Abstract
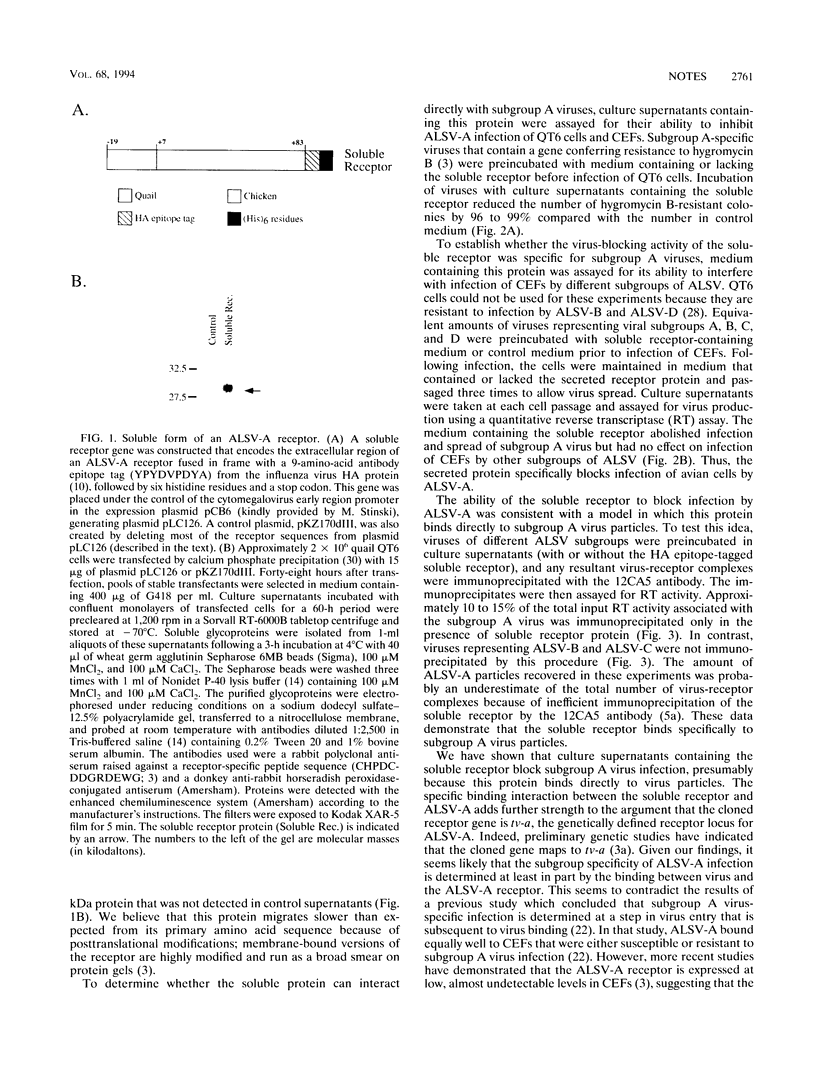
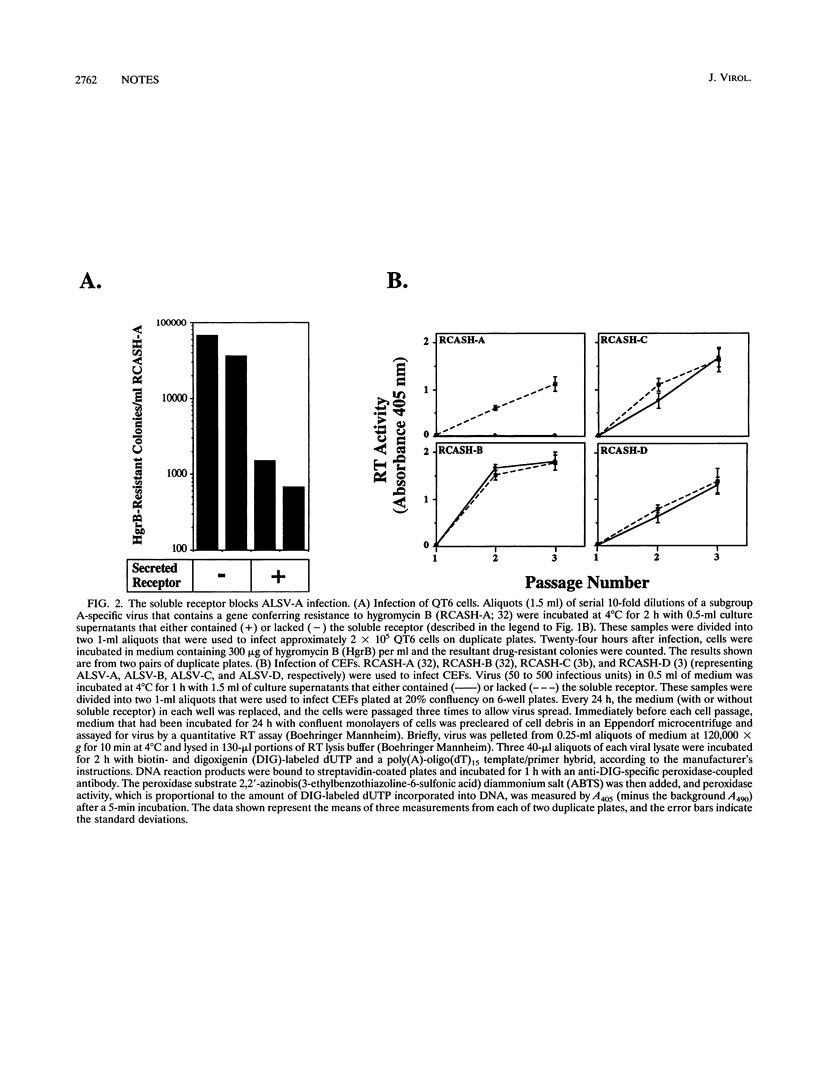
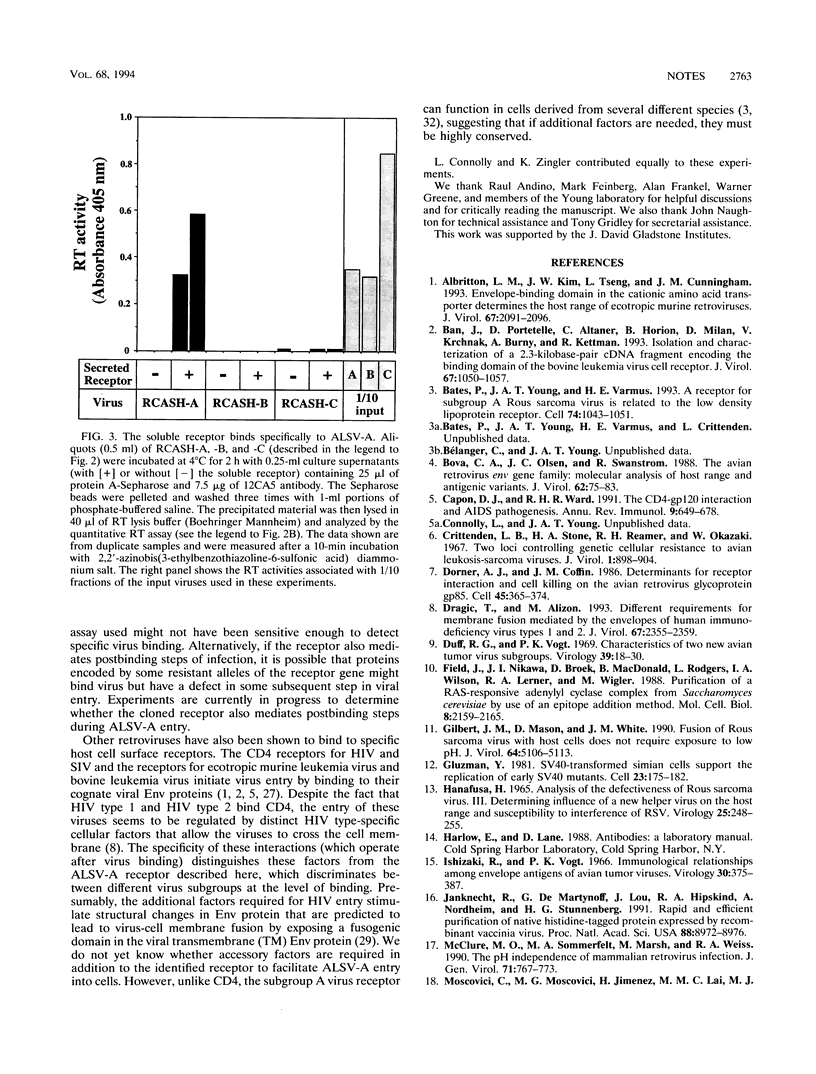
A receptor that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses (ALSV-A) has been described (P. Bates, J. A. T. Young, and H. E. Varmus, Cell 74:1043-1051, 1993). A soluble form of the receptor was generated to determine whether this protein interacts directly with virus particles in the absence of other cell surface factors. The soluble protein comprised the extracellular region of the ALSV-A receptor fused to an antibody epitope tag and six histidine residues. Preincubating this protein with virus led to an efficient block to infection of avian cells by ALSV-A but had no effect on infection by ALSV-B, ALSV-C, or ALSV-D. Furthermore, an antibody directed against the introduced epitope tag immunoprecipitated ALSV-A particles bound to the soluble receptor. In contrast, other ALSV subgroups were not immunoprecipitated by this procedure. These data demonstrate that the cloned receptor interacts directly with ALSV-A and discriminates between different ALSV subgroups at the level of virus binding.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Kim J. W., Tseng L., Cunningham J. M. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J Virol. 1993 Apr;67(4):2091–2096. doi: 10.1128/jvi.67.4.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban J., Portetelle D., Altaner C., Horion B., Milan D., Krchnak V., Burny A., Kettmann R. Isolation and characterization of a 2.3-kilobase-pair cDNA fragment encoding the binding domain of the bovine leukemia virus cell receptor. J Virol. 1993 Feb;67(2):1050–1057. doi: 10.1128/jvi.67.2.1050-1057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P., Young J. A., Varmus H. E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993 Sep 24;74(6):1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- Bova C. A., Olsen J. C., Swanstrom R. The avian retrovirus env gene family: molecular analysis of host range and antigenic variants. J Virol. 1988 Jan;62(1):75–83. doi: 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Ward R. H. The CD4-gp120 interaction and AIDS pathogenesis. Annu Rev Immunol. 1991;9:649–678. doi: 10.1146/annurev.iy.09.040191.003245. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Stone H. A., Reamer R. H., Okazaki W. Two loci controlling genetic cellular resistance to avian leukosis-sarcoma viruses. J Virol. 1967 Oct;1(5):898–904. doi: 10.1128/jvi.1.5.898-904.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dragic T., Alizon M. Different requirements for membrane fusion mediated by the envelopes of human immunodeficiency virus types 1 and 2. J Virol. 1993 Apr;67(4):2355–2359. doi: 10.1128/jvi.67.4.2355-2359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. M., Mason D., White J. M. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J Virol. 1990 Oct;64(10):5106–5113. doi: 10.1128/jvi.64.10.5106-5113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS. 3. DETERMINING INFLUENCE OF A NEW HELPER VIRUS ON THE HOST RANGE AND SUSCEPTIBILITY TO INTERFERENCE OF RSV. Virology. 1965 Feb;25:248–255. doi: 10.1016/0042-6822(65)90203-5. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Janknecht R., de Martynoff G., Lou J., Hipskind R. A., Nordheim A., Stunnenberg H. G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Sommerfelt M. A., Marsh M., Weiss R. A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990 Apr;71(Pt 4):767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- PAYNE L. N., BIGGS P. M. DIFFERENCES BETWEEN HIGHLY INBRED LINES OF CHICKENS IN THE RESPONSE TO ROUS SARCOMA VIRUS OF THE CHORIOALLANTOIC MEMBRANE AND OF EMBRYONIC CELLS IN TISSUE CULTURE. Virology. 1964 Dec;24:610–616. doi: 10.1016/0042-6822(64)90215-6. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Biggs P. M. Genetic basis of cellular susceptibility to the Schmidt-Ruppin and Harris strains of Rous sarcoma virus. Virology. 1966 Jun;29(2):190–198. doi: 10.1016/0042-6822(66)90025-0. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Pani P. K. Ecidence for linkage between genetic loci controlling response of fowl to subgroup A and subgroup C sarcoma viruses. J Gen Virol. 1971 Nov;13(2):253–259. doi: 10.1099/0022-1317-13-2-253. [DOI] [PubMed] [Google Scholar]
- RUBIN H. GENETIC CONTROL OF CELLULAR SUSCEPTIBILITY TO PSEUDOTYPES OF ROUS SARCOMA VIRUS. Virology. 1965 Jun;26:270–276. doi: 10.1016/0042-6822(65)90274-6. [DOI] [PubMed] [Google Scholar]
- VOGT P. K. A HETEROGENEITY OF ROUS SARCOMA VIRUS REVEALED BY SELECTIVELY RESISTANT CHICK EMBRYO CELLS. Virology. 1965 Feb;25:237–247. doi: 10.1016/0042-6822(65)90202-3. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology. 1965 Aug;26(4):664–672. doi: 10.1016/0042-6822(65)90329-6. [DOI] [PubMed] [Google Scholar]
- Wang H., Paul R., Burgeson R. E., Keene D. R., Kabat D. Plasma membrane receptors for ecotropic murine retroviruses require a limiting accessory factor. J Virol. 1991 Dec;65(12):6468–6477. doi: 10.1128/jvi.65.12.6468-6477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Young J. A., Bates P., Varmus H. E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993 Apr;67(4):1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




