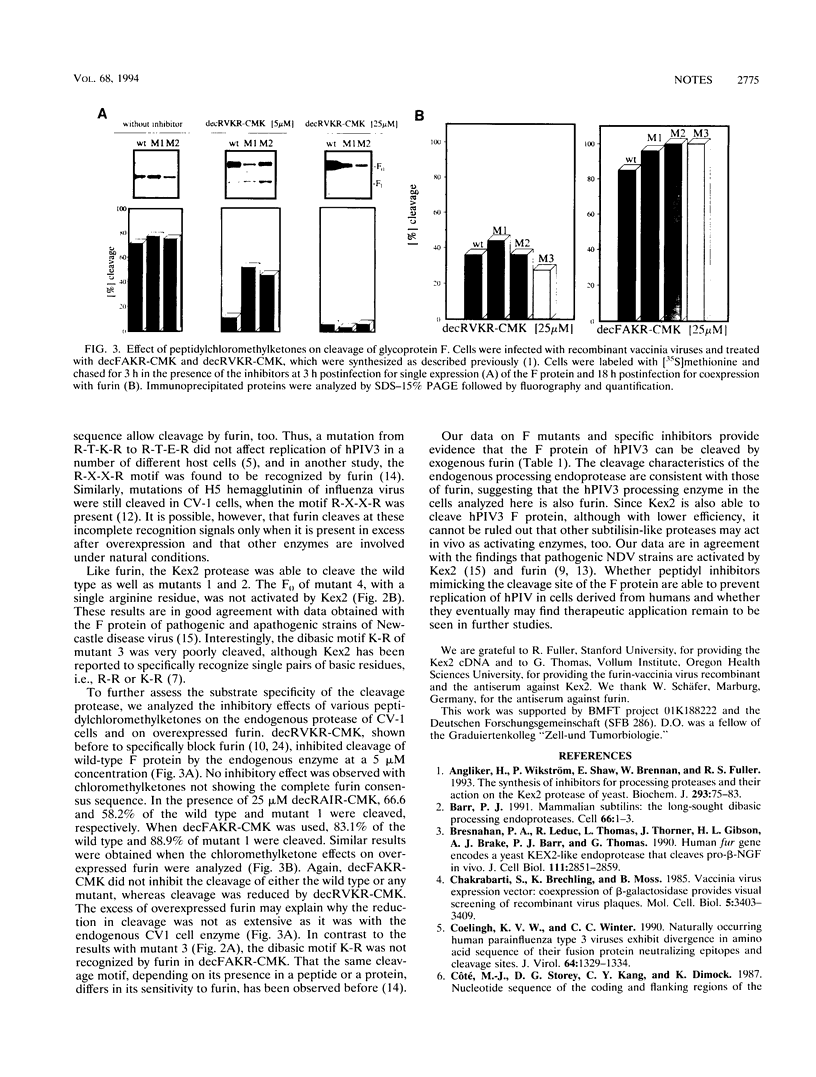
Abstract
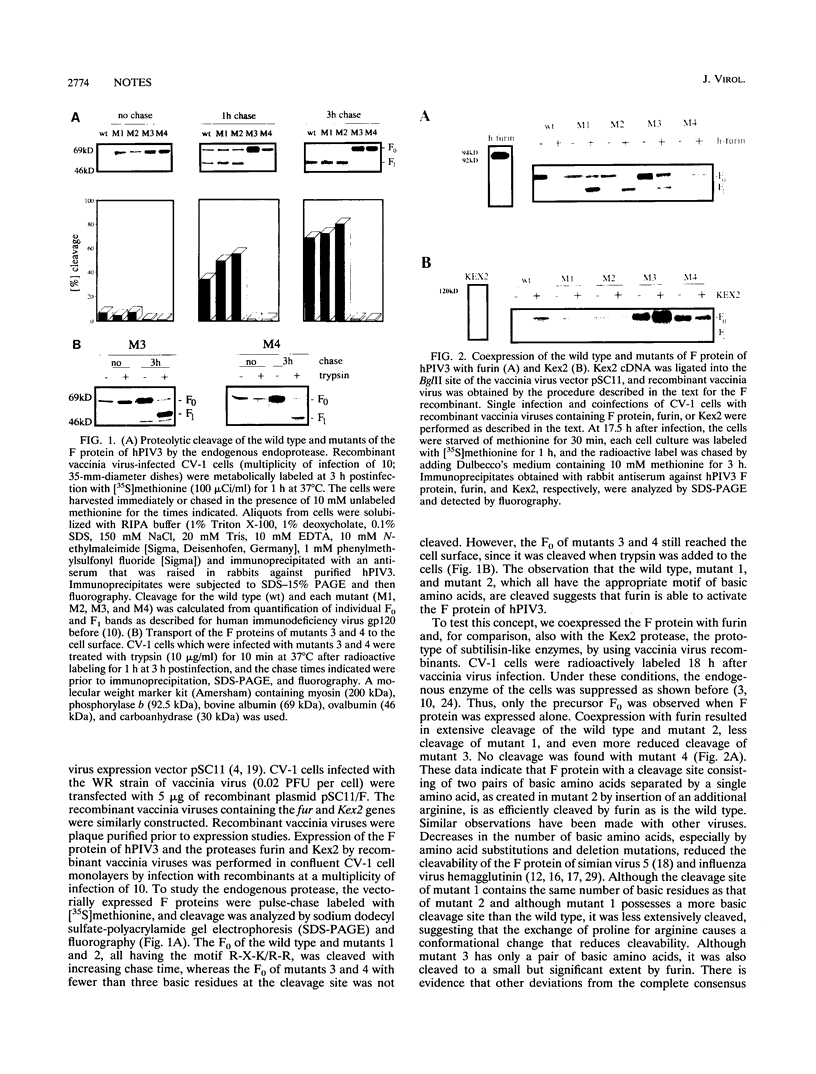
The fusion (F) protein of human parainfluenza virus type 3 contains the tribasic cleavage site R-T-K-R, which was altered by site-directed mutagenesis. Wild-type F protein and various mutants were expressed by recombinant vaccinia viruses. The endogenous endoprotease present in CV-1 cells cleaves F variants containing the furin recognition motif R-X-K/R-R but not variants containing the dibasic site K-R or a single R at the cleavage site. A similar cleavage pattern was obtained when the subtilisin-like endoproteases Kex2 and furin were coexpressed with the wild type and mutants of the F protein. Peptidylchloromethylketone inhibitors mimicking basic cleavage sites prevent cleavage of the precursor Fo by the endogenous protease only when the furin-specific motif is present in the peptidyl portion. The data support the concept that furin is a cellular protease responsible for the activation of the F protein of human parainfluenza virus type 3.
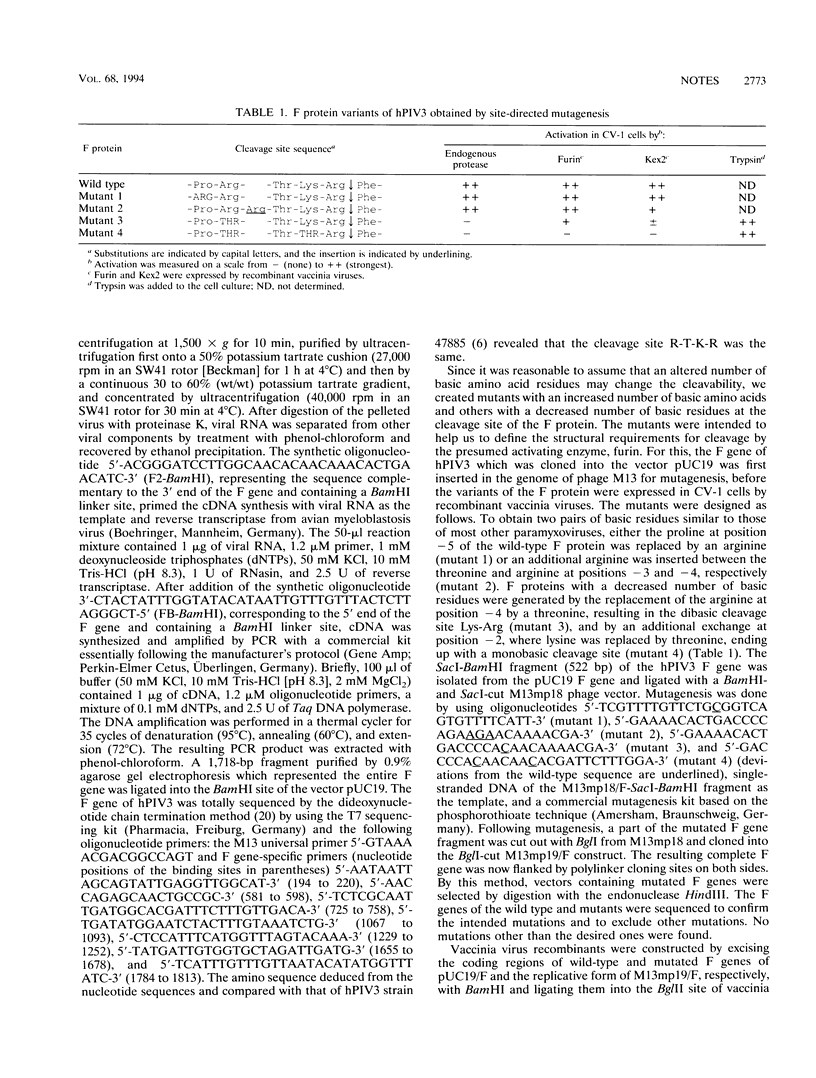
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angliker H., Wikstrom P., Shaw E., Brenner C., Fuller R. S. The synthesis of inhibitors for processing proteinases and their action on the Kex2 proteinase of yeast. Biochem J. 1993 Jul 1;293(Pt 1):75–81. doi: 10.1042/bj2930075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelingh K. V., Winter C. C. Naturally occurring human parainfluenza type 3 viruses exhibit divergence in amino acid sequence of their fusion protein neutralization epitopes and cleavage sites. J Virol. 1990 Mar;64(3):1329–1334. doi: 10.1128/jvi.64.3.1329-1334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M. J., Storey D. G., Kang C. Y., Dimock K. Nucleotide sequence of the coding and flanking regions of the human parainfluenza virus type 3 fusion glycoprotein gene. J Gen Virol. 1987 Apr;68(Pt 4):1003–1010. doi: 10.1099/0022-1317-68-4-1003. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Gotoh B., Ogasawara T., Toyoda T., Inocencio N. M., Hamaguchi M., Nagai Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990 Dec;9(12):4189–4195. doi: 10.1002/j.1460-2075.1990.tb07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh B., Ohnishi Y., Inocencio N. M., Esaki E., Nakayama K., Barr P. J., Thomas G., Nagai Y. Mammalian subtilisin-related proteinases in cleavage activation of the paramyxovirus fusion glycoprotein: superiority of furin/PACE to PC2 or PC1/PC3. J Virol. 1992 Nov;66(11):6391–6397. doi: 10.1128/jvi.66.11.6391-6397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenberger S., Bosch V., Angliker H., Shaw E., Klenk H. D., Garten W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature. 1992 Nov 26;360(6402):358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- Hu X. L., Compans R. W., Matsuoka Y., Ray R. Molecular cloning and sequence analysis of the fusion glycoprotein gene of human parainfluenza virus type 2. Virology. 1990 Dec;179(2):915–920. doi: 10.1016/0042-6822(90)90168-q. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Inocencio N. M., Robertson B. J., Moehring T. J. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J Biol Chem. 1993 Feb 5;268(4):2590–2594. [PubMed] [Google Scholar]
- Molloy S. S., Bresnahan P. A., Leppla S. H., Klimpel K. R., Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992 Aug 15;267(23):16396–16402. [PubMed] [Google Scholar]
- Nagai Y., Inocencio N. M., Gotoh B. Paramyxovirus tropism dependent on host proteases activating the viral fusion glycoprotein. Behring Inst Mitt. 1991 Jul;(89):35–45. [PubMed] [Google Scholar]
- Ohuchi M., Orlich M., Ohuchi R., Simpson B. E., Garten W., Klenk H. D., Rott R. Mutations at the cleavage site of the hemagglutinin after the pathogenicity of influenza virus A/chick/Penn/83 (H5N2). Virology. 1989 Feb;168(2):274–280. doi: 10.1016/0042-6822(89)90267-5. [DOI] [PubMed] [Google Scholar]
- Ohuchi R., Ohuchi M., Garten W., Klenk H. D. Human influenza virus hemagglutinin with high sensitivity to proteolytic activation. J Virol. 1991 Jul;65(7):3530–3537. doi: 10.1128/jvi.65.7.3530-3537.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Shaughnessy M. A., Lamb R. A. Analysis of the relationship between cleavability of a paramyxovirus fusion protein and length of the connecting peptide. J Virol. 1989 Mar;63(3):1293–1301. doi: 10.1128/jvi.63.3.1293-1301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. C., Garten W., Klenk H. D. Role of conserved glycosylation sites in maturation and transport of influenza A virus hemagglutinin. J Virol. 1993 Jun;67(6):3048–3060. doi: 10.1128/jvi.67.6.3048-3060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Stieneke-Gröber A., Vey M., Angliker H., Shaw E., Thomas G., Roberts C., Klenk H. D., Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992 Jul;11(7):2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzu S., Sakai Y., Shioda T., Shibuta H. Nucleotide sequence of the bovine parainfluenza 3 virus genome: the genes of the F and HN glycoproteins. Nucleic Acids Res. 1987 Apr 10;15(7):2945–2958. doi: 10.1093/nar/15.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Yokogoshi Y., Tobita K., Seto J. T., Rott R., Kido H. Tryptase Clara, an activating protease for Sendai virus in rat lungs, is involved in pneumopathogenicity. J Virol. 1992 Dec;66(12):7211–7216. doi: 10.1128/jvi.66.12.7211-7216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Varsanyi T. M., Kövamees J., Norrby E. Molecular cloning and sequence analysis of human parainfluenza type 2 virus mRNA encoding the fusion glycoprotein. J Gen Virol. 1991 Jan;72(Pt 1):89–95. doi: 10.1099/0022-1317-72-1-89. [DOI] [PubMed] [Google Scholar]
- Vey M., Orlich M., Adler S., Klenk H. D., Rott R., Garten W. Hemagglutinin activation of pathogenic avian influenza viruses of serotype H7 requires the protease recognition motif R-X-K/R-R. Virology. 1992 May;188(1):408–413. doi: 10.1016/0042-6822(92)90775-K. [DOI] [PMC free article] [PubMed] [Google Scholar]