Abstract
Background
Stratification variables of age, race, and sex figure prominently in the assessment of cardiovascular disease risk. Similarly, cardiac autonomic regulation, measured by RR interval variability (RRV), is associated with risk. The relationship among these variables is unclear.
Methods
We examined the cross-sectional relationship between RRV and age, race, and sex in 757 subjects from the NHLBI-funded Coronary Artery Disease in Young Adults (CARDIA) Study.
Results
Age was a significant determinant of RRV, despite the narrow range (33–47): participants aged 33–39 years had had greater levels of HF power, LF power, and standard deviation (SD) of RR intervals than did those aged 40–47 years. There was no age effect for the LF/HF ratio. Compared to whites, blacks had lower levels of LF power, SD, and lower LF/HF. Blacks and whites did not differ in HF power. Finally, compared to men, women had lower levels of LF power, SD, and LF/HF but did not differ in HF power.
Conclusions
Data from the CARDIA study suggest that in adults in the 33–47 year age range, indices of RRV were greater in younger compared to older subjects, in men compared to women and in whites compared to blacks. These findings are broadly consistent with those of other large studies examining relationships between RRV and age, sex, and race. However, patterns of associations between RRV and these stratification variables are not entirely consistent with an underlying autonomic physiology linked to cardioprotection.
Keywords: Parasympathetic nervous system, race, sex, age, community study
INTRODUCTION
Stratification variables of age, race, and sex figure prominently in the assessment of cardiovascular disease risk. The incidence of coronary heart disease (CHD) in the US rises steadily with age (Rosamond et al., 2007). Race and sex also influence risk: age-adjusted incidence rates for CHD, ages 35–74, are 4.5 for white men, 5.4 for black men, 1.9 for white women, and 3.4 for black women (National Institutes of Health, 2006).
The mechanisms associated with these differences in risk are only partially understood. One potential pathophysiological mechanism, autonomic nervous system dysfunction, clearly is related to the risk of CHD. Resting heart rate (HR) is associated prospectively with mortality (Kannel et al., 1987). While elevated HR may simply be a marker of hypertension associated with elevated sympathetic nervous system activity, evidence suggests otherwise. First, experimental reduction of HR in animals leads to a reduction in coronary and carotid atherosclerosis (Beere et al., 1984; Beere et al., 1992). Second, in human studies, HR predicts cardiovascular outcomes even in patients with hypertension (Gillman et al., 1993; Thomas et al., 2001). RR interval variability (RRV), a more sensitive index of cardiac autonomic regulation, also is related to the risk of CHD. In the Atherosclerosis Risk in Communities (ARIC) study, the Framingham Offspring study, and the Zutphen study, low RRV predicted the development of CHD in initially healthy men and women (Dekker et al., 2000; Liao et al., 1995; Tsuji et al., 1996a).
The relationship of RRV to stratification variables age, sex, and race is less clear. Most studies agree that age is inversely associated with RRV (Crasset et al., 2001; Gregoire et al., 1996; Kuo et al., 1999; Tsuji et al., 1996b; Yeragani et al., 1997). The data are less consistent on the impact of sex and race. Some studies have reported that women have greater levels of HF power (Kuo et al., 1999; Ryan et al., 1994) while others report no sex difference (Evans et al., 2001; Laitinen et al., 1998; Pikkujamsa et al., 2001; Ramaekers et al., 1998). Fewer studies still report on the impact of race on RRV. In a study of young (early 20s) men, we demonstrated that black subjects (N = 32) had lower HF power compared to non-blacks (N = 29), a group that included whites, Asians, and Latinos (Zion et al., 2003). In this study, the LF/HF ratio was greater in the blacks. In contrast, Guzzetti et al. (Guzzetti et al., 2000) reported that 24-hour normalized LF power and the LF/HF ratio were lower in blacks compared to whites but the study was small (N = 52) and all subjects were untreated hypertensives. In another small study (N = 39) of males, the LF/HF ratio was lower and pNN50, an index of cardiac vagal modulation, was higher in blacks compared to whites (Urbina et al., 1998).
Most of these studies used small convenience samples that may account for these inconsistencies. To address this matter, we examined the influence of age, sex, and race on RRV using data from the NHLBI-funded multi-center study of Coronary Artery Risk Development in Young Adults (CARDIA) study.
MATERIALS AND METHODS
Study Population
CARDIA is a biethnic, prospective, multicenter epidemiological study of the evolution of cardiovascular risk development in young adulthood. In 1985–1986, 5115 black and white men and women, aged 18 to 30 years, were recruited at Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA, to achieve a balance at each site by race (black, white), sex, education (high school degree or less, more than high school), and age (18–24 years, 25–30 years) (Cutter et al., 1991). Participants were examined at study entry and years 2, 5, 7, 10, and 15 with re-examination rates among surviving cohort members of 90.5%, 85.7%, 80.6%, 78.5%, and 73.5%, respectively. Comparisons of CARDIA subjects who participated in the Year 15 exam with those who did not indicated that the latter participants were more likely to be black, younger, less educated and smokers (data not shown). Site institutional review committee approval and informed consent were obtained for each examination.
At the Year 15 exam, subjects seen at the Oakland, CA and Chicago, IL sites (and living within 50 miles of the clinic; N = 721 and 615 respectively) were asked to participate in a substudy of socioeconomic status and development of biological risk, including assessments of RRV. Of the 1,336 subjects who were eligible for the substudy, 789 (59%) agreed to participate in the RRV component of the substudy.
Data Collection
Standardized questionnaires were used to assess age, race, and sex at the time of the initial CARDIA examination in 1985–86.
RRV Assessments
Participants arrived at the laboratory having eaten a light breakfast but abstaining from caffeinated beverages and smoking that morning. Study protocols were explained and written consent was obtained. The RRV protocol was explained and ECG electrodes were attached. Subjects then rested quietly in the seated position for a two-minute period after which data were collected for 10 min. Subjects were asked to sit quietly without moving or talking.
Acquisition and Processing of ECG Signals
ECG data were collected continuously throughout the protocol. ECG electrodes were placed on the right shoulder, on the left anterior axillary line at the 10th intercostal space and in the right lower quadrant. Analog ECG signals were digitized at 500 Hz by a National Instruments A/D board and stored on a microcomputer. The ECG waveform was submitted to a specially written R-wave detection routine, resulting in a time series of RR interval (RRI). Errors in marking of R-waves were corrected interactively.
Heart Rate and RRV
The mean heart rate (HR) and the standard deviation of all RRIs (SDRR) in each experimental period were computed for all subjects. Spectral power in the low (0.04–0.15 Hz (LF)) and high (0.15–0.50 Hz (HF)) frequency bands and the LF/HF ratio were computed as recommended by the Task Force Report on Heart Rate Variability (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996).
Spectra were calculated on 300-second epochs using an interval method for computing Fourier transforms similar to that described by DeBoer, Karemaker, and Strackee (deBoer et al., 1984). Prior to computing Fourier transforms, the mean of the SBP and DBP series were subtracted from each value in the series and the residual series then was filtered using a Hanning window (Harris, 1978) and the spectral power, i.e., variance (in msec2), over the LF and HF bands was summed. Estimates of spectral power were adjusted to account for attenuation produced by this filter (Harris, 1978).
In cases in which 300-second epochs of data were compromised by electronic artifact or subject movement, identification of all R waves was impossible. RR intervals associated with these artifacts were fixed using established procedures if possible (Berntson et al., 1990). If not, the file was excluded from spectral analysis.
For each subject, RRV was computed as the mean of the two 5-min epochs. Prior to statistical analysis, estimates of RRV were log transformed to correct for skewness.
Statistical Analyses
Separate analysis of covariance (ANCOVA) models were run to obtain age, sex and race-specific mean scores for five RRV parameters (i.e. HF, LF, LF/HF ratio, HR, SD). All models included age, sex and race, providing estimates of mean scores for each of these 3 demographic characteristics, adjusted for the other two. All two-way interactions between age, sex, and race were evaluated within the models in addition to the three-way interaction. Trimmed models are presented in which only interactions with p-value ≤ 0.05 were retained in the model.
Additional analyses were conducted to examine the effects of covariates including smoking, systolic blood pressure (SBP), and body mass index (BMI). We also ran analyses on the subset of participants who reported that they did not take medications for cardiovascular disease, asthma, and diabetes.
RESULTS
Of the 789 subjects who agreed to participate, 757 had technically adequate data. Table I presents general characteristics of this cohort. 44% were white and 42% were men. Mean age of the cohort was 40.0 ± 3.7 years.
Participants in the substudy differed from the overall CARDIA sample in some of the covariates. Substudy participants were significantly more likely to be in the lower income category (p < .001). They had higher BMIs (p < .0001) and higher SBP and DBP (p < .01).
RRV, Race, Sex, and Age
Data on RRV and race, sex, and age are presented in Figures 1 and 2 for HF and LF power respectively. In each of these figures, RRV for one factor has been adjusted for the other two factors.
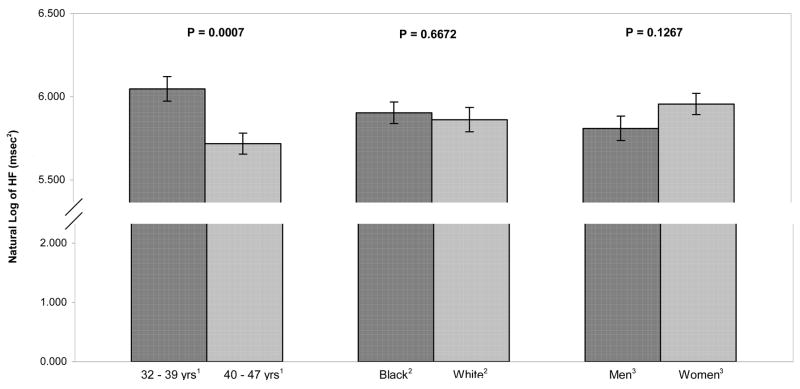
Figure 1.
Adjusted means of high frequency (HF) RR interval variability (natural log) by age, race, and sex. HF power was significantly greater in younger participants compared to older ones. There were no effects of race and sex on HF power.
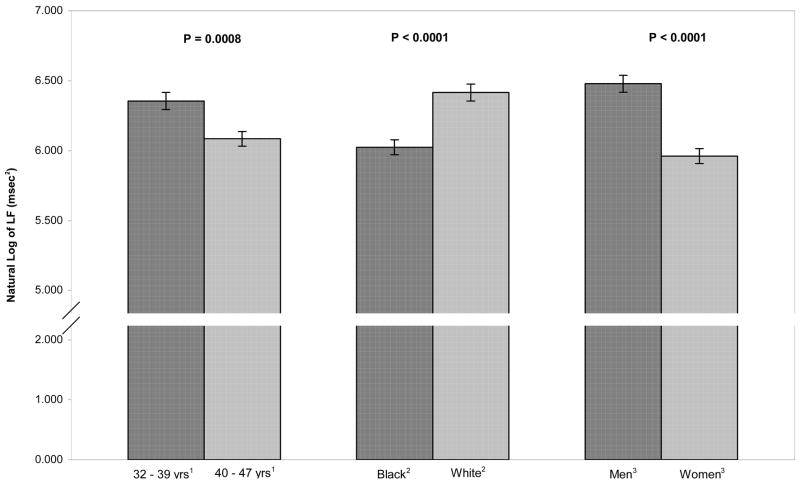
Figure 2.
Adjusted means of low frequency RR interval variability (natural log) by age, race, and sex. LF power was significantly greater in younger participants compared to older ones, in whites compared to blacks, and in men compared to women.
Despite the relatively narrow age range, older subjects (40–47 years) had significantly lower HF power compared to younger ones (33–39 years) (6.05 vs. 5.72 msec2, p < .001). HF power did not differ between blacks and whites (5.90 vs. 5.86 msec2, ns) or between men and women (5.81 vs. 5.96 msec2, ns).
The effect of age also was significant for LF power, with younger subjects having higher levels (6.35 vs. 6.08 msec2, p < .001). Blacks had lower LF power compared to whites (6.02 vs. 6.42 msec2, p < .001) and men had higher levels of LF power compared to women (6.48 vs. 5.96 msec2, p < .001).
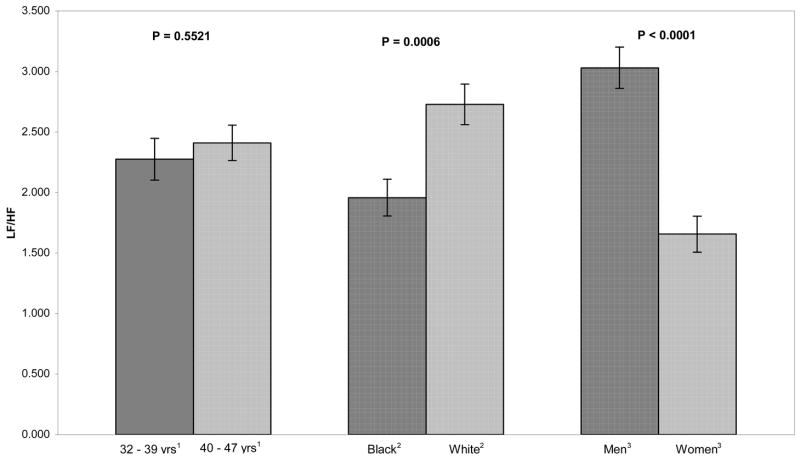
Figures 3, 4, and 5 present the data for the LF/HF ratio, SD, and HR. The LF/HF ratio did not differ between younger and older subjects (2.28 vs. 2.41, ns). The ratio was lower in blacks than whites (1.96 vs. 2.73, p < .001) and higher in men than in women (3.03 vs. 1.66, p < .001).
Figure 3.
Adjusted means of the LF/HF ratio by age, race, and sex. The LF/HF ratio was significantly greater in whites compared to black and in men compared to women.
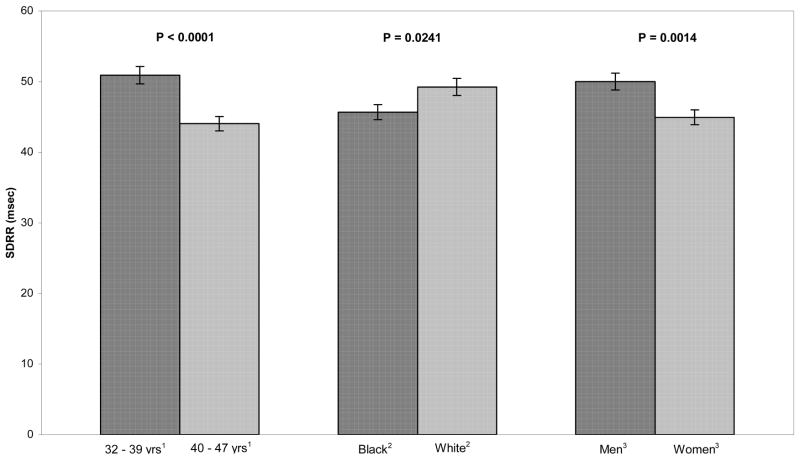
Figure 4.
Adjusted means of the standard deviation of RR intervals by age, race, and sex. SDRR was significantly greater in younger participants compared to older ones, in whites compared to blacks, and in men compared to women.
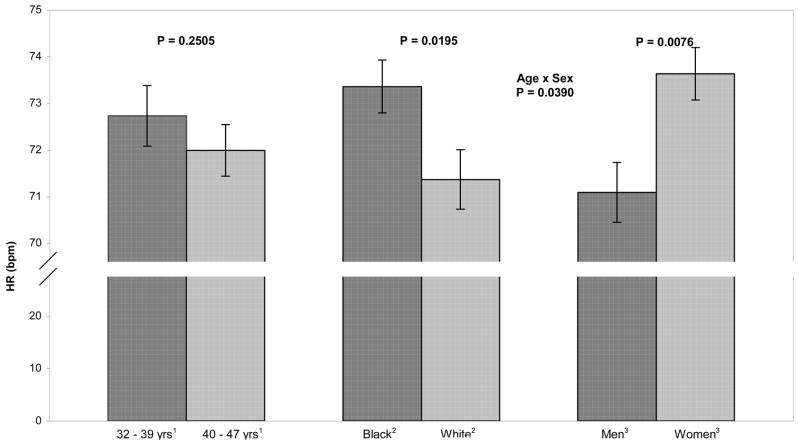
Figure 5.
Adjusted means of heart rate by age, race, and sex. HR was significantly greater in blacks compared to whites. There was a significant age X sex interaction: HR was significantly lower in older men compared to younger men, older women, and younger women.
Younger subjects had greater SDRR than older ones (50.89 vs. 44.02 msec, p < .001). SDRR was lower in black subjects compared to whites (45.67 vs. 49.24 msec, p = .02). In men, SDRR was greater than in women (49.99 vs. 44.93 msec, p = .001).
Finally, HR did not differ between the younger and older age groups (72.70 vs. 71.99 bpm, ns). In blacks, HR was higher than in whites (73.36 vs. 71.36 bpm, p = .02). In men, HR was lower than in women (71.09 vs. 73.63 bpm, p = .003). However, the age X sex interaction was significant (F(1,752) = 4.28, p < .05): HR was lower in older men compared to the other three groups.
Addition of covariates of smoking, SBP, and BMI to analyses produced some differences in these findings. Effects of race and sex emerged for HF power, with blacks having greater levels than whites and women having greater levels compared to men. On the other hand, addition of covariates eliminated the race differences observed for SD and HR in the above analyses.
Analyses Excluding Participants Taking Medications
We conducted supplementary analyses excluding participants taking medications for cardiovascular disease, asthma, and diabete. With few exceptions, results on this smaller sample (N = 585) were identical to those from the full sample. For SDRR, the significant effect of race became only marginally significant and a significant race X sex interaction emerged, with white men having greater SDRR than all other groups. In addition, the age X sex interaction for HR in the full sample disappeared.
DISCUSSION
Stratification variables of age, race, and sex figure prominently in assessment of cardiovascular risk. One significant contributor to this risk is autonomic regulation of the heart, as indexed by RR interval variability. In the Atherosclerosis Risk in Communities (ARIC) study, low levels of HF-RRV measured at study entry predicted incident CHD in 2252 initially healthy participants at 3-year follow-up (Liao et al., 1997). In the Framingham Offspring study, low values of several frequency domain indices of RRV including HF, LF, and VLF but not the LF/HF ratio predicted onset of new CHD in 2051 subjects after 3.5-year follow-up (Tsuji et al., 1996a). In the Zutphen study of 1763 men, low standard deviation of RR intervals predicted all cause mortality after 5-year follow-up (Dekker et al., 1997). These data suggest that reduced autonomic regulation of the heart contributes to the development of CHD.
The possibility that autonomic dysfunction may partially account for the age-, sex-, and race-related differences in CHD risk depends on evidence that these stratification variables are linked to RRV in a manner consistent with this risk. Although previous research has examined such relationships in middle and older aged adults, the extent to which relationships are seen even in younger adults has received less attention. To address this matter, we analyzed data from 756 participants in the CARDIA study of the evolution of cardiovascular risk development in young adulthood. Analyses revealed that HF-RRV was higher in younger compared to older subjects., HF power was greater in blacks compared to whites and in women compared to men, after adjustment for covariates. LF power was greater in young subjects, whites, and men. The LF/HF ratio did not differ as a function of age but was higher in whites and men. We also found that standard deviation of RR intervals was greater in younger subjects and men, with no difference between blacks and whites. In analyses excluding participants taking medications for cardiovascular disease, asthma, and diabetes, SDRR was greater in white men compared to white women and black men and women. Finally, HR was greater in women but there were no age or racial differences. An age X sex interaction in the full sample disappeared in participants not taking medications.
Several other large (N>200 participants) studies have examined relationships between some of these variables and RRV (Antelmi et al., 2004; Bigger et al., 1995; Kuo et al., 1999; Lampert et al., 2005; Liao et al., 1995; Pikkujamsa et al., 2001; Ramaekers et al., 1998; Umetani et al., 1998). In general, these findings are quite consistent with each other and with data from CARDIA. RRV is greater in younger subjects. Many studies have demonstrated the inverse association between age and RRV and this association is uncontroversial, even within the relatively narrow age range in the CARDIA study.
Associations between RRV and sex are less clear. Of these large studies, four reported no difference between men and women in HF power (Bigger et al., 1995; Lampert et al., 2005; Liao et al., 1995; Pikkujamsa et al., 2001; Ramaekers et al., 1998), two reported greater HF power in women (Antelmi et al., 2004; Kuo et al., 1999), and one reported greater HF power in men (Umetani et al., 1998). In most of these studies, LF power was greater in men. Three reported greater SDRR in men (Pikkujamsa et al., 2001; Ramaekers et al., 1998; Umetani et al., 1998) and three reported no sex difference (Antelmi et al., 2004; Bigger et al., 1995; Kuo et al., 1999). The LF/HF ratio tended to be greater in men. Finally, HR was greater in women in most studies.
Evidence about race and RRV is more limited, with few large studies. For HF-RRV, there was no consistency in the findings, with one large study reporting greater levels in blacks (Liao et al., 1995) and another greater levels in whites (Lampert et al., 2005). For other indices, RRV generally is greater in whites (Lampert et al., 2005; Liao et al., 1995). In a small sample of healthy young men, black participants had lower HF power and a higher LF/HF ratio compared to non-blacks, a group that included whites, Asians, and Latinos (Zion et al., 2003). In contrast, some studies of adolescents have found greater HF power in blacks compared to whites (Gutin et al., 2005; Wang et al., 2005).
To summarize, RRV is greater in younger subjects but no clear pattern emerges for sex. RRV is greater in whites compared to blacks, with HF power the exception. The consistency of these data is quite striking, especially in light of different measurement conditions (brief vs. 24-hour recordings), the physical position during brief recordings (seated vs. supine), and study designs (community studies vs. convenience samples).
Are these patterns of RRV consistent with the risk of CHD associated with age, sex, and race? The answer is unclear. Indices of RRV are greater in the younger participants and with the exception of HF power, in whites, suggesting a cardioprotective effect consistent with evidence from community studies that high levels of RRV predicted lower incidence of CHD (Liao et al., 1997; Tsuji et al., 1996a). However, men, at higher risk of CHD, also generally had higher levels of RRV, again with the exception of HF power.
This reduced risk of CHD associated with higher levels of RRV has been attributed to increased parasympathetic regulation of the heart and most evidence supports this association. HF power is most clearly linked to cardiac vagal modulation (Hammer et al., 2005; Pomeranz et al., 1985; Saul et al., 1991). Unfortunately, the associations of HF power and the stratification variables of sex and race were weaker than for other indices of RRV.
Understanding the associations between LF power and age, sex, and race as they relate to the risk of CHD require consideration of the physiological significance of LF-RRV. Some have suggested that it reflects cardiac sympathetic modulation (Pagani et al., 1986), most evidence does not support this claim (Cooke et al., 1999; Kingwell et al., 1994; Sloan et al., 1996). The sympathetic nervous system may contribute to LF power but this association is clearly dependent on a number of factors including physical position. In the supine position in humans, virtually all RRV is parasympathetic in origin (Levy et al., 1984; Saul et al., 1991). In the upright position, there is significant vagal and sympathetic contribution to LF power (Pomeranz et al., 1985; Saul et al., 1991). The physiological significance of LF power in the seated position, in which some of these studies RRV data were collected, is unclear but findings from Pikkujamsa et al. (Pikkujamsa et al., 2001) and Tulen et al. (Tulen, 1999) suggest little difference in RRV between the supine and seated positions. Moreover, Taylor et al. demonstrated that atropine eliminated LF power in the 40° tilted position, intermediate between the supine and standing positions, but that atenolol had no effect (Taylor et al., 1998). They also showed that LF power did not change from the supine to the 40° upright position whereas HF power fell significantly. These data suggest that like HF power, LF power also reflects cardiac parasympathetic modulation.
Even accepting the assumptions that most RRV is vagal in origin and that increased cardiac vagal regulation is cardioprotective, the association of RRV with the stratification variables is consistent for age and race but not for sex. Younger subjects and whites are at reduced risk of CHD (National Institutes of Health, 2006) and have higher RRV, while men, despite greater risk of CAD, generally have higher RRV,.
The associations of age, RRV as an index of cardiac vagal modulation, and risk of CHD are supported by the underlying physiology: the function of many hormone and neurotransmitters systems decline with age (Roth, 1979). While evidence of this decline is more abundant for the adrenergic system (Lakatta, 1993), recent reports now indicate a similar effect on the human cholinergic system. For example, pirenzipine, an M1 muscarinic receptor antagonist, caused an age-dependent effect, increasing resting HR in young subjects more than in older ones (Poller et al., 1997), suggesting greater parasympathetic tone in younger subjects. This age-dependent decrease in parasympathetic activity appears to be based on a decrease in right atrial M2 receptor density and function (Brodde et al., 1998). Other indices of cardiac vagal regulation, e.g., the Valsalva response, reveal an age-related decline in parasympathetic modulation (Low et al., 1997). Thus, the evidence from studies of RRV, age, and risk of CHD is consistent with the impact of aging on the cholinergic system.
Greater RRV in white compared to black participants also is consistent with the reduced risk of CHD in whites. However, there is little evidence linking racial differences to variations in parasympathetic nervous system functioning other than the few studies examining RRV.
The cardioprotective effects of estrogen are thought to be responsible for the lower risk of heart disease in pre-menopausal women compared to men and estrogen enhances vagal regulation of the heart. Nonetheless, with most large studies reporting that RRV generally is greater in men compared to women, the RRV data are not consistent with this sex-related differential risk of CHD or exposure to estrogen. In some of these studies, women were likely to be peri- or post-menopausal. But even among pre-menopausal women, the evidence is mixed. In CARDIA participants (age 33–45 years) after control for covariates and in participants under 50 years of age in two other studies (Antelmi et al., 2004; Kuo et al., 1999), women had higher HF power than men. However, in the 18–39 year old and 30–49 year old subjects in the studies of Ramaekers et al. and Umetani et al. respectively (Ramaekers et al., 1998; Umetani et al., 1998), there was no HF difference between men and women. Other RRV indices generally were greater in men than in women. Clearly many questions remain regarding how men and women differ in patterns of age-related change in parameters of RRV and how this relates to their cardiovascular health risks.
Limitations
As a cross-sectional study of young adults, we lack information on the temporal stability of the relationships between RRV and stratification variables. It is conceivable that the relationships between RRV and race or sex may differ as our CARDIA subjects age. However, in most of the large studies examining stratification variables and RRV, the relationship between sex and RRV is relatively constant across the age spectrum. To few studies exist to examine an age-differential with respect to the race-RRV relationship.
A second limitation is our reliance on a brief ECG recording. Longer recordings may provide more stable estimates of RRV. However, several studies have demonstrated a significant relationship between estimates of RRV from brief and 24-hour recordings (Bigger et al., 1993; Sloan et al., 1994). Moreover, the relationships between RRV and age, sex, and race reviewed above did not differ depending on recording length.
Conclusion
Data from the CARDIA study of the development of heart disease in young adults suggest that in adults in the 33–47 year age range, indices of RRV were greater in younger compared to older subjects, in men compared to women and in whites compared to blacks. These findings are broadly consistent with those of other large studies examining relationships between RRV and age, sex, and race. However, patterns of associations between RRV and these stratification variables are not entirely consistent with an underlying autonomic physiology linked to cardioprotection.
Table 1.
Characteristics of the Cohort
| All
|
Black Women
|
Black Men
|
White Women
|
White Men
|
Race – Sex Differences
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable* | Mean or N | SD or % | Mean or N | SD or % | Mean or N | SD or % | Mean or N | SD or % | Mean or N | SD or % | |
| Age | 40.0 | 3.7 | 39.6 | 3.9 | 39.5 | 3.6 | 40.7 | 3.3 | 40.2 | 3.6 |
p < 0.0001
Blacks younger than whites |
| Ethnicity | |||||||||||
| Black | 421 | 55.6 | |||||||||
| White | 336 | 44.4 | |||||||||
| Gender | |||||||||||
| Female | 436 | 57.6 | |||||||||
| Male | 321 | 42.4 | |||||||||
| Education |
p < 0.0001
Whites higher than blacks, BW > WM |
||||||||||
| <= High school | 164 | 21.7 | 69 | 27.0 | 51 | 30.9 | 21 | 11.7 | 23 | 14.7 | |
| > High school | 593 | 78.3 | 187 | 73.0 | 114 | 69.1 | 159 | 88.3 | 133 | 85.3 | |
| BMI (kg/m2) | 29.3 | 7.3 | 32.0 | 8.2 | 29.7 | 6.4 | 26.6 | 7.3 | 27.7 | 4.7 |
p < 0.0001
BW > BM > WM > WW (all pairwise sig) |
| SBP (mmHg) | 114.1 | 14.1 | 117.6 | 15.2 | 117.4 | 12.5 | 107.5 | 13.6 | 112.4 | 11.3 |
p < 0.0001
BM > BW > WM > WW (all pairwise sig) |
| DBP (mmHg) | 75.5 | 10.6 | 76.8 | 11.3 | 78.0 | 10.9 | 71.6 | 9.4 | 75.4 | 8.9 |
p < 0.0001
BM > BW,WM > |
| Heart Rate (bpm) | 72.6 | 11.6 | 74.8 | 10.7 | 71.8 | 11.8 | 72.2 | 11.7 | 70.3 | 12.0 | |
| Diabetes mellitus | 45 | 5.9 | 19 | 7.4 | 9 | 5.5 | 11 | 6.1 | 6 | 3.9 |
p < 0.0001
B > W, M > W |
| Hypertension | 133 | 17.6 | 63 | 24.6 | 33 | 20.0 | 21 | 11.7 | 16 | 10.3 |
p < 0.0001
B > W |
| Smoking status |
p < 0.0001
B > W, WW highest ex-smoker |
||||||||||
| never | 486 | 64.3 | 152 | 59.6 | 102 | 61.8 | 112 | 62.2 | 120 | 76.9 | |
| ex-smoker | 128 | 16.9 | 41 | 16.1 | 20 | 12.1 | 49 | 27.2 | 18 | 11.5 | |
| current | 142 | 18.8 | 62 | 24.3 | 43 | 26.1 | 19 | 10.6 | 18 | 11.5 | |
| HF Power(msec2) | 771.74 | 1272.90 | 974.14 | 1748.03 | 726.81 | 1140.36 | 649.04 | 855.07 | 628.71 | 740.06 | |
| LF Power(msec2) | 826.70 | 1106.71 | 605.71 | 832.4 | 903.29 | 1137.19 | 695.38 | 998.17 | 1259.86 | 1421.02 | |
| LF/HF Ratio | 2.205 | 3.139 | 1.276 | 1.635 | 2.645 | 4.380 | 2.063 | 2.516 | 3.427 | 3.590 | |
| SD (msec) | 46.33 | 21.79 | 43.74 | 23.27 | 46.77 | 22.75 | 44.22 | 18.18 | 52.56 | 21.00 | |
Note: B = black and W = white in racial comparisons; M = men and W = Women in comparison of sex differences
Acknowledgments
Work on this manuscript was supported (or partially supported) by contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050 and N01-HC-95095 from the National Heart, Lung and Blood Institute, the MacArthur Research Network on SES and Health through grants from the John D. and Catherine T. MacArthur Foundation, by Independent Scientist Award K02 MH01491 (Sloan) from the National Institute of Mental Health, and the Nathaniel Wharton Fund. The authors are indebted to Maria-Paola Pacifici for her expert technical contributions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226:180–182. doi: 10.1126/science.6484569. [DOI] [PubMed] [Google Scholar]
- Beere PA, Glagov S, Zarins CK. Experimental atherosclerosis at the carotid bifurcation of the cynomolgus monkey. Localization, compensatory enlargement, and the sparing effect of lowered heart rate. Arterioscler Thromb. 1992;12:1245–1253. doi: 10.1161/01.atv.12.11.1245. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Jang JF, Boysen ST. An approach to artifact identification: application to heart period data. Psychophysiology. 1990;27:586–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Schneider WJ, Stein PK. RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation. 1995;91:1936–1943. doi: 10.1161/01.cir.91.7.1936. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Konschak U, Becker K, Ruter F, Poller U, Jakubetz J, Radke J, Zerkowski HR. Cardiac muscarinic receptors decrease with age. In vitro and in vivo studies. J Clin Invest. 1998;101:471–478. doi: 10.1172/JCI1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human responses to upright tilt: A window on central autonomic integration. Journal of Physiology. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasset V, Mezzetti S, Antoine M, Linkowski P, Degaute JP, van de Borne P. Effects of Aging and Cardiac Denervation on Heart Rate Variability During Sleep. Circulation. 2001;103:84–88. doi: 10.1161/01.cir.103.1.84. [DOI] [PubMed] [Google Scholar]
- Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Manolio TA, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events, particularly for heart rate variability spectra. IEEE Trans Biomed Eng BME-31. 1984:384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- Gregoire J, Tuck S, Yamamoto Y, Hughson RL. Heart rate variability at rest and exercise: Influence of age, gender, and physical training. Canadian Journal of Applied Physiology. 1996;21:455–470. doi: 10.1139/h96-040. [DOI] [PubMed] [Google Scholar]
- Gutin B, Howe C, Johnson MH, Humphries MC, Snieder H, Barbeau P. Heart rate variability in adolescents: relations to physical activity, fitness, and adiposity. Med Sci Sports Exerc. 2005;37:1856–1863. doi: 10.1249/01.mss.0000175867.98628.27. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Mayet J, Shahi M, Mezzetti S, Foale RA, Sever PS, Poulter NR, Porta A, Malliani A, Thom SA. Absence of sympathetic overactivity in Afro-Caribbean hypertensive subjects studied by heart rate variability. J Hum Hypertens. 2000;14:337–342. doi: 10.1038/sj.jhh.1001009. [DOI] [PubMed] [Google Scholar]
- Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1637–1648. doi: 10.1152/ajpregu.00050.2004. [DOI] [PubMed] [Google Scholar]
- Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proceedings of the IEEE. 1978;66:51–83. [Google Scholar]
- Kannel WB, Cannel C, Paffenbarger RS. Heart rate and cardiovascular mortality: The Framingham study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Lansimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. Journal of Applied Physiology. 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: A potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150:153–160. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Levy MN, Martin P. Parasympathetic control of the heart, Nervous Control of Cardiovascular Function. Oxford; New York: 1984. pp. 68–94. [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Sorlie P, Heiss G. Age, race and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability - the ARIC Study. Am J Cardiol. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle & Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Incidence and Prevalence: 2006 Chart Book on Cardiovascular and Lung Diseases NIH. Bethesda MD: 2006. [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pikkujamsa SM, Makikallio TH, Airaksinen KEJ, Huikuri HV. Determinants and interindividual variation of R-R interval dynamics in healthy middle-aged subjects. Am J Physiol. 2001;280:H1400–1406. doi: 10.1152/ajpheart.2001.280.3.H1400. [DOI] [PubMed] [Google Scholar]
- Poller U, Nedelka G, Radke J, Ponicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. J Am Coll Cardiol. 1997;29:187–193. doi: 10.1016/s0735-1097(96)00437-8. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, Macaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur heart J. 1998;19:1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y for the American Heart Association Statistics Committee, Stroke Statistics S. Heart Disease and Stroke Statistics--2007 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Roth GS. Hormone receptor changes during adulthood and senescence: significance for aging research. Fed Proc. 1979;38:1910–1914. [PubMed] [Google Scholar]
- Ryan SM, Goldberger AL, Pincus SM, Joseph M, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: Are women more complex than men? Journal of the American College of Cardiology. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: Unique insights into cardiovascular regulation. Am J Physiol. 1991;30:H1231–H1245. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Bigger JT, Lo ES, Gorman JM. Relationship between circulating catecholamines and low frequency heart period variability as indices of cardiac sympathetic activity during mental stress. Psychosomatic Medicine. 1996;58:25–31. doi: 10.1097/00006842-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bagiella E, Myers MM, Bigger JT, Steinman RC, Gorman JM. Brief interval HPV by different methods of analysis correlates highly with 24-hour analyses in normals. Biol Psychology. 1994;38:133–142. doi: 10.1016/0301-0511(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Thomas F, Rudnichi A, Bacri AM, Bean K, Guize L, Benetos A. Cardiovascular mortality in hypertensive men according to presence of associated risk factors. Hypertension. 2001;37:1256–1261. doi: 10.1161/01.hyp.37.5.1256. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation. 1996a;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Determinants of heart rate variability. Journal of the American College of Cardiology. 1996b;28:1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- Tulen JHM, Boomsma F, Man AJ. Cardiovascular control and plasma catecholamines during rest and mental stress: effects of posture. Clinical Science. 1999;96:567–576. [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-Four Hour Time Domain Heart Rate Variability and Heart Rate: Relations to Age and Gender Over Nine Decades. Journal of the American College of Cardiology. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- Urbina EM, Bao W, Pickoff AS, Berenson GS. Ethnic (black-white) contrasts in heart rate variability during cardiovascular reactivity testing in male adolescents with high and low blood pressure: the Bogalusa Heart Study. Am J Hypertens. 1998;11:196–202. doi: 10.1016/s0895-7061(97)00314-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Thayer JF, Treiber F, Snieder H. Ethnic Differences and Heritability of Heart Rate Variability in African- and European American Youth. The American Journal of Cardiology. 2005;96:1166–1172. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Sobolewski E, Kay J, Jampala VC, Igel G. Effect of age on long-term heart rate variability. Cardiovascular Research. 1997;35:35–42. doi: 10.1016/s0008-6363(97)00107-7. [DOI] [PubMed] [Google Scholar]
- Zion AS, Bond V, Adams RG, Williams D, Fullilove RE, Sloan RP, Bartels MN, Downey JA, De Meersman RE. Low arterial compliance in young African-American males. American Journal of Physiology Heart and Circulatory Physiology. 2003;285:H457–462. doi: 10.1152/ajpheart.00497.2002. [DOI] [PubMed] [Google Scholar]