Abstract
Because neither the degree of constriction of the spinal canal considered to be symptomatic for lumbar spinal stenosis nor the relationship between the clinical appearance and the degree of a radiologically verified constriction is clear, a correlation of patient’s disability level and radiographic constriction of the lumbar spinal canal is of interest. The aim of this study was to establish a relationship between the degree of radiologically established anatomical stenosis and the severity of self-assessed Oswestry Disability Index in patients undergoing surgery for degenerative lumbar spinal stenosis. Sixty-three consecutive patients with degenerative lumbar spinal stenosis who were scheduled for elective surgery were enrolled in the study. All patients underwent preoperative magnetic resonance imaging and completed a self-assessment Oswestry Disability Index questionnaire. Quantitative image evaluation for lumbar spinal stenosis included the dural sac cross-sectional area, and qualitative evaluation of the lateral recess and foraminal stenosis were also performed. Every patient subsequently answered the national translation of the Oswestry Disability Index questionnaire and the percentage disability was calculated. Statistical analysis of the data was performed to seek a relationship between radiological stenosis and percentage disability recorded by the Oswestry Disability Index. Upon radiological assessment, 27 of the 63 patients evaluated had severe and 33 patients had moderate central dural sac stenosis; 11 had grade 3 and 27 had grade 2 nerve root compromise in the lateral recess; 22 had grade 3 and 37 had grade 2 foraminal stenosis. On the basis of the percentage disability score, of the 63 patients, 10 patients demonstrated mild disability, 13 patients moderate disability, 25 patients severe disability, 12 patients were crippled and three patients were bedridden. Radiologically, eight patients with severe central stenosis and nine patients with moderate lateral stenosis demonstrated only minimal disability on percentage Oswestry Disability Index scores. Statistical evaluation of central and lateral radiological stenosis versus Oswestry Disability Index percentage scores showed no significant correlation. In conclusion, lumbar spinal stenosis remains a clinico-radiological syndrome, and both the clinical picture and the magnetic resonance imaging findings are important when evaluating and discussing surgery with patients having this diagnosis. MR imaging has to be used to determine the levels to be decompressed.
Keywords: Spine, abnormalities; Spine, MR; Lumbar spinal stenosis; Oswestry Disability Index
Introduction
As a greater percentage of the general population become older, lumbar spinal stenosis (LSS) is a frequently encountered painful and potentially disabling condition [2, 28]. Degenerative LSS is defined as narrowing of the spinal canal, the lateral nerve root canals or the intervertebral neural foramina due to progressive hypertrophy of any of the surrounding osteocartilaginous and ligamentous elements, and may result in neurogenic or vascular compression of the contents of the spinal canal at one or more levels. The spinal canal demonstrates narrowing, attributed most frequently to acquired degenerative or arthritic changes such as hypertrophy of the articulations surrounding the canal, intervertebral disc herniation or bulges, hypertrophy of the ligamentum flavum, osteophyte formation and degenerative spondylolisthesis [2, 3, 19].
Anatomically, spinal stenosis is classified as (1) central, when it affects the spinal canal and dural sac, (2) foraminal, when it affects the spinal foramina, or (3) lateral, when it affects the lateral recess [14, 28, 29]. Although classically central and lateral stenoses are described as distinct entities, central and lateral lesions are linked in the genesis of the complaints in elderly patients with marked degenerative changes [29, 30]. In addition to its structural aspects, the pathology of LSS also has a dynamic component. Extension of the spine and axial loading contribute to further narrowing of both the central and lateral canals [20, 21, 26, 27].
In patients with symptomatic LSS, it is common to observe three or four different syndromes, including neurogenic claudication, nerve root compression, central lower back pain and non-radicular referred lower extremity pain [31]. The classic presentation of LSS is neurogenic claudication [4, 30]. Substantial reduction in walking tolerance because of neurogenic claudication is often the reason for seeking medical intervention [1, 22, 31].
The diagnosis is often delayed due to the insidious onset and slow progression of the disease and further complicated by frequent concomitant pathologies that coexist in the aging population, obscuring differential diagnosis [29]. Although there is a wide range of conservative treatments, the goal of surgery is to decompress and stabilize the stenotic area determined in the radiological examination to relieve pressure on the neurovascular structures. Thus, accurate diagnosis is critical to appropriate selection of therapy [19]. Since the degree of constriction of the spinal canal considered to be symptomatic for LSS is not clear, and the relationship between the clinical appearance and the degree of radiologically verified constriction is also not well understood, a correlation of a patient’s disability level and radiographic constriction of the lumbar spinal canal is of interest [13].
The sensitivity and specificity of magnetic resonance (MR) imaging for assessing individuals with LSS are reported to be higher than those of competing methods, with MR imaging outperforming computed tomography and myelography [9]. Measurements of the cross-sectional area of the dural sac are considered more effective in diagnosing central stenosis than measurements of the osseous spinal canal [25].
The Oswestry Disability Index (ODI) is a self-completed questionnaire by the patient that examines perceived levels of disability in 10 everyday activities of daily living to assign a subjective score of level of function. It is a simple condition-specific preferred multidimensional tool with advantages of easy patient comprehension, self-assessment and encompasses a wide domain of function, pain and limitation in health status [7, 8, 24].
The key question in this study was to determine the relationship between the degree of radiologically demonstrated anatomical stenosis and the patient’s disability level.
Materials and methods
Sixty-three consecutive patients with degenerative LSS scheduled for elective surgery were enrolled in this retrospective study. All patients included in the current study were referred for MR imaging with a clinical suspicion of spinal stenosis. The presenting symptoms were neurologic claudication or unilateral/bilateral sciatic pain, numbness or weakness with or without lower back pain. The duration of neurologic claudication was noted for each patient. All patients included in the study had either multilevel spinal stenosis (n = 52) or central and lateral stenosis at a single intervertebral level (n = 11). Additional anomalies of the lumbar spine such as additional lumbar vertebra or incomplete fusion of the dorsal arches were exclusion criteria, but there were no such patients.
All 63 patients underwent routine lumbar spine MR imaging using a 1.5 T magnet (Sonata, Siemens, Erlangen, Germany) with a dedicated receive-only spinal coil. The patients were placed in supine position with a cushion under both knees. The protocol comprised sagittal T1 (500–750/19 repetition time ms/echo time ms) and T2 weighted (3,000–6,000/99) turbo spin echo (TSE) imaging of the entire lumbar spine. The image matrix was 320 × 256, the field of view was 280 × 100 mm2, the section thickness was 3 mm, the intersection gap was 0.6–1.3 mm and the echo train lengths were 7 and 25 for T1 and T2 weighted imaging, respectively. In addition, transverse oblique T1 and T2 weighted TSE images (500–750/17 and 3,000–6,000/115, respectively) of intervertebral spaces were obtained. The image matrix was 320 × 225, the field of view was 200 × 100 mm2, the section thickness was 4 mm, the intersection gap was 1.2–1.8 mm and the echo train lengths were 7 and 25 for T1 and T2 weighted imaging, respectively.
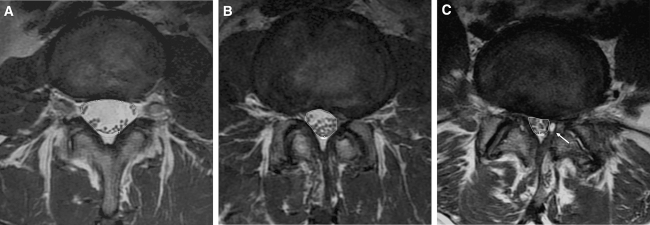
Quantitative and qualitative image evaluation for LSS was performed. The cross-sectional area of the dural sac was measured on the transverse angled sections through the central part of the disc on conventional MR images. The dural sac cross-sectional area (DSCSA) was calculated by using a measurement program on a diagnostic work-station (Magic View 1000; Siemens, Erlangen, Germany), as shown in Fig. 1. Nerve root compromise was subjectively analyzed in the lateral recess and foramina of the selected lumbar intervertebral levels independently by two observers.
Fig. 1.
Illustration of the dural sac cross-sectional area measurement technique. a Normal central spinal canal with a dural sac area of 222 mm2. b There is bulging of the disc and facet arthrosis causing a moderate spinal stenosis with a dural sac area of 98 mm2. c In addition to discal bulging and facet arthropathy, there is a left-sided synovial cyst (arrow) which further increases the spinal stenosis and dural sac compression (dural sac area of 57 mm2)
The quantitative criteria used for central anatomical LSS were as follows:
The DSCSA greater than 100 mm2 was considered normal; 76 to 100 mm2 was considered to be moderately stenotic and less than 76 mm2 was classified as severely stenotic. Nerve root compromise in the lateral recess was graded as follows: Grade 0, no contact of the disc with the nerve root; Grade 1, contact without deviation; Grade 2, nerve root deviation; Grade 3, nerve root compression. Nerve root compression was considered to be present when the root was deformed [32]. Criteria for foraminal qualitative assessment were as follows: Grade 0, normal foramina with normal dorsolateral border of the intervertebral disk and normal form of the foraminal epidural fat (oval or inverted pear shape); Grade 1, slight foraminal stenosis and deformity of the epidural fat with the remaining fat still completely surrounding the exiting nerve root; Grade 2, marked foraminal stenosis and deformity of the epidural fat with the remaining fat only partially surrounding the exiting nerve root; and Grade 3, advanced stenosis with obliteration of the epidural fat [32, 33].
After completion of the MR examination, all patients were subsequently instructed to duly answer and complete the national translation of the ODI questionnaire. The questionnaire contained six statements (denoted levels 0 to 5) in each of the 10 sections related to impairments like pain, and abilities such as personal care, lifting, walking, sitting, standing, sleeping, sex life, social life and traveling. In each section, the patient chose the statement that best described his/her status. If the limitation fell between two levels, the higher point value was selected. The chosen statements received scores 0 to 5 corresponding to the level indicated. The total scores could range from 0 (highest level of function) to 50 (lowest level of function). To accommodate patients who did not respond to every section, a percentage disability was calculated on the basis of the total possible points. Upon adding up all of the points, the total score was divided by 50 and multiplied by 100 to calculate the percentage disability: Total Points/50 × 100 = %Disability
Interpretation of results
Percentage disability was scored as follows: 0–20% was considered to be minimally disabled such that the patient could cope with most living activities. Usually no treatment is indicated apart from advice on lifting, sitting and exercise. A percentage of 21 to 40 were classified as moderate disability such that the patient experiences more pain and difficulty with sitting, lifting and standing. Travel and social life are more difficult and the patient may be prevented from working. Personal care, sexual activity and sleeping are not grossly affected, and the patients can generally be managed by conservative means. A percentage of 41 to 60 were considered to be severely disabled, with pain accounting for the main problems and impacting daily living. These patients require a detailed investigation. Sixty-one to −80% were classified as being crippled, where back pain impinged on all aspects of the patient’s life and required positive intervention. A percentage of 81 to 100 included all bedridden or exaggerating patients.
Statistical analysis of the data was performed to correlate quantitative and qualitative radiological stenosis and percentage disability recorded by the ODI. When the patients had multilevel spinal stenosis, the level with the greatest stenosis was selected for the correlation. Statistical calculations were performed using the GraphPad Prisma V.3 program. The chi square test was performed during the evaluation of qualitative data. Qualitative grading of nerve root compromise was analyzed with Kappa statistics for the evaluation of intra- and interobserver differences.
The study was approved by the Institutional Review Board, and informed consent was obtained from all patients.
Results
The results obtained in this study must be viewed in light of the selection bias of our study group. The 63 patients suffering from degenerative lumbar spinal stenosis included in this study ranged in age from 43 to 85 with a mean age of 69.2. There was a female predominance with a male to female ratio of 22:41.
In these 63 patients, a total of 290 intervertebral spaces and 580 lateral recesses and foramina were analyzed for stenosis. The cross-sectional area of the dural sac varied between 18 and 232 mm2 in the supine position. Of the 290 evaluated levels, 59 revealed moderate and 80 revealed severe central stenosis (Table 1). Of the 580 evaluated nerve roots, 318 did not have any contact with adjacent discs, while contact without deviation was found in 187 instances, contact with deviation was found in 61 instances and compression of nerve roots was seen at 14 sites (Table 2). Of the 580 foramina evaluated, 103 were completely normal; 272 demonstrated grade 1 foraminal stenosis; 156 demonstrated grade 2 foraminal stenosis; and 49 demonstrated grade 3 foraminal stenosis (Table 3).
Table 1.
Dural sac stenosis
| Stenosis | Number of levels |
|---|---|
| Absent | 151 |
| Moderate | 59 |
| Severe | 80 |
| Total | 290 |
Number of patients = 63. Number of levels evaluated = 290
Table 2.
Nerve root compromise in lateral recess
| Grade | Number of nerve roots | |
|---|---|---|
| Observer 1 | Observer 2 | |
| 0 | 318 | 314 |
| 1 | 187 | 177 |
| 2 | 61 | 73 |
| 3 | 14 | 16 |
| Total | 580 | 580 |
Number of patients = 63. Number of nerve roots evaluated = 580
Table 3.
Foraminal stenosis
| Grade | Number of nerve roots | |
|---|---|---|
| Observer 1 | Observer 2 | |
| 0 | 103 | 112 |
| 1 | 272 | 264 |
| 2 | 156 | 154 |
| 3 | 49 | 50 |
| Total | 580 | 580 |
Number of patients = 63. Number of foramina evaluated = 580
On the basis of previous data, evaluating the highest degree of stenosis showed that 33 and 27 of the 63 patients, respectively, had moderate and severe central dural sac stenosis, while 34 and 26 patients had grade 2- and grade 3-level lateral stenosis, respectively. Of the lateral stenosis group, 27 and 11 patients had grade 2- and grade 3-level stenosis, respectively, in the lateral recess; 37 and 22 patients had grade 2- and grade 3-level stenosis, respectively, in the foramina.
In 18 of the patients (3 men, 15 women), there was degenerative spondylolisthesis (one occurred at L2–L3 level, two occurred at L3–L4 level, 15 occurred at L4–L5 level; grade I in 14 and grade II in 4 cases). In 16 of the 18 patients, the narrowest site was the level of spondylolisthesis. The average DSCSA was 58 mm2 at the levels of spondylolisthesis compared with 61 mm2 at the most severe stenotic levels in patients without spondylolistesis.
Assessment of the Oswestry scores showed that 63 patients completed the ODI questionnaire. Each questionnaire had 10 questions correlating to 630 possible responses. Of these, 595 responses were received, giving an overall response rate of 94%. There was no response given to the sections on sex life, traveling and sleeping by 32, 3, and 1 of the 63 patients, respectively. In our study, the response rate for sex life was 49%, traveling was 95%, and sleeping was 98%. The maximum number of patients responded as level 3 (in a range of 0–5). If the higher levels of disability (i.e., 3, 4, or 5) were grouped together, 40, 39, 39, and 33 of the total number of patients (63) had higher levels of disability in standing, walking, social life and lifting, respectively.
On the basis of the percentage disability score of the ODI, out of the 63 patients, 10 patients demonstrated mild disability; 13 patients moderate disability, 25 patients severe disability; 12 patients were crippled and 3 patients were bedridden. Radiologically, eight patients with severe central stenosis and nine patients with moderate lateral stenosis demonstrated only minimal disability by percentage ODI scores.
Initially, ODI percentage scores were compared with numeric values of DSCSA and no correlation was found. In addition, a second comparison was performed between the subdivisions of the degree of central canal stenosis (three groups: normal, moderately stenotic and severely stenotic) and the ODI outcome which was also presented in 20 percentiles. This comparison also showed no correlation (Table 4). Moreover, upon statistical evaluation of qualitative lateral radiological stenosis versus ODI percentage scores, no significant correlation was established (Table 5). Subdivision of the patients into the two categories of spondylolisthesis and non-spondylolisthesis also had no effect. There was no correlation between ODI scores and degree of narrowing in patients with and without spondylolisthesis in our patient group. The number of levels that were defined as stenotic also had no correlation with the ODI scores. In addition, duration of neurologic claudication did not correlate with the radiological severity of the patients’ spinal stenosis.
Table 4.
Statistical evaluation: central stenosis versus ODI
| Severity of ODI | Central stenosis | |||||
|---|---|---|---|---|---|---|
| Normal | Moderate | Severe | ||||
| Mild | 1 | 25.00% | 1 | 6.70% | 8 | 18.20% |
| Moderate | 0 | 0.00% | 5 | 33.30% | 8 | 18.20% |
| Severe | 2 | 50.00% | 5 | 33.30% | 18 | 40.90% |
| Crippled | 1 | 25.00% | 4 | 26.70% | 7 | 15.90% |
| Bedridden | 0 | 0.00% | 0 | 0.00% | 3 | 6.80% |
There is no statistically significant relationship between the distribution of ODI classification and the central stenosis classification (P = 0.690); χ2 = 5.61, P = 0.690
Table 5.
Statistical evaluation: lateral stenosis versus ODI
| Severity of ODI | Lateral stenosis | |||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| Mild | 0 | 0.00% | 9 | 26.50% | 1 | 3.80% |
| Moderate | 0 | 0.00% | 5 | 14.70% | 8 | 30.80% |
| Severe | 3 | 100.00% | 11 | 32.40% | 11 | 42.30% |
| Crippled | 0 | 0.00% | 6 | 17.60% | 6 | 23.10% |
| Bedridden | 0 | 0.00% | 3 | 8.80% | 0 | 0.00% |
There is no statistically significant relationship between the distribution of ODI classification and the lateral stenosis classification (P = 0.072); χ2 = 14.38, P = 0.072
The intra- and interobserver variation with regard to lateral recess and foraminal grading was good (k = 0.79 and k = 0.62, respectively).
Discussion
With increasing longevity of life and aging populations, the prevalence and associated clinical disability related to degenerative lumbar spinal stenosis is on the rise. Spinal stenosis is most frequently a consequence of severe spinal degeneration. Frequent concomitant pathologies coexisting in the aging population make the differential diagnosis of LSS even more challenging [29]. Since the degree of constriction of the spinal canal considered to be symptomatic for LSS is not clear, the diagnostic accuracy of imaging techniques, findings from clinical examination and appropriate outcome measures need to be identified to ensure careful patient and treatment selection for successful and lasting results. These questions warrant attention to improve the management of patients with LSS [9, 19].
In regard to the choice of the measure of disability, the ODI, as previously reported by other studies, has proven to be a simple, condition specific, preferred multidimensional tool with the advantage of easy patient comprehension and compliance. This self-assessment test takes less than 5 min to complete and 1 min to score, with no training, equipment or cost requirements; and it covers a wide range of function, pain and role limitation. The national translated version of the ODI questionnaire used in our study was easily comprehended and had a response rate of 94%. Studies have reported that this short, self-administered questionnaire is reproducible, reliable, internally consistent, valid and is an adequately useful instrument for the assessment of disability in patients with lower back pain [11].
Although a 1998 report states that disability assessed using the ODI correlates significantly with severity of stenosis [12], no such significant correlation could be established in our study between the radiologically depicted anatomical lumbar stenosis and the ODI. Various authors have also noted a lack of correlation between radiographically detected stenosis and clinical findings, and the presence and/or absence and intensity of symptoms and signs [1, 13, 17]. Although patients with narrower spinal canals are more likely to develop some symptoms of LSS, the radiographic changes were more extensive than expected from the clinical picture. Difficulties associated with finding such correlations include the presence of a large number of patients with spinal narrowing and a complete lack of symptoms [5, 30], variations in canal size throughout the population and a lack of an accepted system for quantifying the degree of narrowing. Obviously, patients can have changes on MR imaging compatible with the morphological diagnosis of spinal stenosis but be asymptomatic. Another question is whether the reverse situation may occur and, if so, whether it is caused by different susceptibility of the nerves for narrowing of the spinal canal, or by dynamic stenosis. The extent of narrowing is also dynamic and is likely to change with the posture of the patient. For example, extension significantly decreases the canal area, whereas flexion has the opposite effect [21]. Therefore, a static image of the canal dimensions may not be predictive of a patient’s symptoms. The lack of correlation in our study may also be related to the observation that symptoms tend to fluctuate considerably over time, and there is a wide variability in lumbar dimensions among patients who do not have clinical spinal stenosis [1].
The age at which patients with degenerative stenosis become symptomatic is usually in the fifth through sixth decades of life [4]. In our study, the mean age of patients was 69. Most series demonstrate a male predominance of 56% [15], whereas our series had a 41:22 female predominance. The reason for the discrepancy may be related to the higher risk of degenerative spondylolisthesis present in females [15]. Degenerative spondylolisthesis, described by McNab [18] as “spondylolisthesis with an intact neural arch,” is highly associated with degenerative facet joint arthritis and occurs more commonly at L4–L5 level. It may cause a pronounced canal narrowing and also may contribute to narrowing of the neural foramina [16]. Degenerative spondylolisthesis is usually at one level and contradicts the theory of multiple level pathogenesis described by Porter [23]. In our study group, patients with degenerative spondylolisthesis did not have a more pronounced narrowing of the spinal canal; average DSCSA was 58 mm2 at the levels with displacement compared with 61 mm2 at the most severe stenotic levels in patients without spondylolisthesis. There was also a non-correlation between ODI scores and degree of narrowing in patients with spondylolisthesis, similar to patients without spondylolisthesis, in our patient group.
Four patients without central stenosis were included in the study and scheduled for surgery due to symptoms of severe disability and crippling (Table 4). These patients with normal values of DSCSA concerning central stenosis had grade 2 or 3 lateral stenosis. It would be of interest to document whether the combined results of central and lateral stenosis correlate better to the ODI. However, the authors of this study could neither find a convenient classification in combining the two types of stenosis, nor found such a combined classification in previous presentations in the literature.
A range of morphologic and psychosocial variables may also play a role [6]. Hazard et al. [10] have shown that both psychologically disturbed and depressed patients have higher Oswestry scores than normal patients, when self-reported disability is compared with objective physical measurements. This may explain the high ODI scores of some of our patients that did not correspond to their degree of radiologically shown spinal stenosis. Although anatomical evidence of cauda equina compression is necessary, it is by no means sufficient to establish the diagnosis. Therapy must be targeted to the symptoms that bother the patient and not rely solely on the degree of narrowing.
This study has bias in patient selection, because it is a subset of patients selected for surgery. There may be a correlation between imaging appearances and level of disability if healthy subjects and patients with less intense spinal stenosis, not planned for surgery, are included. It would also be of interest to see if other aspects of the disease correlate with MR imaging. In this retrospective study, the authors were not able to include other preoperative clinical evaluations such as pain on the visual analogue scale, quality of life scores, and other subjective parameters documenting the disease due to lack of available data in the medical reports of the patients. The authors are planning to present such facts in the future studies.
In conclusion, data collected and analyzed in the current study demonstrate no significant correlation between imaging appearances and levels of disability in patients with LSS. The fact that in some patients the radiological changes were more extensive than expected from the clinical picture and the degree of narrowing did not correspond to the severity of ODI percentage disability further establishes that degenerative LSS is a clinico-radiological syndrome. When evaluating and discussing surgery in patients with this diagnosis, both clinical symptoms and MR imaging are important, especially to determine the levels to be decompressed.
References
- 1.Amundsen T, Weber H, Lilleas F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis. Clinical and radiologic features. Spine. 1995;20:1178–1186. doi: 10.1097/00007632-199505150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Arbit E, Pannullo S. Lumbar stenosis. A clinical review. Clin Orthop Relat Res. 2001;384:137–143. doi: 10.1097/00003086-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, Gargano FP, Jacobson RE, Kirkaldy-Willis WH, Kurihara A, Langenskiold A, Macnab I, McIvor GW, Newman PH, Paine KW, Russin LA, Sheldon J, Tile M, Urist MR, Wilson WE, Wiltse LL. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976;115:4–5. [PubMed] [Google Scholar]
- 4.Atlas SJ, Delitto A. Spinal stenosis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:198–207. doi: 10.1097/01.blo.0000198722.70138.96. [DOI] [PubMed] [Google Scholar]
- 5.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 6.Carragee EJ, Alamin TF, Miller JL, Carragee JM. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24–35. doi: 10.1016/j.spinee.2004.05.250. [DOI] [PubMed] [Google Scholar]
- 7.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 8.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 9.Fritz JM, Delitto A, Welch WC, Erhard RE. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil. 1998;79:700–708. doi: 10.1016/S0003-9993(98)90048-X. [DOI] [PubMed] [Google Scholar]
- 10.Hazard RG, Bendix A, Fenwick JW. Disability exaggeration as a predictor of functional restoration outcomes for patients with chronic low-back pain. Spine. 1991;16:1062–1067. doi: 10.1097/00007632-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Herno A, Airaksinen O, Saari T. Computed tomography after laminectomy for lumbar spinal stenosis. Patients’ pain patterns, walking capacity, and subjective disability had no correlation with computed tomography findings. Spine. 1994;19:1975–1978. doi: 10.1097/00007632-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Hurri H, Slatis P, Soini J, Tallroth K, Alaranta H, Laine T, Heliovaara M. Lumbar spinal stenosis: assessment of long-term outcome 12 years after operative and conservative treatment. J Spinal Disord. 1998;11:110–115. doi: 10.1097/00002517-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson B, Annertz M, Sjoberg C, Stromqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part I: clinical features related to radiographic findings. Spine. 1997;22:2932–2937. doi: 10.1097/00007632-199712150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Karantanas AH, Zibis AH, Papaliaga M, Georgiou E, Rousogiannis S. Dimensions of the lumbar spinal canal: variations and correlations with somatometric parameters using CT. Eur Radiol. 1998;8:1581–1585. doi: 10.1007/s003300050590. [DOI] [PubMed] [Google Scholar]
- 15.Katz JN, Dalgas M, Stucki G, Lipson SJ. Diagnosis of lumbar spinal stenosis. Rheum Dis Clin North Am. 1994;20:471–483. [PubMed] [Google Scholar]
- 16.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726–733. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 17.Lohman CM, Tallroth K, Kettunen JA, Lindgren KA. Comparison of radiologic signs and clinical symptoms of spinal stenosis. Spine. 2006;31:1834–1840. doi: 10.1097/01.brs.0000227370.65573.ac. [DOI] [PubMed] [Google Scholar]
- 18.McNab I. Spondylolisthesis with an intact neural arch: the so-called pseudo-spondylolisthesis. J Bone Joint Surg Br. 1950;32:325–333. doi: 10.1302/0301-620X.32B3.325. [DOI] [PubMed] [Google Scholar]
- 19.Panagiotis ZE, Athanasios K, Panagiotis D, Minos T, Charis M, Elias L. Functional outcome of surgical treatment for multilevel lumbar spinal stenosis. Acta Orthop. 2006;77:670–676. doi: 10.1080/17453670610012773. [DOI] [PubMed] [Google Scholar]
- 20.Panjabi MM, Takata K, Goel VK. Kinematics of the lumbar intervertebral foramen. Spine. 1983;8:348–357. doi: 10.1097/00007632-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy. A dynamic CT-myelographic study. Spine. 1987;12:488–500. doi: 10.1097/00007632-198706000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046–2052. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- 23.Porter RW, Ward D. Cauda equina dysfunction: the significance of multiple level pathology. Spine. 1992;17:9–15. doi: 10.1097/00007632-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Pratt RK, Fairbank JC, Virr A. The reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the assessment of patients with lumbar spinal stenosis. Spine. 2002;27:84–91. doi: 10.1097/00007632-200201010-00020. [DOI] [PubMed] [Google Scholar]
- 25.Schonstrom N, Boleander NF, Spengler DM. The pathomorphology of spinal stenosis as seen on CT scans of the lumbar spine. Spine. 1985;10:806–811. doi: 10.1097/00007632-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Schonstrom N, Lindahl S, Willen J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989;7:115–121. doi: 10.1002/jor.1100070116. [DOI] [PubMed] [Google Scholar]
- 27.Sortland O, Magnaes B, Hauge T. Functional myelography with metrizamide in the diagnosis of lumbar spinal stenosis. Acta Radiol Suppl. 1977;355:42–54. [PubMed] [Google Scholar]
- 28.Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am. 1998;80:1053–1066. doi: 10.2106/00004623-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Szpalski M, Gunzburg R. Lumbar spinal stenosis: clinical features and new trends in surgical treatment. Geriatr Times. 2004;5(4):11. [Google Scholar]
- 30.Szpalski M, Gunzburg R. Lumbar spinal stenosis in the elderly: an overview. Eur Spine J. 2003;12(Suppl 2):170–175. doi: 10.1007/s00586-003-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral column. A review of twenty seven years experience. J Bone Joint Surg Br. 1977;59:181–188. doi: 10.1302/0301-620X.59B2.141452. [DOI] [PubMed] [Google Scholar]
- 32.Weishaupt D, Schmid MR, Zanetti M, Boos N, Romanowski B, Kissling RO, Dvorak J, Hodler J. Positional MR Imaging of the lumbar spine: does it demonstrate nerve root compromise not visible at conventional MR Imaging? Radiology. 2000;215:247–253. doi: 10.1148/radiology.215.1.r00ap06247. [DOI] [PubMed] [Google Scholar]
- 33.Wildermuth S, Zanetti M, Duewell S, Schmid MR, Romanowski B, Benini A, Boni T, Hodler J. Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology. 1998;207:391–398. doi: 10.1148/radiology.207.2.9577486. [DOI] [PubMed] [Google Scholar]