Abstract
Severe malaria is commonly misdiagnosed in Africa, leading to a failure to treat other life-threatening illnesses. In malaria-endemic areas, parasitemia does not ensure a diagnosis of severe malaria because parasitemia can be incidental to other concurrent disease. The detection of malarial retinopathy is a candidate diagnostic test for cerebral malaria. Malarial retinopathy consists of a set of retinal abnormalities that is unique to severe malaria and common in children with cerebral malaria. Its presence and severity are related to risk of death and length of coma in survivors. A large, prospective autopsy study of children dying with cerebral malaria in Malawi found that malarial retinopathy was better than any other clinical or laboratory feature in distinguishing malarial from non-malarial coma. However, visualization has to date relied on specialist examination techniques. Further studies are planned to evaluate the usefulness of funduscopy by general clinicians in a variety of settings across Africa. Studies of the retina and retinal blood vessels provide an unparalleled opportunity to visualize an infected microvasculature and its effect on neural tissue in vivo. This report reviews current knowledge of malarial retinopathy, including its use as a diagnostic test in the comatose child, and its value as a tool for research into the pathophysiology of cerebral malaria.
INTRODUCTION
African children bear 90% of the burden of mortality from malaria, with estimates exceeding a million deaths every year.1 However, recent data from Africa suggest that malaria is commonly misdiagnosed, leading to a failure to treat other causes of life-threatening disease.2 Making a definite diagnosis of severe malaria in disease-endemic areas is complicated by the fact that parasitemia may be incidental to other concurrent severe disease. In areas of high transmission, the prevalence of asymptomatic parasitemia in the population may be as high as 40–70%,3,4 and the signs of severe malaria are non-specific.5,6 Therefore, parasitemia in a critically ill patient is insufficient basis for the diagnosis of severe malaria in disease-endemic areas. Unfortunately, it is in these under-resourced areas where the exclusion of other causes of illness is often difficult. Even in research settings where more precise diagnoses are possible, other causes of coma cannot always be excluded in patients who satisfy the clinical definition of cerebral malaria, and this can result in misdiagnoses.7
Malarial retinopathy is a recently described set of retinal signs, which can be accompanied by papilledema. Some of the retinal signs are unique to malaria and their detection is a candidate diagnostic test for severe malaria.
The retina is embryologically part of the central nervous system with an analogous cellular structure and blood-tissue barrier. Therefore, examination of the retina provides a unique opportunity to study an infected microvasculature and its effects on neurologic tissue in vivo. In this report, we review the features of malarial retinopathy, how it can be best identified, and its use in the diagnosis of cerebral malaria. We also summarize research on the pathophysiology of retinal signs and the insights gained into the pathogenesis of cerebral malaria.
METHODS
We systematically searched PubMed to identify relevant studies by combining the term malaria with retinopathy, retinal hemorrhage, papilledema, retina, and eye. These searches were conducted most recently on January 26, 2006. Abstracts were reviewed for relevance, and pertinent articles were reviewed in full. All studies that systematically recorded ocular signs in severe malaria are included in this review. We searched article bibliographies to identify further relevant work. Case reports and articles on drug-induced retinopathy were excluded.
FEATURES OF MALARIAL RETINOPATHY
Unusual retinal signs in children with cerebral malaria were first described by Lewallen and others using direct and indirect ophthalmoscopy in Malawi in 1993.8 These signs have since been further characterized, and also described in Kenyan and Gambian children, with fundus photographs documenting their distinctive nature.9–12 The largest series now includes dilated funduscopy in more than 1,000 children with cerebral malaria.13
Malarial retinopathy consists of four main components: retinal whitening, vessel changes, retinal hemorrhages, and papilledema (Table 1). The first two of these abnormalities are specific to malaria, and are not seen in other ocular or systemic conditions.
TABLE 1.
Components of malarial retinopathy
| Retinal whitening |
| Macular |
| Peripheral |
| Vessel changes |
| Whitening (including orange vessels and tramlining) |
| Capillary whitening |
| Retinal hemorrhages, predominantly white-centered |
| Papilledema |
| Cotton wool spots |
Retinal whitening
Retinal whitening affecting the macula (which approximates to the area within the temporal vascular arcades) is termed macular whitening. When this feature occurs outside the macula, it is termed peripheral whitening. In some early reports, retinal whitening was referred to as retinal edema. Macular whitening is a patchy opacification of the retina centered on the fovea, but it spares the central fovea, or foveola (Figures 1 and 2).11 It frequently extends temporally between the vascular arcades (Figure 2).
FIGURE 1.
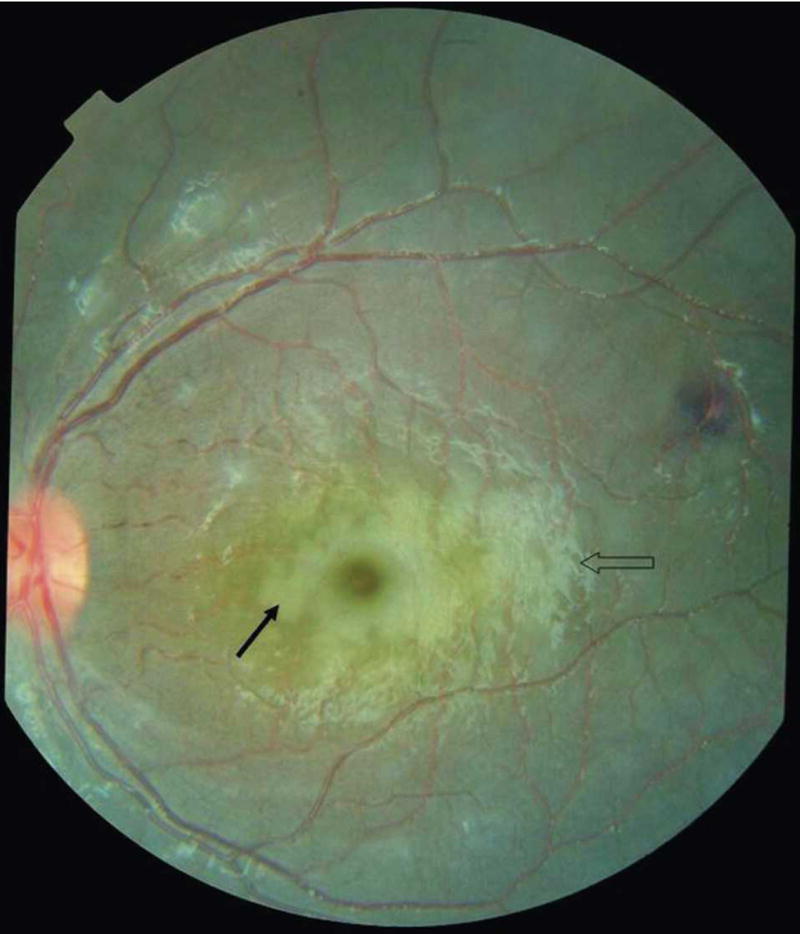
Severe macular whitening (solid arrow) completely surrounding the foveola of a Malawian child with cerebral malaria. Papilledema is present as well as a white-centered hemorrhage temporal to the macula and cotton wool spots above superior temporal arcade. The open arrow indicates glare (photographs provided by Nicholas A. V. Beare).
FIGURE 2.
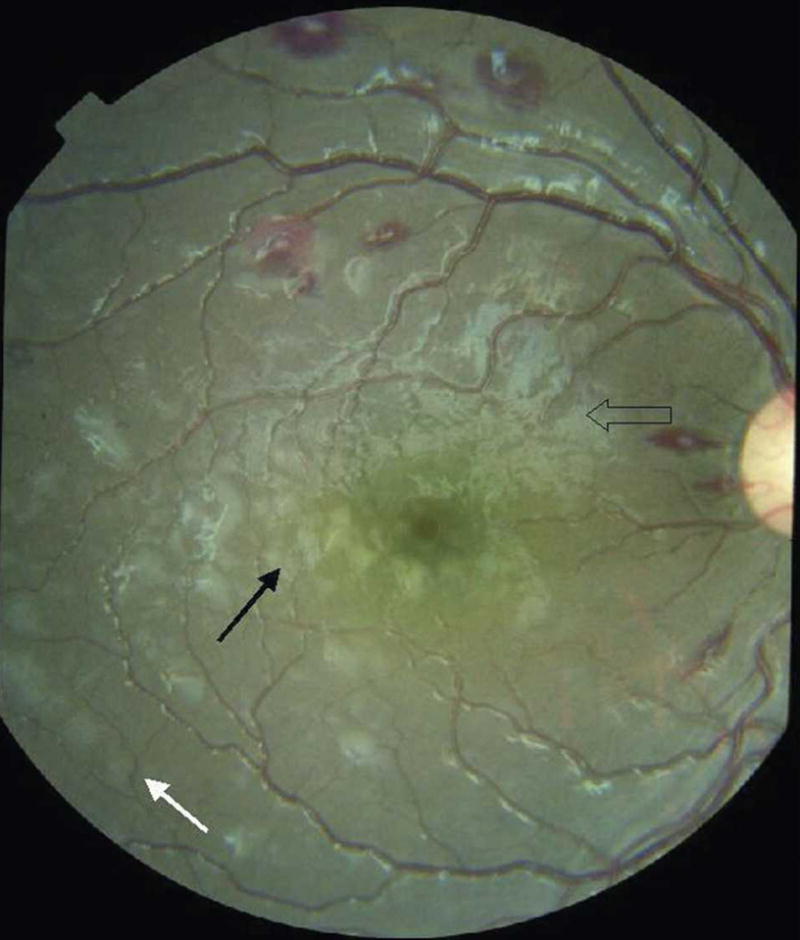
Macular whitening around inferior fovea and temporal macula (solid black arrow). White-centered hemorrhages are temporal to the disc and on the superior macula. Peripheral whitening is outside the vascular arcades (solid white arrow). Open arrow indicates glare.
Peripheral whitening is similar in appearance to macular whitening but outside the temporal vascular arcades (Figure 2), or it can be in a more mosaic pattern in the peripheral fundus. The appearance is distinct from cotton wool spots, which also occur, but less frequently. Patches of peripheral whitening associated with severe malaria are less well demarcated, less brightly white, and more widely distributed than cotton wool spots. Retinal whitening is similar in appearance to patchy ischemic retinal whitening, an uncommon finding in central retinal vein occlusion,14 but has a different retinal distribution.
Vessel changes
Vessel changes are manifest as discoloration of retinal vessels to orange or white, mainly in the peripheral fundus (Figure 3). Either discrete sections of vessels, or peripheral trees, can be involved. White or orange tramlining within larger vessels can occur (continuous or interrupted), delineating an apparently narrowed blood column (Figure 4). Capillary whitening refers to whitening of retinal capillaries and post-capillary venules such that they become prominent against the choroidal background. When viewed with a high magnification indirect lens (× 5.5), capillary whitening appears to be more widely distributed in the fundus than has been previously recognized.
FIGURE 3.
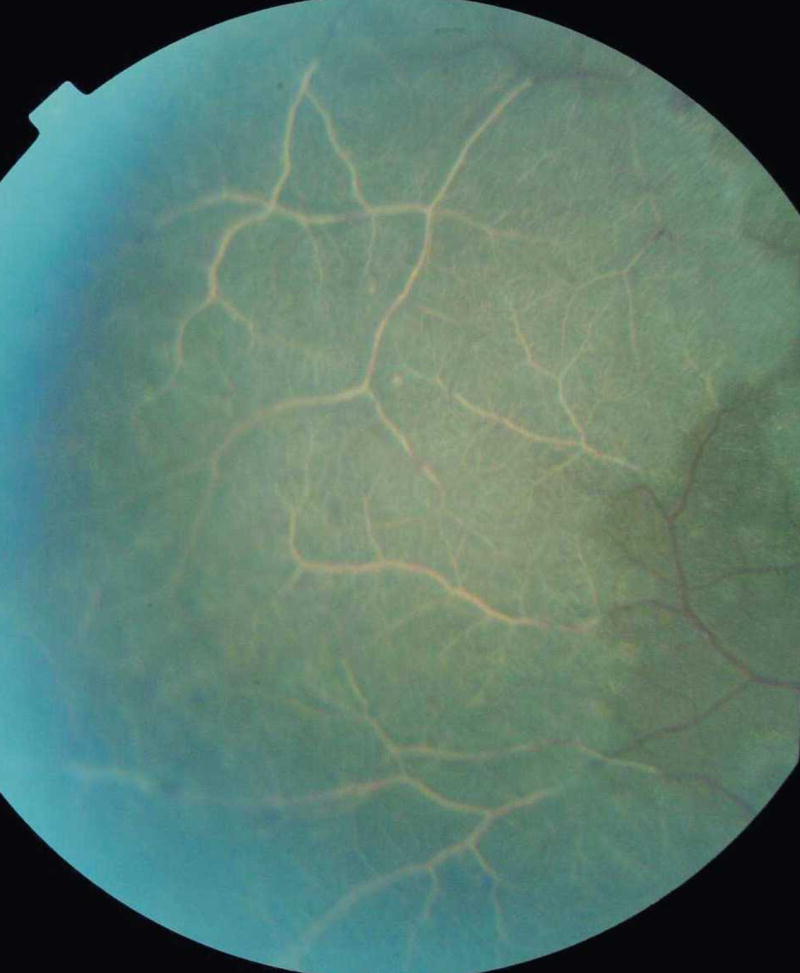
White retinal vessels in an area of confluent peripheral retinal whitening.
FIGURE 4.
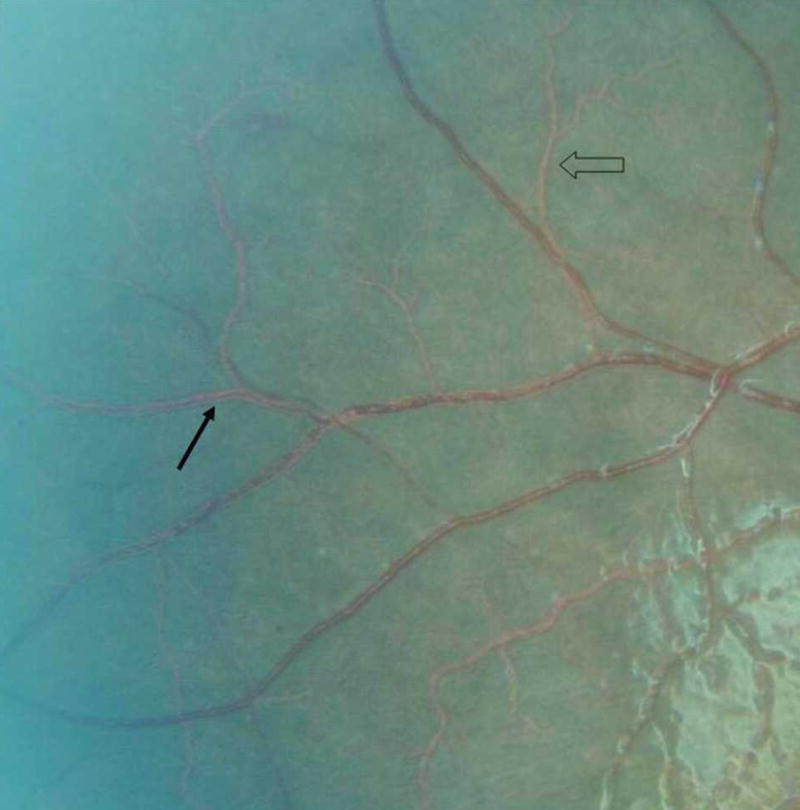
Vessel changes in same child as in Figure 1, including examples of tramlining (solid arrow) and orange vessel (open arrow).
Hemorrhages
Retinal hemorrhages are predominantly white-centered, intra-retinal, blot hemorrhages similar to Roth spots (Figure 2). In severe cases, these can be extremely numerous (>120 in each eye) and overlapping (Figure 5). Flame and large blot hemorrhages also occur frequently. Occasionally, hemorrhages can extend into the pre-retinal space.
FIGURE 5.
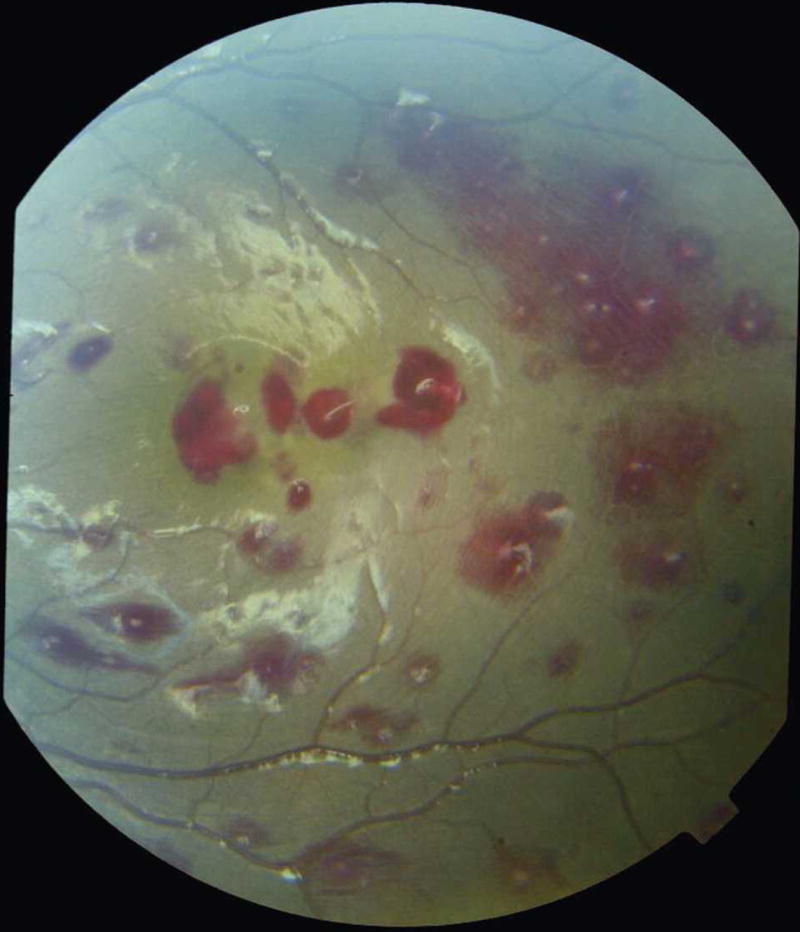
Large number of retinal hemorrhages in a child with cerebral malaria.
Papilledema
Papilledema is not specific to malaria and can occur in many other conditions that cause coma. It accompanies retinal features of cerebral malaria in a proportion of cases and independently increases the risk of fatal outcome.9,12 When papilledema is present without retinal whitening, vessels changes, or white-centered hemorrhages, the examiner should consider other causes of raised intracranial pressure.
Prognostic significance and outcome
A large prospective study of Malawian children with cerebral malaria has found that the severity of retinal signs, including the number of retinal hemorrhages, is related to fatal outcome and length of coma in survivors.12 The relative risk of death conferred by the presence of each fundus sign is shown in Table 2. This concurred with earlier studies that found that ocular fundus signs were associated with an increased risk of death.8,9 In a smaller number of Kenyan children with cerebral malaria, retinal hemorrhages were associated with deep coma and severe anemia.10 In children in Mali with malaria, retinopathy was related to severity, but this study is difficult to compare with other studies in African children because it does not state the method of ophthalmoscopy used and uses a more inclusive definition of cerebral malaria.15
TABLE 2.
Prevalence and relative risk of death associated with ocular fundus signs in children with cerebral malaria in Malawi12*
| Relative risk of death (95% confidence interval)
|
|||
|---|---|---|---|
| Fundal sign | Prevalence in cerebral malaria (%) | In presence of sign | Moderate or severe manifestation |
| Any retinopathy | 61 | 3.7 (1.6–8.5) | NA |
| Papilledema | 15 | 4.5 (2.7–7.6) | NA |
| Hemorrhages | 46 | 2.0 (1.1–3.6) | 3.4 (2.0–5.8) |
| Vessel changes | 32 | 2.4 (1.4–4.3) | 3.0 (1.7–5.1) |
| Macular whitening | 46 | 1.5 (0.9–2.7) | 2.3 (1.3–4.0) |
| Peripheral whitening | 44 | 1.6 (0.9–2.8) | 1.6 (0.9–2.9) |
NA = not applicable.
Malarial retinopathy resolves some time after the resolution of coma in cerebral malaria and with no persisting retinal abnormalities.12 A study on its effect on vision found no detectable effect of retinopathy on visual acuity in the first month after discharge.16 However, this study made use of relatively crude measures of visual acuity in a young age group in whom accurate assessment of vision is notoriously difficult.
DETECTION OF MALARIAL RETINOPATHY
Malaria is common, and the ophthalmoscope was invented in 1851, so why has the entity of malarial retinopathy escaped notice until relatively recently? The answer lies with different methods of funduscopy. Papilledema and retinal hemorrhages had been previously described by investigators using direct ophthalmoscopy,17–19 but the components specific to severe malaria are best seen by indirect ophthalmoscopy through dilated pupils (Figure 6). Pupil dilation can be achieved with tropicamide 0.5% or 1% eye drops, with the addition of 2.5% phenylephrine if required. The wider field of view afforded by the indirect ophthalmoscope allows visualization of the peripheral retina where the unique vascular changes are mostly situated, beyond the view of the direct ophthalmoscope. This perhaps explains how they had evaded attention.
FIGURE 6.
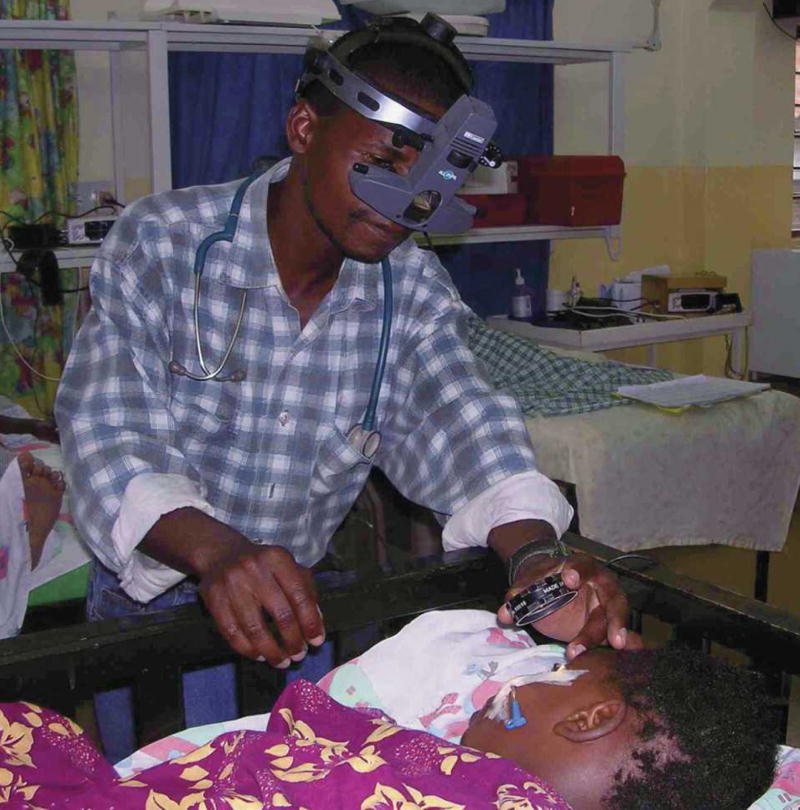
Clinician performing indirect ophthalmoscopy. This figure appears in color at www.ajtmh.org.
At present, the specialist equipment and skill required for indirect ophthalmoscopy remain barriers to wider use of this sign in clinical practice. Although retinal hemorrhages, papilledema, and macular whitening are detectable with direct ophthalmoscopy, studies recognizing a high incidence of malarial retinopathy have used indirect ophthalmoscopy.9,10,12 In comparison to indirect ophthalmoscopy, direct ophthalmoscopy showed retinopathy with a sensitivity of 73% in the hands of an ophthalmologist in Gambia, but this decreased to 37% in those with no specific training and without consistent pupil dilation.20 When physicians have been trained in indirect ophthalmoscopy, as they have been in Blantyre, Malawi, then the sensitivity of detection improves to 95% compared with an ophthalmologist (T. E. Taylor, unpublished data). The interobserver concordance between ophthalmologists for grading the components of malarial retinopathy has proved satisfactory.21
Pharmacologic dilatation of the pupils of patients with acute neurologic illness may be a concern to physicians who are then temporarily unable to monitor pupil size and reaction. The pupil reactions should be carefully examined and recorded prior to instilling dilating drops. Clinicians should be made aware that drops have been given, and the time of administration recorded. The effect of tropicamide wears off after approximately two hours. Since the severity of malarial retinopathy is usually similar in both eyes, most information can be gained by dilating one pupil.
Evidence for the utility of examining pupillary responses comes mostly from head injury and intracranial hemorrhage.22–26 In contrast, cerebral malaria is a diffuse cerebral disease in which the role of tentorial herniation is as yet unreported. Classic studies suggest that pupil responses are less helpful in diffuse parenchymal lesions (which may cause central transtentorial herniation) than in lateralized, rapidly expanding lesions such as hematomas, which lead to uncal herniation.27 It should also be noted that pupil dilation in coma may be due to mechanisms other than compression of the oculomotor nerve by herniation, such as brain stem ischemia.22,28,29 In medical comas, pupil dilation is a late event preceded or accompanied by other signs of brainstem dysfunction, such as deepening of coma, abnormal posturing, abnormal oculocephalic (Doll’s eye) responses, and disordered respiratory patterns. These signs should alert the clinical team to a serious deterioration in a patient’s condition.
Studies using undilated funduscopy are likely to miss many abnormalities and all those peripheral to the posterior pole.19,30 In a parasitemic, comatose child, the additional diagnostic information obtained from dilated funduscopy is arguably of greater utility than maintaining constant pupil monitoring.7 There is no additional intervention that is likely to be of benefit in a patient diagnosed with cerebral malaria should they develop pupil abnormalities. Therefore, we believe the diagnostic benefit gained by dilated funduscopy out-weighs the disadvantage of temporarily interfering with pupil signs pharmacologically after the initial pupil examination. More than 1,000 children with cerebral malaria in Malawi have had pupils dilated for retinal examination, without identifiable detriment to clinical decision-making.13
DIAGNOSTIC VALUE OF RETINOPATHY
Diagnostic difficulty in severe malaria
In children, severe malaria presents as three syndromes that commonly overlap: cerebral malaria, severe malarial anemia, and respiratory distress or metabolic acidosis. The clinical diagnosis of severe malaria based on World Health Organization (WHO) criteria requires Plasmodium falciparum parasitemia and a measure of severe disease, such as impaired consciousness or severe anemia, with the exclusion of other causes.1 In malaria-endemic areas, reliably establishing parasitemia and excluding other causes of severe disease can be difficult.
A large study in 10 Tanzanian district hospitals showed that 54% of people treated for severe malaria had no parasitemia.2 In this study, most children (55%) less than five years of age in moderate and low transmission areas thought to have severe malaria were misdiagnosed because blood films were inaccurate or ignored. The rate of malaria diagnosis without parasitemia was lower in high transmission areas (31%), probably because a higher proportion of children who came to the hospital were parasitemic.
Limitations of current diagnostic methods
Since severe malaria without peripheral parasitemia is rare, a correctly examined blood film provides an important contribution to diagnosis. Given the required microscope, reagents, and trained technicians, the limitation of correctly identifying a parasitemia is that it may be incidental to another cause of the severe illness.
Rapid diagnostic tests based on the detection of Plasmodium-specific proteins are simple to use, but less simple to interpret. These tests rely on detecting different parasite proteins, and vary in sensitivity between malaria species. They do not give any information on the density of infection, and have lower sensitivities at low parasite densities.31 Fundamentally, they cannot distinguish a parasitemia causing a malarial illness from an incidental parasitemia.
In a malaria-endemic area, any child with signs of severe disease may have a parasitemia that is not the cause of the critical illness. Recent autopsy data suggests that this situation may be more common than is generally recognized.7 The parasitemia obviously requires treatment, but a child will not recover unless the main pathology is first suspected, and then addressed. Most of the clinical features of severe malaria in children, notably coma, convulsions, abnormal posturing, respiratory distress, anemia, and jaundice, are not specific. There is considerable overlap between the clinical signs of severe malaria and pneumonia for instance, and studies in east African children have concluded that they cannot be reliably distinguished on clinical grounds.5,6
Most children who die of malaria in Africa have cerebral malaria manifest as profound coma.32 Even in tertiary African hospitals facilities are often not available to reliably exclude other causes of coma in a parasitemic child. In a prospective clinicopathologic study of Malawian children diagnosed with cerebral malaria, 23% of the 31 examined postmortem had an alternative cause of death, with no histopathologic findings typical of cerebral malaria.7 Since all these children satisfied the clinical case definition of cerebral malaria and would have been classed as such had an autopsy not been performed, this number of other diagnoses was unexpected. Of all the clinical and laboratory features documented, only malarial retinopathy distinguished malarial from non-malarial coma.
Malarial retinopathy
In this Malawi autopsy study, 10 different ophthalmologists demonstrated a collective sensitivity of 95% and specificity of 90% when using the detection of retinopathy as a diagnostic test for cerebral malaria in subsequently fatal cases of coma.7 This compares with a specificity of 61% for the diagnosis of cerebral malaria using all other available clinical and laboratory data applied to WHO diagnostic criteria for strictly defined cerebral malaria. The reference standard used in this study was sequestered, parasitized erythrocytes in cerebral blood vessels at post-mortem examination. Patients with parasitemia, but no malarial retinopathy, were found at post mortem to have evidence of alternative causes of death and no significant cerebral sequestration. One patient with a non-malarial coma was deemed to have a trace of retinal whitening and no other retinal abnormalities. If one considers only parasitemic patients, detection of any malarial retinopathy improved the specificity of the cerebral malaria diagnosis from 61% to 100%. Overall, when using malarial retinopathy to diagnose cerebral malaria in any fatal coma, these data produce a positive predictive value (PPV) of 95% and a negative predictive value of 90%, compared with a PPV of 77% for parasitemia with exclusion of other causes.
The combination of retinal abnormalities associated with severe malaria is unique. To our knowledge, retinal whitening and vessel changes are not seen in any other ocular or systemic condition. None of these changes have been seen in Malian febrile controls,15 critically anemic Malawian children without malaria (hematocrit = 7–8%), or Malawian children with meningitis.12 The definite presence of malarial retinopathy of any severity confirms a diagnosis of severe malaria, and is particularly useful in a parasitemic child in a coma (Figure 7).
FIGURE 7.
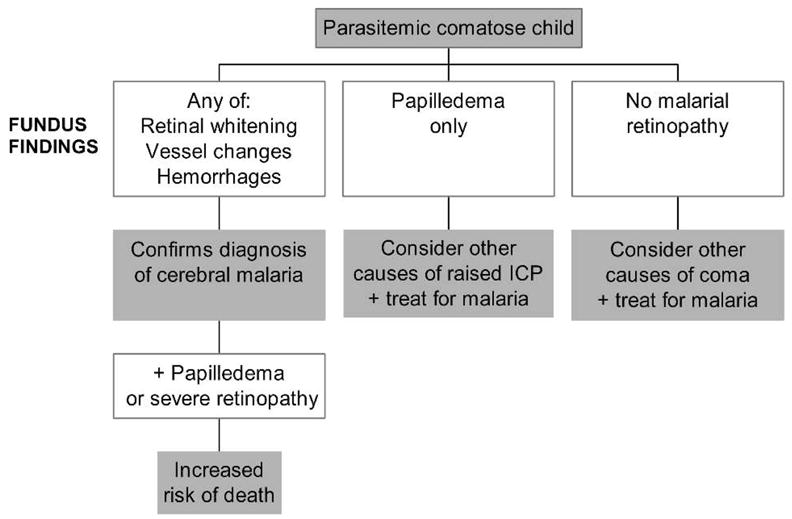
Proposed algorithm for use of funduscopic findings in a parasitemic comatose child in a malaria-endemic area. This algorithm will be tested by a multicenter SMAC trial. ICP = intracranial pressure; SMAC = severe malaria in African children clinical network.
Malarial retinopathy is also seen in other manifestations of severe malaria in children, without profound coma, but the retinopathy is generally less severe, with fewer of the component changes.12 Occasional retinal hemorrhages can be seen in children with non-severe malaria15 and on their own are non-specific.
Malarial retinopathy is absent in approximately one-third of children diagnosed with cerebral malaria. From autopsy data of Taylor and others, in which other causes of death were established,7 it can be deduced that the cause of coma in some of these patients with normal fundi may not be malaria. Alternatively, they may have a prolonged postictal state after a febrile convulsion caused by otherwise uncomplicated malaria. Without a reliable reference standard applicable during life, assessing the sensitivity of malarial retinopathy in non-fatal cases is difficult. However, a normal fundus in children meeting the clinical definition of cerebral malaria is associated with a shorter duration of coma, and a lower risk of death.12 The absence of malarial retinopathy should alert the clinician to the possibility of other causes of coma, particularly if the coma is prolonged.
Attempting to use malarial retinopathy to predict children who might deteriorate is difficult because fundus examination in alert children is more demanding, and only a small minority will develop cerebral complications on treatment. In a study in Gambia, retinopathy on admission was associated with later convulsions, but this association was confounded by pre-admission convulsions.20 We have found that a large increase in the number of retinal hemorrhages can be related to clinical deterioration and even death.12
RETINAL CHANGES IN ADULTS
The clinical features of severe malaria in adults are different from those in children, with multi-system involvement including renal failure, hepatic dysfunction, pulmonary edema, and disseminated intravascular coagulation being more common. Severe malaria is uncommon in adults in disease-endemic areas of Africa because partial immunity develops from repeated infection. Cerebral malaria is nevertheless commonly over-diagnosed in African adults, with under-treatment of other causes of coma.33,34
Cerebral malaria in adults is common in southeast Asia and India, where studies of ocular findings have been undertaken. Retinal hemorrhages have been found to be less frequent than in children, and to be associated with severe disease, but not death, in Thai and Indian adults.17,35,36 Retinal edema and exudates have been described as infrequent findings, but these terms presume etiologies that are unproven, and it is not clear whether they refer to retinal whitening seen in children. Macular retinal whitening has been observed in two Malawian adults with cerebral malaria in a prospective study of patients admitted with fever.37 However, malarial retinopathy, as described in African children, does not appear to be a prominent feature of severe malaria in adults. In particular, the unique vessel changes have not been documented. Systematic studies are required to establish the prevalence and significance of malarial retinopathy in adults.
PATHOGENESIS OF RETINOPATHY
Histopathologic studies of malarial retinopathy have shown correlations with cerebral pathology. The characteristic histopathologic feature of P. falciparum malaria is sequestration of parasitized erythrocytes in microvessels by cytoadherence. Histologic examination of the retina suggests that vessel abnormalities are caused by sequestered erythrocytes: eyes that had vessel whitening in life had sequestration in retinal vessels at autopsy, with cytoadherent erythrocytes containing late stages of P. falciparum and little hemoglobin.38 It is only the blood column of retinal vessels that is normally seen at ophthalmoscopy, and erythrocyte hemoglobin is the pigment responsible for their red color. We believe that sequestered erythrocytes whose hemoglobin has been consumed by parasites account for tramlining, and for the orange and white appearance of vessels. However, sequestration with parasitized erythrocytes has not yet been demonstrated in individual retinal vessels with these abnormalities.
The number of retinal hemorrhages seen on funduscopic examination correlates with the number of cerebral hemorrhages in fatal cerebral malaria.39 In common with cerebral hemorrhages, fibrin thrombi are seen in the small vessel at the center of hemorrhages and hemorrhaged red blood cells rarely contain parasites.
Functional studies have been conducted to investigate the pathophysiology of retinal signs.10,16,40 These suggest that macular whitening is caused by oncotic swelling of second-order neurones in the inner retina due to metabolic or hypoxic stress. A study using fundus fluorescein angiography suggests that metabolic steal by intravascular parasites may play a role, rather than capillary obstruction, which was not seen in association with macular whitening.10 Hero and others performed fluorescein angiography on 12 Kenyan children with cerebral malaria and also found that fluorescein did not breach the blood retina barrier or accumulate as extracellular edema.10 The typical distribution of macular whitening, in sites of high metabolic demand and in vascular watershed zones, supports causation by metabolic steal or hypoxia over toxic malarial products, cytokines, or nitric oxide.
Further histopathologic studies of malaria retinopathy are underway or planned, including the electron microscopic appearances of retinal whitening, and detailed comparison of retinal photographs to flat-mounted, whole retinal specimens. These are in conjunction with cerebral histologic examination. However, an important benefit of studying retinopathy in severe malaria is that it can be done in vivo. Vessels with sequestration can be studied in children at any time point, repeatedly, in those who survive as well as those who die. With this in mind, a study using digital fluorescein angiography is underway to investigate retinal perfusion and integrity of the blood retina barrier in Malawian children with cerebral malaria. In contrast to Hero and others, this study benefits from the advantages of digital over film-based technology, and the improved resolution of a modern fundus camera.
FUTURE DEVELOPMENTS
Further studies are planned at five centers across Africa that are part of the Severe Malaria in African Children (SMAC) network. These aim to determine the effectiveness of trained physicians in detecting malarial retinopathy using indirect as well as direct ophthalmoscopy. If funduscopy does prove useful, it could help to improve the efficiency of pivotal studies of malaria pathogenesis, treatment, and prevention. Accurate diagnosis is critical to ensure that studies are correctly powered.
We hope that funduscopy will become more widely used to improve the diagnosis of severe malaria in Africa. An inexpensive, lens-free direct ophthalmoscope has recently been developed and launched in the United Kingdom.41 This may enable increased use of direct ophthalmoscopy in resource-poor settings. It remains to be established how effective direct ophthalmoscopy alone can be in detecting malarial retinopathy when pupils are consistently dilated, and when observers undergo specific training. Detection of malarial retinopathy is potentially a low-cost, bedside, diagnostic method, which could improve the precision of the clinical diagnosis of severe malaria.
CONCLUSIONS
Since malarial retinopathy was first reported as an unusual cluster of retinal signs with prognostic value in cerebral malaria, strong evidence of its diagnostic value has been published. Although this is predominantly in cerebral malaria in children, malarial retinopathy is also present in other forms of severe malaria. Two of the components of malarial retinopathy, retinal whitening and vessel changes, are unique to severe malaria. Autopsy data suggest that the recognition of malarial retinopathy improves on existing diagnostic criteria by reducing reliance on the exclusion of other causes of severe disease and by making the diagnosis on a positive basis. The sensitivity in non-fatal cases of cerebral malaria is not yet established, but its presence is associated with the most severe cases, the group for whom early interventions aimed at reducing mortality need to be developed.
Studies on malarial retinopathy have been conducted mainly in Malawi, Kenya, and Gambia, all with involvement of ophthalmologists. There are not enough ophthalmologists in Africa to perform these examinations. Further work is required to assess whether fatal coma with parasitemia has a similar rate of alternative diagnoses at other settings and transmission intensities, and whether detection of malarial retinopathy can improve diagnosis when fundus examination is performed by non-ophthalmologists.
To a clinician detecting malarial retinopathy, its presence confirms a diagnosis of severe malaria, and in cerebral malaria the likelihood of death is strongly related to its severity. If retinopathy is absent in a child thought to have cerebral malaria, the clinician should bear in mind the possibility of other causes of coma, particularly if the coma is prolonged. This knowledge could improve care of critically ill patients with parasitemia in disease-endemic areas, and also improve the power of studies to assess interventions aimed at reducing the persistently high mortality of severe malaria.
Acknowledgments
Nicholas A. V. Beare is supported by a Wellcome Trust project grant 074125/Z/04/Z. Malcolm E. Molyneux is supported by the Wellcome Trust grant 074124. Terrie E. Taylor is supported by the National Institutes of Health grant no. RO1 AI34969. The American Committee on Clinical Tropical Medicine and Travellers’ Health (ACCTMTH) assisted with publication expenses.
Footnotes
Disclosure: The Wellcome Trust and the National Institutes of Health NIH, while providing financial support, played no direct part in the preparation, review, or approval of this article. There are no competing financial interests.
References
- 1.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:S1–S90. [PubMed] [Google Scholar]
- 2.Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Green-wood BM, Whitty CJ. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212–1217. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith T, Charlwood JD, Kihonda J, Mwankusye S, Billingsley P, Meuwissen J, Lyimo E, Takken W, Teuscher T, Tanner M. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993;54:55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]
- 5.Kallander K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia–policy implications for home management strategies. Acta Trop. 2004;90:211–214. doi: 10.1016/j.actatropica.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 6.English M, Punt J, Mwangi I, McHugh K, Marsh K. Clinical overlap between malaria and severe pneumonia in Africa children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–662. doi: 10.1016/s0035-9203(96)90423-x. [DOI] [PubMed] [Google Scholar]
- 7.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 8.Lewallen S, Taylor TE, Molyneux ME, Wills BA, Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100:857–861. doi: 10.1016/s0161-6420(93)31563-0. [DOI] [PubMed] [Google Scholar]
- 9.Lewallen S, Bakker H, Taylor TE, Wills BA, Courtright P, Molyneux ME. Retinal findings predictive of outcome in cerebral malaria. Trans R Soc Trop Med Hyg. 1996;90:144–146. doi: 10.1016/s0035-9203(96)90116-9. [DOI] [PubMed] [Google Scholar]
- 10.Hero M, Harding SP, Riva CE, Winstanley PA, Peshu N, Marsh K. Photographic and angiographic characterization of the retina of Kenyan children with severe malaria. Arch Ophthalmol. 1997;115:997–1003. doi: 10.1001/archopht.1997.01100160167005. [DOI] [PubMed] [Google Scholar]
- 11.Lewallen S, Harding SP, Ajewole J, Schulenburg WE, Molyneux ME, Marsh K, Usen S, White NJ, Taylor TE. A review of the spectrum of clinical ocular fundus findings in P. falciparum malaria in African children with a proposed classification and grading system. Trans R Soc Trop Med Hyg. 1999;93:619–622. doi: 10.1016/s0035-9203(99)90071-8. [DOI] [PubMed] [Google Scholar]
- 12.Beare NA, Southern C, Chalira C, Taylor TE, Molyneux ME, Harding SP. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol. 2004;122:1141–1147. doi: 10.1001/archopht.122.8.1141. [DOI] [PubMed] [Google Scholar]
- 13.Hirneiss C, Klauss V, Wilke M, Kampik A, Taylor TE, Lewallen S. Ocular changes in tropical malaria with cerebral involvement: results from the Blantyre Malaria Project. Klin Monatsbl Augenheilkd. 2005;222:704–708. doi: 10.1055/s-2005-858445. [DOI] [PubMed] [Google Scholar]
- 14.Browning DJ. Patchy ischemic retinal whitening. Ophthalmology. 2004;111:606–607. doi: 10.1016/j.ophtha.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Schemann JF, Doumbo O, Malvy D, Traore L, Kone A, Sidibe T, Keita M. Ocular lesions associated with malaria in children in Mali. Am J Trop Med Hyg. 2002;67:61–63. doi: 10.4269/ajtmh.2002.67.61. [DOI] [PubMed] [Google Scholar]
- 16.Beare NA, Southern C, Kayira K, Taylor TE, Harding PS. Visual outcomes in children in Malawi following retinopathy of severe malaria. Br J Ophthalmol. 2004;88:321–324. doi: 10.1136/bjo.2003.025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looareesuwan S, Warrell DA, White NJ, Chanthavanich P, Warrell MJ, Chantaratherakitti S, Changswek S, Chongmankongcheep L, Kanchanaranya C. Retinal hemorrhage, a common sign of prognostic significance in cerebral malaria. Am J Trop Med Hyg. 1983;32:911–915. doi: 10.4269/ajtmh.1983.32.911. [DOI] [PubMed] [Google Scholar]
- 18.Newton CR, Winstanley PA, Marsh K. Retinal haemorrhages in falciparum malaria. Arch Dis Child. 1991;66:753. doi: 10.1136/adc.66.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslett P. Retinal haemorrhages in Zambian children with cerebral malaria. J Trop Pediatr. 1991;37:86–87. doi: 10.1093/tropej/37.2.86. [DOI] [PubMed] [Google Scholar]
- 20.Burton M, Nyong’o O, Burton K, John W, Inkoom E, Pinder M, Corrah T, Johnson G, Bailey R. Retinopathy in Gambian children admitted to hospital with malaria. Trop Doct. 2004;34:214–218. doi: 10.1177/004947550403400409. [DOI] [PubMed] [Google Scholar]
- 21.Beare NA, Southern C, Lochhead J, Molyneux ME, Lewallen S, Harding SP. Inter-observer concordance in grading retinopathy in cerebral malaria. Ann Trop Med Parasitol. 2002;96:105–108. doi: 10.1179/000349802125000565. [DOI] [PubMed] [Google Scholar]
- 22.Ritter AM, Muizelaar JP, Barnes T, Choi S, Fatouros P, Ward J, Bullock MR. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 1999;44:941–948. doi: 10.1097/00006123-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Carter BG, Butt W. Are somatosensory evoked potentials the best predictor of outcome after severe brain injury? A systematic review. Intensive Care Med. 2005;31:765–775. doi: 10.1007/s00134-005-2633-1. [DOI] [PubMed] [Google Scholar]
- 24.Krieger DH, Adams P, Schwarz S, Rieke K, Aschoff A, Hacke W. Prognostic and clinical relevance of pupillary responses, intracranial pressure monitoring, and brainstem auditory evoked potentials in comatose patients with acute supratentorial mass lesions. Crit Care Med. 1993;21:1944–1950. doi: 10.1097/00003246-199312000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- 26.Woodward H, Winterhalther K, Donders J, Hackbarth R, Kuldanek A, Sanfilippo D. Prediction of neurobehavioral outcome 1–5 years post pediatric traumatic head injury. J Head Trauma Rehabil. 1999;14:351–359. doi: 10.1097/00001199-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Plum F, Posner JB. The Diagnosis of Stupor and Coma. 3. Philadelphia: F.A. Davis Company; 1984. Supratentorial causes of coma; pp. 87–133. [Google Scholar]
- 28.Marshman LA. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 2002;51:848–849. [PubMed] [Google Scholar]
- 29.Bullock MR. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 2002;51:849. [PubMed] [Google Scholar]
- 30.Olumese PE, Adeyemo AA, Gbadegesin RA, Walker O. Retinal haemorrhage in cerebral malaria. East Afr Med J. 1997;74:285–287. [PubMed] [Google Scholar]
- 31.Hanscheid T. Current strategies to avoid misdiagnosis of malaria. Clin Microbiol Infect. 2003;9:497–504. doi: 10.1046/j.1469-0691.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 32.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 33.Makani J, Matuja W, Liyombo E, Snow RW, Marsh K, Warrell DA. Admission diagnosis of cerebral malaria in adults in an endemic area of Tanzania: implications and clinical description. QJM. 2003;96:355–362. doi: 10.1093/qjmed/hcg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okubadejo NU, Danesi MA. Diagnostic issues in cerebral malaria: a study of 112 adolescents and adults in Lagos, Nigeria. Niger Postgrad Med J. 2004;11:10–14. [PubMed] [Google Scholar]
- 35.Kochar DK, Shubhakaran, Kumawat BL, Thanvi I, Joshi A, Vyas SP. Ophthalmoscopic abnormalities in adults with falciparum malaria. QJM. 1998;91:845–852. doi: 10.1093/qjmed/91.12.845. [DOI] [PubMed] [Google Scholar]
- 36.Kochar DK, Shubhakaran, Kumawat BL, Vyas SP. Prognostic significance of eye changes in cerebral malaria. J Assoc Physicians India. 2000;48:473–477. [PubMed] [Google Scholar]
- 37.Beare NA, Lewis DK, Kublin JG, Harding SP, Zijlstra EE, Molyneux ME. Retinal changes in adults with cerebral malaria. Ann Trop Med Parasitol. 2003;97:313–315. doi: 10.1179/000349803235001859. [DOI] [PubMed] [Google Scholar]
- 38.Lewallen S, White VA, Whitten RO, Gardiner J, Hoar B, Lindley J, Lochhead J, McCormick A, Wade K, Tembo M, Mwenechanyana J, Molyneux ME, Taylor TE. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol. 2000;118:924–928. [PubMed] [Google Scholar]
- 39.White VA, Lewallen S, Beare N, Kayira K, Carr RA, Taylor TE. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg. 2001;95:618–621. doi: 10.1016/s0035-9203(01)90097-5. [DOI] [PubMed] [Google Scholar]
- 40.Harding SP, Lochhead J, Movaffaghy A, Falsini B, Riva CE, Molyneux ME. Electroretinography of severe malaria in Malawian children. [Accessed April 12, 2006];Invest Ophthalmol Vis Sci. 1999 40:S148.41. www.ophthalmos.co.uk.