Abstract
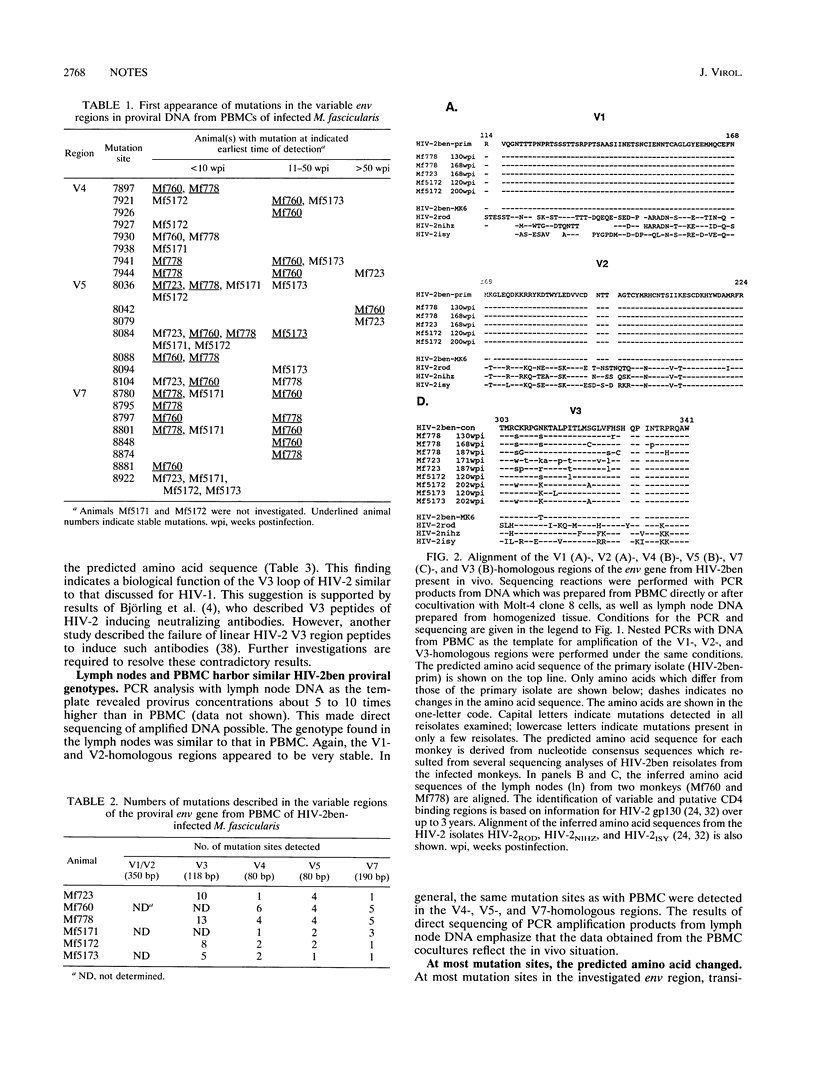
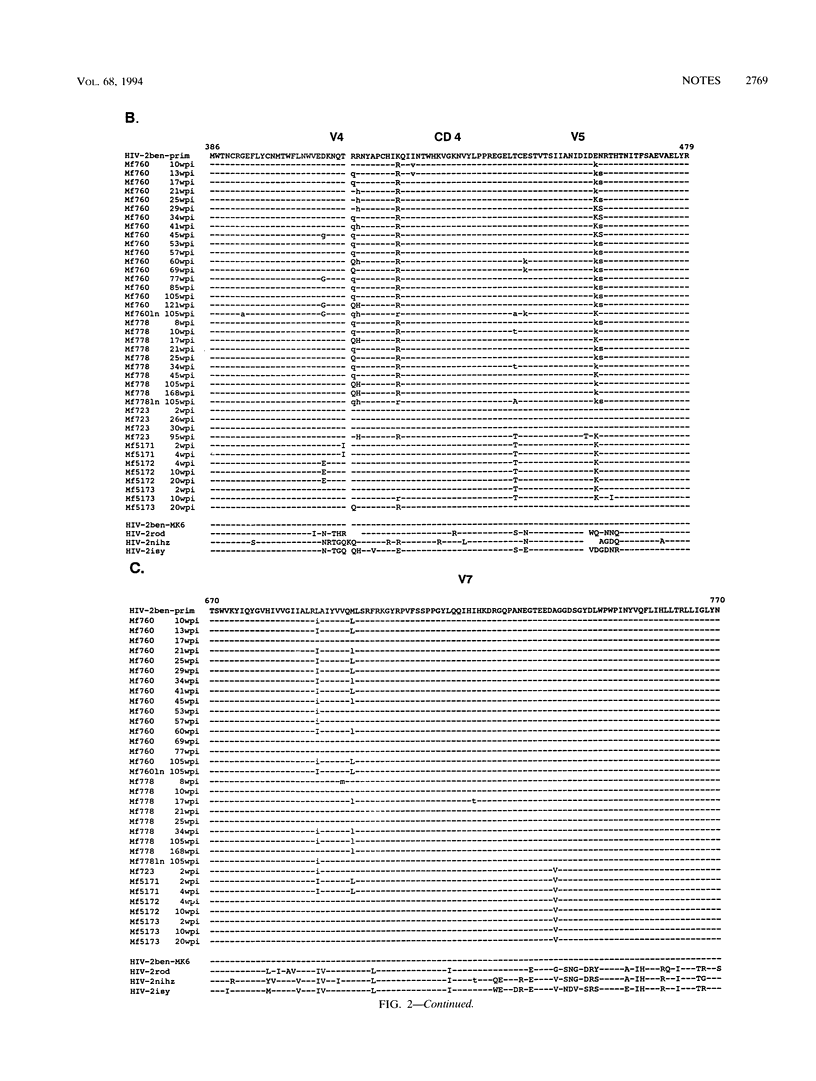
The sequence variability of distinct regions of the proviral env gene of human immunodeficiency virus type 2 strain ben (HIV-2ben) isolated sequentially over 3 to 4 years from six experimentally infected macaques was studied. The regions investigated were homologous to the V1, V2, V3, V4, V5, and V7 hypervariable regions identified in the env genes of HIV-1 and simian immunodeficiency virus SIVmac, respectively. In contrast to findings with HIV-1 and SIVmac, the V1- and V2-homologous regions were found to be highly conserved during the course of the HIV-2ben infection in macaques. The V3-homologous region showed a degree of variation comparable to that of HIV-1 but not of SIV. In the V4-, V5-, and V7-homologous regions, mutation hot spots were detected in most reisolates of the infected monkeys. Most of these mutations occurred during the first 10 weeks after infection. After 50 weeks, new mutations were rarely detected. At most mutation sites, a dynamic equilibrium between the mutated viral isotype and the infecting predominant wild type was present. This equilibrium might prevent an accumulation of mutations in isolates later in the course of infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Almond N., Jenkins A., Slade A., Heath A., Cranage M., Kitchin P. Population sequence analysis of a simian immunodeficiency virus (32H reisolate of SIVmac251): a virus stock used for international vaccine studies. AIDS Res Hum Retroviruses. 1992 Jan;8(1):77–88. doi: 10.1089/aid.1992.8.77. [DOI] [PubMed] [Google Scholar]
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björling E., Broliden K., Bernardi D., Utter G., Thorstensson R., Chiodi F., Norrby E. Hyperimmune antisera against synthetic peptides representing the glycoprotein of human immunodeficiency virus type 2 can mediate neutralization and antibody-dependent cytotoxic activity. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6082–6086. doi: 10.1073/pnas.88.14.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri E., Giri A., Lillo F., Ferrari G., Varnier O. E., Ferro A., Sabbatani S., Saxinger W. C., Franchini G. In vivo genetic variability of the human immunodeficiency virus type 2 V3 region. J Virol. 1992 Jul;66(7):4546–4550. doi: 10.1128/jvi.66.7.4546-4550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. P., Desrosiers R. C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991 Apr;65(4):1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Emerman M., Tiollais P., Sonigo P. The cytoplasmic domain of simian immunodeficiency virus transmembrane protein modulates infectivity. J Virol. 1989 Oct;63(10):4395–4403. doi: 10.1128/jvi.63.10.4395-4403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Dormont D., Livartowski J., Chamaret S., Guetard D., Henin D., Levagueresse R., van de Moortelle P. F., Larke B., Gourmelon P., Vazeux R. HIV-2 in rhesus monkeys: serological, virological and clinical results. Intervirology. 1989;30 (Suppl 1):59–65. doi: 10.1159/000150125. [DOI] [PubMed] [Google Scholar]
- Dunning A. M., Talmud P., Humphries S. E. Errors in the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 11;16(21):10393–10393. doi: 10.1093/nar/16.21.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis P. D., Zemmour J., Salter R. D., Parham P. Rapid cloning of HLA-A,B cDNA by using the polymerase chain reaction: frequency and nature of errors produced in amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2833–2837. doi: 10.1073/pnas.87.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyö E. M., Albert J., Asjö B. Replicative capacity, cytopathic effect and cell tropism of HIV. AIDS. 1989;3 (Suppl 1):S5–12. doi: 10.1097/00002030-198901001-00002. [DOI] [PubMed] [Google Scholar]
- Franchini G., Fargnoli K. A., Giombini F., Jagodzinski L., De Rossi A., Bosch M., Biberfeld G., Fenyo E. M., Albert J., Gallo R. C. Molecular and biological characterization of a replication competent human immunodeficiency type 2 (HIV-2) proviral clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2433–2437. doi: 10.1073/pnas.86.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Markham P., Gard E., Fargnoli K., Keubaruwa S., Jagodzinski L., Robert-Guroff M., Lusso P., Ford G., Wong-Staal F. Persistent infection of rhesus macaques with a molecular clone of human immunodeficiency virus type 2: evidence of minimal genetic drift and low pathogenetic effects. J Virol. 1990 Sep;64(9):4462–4467. doi: 10.1128/jvi.64.9.4462-4467.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow M., Huet T., Saurin W., Kwok S., Sninsky J., Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr. 1989;2(4):344–352. [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Edmondson P., Murphey-Corb M., Arbeille B., Johnson P. R., Mullins J. I. SIV adaptation to human cells. Nature. 1989 Oct 19;341(6243):573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Hirsch V. M. Genetic variation of simian immunodeficiency viruses in nonhuman primates. AIDS Res Hum Retroviruses. 1992 Mar;8(3):367–372. doi: 10.1089/aid.1992.8.367. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Henkel R. D., Pauletti D., Allan J. S., Lee T. H., Essex M., Dreesman G. R. Antiserum to a synthetic peptide recognizes the HTLV-III envelope glycoprotein. Science. 1986 Mar 28;231(4745):1556–1559. doi: 10.1126/science.3006246. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F., Jentsch K. D., Bachmann B., Stuke A., Laloux C., Lüke W., Stahl-Hennig C., Schneider J., Nieselt K., Eigen M. A novel proviral clone of HIV-2: biological and phylogenetic relationship to other primate immunodeficiency viruses. Virology. 1990 Jul;177(1):305–311. doi: 10.1016/0042-6822(90)90484-9. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F., Jentsch K. D., Stuke A., Mous J., Hunsmann G. Genomic divergence of an HIV-2 from a German AIDS patient probably infected in Mali. AIDS. 1990 Sep;4(9):847–857. doi: 10.1097/00002030-199009000-00003. [DOI] [PubMed] [Google Scholar]
- Kodama T., Wooley D. P., Naidu Y. M., Kestler H. W., 3rd, Daniel M. D., Li Y., Desrosiers R. C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989 Nov;63(11):4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S. M., Weeger M., Stahl-Hennig C., Coulibaly C., Hunsmann G., Müller J., Müller-Hermelink H., Fuchs D., Wachter H., Daniel M. M. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993 Feb;67(2):902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüke W., Voss G., Stahl-Hennig C., Coulibaly C., Putkonen P., Petry H., Hunsmann G. Protection of cynomolgus macaques (Macaca fascicularis) against infection with the human immunodeficiency virus type 2 strain ben (HIV-2ben) by immunization with the virion-derived envelope glycoprotein gp130. AIDS Res Hum Retroviruses. 1993 May;9(5):387–394. doi: 10.1089/aid.1993.9.387. [DOI] [PubMed] [Google Scholar]
- Marlink R. G., Ricard D., M'Boup S., Kanki P. J., Romet-Lemonne J. L., N'Doye I., Diop K., Simpson M. A., Greco F., Chou M. J. Clinical, hematologic, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2). AIDS Res Hum Retroviruses. 1988 Apr;4(2):137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M., Papenhausen M. D., Benveniste R. E., Morton W. R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991 Dec;65(12):7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkonen P., Böttiger B., Warstedt K., Thorstensson R., Albert J., Biberfeld G. Experimental infection of cynomolgus monkeys (Macaca fascicularis) with HIV-2. J Acquir Immune Defic Syndr. 1989;2(4):366–373. [PubMed] [Google Scholar]
- Robert-Guroff M., Aldrich K., Muldoon R., Stern T. L., Bansal G. P., Matthews T. J., Markham P. D., Gallo R. C., Franchini G. Cross-neutralization of human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus isolates. J Virol. 1992 Jun;66(6):3602–3608. doi: 10.1128/jvi.66.6.3602-3608.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensey L. M., Papenhausen M. D., Overbaugh J. Replication and persistence of simian immunodeficiency virus variants after passage in macaque lymphocytes and established human cell lines. J Virol. 1993 Mar;67(3):1727–1733. doi: 10.1128/jvi.67.3.1727-1733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl-Hennig C., Herchenröder O., Nick S., Evers M., Stille-Siegener M., Jentsch K. D., Kirchhoff F., Tolle T., Gatesman T. J., Lüke W. Experimental infection of macaques with HIV-2ben, a novel HIV-2 isolate. AIDS. 1990 Jul;4(7):611–617. doi: 10.1097/00002030-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Rutledge R. A., Dias S., Folks T., Theodore T., Buckler C. E., Martin M. A. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5038–5042. doi: 10.1073/pnas.83.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]



