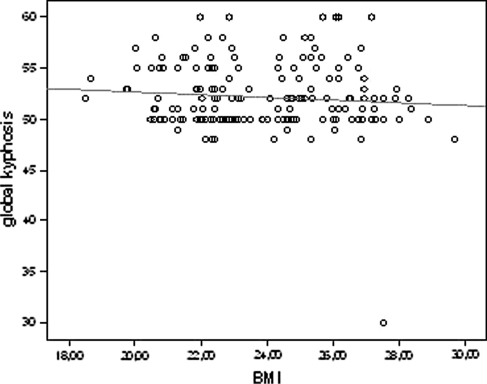
However, there was practically no correlation between weight (r = –0.019, p = 0.804), height (r = 0.053, p = 0.484) and BMI (r = –0.177, p = 0.019) with the magnitude of kyphotic curve (Figs. 2–4).
Fig. 3.
Correlation of height and global kyphosis (scatter plot with linear fit line)
Fig. 2.
Correlation of weight and global kyphosis (scatter plot with linear fit line)
Fig. 4.
Correlation of BMI (body mass index) and global kyphosis (scatter plot with linear fit line)
The correlation concerning BMI was statistically significant, however, the correlation coefficient was too low and negative and as a result, with no clinical significance.
There was also no significant correlation between the weight of children with Scheuermann’s disease (r = –0.27, p = 0.722), height (r = –0.025, p = 0.744) and BMI (r = –0.038, p = 0,619) with Voutsinas index. Age had very little effect on the strength of relationship between these variables (Table 2).
Table 2.
Correlations of height, weight and BMI with global kyphosis and Voutsinas index when controlling for age
| Weight | Height | BMI | |
|---|---|---|---|
| Global kyphosis | |||
| r | 0.006 | 0.109 | –0.140 |
| p | 0.941 | 0.152 | 0.065 |
| Voutsinas index | |||
| r | 0.053 | 0.112 | 0.050 |
| p | 0.490 | 0.142 | 0.516 |
Our measurements repeated for many other comparable groups concerning size and age taken randomly from the initial group of healthy children were of the same significance.
Discussion
Even though several theories have been proposed and many factors have been associated with the development of Scheuermann’s disease, both its exact pathogenetic mechanism as well as the existence of possible contributing factors to its development remain at the moment, more or less, unclear [1, 2, 10, 13, 23].
Hereditary and mechanical factors are suspected to be mainly responsible for the pathogenesis of the disease; although, they are probably intermingled, the role of each of these components is still a matter of interest [20].
It is mentioned before, but it is not defined whether children with Scheuermann’s disease are taller and heavier than normal ones. Scogland et al. [17] reported that patients with Scheuermann’s disease were taller than their normal peers. Ascani et al. [19] also noted a similar difference in height of healthy persons and affected ones. On the contrary, Scoles et al. [18] reported that there were no significant differences between the mean height of normal and affected individuals. This study also indicated that the mean weight of children with Scheuermann’s disease was rather greater than this of healthy group. Last but not least, these studies are deprived of statistical analysis that would allow reaching secure conclusions about the weight and height of children with Scheuermann’s disease.
In addition, most investigators agree that mechanical factors (repetitive strain, weight) have a significant role in the pathogenesis of Scheuermann’s kyphosis [10, 11, 18, 20]. However, it is obscure if the weight or the height of patients with SD is a parameter correlating with the genesis or the severity of the kyphotic deformity. Scheuermann himself theorized that mechanical factors were responsible for the kyphotic deformity, noting that, an increased incidence occurred in manual laborers [1]. Van Linthoudt et al. [20] reported identical twins presenting similar radiological lesions of localized Scheuermann’s disease, which were worse in one of a twin that was heavier and practiced strenuous sport activities. In contrast Graat et al. [6] reported monozygotic twins, with almost identical weight and height that had an intertwine variation of the kyphotic deformity.
There are no studies in the literature that examined the exact relation between the weight and height of patients with Scheuermann’s disease and the magnitude of the kyphotic curve; these are usually simple recordings of patients’ weight and height. None of them is a true comparative study between a group of children with Scheuermann’s disease and a control group of healthy ones. Our study is the first in the English literature that examines and compares the weight and height of adolescents with Scheuermann’s disease with a comparable group of healthy ones and also correlates the height and weight of patients with the magnitude and morphology of the kyphotic curve.
Our study confirmed that patients with SD are heavier and taller than healthy ones of the same sex and age. However, if this is a part of the pathogenetic mechanism of SD or a result of this is not clear.
A previous study has postulated that the spinal changes associated with Scheuermann’s kyphosis are the result of increased exerted stress on the anterior portion of the growth plate [18]. The greater weight increases the compressive forces on the anterior portion of thoracic vertebral bodies because in the thoracic region, the posterior components grow at a faster pace than their anterior counterparts, resulting in an uneven distribution of forces [5]. Probably, the greater height increases the compressive forces in the anterior portion of thoracic vertebrae, as well. Most of the tall children usually bend their trunk in daily activities more than required.
However, in our study, practically no statistically significant correlation was found between weight (r = –0.019, p = 0.804), height (r = 0.053, p = 0.484) and BMI (r = –0.177, p = 0.019) with the magnitude of kyphotic curve.
Voutsinas index is a measurement completing our knowledge about the morphology of the kyphotic curve. Two spinal curvatures may be quite different in magnitude but when we are using Cobb’s method to measure the deformities, the degrees of curvatures could be identical. The differences in the curves are more accurately reflected when the length of the curves (L) and their respective widths (W) are taken into consideration, as it was evaluated by Voutsinas index [21]. In our study, no statistically significant correlation was found between the weight of children with Scheuermann’s disease (r = –0.27, p = 0.722), height (r = –0.025, p = 0.744) and BMI (r = –0,038, p = 0.619) with Voutsinas index.
It is true that this study has certain limitations. The most important one is which radiographic criteria followed, in order to diagnose Scheuermann’s disease. A thorough search of the literature provides an evolvement of these criteria over time. Sorenson in 1964 suggested that three or more contiguous apical vertebrae that are wedged of a minimum of 5° each are required for diagnosis [1]. Drummond in 1987 suggested that two or more contiguous apical wedged vertebrae are enough for the diagnosis [1]. Finally, Sachs et al. [16] reduced the requirement to one or more vertebrae wedged 5° or more, for diagnosis of the disease.
In our study, the Sachs’ criteria for the diagnosis of disease were followed. The hypothesis of reducing the number of wedged vertebrae for diagnosis is that the number of affected vertebrae usually increases, as the disease and the deformity progress [1]. Therefore, reducing the requirement for diagnosis to one wedged vertebra leads to earlier diagnosis and no loss of affected child occurs.
Other limitations are the radiographic technique used, the range of vertebrae used for the Cobb angle measurement and the definition of normal kyphosis. All lateral X-ray films were taken using standard radiographic technique, with the student standing with his or her hands resting on a bar in front of the subject at the shoulder level. In a pilot study, this mode of placement proved not to change the configuration of the spine significantly [21]. We measured the thoracic kyphosis from the upper vertebral end plate of T3 to the lower vertebral end plate of T12 and the standard Cobb’s method was used for this measurement. In few cases in which T3 vertebra was not visible, the magnitude of the kyphotic curve was estimated from the upper surface of T4 to lower surface of T12 [3, 7]. We adopted the range of normal kyphosis in the growing adolescents as 20°–40°, as stated by the Scoliosis Research Society [19]. The range of these limitations affects the incidence of Scheuermann’s disease. In our study, the incidence of the disease was 1.7%. The prevalence of Scheuermann’s disease varies depending on inclusion criteria and has been reported to be between 0.4 and 8% [8, 14]. One large school-screening study involving nearly 20,000 students reported a rate of about 1% of students with Scheuermann’s disease [1].
Scheuermann’s disease is probably a multifactorial skeletal deformity. Children with Scheuermann’s disease seem to be heavier and taller in comparison with healthy ones. However, this parameter does not seem to affect the magnitude and morphology of the main kyphotic curve. It seems probably that this observation is not part of the pathogenetic mechanism of SD but a result of its cascade. The increased weight and height of these patients may be the secondary result of other disturbances (i.e. hormonal), which may play more crucial role in Scheuermann’s disease pathogenesis. A possible hypothesis concerns sex hormones or their precursors, which have been already measured in other pathological conditions of unknown origin [15].
Nevertheless, it is our belief that more studies may be needed in order to understand, the potential connection (if there is any) between weight, height and the magnitude of kyphotic curve, better.
References
- 1.Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70–75. doi: 10.1097/00008480-199902000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Axenovich TI, Zaidman AM, Zorkoltseva IV, Kalashnikova EV, Borodin PM. Segregation analysis of Scheuermann disease in ninety families from Siberia. Am J Med Genet. 2001;100:275–279. doi: 10.1002/ajmg.1290. [DOI] [PubMed] [Google Scholar]
- 3.Bernhardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine. 1989;14:717–721. doi: 10.1097/00007632-198907000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Damborg F, Engell V, Andersen M, Kyvik KO, Thomsen K. Prevalence, concordance and heritability of Scheuermann kyphosis based on a study of twins. J Bone Joint Surg Am. 2006;88(10):2133–2136. doi: 10.2106/JBJS.E.01302. [DOI] [PubMed] [Google Scholar]
- 5.Dimeglio A. Growth in pediatric orthopaedics. In: Morrissy RT, Weinstein SL, editors. Lovell and Winter’s pediatric orthopaedics. 5th edn. Philadelphia: JB Lippincott Company; 2001. pp. 33–59. [Google Scholar]
- 6.Graat HC, Rhijn LW, Schrander-Stumpel CT, Ooij A. Classical Scheuermann disease in male monozygotic twins: further support for the genetic etiology hypothesis. Spine. 2002;27(22):485–487. doi: 10.1097/00007632-200211150-00020. [DOI] [PubMed] [Google Scholar]
- 7.Harrison DE, Janik TJ, Harrison DD, Cailliet R, Harmon SF. Can the thoracic kyphosis be modeled with a simple geometric shape. J Spinal Disord. 2002;15(3):213–220. doi: 10.1097/00024720-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Heithoff KB, Gundry CR, Burton CV, Winter RB. Juvenile discogenic disease. Spine. 1994;19(3):335–340. doi: 10.1097/00007632-199402000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Lemire JJ, Mierau DR, Crawford CM, Dzus AK. Scheuermann’s juvenile kyphosis. J Manipulative Physiol Ther. 1996;19(3):195–201. [PubMed] [Google Scholar]
- 10.Lowe TG. Scheuermann disease. J Bone Joint Surg Am. 1990;72:940–945. [PubMed] [Google Scholar]
- 11.Lowe TG. Scheuermann’s disease. Orthop Clin. 1999;30(3):475–485. doi: 10.1016/S0030-5898(05)70100-0. [DOI] [PubMed] [Google Scholar]
- 12.Mc Kenzie L, Sillence D. Familial Scheuermann disease: a genetic and linkage study. J Med Genet. 1992;29:41–45. doi: 10.1136/jmg.29.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann kyphosis. J Bone Joint Surg. 1993;75-A:236–248. doi: 10.2106/00004623-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Nissinen M. Spinal posture during pubertal growth. Acta Paediatr. 1995;84:308–312. doi: 10.1111/j.1651-2227.1995.tb13634.x. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport R, Forest MG, Bayard F, Duval-Beaupere G, Blizzard RM, Migeon CJ. Plasma androgens and LH in scoliotic patients with premature pubarche. J Clin Endocrinol Metab. 1974;38(3):401–406. doi: 10.1210/jcem-38-3-401. [DOI] [PubMed] [Google Scholar]
- 16.Sachs B, Bradford D, Winter R, Lonstein J, Moe J, Willson S. Scheuermann kyphosis. J Bone Joint Surg Am. 1987;69(1):50–57. [PubMed] [Google Scholar]
- 17.Scogland LB, Steen H, Trygstad O. Spinal deformities in tall girls. Acta Orthop Scand. 1985;56(2):155–157. doi: 10.3109/17453678508994344. [DOI] [PubMed] [Google Scholar]
- 18.Scoles PV, Latimer BM, DiGiovanni BF, Vargo E, Bauza S, Jellema LM. Vertebral alterations in Scheuermann’s kyphosis. Spine. 1991;16(5):509–515. doi: 10.1097/00007632-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Tribus CB. Scheuermann’s kyphosis in adolescents and adults: diagnosis and management. J Am Acad Orthop Surg. 1998;6:36–43. doi: 10.5435/00124635-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Linthoudt D, Revel M. Similar radiologic lesions of localized Scheuermann’s disease of the lumbar spine in twin sisters. Spine. 1994;19(8):987–989. doi: 10.1097/00007632-199404150-00020. [DOI] [PubMed] [Google Scholar]
- 21.Voutsinas SA, MacEwen GD. Sagittal profiles of the spine. Clin Orthop Relat Res. 1986;210:235–242. [PubMed] [Google Scholar]
- 22.Warner WC, Kyphosis (2001) In: Morrissy RT, Weinstein SL (eds) Lovell and Winter’s pediatric orthopaedics, vol 2, 5th edn. JB Lippincott Company, Philadelphia, pp 750–757
- 23.Wenger DR, Frick SL. Scheuermann kyphosis. Spine. 1999;24(24):2630–2639. doi: 10.1097/00007632-199912150-00010. [DOI] [PubMed] [Google Scholar]