Abstract
Background
Polybrominated diphenyl ethers (PBDEs) are widely found in the environment, and they may act as endocrine disruptors.
Objective
Our goal in this study was to test the PBDE mixture DE-71 for estrogenic activity.
Methods
We used proliferation of cultured breast cancer cells (MCF-7) and trophic effects in the reproductive tracts of ovariectomized mice as estrogen bioassays. DE-71 was administered to mice by subcutaneous injection (sc) or oral gavage (po), alone or in combination with estradiol, for 3 or 34 days. Liver weights and cytochrome P450 enzyme activities were also measured.
Results
DE-71 increased MCF-7 cell proliferation, and this was prevented by antiestrogen. DE-71 cotreatment reduced the effect of estradiol in MCF-7 cells. In the mouse 3-day assay, DE-71 administered alone had no effect on uterine weight, uterine epithelial height (UEH), or vaginal epithelial thickness (VET); however, when DE-71 was administered as a cotreatment, it potentiated estradiol’s effect on uterine weight. DE-71 administered sc to BALB/c mice for 34 days slightly increased UEH and VET, and attenuated the estradiol-induced increase in UEH; these effects were not seen in BALB/c mice treated po or in C57BL/6 mice treated sc. DE-71 increased liver weight in BALB/c, C57BL/6, and estrogen receptor-α knockout mice. We also found an increase in liver cytochrome P450 1A (CYP1A) and CYP2B activities when DE-71 was administered po, but only CYP2B increased after sc treatment.
Conclusion
DE-71 behaves as a weak estrogen. In mice, the treatment route and duration determined if DE-71 was estrogenic. BALB/c mice are more susceptible to DE-71 effects in estrogen target tissues than C57BL/6 mice. DE-71 increased liver weight independently of estrogen receptor-α.
Keywords: CYP1A, CYP2B, DE-71, endocrine disruptors, estrogens, MCF-7, mice, ovariectomized, PBDEs, polybrominated diphenyl ethers
The polybrominated diphenyl ethers (PBDEs) are a series of 209 possible brominated diphenyl ethers (BDEs) that differ in the number and position of bromine atoms [Agency for Toxic Substances and Disease Registry (ATSDR) 2004]. Three kinds of PBDE blends were manufactured and marketed as standard mixtures: Penta-BDE (containing penta-, tetra-, and some hexabrominated congeners), Octa-BDE (containing from hexa- to decabrominated congeners), and Deca-BDE (97% deca-BDE and 3% nonbrominated congeners). Standard mixtures of PBDEs have been used extensively as flame retardants over the past 30 years in a variety of consumer products (e.g., plastics, foams, electronics). The PBDEs are very stable compounds and are not chemically bonded to material they are intended to protect from burning. Therefore, it is not unexpected that PBDEs are being found more and more in environmental media [reviewed by Domingo (2004), Hites (2004), and Law et al. (2006)], and possible exposure to them has become a public health concern.
The endocrine-disrupting potential of PBDEs has been studied, mostly in regard to their effect on thyroid hormone homeostasis. Commercial PBDE formulations reduce thyroid hormone levels and induce thyroid hyperplasia in rodents (Darnerud et al. 2007; Stoker et al. 2004). Increased thyroid hormone clearance by induction of metabolic enzymes, and displacement of thyroxine from its transport protein have been suggested as mechanisms of thyroid disruption (reviewed by Siddiqi et al. 2003). Neurobehavioral alterations were observed in rodents treated neonataly with PBDEs (Viberg et al. 2002, 2003), but these alterations have not been linked to hormonal activity. Recent studies have linked PBDE to estrogenic effects in vitro (Meerts et al. 2001) and to adverse effects in sexual development and behavior in rodents (Ceccatelli et al. 2006; Kuriyama et al. 2005; Lilienthal et al. 2006; Stoker et al. 2004).
In vivo estrogen bioassays are classically based on responses of reproductive organ tissues in rodents. Estrogens induce many physiological changes in the mammalian female reproductive tract and mammary glands. Immature or ovariectomized (OVX) adult rodents have served as the standard bioassay model for estrogenic activity. In the adult mouse, estrogen target organ size and histologic characteristics regress to a nonstimulated state after ovariectomy, thus providing a model free of endogenous estrogens in which their physiologic effects can be studied. In OVX animals, estrogens increase uterine wet weight (Evans et al. 1941; Gordon et al. 1986) and uterine epithelial height (UEH) and vaginal epithelial thickness (VET) (Suzuki et al. 1996; Ulrich et al. 2000) after a few days of treatment. In the uterus, the columnar epithelial cells become taller, with a concomitant increase in cytoplasmic volume; they also proliferate, causing overcrowding and a pseudostratified appearance. In the vagina, the single squamous epithelial layer of the OVX mouse becomes a multicell layer after estrogen treatment.
Breast cancer cells in culture also serve as an estrogen bioassay. Although most normal mammary epithelial cells have no estrogen receptors (ERs) and depend on stromal interactions in their response to estrogen, carcinomas often express ERs, and estrogens induce their growth (Hahnel and Twaddle 1971; Osborne et al. 1985; Welsch et al. 1981). The fact that estrogens increase proliferation of neoplastic mammary epithelium in vitro is the basis of cell proliferation assays (Weichselbaum et al. 1978).
In vivo uterotrophic assays and in vitro breast cancer cell proliferation assays have been used extensively to assess the estrogenicity of environmental chemicals (Clode 2006; Soto et al. 1995). In the present study, we used an estrogen-responsive human cancer cell line, MCF-7, and the OVX mouse as bioassay models to assess the estrogenicity of DE-71, a standard Penta-BDE mixture of PBDEs commonly used in consumer goods.
Materials and Methods
Test chemicals
We purchased dimethyl sulfoxide (DMSO), 1,3,5[10]estratriene-3,17-β-diol [17β-estradiol, (E2)], and β-estradiol-3-benzoate (EB) from Sigma-Aldrich (St. Louis, MO). Corn oil was purchased from ICN Biomedicals Inc. (Aurora, OH). Fulvestrant [ICI 182 780 (ICI)] was a gift from Astra Zeneca (Macclesfield, Cheshire, UK). The PBDE congener mixture DE-71 (lot no. 9550OF05A) was a gift from the Great Lakes Chemical Corporation (West Lafayette, IN); the congener composition was reported previously (Qiu et al. 2007). There were no detectable dioxins in the DE-71 lot used, but it was not tested for furan content (Hites R, Qiu X, personal communication). DMSO was used as the primary solvent for all treatment chemicals, and the DMSO solutions were further diluted in corn oil for animal treatments. DE-71 remained in solution in DMSO at all doses used, but vials were still vigorously vortexed for several seconds immediately before treatment. DE-71 prepared for in vivo treatments was maintained in suspension in corn oil by continuous stirring while the needle was filled, and animals were dosed immediately.
Cell proliferation measurements
To measure cell proliferation, we used the colorimetric assay developed by Mosmann (1983) with minor modifications. Cells were regularly maintained in growth medium [i.e. minimum essential media (MEM; Gibco/Invitrogen; Carlsbad, CA) supplemented with l-glutamine (2 mM; Gibco/Invitrogen), nonessential amino acids (0.1 mM; Gibco/Invitrogen), HEPES buffer (10 mM, Gibco/Invitrogen), and 5% vol/vol bovine growth serum (Hyclone, Logan, UT)]. To minimize basal hormonal activity during assays, cells were incubated in basal medium (i.e., phenol red free MEM supplemented with 2 mM l-glutamine, 0.1 mM nonessential amino acids, 10 mM HEPES, and 3% vol/vol dextran-coated charcoal-stripped bovine growth serum).
For the cell proliferation assay, cells cultured with growth medium were plated in 24-well dishes (15,000 cells/well). The day after plating, culture medium was changed to basal medium. Starting 2 days after changing to basal medium, cells were cultured in treatment medium (basal medium plus treatment) for a total of 10 days, changing the treatment medium every 2 days. On day 10, cells were incubated in tetrazolium MTT [3-(4,5-dimethylthiazolyl-2)2,5-diphenyltetrazolium bromide] for 2 hr, then lysed in acid iso-propanol. Reduced (blue) MTT absorbance was read at 570 nm.
To confirm the MTT absorbance correlated with cell growth and not increased metabolic activity, in some experiments we included additional plates treated in parallel for DNA determination. After 10 days of treatment, cells were washed twice with phosphate-buffered saline and incubated for 10 sec in cold methanol. After removing the methanol, we allowed cells to dry at room temperature. Cells were then dissolved in 0.5 M sodium hydroxide by rocking in a humidified 37°C chamber for 30 min. Samples were then transferred to microtubes and incubated at 65°C for 1 hr. Sample (100 μL) was diluted in TNE buffer (10 mM Tris, 0.1 M NaCl, 1 mM EDTA, pH 7.4) containing 0.1 mg/mL Hoescht 33258 dye (Polysciences, Warrington PA), and neutralized with an equimolar amount of hydrochloric acid. Fluorescence was measured in a Hoefer TKO 100 DNA Fluorometer (Hoefer Scientific Instruments, San Francisco, CA) against a salmon sperm DNA standard (Invitrogen, Carlsbad, CA).
Animal treatments
All procedures performed on animals were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. Animals were treated humanely and with regard for the alleviation of suffering. Adult BALB/c and wild-type (WT) C57BL/6 mice were purchased from Harlan (Indianapolis); ER-αKO mice (in C57BL/6 background for > 10 generations) were derived from an in-house colony. Animals were ovariectomized at 6–8 weeks of age, and 3 weeks later, they were treated for either 3 or 34 days with vehicle or test compound. In brief, groups of five or six animals were treated daily by either subcutaneous injection (sc) or oral gavage (po) with either vehicle control, EB (10 μg/kg), or DE-71 (50 mg/kg for 34 days or with 75, 150, or 300 mg/kg for 3 days). Some groups were cotreated with EB plus DE-71. Chemicals were first dissolved in DMSO and then diluted in corn oil and administered at 0.1 mL for po or at 10–20 μL for sc. Doses were prepared based on the average body weight measured for each group on the first day of treatment. On the day after the last treatment, animals were sacrificed by decapitation, and blood was collected by exsanguination. Serum was collected and stored at –20°C until analysis for individual BDE congeners and their hydroxylated metabolites [reported by Qiu et al. (2007)]. The liver was perfused in place with phosphate-buffered saline through the hepatic portal vein. The uterus and liver were weighed and expressed on a per gram of body weight basis. One uterine horn and the vagina were fixed in Bouin’s solution overnight. The liver was flash-frozen in liquid nitrogen and then stored at –70°C. The fixed uterus and vagina were embedded in paraffin, and 5-μm cross-sections were stained with hematoxylin and eosin for analysis by light microscopy. Using an image analysis program (IPLab, version 3.5 imaging software; Scanalytics Inc., Fairfax, VA), UEH and VET were measured as estrogen-sensitive end points.
Cytochrome P450 (CYP) activity assays
For each animal, about 0.2 g of frozen liver was homogenized in 1 mL high-sucrose buffer [0.15 M potassium chloride, 0.5 M Tris, 1 mM EDTA, 0.25 M sucrose, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 20 μM butylated hydroxytoluene (BHT), pH 7.4] and centrifuged at 9,000 × g for 20 min at 4°C. To obtain the microsomal fraction, we centrifuged the resulting supernatant at 105,000 × g and 4°C for 60 min; the pellet was washed in potassium pyrophosphate buffer (0.1 M potassium pyrophosphate, 1 mM EDTA, 0.2 mM PMSF, 20 μM BHT, pH 7.4) by resuspending it using disposable homogenizing microtubes and pestles (Kontes Glass Company, Vineland, NJ). The protein content of the microsomal preparation was determined using the Pierce BCA Protein Assay Kit (Pierce, Rockford, IL) against a bovine serum albumin standard. The liver samples from each animal were assayed in duplicate.
We measured 7-ethoxyresorufin O-dealkylation (EROD; CYP1A) activity and 7-pentoxy-resorufin O-dealkylation (PROD; CYP2B) activity by mixing 5 μL of sample with 5 μL 250 μM NADPH and 3.4 μL 0.6 mM 7-ethoxyresorufin or 7-pentoxyresorufin in 1.2 mL 0.1 Tris buffer at 37°C. After allowing the mixture to equilibrate for 1 min, fluorescence was measured at 530 nm excitation and 585 nm emission in a fluorometer at 1-sec intervals over the course of 1 min. Similar measurements were made with six different resorufin concentrations to determine a standard curve. The slope of the linear range for each activity assay (Δfluorescence/sec) was converted to moles of resorufin per gram per second (mol/g*sec) using the standard curve and the protein content of each sample.
Statistics
All statistics were performed using GraphPad Prism, version 3.0a, for Macintosh (GraphPad Software, San Diego, CA). For each statistical analysis, we used Bartlett’s test to determine if groups had unequal variances. Group averages with equal variances were compared to each other by either one-way analysis of variance (ANOVA) with Tukey post-test or unpaired t-test as appropriate. Group averages with unequal variances were compared to each other by t-test with Welch’s correction. Groups treated with DE-71 alone were analyzed against vehicle controls. Groups cotreated with DE-71 and EB were analyzed against controls treated with EB alone. All values are expressed as mean ± SE. Groups were considered statistically different if the result of ANOVA with Tukey post-test or t-test (two-tailed) had p < 0.05; however, in some instances, means were considered of borderline statistical significance if 0.05 < p < 0.10. DE-71 dose–response studies were also subjected to regression analysis using three curve fitting models: a) linear: response = (slope × dose) + intercept; b) sigmoidal: response = minimum + (maximum – minimum) ÷ {1 + 10[Log(EC50) – Log(dose)] × Hillslope}; c) modified Gaussian distribution: response = minimum + ({maximum – minimum} × exp{–[log(dose) – A] ÷ slope}2, where A = Log(EC50) + 0.833 × slope, and the EC50 is the median effective concentration. The best fitting curve appears in graphs if R2 > 0.8.
Results
Cell proliferation assays
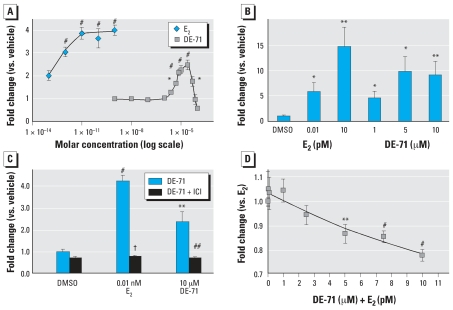
We compared the capacity of DE-71 to increase breast cancer cell (MCF-7) proliferation with that of E2. Both E2 and DE-71 were able to significantly increase cell number, as determined by the MTT assay (Figure 1A). DNA assays confirmed this finding, indicating that the increase in reduced MTT was caused by an increase in cell number and was not merely an effect on the cell redox systems (Figure 1B). The effects of both E2 and DE-71 were negated by cotreatment with the estrogen antagonist ICI (Figure 1C). DE-71 produced a biphasic dose–response curve, suggesting that it may have been toxic to MCF-7 cells at concentrations > 2.5 × 10–5 M (Figure 1A). At those same concentrations, we observed an accumulation of a white precipitate, suggesting a solubility problem. Cotreatment of cells with both E2 and DE-71 resulted in a lesser increase in cell proliferation compared with E2 alone, suggesting an antagonistic effect of DE-71 on E2-induced cell proliferation (Figure 1D). Antagonism was dose dependent; the highest dose tested corresponds to that which produced maximal proliferative effect when cells were treated with DE-71 alone.
Figure 1.
DE-71 induced proliferation of MCF-7 cells treated for 10 days with DE-71 and/or E2 at the indicated concentrations. Proliferation was measured using the MTT assay (A, C, D) or a DNA assay (B). The antiestrogen fulvestrant (ICI, 10 nm) blocked the effect of DE-71 (C). DE-71 blocked the effect of E2 in a dose-dependent manner (D). Values shown are mean ± SE of three to seven independent assays.
*p < 0.05, **p < 0.01, and #p < 0.001 vs. vehicle; ##p < 0.01, and †p < 0.001 vs. treatment without ICI.
Three-day mouse estrogenic end points
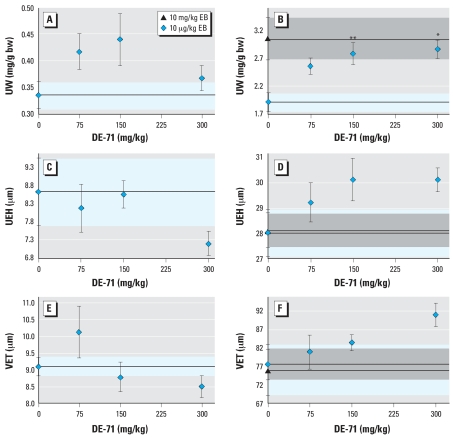
We used a 3-day treatment regimen in OVX BALB/c mice to assess DE-71 effects at three doses (75, 150, and 300 mg/kg); groups of animals were also treated with 10 μg/kg EB plus DE-71 at each of these doses. Uterine wet weight, UEH, and VET were used as estrogenic end points. DE-71 administration by sc alone had no statistically significant effect (Figure 2). However, the increase in uterine wet weight induced by 10 μg/kg EB was enhanced by DE-71 in a dose-dependent manner; this enhanced response was equivalent to the maximal estrogen effect produced by 10 mg/kg EB (Figure 2B). Oral DE-71 administration had no effect on any of the estrogenic parameters measured (data not shown).
Figure 2.
Dose effects of DE-71 on uterus and vagina in ovariectomized BALB/c mice treated sc for 3 days with DE-71 alone at the indicated doses (A,C,E) or with 10 μg/kg EB plus DE-71 (B,D,F). Abbreviations: bw, body weight; UW, uterine weight. (A,B) UW. (C,D) UEH. (E,F) VET. For (A–F), control means are shown as a dashed line; blue shaded areas indicate SE. Effects after 3 days of treatment with 10 mg/kg of EB are shown as a reference for maximal estrogen effect on uterine weight (B), UEH (D), and VET (F); dashed lines indicate the mean; gray shaded areas indicate SE. Values represent means ± SE of 8–10 mice per group.
*p < 0.05, and **p < 0.01 indicate that individual UWs were significantly different from EB controls.
Subcutaneous DE-71 treatment alone had no effect on UEH or VET (Figure 2C, 2E). When mice were cotreated with DE-71 and EB, there was an increasing dose–response trend for UEH and VET (Figure 2D, 2F). However, only borderline statistical significance was achieved for UEH in the highest dose group, and neither trend yielded a good fit by regression analysis.
Thirty-four-day mouse estrogenic end points
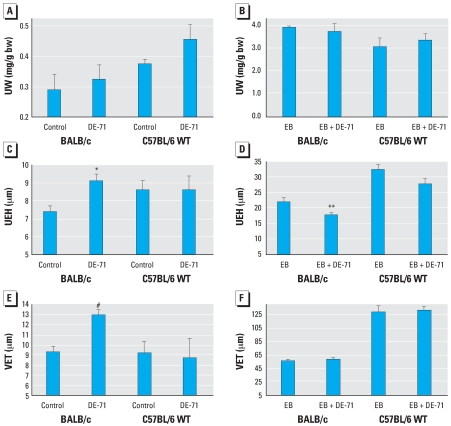
We determined estrogenic effects in ovariectomized BALB/c and C57BL/6 WT and ER-αKO mice treated long-term (34 days). We examined uterine and vaginal parameters as in the 3-day assay. As with the 3-day assay, oral DE-71 administration had no effect on any of the estrogenic parameters measured in BALB/c mice (data not shown); C57Bl/6 mice were not treated orally.
Treatment for 34 days with sc DE-71 alone produced no significant change in uterine weight (Figure 3A). There was a large increase (8- to 12-fold; p < 0.001) in uterine weight of WT mice after E2 treatment. The uterine weight response to 10 μg/kg EB at 34 days was similar to the increase produced by 10 mg/kg EB for 3 days; that is, the 34-day EB treatment produced the maximal uterotrophic effect. EB-induced uterine weight was unaffected by cotreatment with DE-71 (Figure 3A).
Figure 3.
Effects of DE-71 in uterus and vagina of ovariectomized BALB/c and C57BL/6 WT mice treated for 34 days with DE-71 alone (A,C,E) or E2 plus DE-71 (B,D,E). Abbreviations: bw, body weight; UW, uterine weight. (A,B) Relative UW. (C, D) UEH. (E,F) VET. Values shown are mean ± SE of 5–10 mice per group.
*p < 0.05, **p < 0.01, and #p < 0.001 vs. corresponding control.
In BALB/c mice, DE-71 administered sc for 34 days caused a 23% increase in UEH (Figure 3C) and a 33% increase in VET (Figure 3E). When administered alone, DE-71 had no effect on these parameters in C57BL/6 mice. In contrast, we observed a small but statistically significant decrease in the estrogen-induced UEH increase in BALB/c mice cotreated with sc DE-71 (Figure 3D). The similar decrease in UEH in cotreated C57BL/6 mice was not statistically significant. Cotreatment did not alter the EB-induced increase in VET (Figure 3F).
As expected, EB treatment had no effect on ER-αKO uterine weights, UEH, or VET, nor did DE-71 have an effect on any of the uterine or vaginal parameters in either WT C56BL/6 mice (Figure 3) or ER-αKO animals (data not shown).
Liver end points
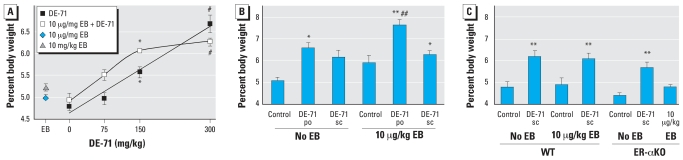
We determined liver weights and activities of CYP1A and CYP2B enzymes. We found a 20–51% increase in liver weight in BALB/c mice treated with DE-71 for 34 days compared with vehicle control, whereas livers of 3-day treated mice increased in weight up to 39% in a dose-dependent manner (Figure 4).
Figure 4.
Effects of DE-71 on liver weight of mice were treated for either 3 (A) or 34 (B,C) days with DE-71 alone or with E2 and DE-71. Controls for the 34-day time point were treated po with vehicle. Values shown are mean ± SE (n = 5–10 mice per sc group; n = 4–5 mice per po group).
*p < 0.05, **p < 0.01, and #p < 0.001 vs. vehicle. ##p < 0.05 vs. DE-71 at the same dose without EB.
EB treatment did not increase liver weight in BALB/c mice. However, EB potentiated the effect of po DE-71 but had no effect on sc DE-71 treatments (Figure 4B). As with BALB/c mice, the livers of DE-71 treated C57BL/6 WT mice were 27–29% larger than vehicle-treated controls. E2 administered alone or in combination with DE-71 had no effect on liver weights of C57BL/6 WT mice. The size of ER-αKO mouse livers increased 30% after DE-71 treatment (Figure 4C).
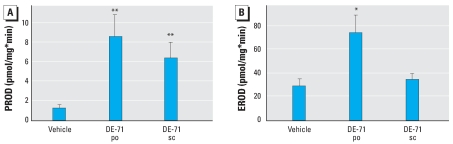
We found a large increase in PROD activity in DE-71–treated BALB/c mice compared with controls, about 7-fold in the po-treated group and 5-fold in the sc-treated group (Figure 5A). Still, PROD activity in the DE-71–induced animals was much lower than EROD activity in vehicle-treated animals (i.e., the maximal PROD was about one-third the minimal EROD activity). Liver microsomal EROD activity also increased (2.5-fold), but only for po-treated mice (Figure 5B). EB treatment had no effect on either EROD or PROD activity (data not shown).
Figure 5.
Effects of DE-71 on liver microsomal CYP activity in BALB/c mice treated for 34 days with DE-71 alone or with EB plus DE-71. (A) PROD (CYP2B) activity. (B) EROD (CYP1A) activity. Values shown are mean ± SE. n = 8 mice per group.
*p < 0.05, and **p < 0.01 vs. vehicle.
Discussion
DE-71 exhibited both estrogenic and anti-estrogenic effects in the MCF-7 cell proliferation assay and in the adult ovariectomized mouse model, a behavior expected from a weak ER agonist (reviewed by Lerner and Jordan 1990). The observation that ICI prevented DE-71 from increasing cell number in the MCF-7 bioassay suggests the involvement of an ER.
To date, this is the first report of a study in which the estrogenic action of a PBDE mixture has been examined using standard rodent bioassay end points. The magnitude of the effects seen in our in vivo studies was similar to those produced by treatment of ovariectomized rats with 200 mg/kg BDE-47, a major component of DE-71 (Dang et al. 2007). The type of response observed (agonist or antagonist) was dependent upon the duration of exposure to DE-71. In the 3-day assay DE-71 administered alone produced no estrogenic effects; however, when it was administered for 34 days, it produced hypertrophy of the uterine epithelium and hyperplasia of the vaginal epithelium. When administered with EB in the 3-day treatment schedule, DE-71 enhanced the estrogen effect, whereas in the 34-day treatment schedule DE-71 produced small anti-estrogenic effects. Thus, pharmacokinetic considerations are paramount when designing further studies of PBDE action in vivo.
We used the ER-αKO mouse to determine if in vivo effects of DE-71 were mediated by ER-α; however, because there was a lack of any estrogenic effects of DE-71 in C57BL/6 WT mice, this experiment was not informative. The observation that DE-71 could enhance estrogenic effects beyond those produced by a saturating dose of EB suggests that PBDEs modify estrogen action through nonclassical pathways, as has been proposed for other xenobiotics such as β-hexachlorocyclohexane (Hatakeyama et al. 2002; Steinmetz et al. 1996).
PBDEs are suspected to behave as estrogens because of the similarity of their chemical structure and properties to other xenobiotics, mainly the polychlorinated biphenyls [reviewed by Hooper and McDonald (2000), Meerts et al. (2001), and Pijnenburg et al. (1995)]. Furthermore, hydroxylated metabolites of PCBs have been shown to exert estrogenic effects [reviewed by Bigsby et al. (2005) and Blair et al. (2000)]; therefore, it may be reasonable to expect that hydroxylated forms of PBDEs would also be estrogenic. Several researchers have shown that individual BDE congeners or certain synthetic hydroxylated congeners could exert estrogenic effects in cultured cells. In estrogen-responsive transcription reporter assays, BDE-28 and BDE-100, as well as the 4′-hydroxy forms of BDE-30 and BDE-119, proved to be estrogenic (Meerts et al. 2001). In addition, several BDE congeners found in DE-71 were mildly anti-estrogenic in the same assay. Likewise, Hamers et. al. (2006) observed weak estrogenic activity by several low-brominated BDEs, weak anti-estrogenic activity for tetra- and heptabrominated BDEs and 6OH-BDE-47, and neither activity for the DE-71 mixture.
Our results show that in the OVX mouse sc DE-71 produced very small estrogenic effects, whereas po DE-71 had no effect. Because DE-71 had estrogenic effects in cell culture, the lack of effect in po-treated mice suggests rapid clearance via liver metabolism. However, when we analyzed plasma of mice that had been treated with DE-71 for 34 days, we found similar amounts of parent compounds and metabolites in the blood of either sc- or po-treated animals, with the exception of BDE-153, which was 5 times higher in the po group (Qiu et al. 2007). Furthermore, the concentration of total DE-71 congeners was approximately 1,000–2,000 ng/mL, similar to what would be achieved with 2–4 μM treatment in culture, where 1 μM was minimally effective and 5 μM showed an effect that was approximately 50% of the maximal E2-induced effect. Staskal et al. (2005) found that non-metabolized BDE-47 is rapidly cleared in the mouse. This process is mediated by urinary proteins and may be saturated after repeated exposure (Diliberto et al. 2007; Staskal et al. 2006). Because the animals were treated long-term with a high dose of DE-71, it is possible that the concentrations we found in blood represent a steady-state level reached when urinary clearance is at its maximum capacity. Although this may explain why the po- and sc-treated animals had similar blood concentrations of most PBDEs, it does not explain why the sc route produced estrogenic effects while the po route did not. Another explanation is that more PBDE congeners may reach estrogen targets when administered sc rather than po. It is possible that sc-administered PBDEs reached higher levels in peripheral tissue than liver compared with po-administered PBDEs by avoiding the high activity of conjugating (phase II) enzymes in the liver and gut (Cassidy and Houston 1984; Li et al. 2004). It is also possible that PBDEs are activated by CYP in peripheral tissues such as adipose or at the estrogen-target tissues (Shimada et al. 2003, Yoshinari et al. 2006). Others have also seen higher efficacy of sc dosing over po dosing of estrogens, as is the case of the xenoestrogen bisphenol A (Berger et al. 2007) and steroidal estrogen (Savvas et al. 1992).
The MCF-7 cell proliferation assay used here to test DE-71 is probably more sensitive than the reporter gene expression system used by others (Hamers et al. 2006; Legler et al. 1999) due to the longer time of incubation with the chemical (10 days vs. 24 hr), thereby allowing accumulation of both the PBDE congeners inside the cell [increased intracellular concentration (Mundy et al. 2004)] and the estrogenic effect (cell growth). Alternatively, MCF-7 cells are known to express CYP enzymes (Barber et al. 2006; Peters et al. 2004), and it may be that during the 10-day incubation they metabolically convert BDE congeners to more active hydroxylated forms.
Other researchers have shown that both BDE-99 and DE-71 interfere with rodent sexual development after prenatal exposure (Ceccatelli et al. 2006; Kuriyama et al. 2005; Lilienthal et al. 2006), but specific hormonal activity involved was not demonstrated. As noted by Ceccatelli et al. (2006), PBDE remains in the offspring for months after birth, making it impossible to determine if the observed increase in expression of estrogen target genes during adulthood was due to developmental defects or adult hormone-like effects (or a combination of both). Although such research is suitable to assess the sensitivity to developmental effects, it does not define these effects as estrogenic. The pubertal development protocol used by Stoker et al. (2004) and the adult gonadectomized rodent model used here are more suitable to assess estrogenic activity by looking at well-known responses to estrogen after chemical challenge. Classic estrogenic responses such as increased uterine weight, UEH, and VET in the adult OVX mouse are a strong indication of the involvement of estrogen-signaling pathways and are standard methods to assess estrogenicity of a chemical (Clode et al. 2006).
We found differences in responses between mouse strains: The uterine and vaginal epithelium seems to be more sensitive to the effects of DE-71 in BALB/c mice than in C57BL/6 mice. Others have shown that C57BL/6 mice exhibit higher sensitivity to estrogen compared with other strains (Silberberg and Silberberg 1951; Spearow et al. 1999, 2001), but BALB/c mice were not included in those comparisons. Although our findings may illustrate a real difference in estrogen sensitivity between the BALB/c and C57BL/6 strains, an alternative explanation is that between-assay variability makes it difficult to measure small estrogenic effects (Ashby et al. 2004; Thigpen et al. 2002; Tinwell et al. 2000).
DE-71 increased liver weight in both BALB/c and C57BL/6 mice treated for 34 days, an effect shown previously in rats (Stoker et al. 2004; Zhou et al. 2001, 2002) and mink (Martin et al. 2007). The fact that a similar increase in liver weight occurred in ER-αKO mice indicates that this is not dependent on ER-α signaling. An increase in liver weight may occur in parallel with histologic changes such as hepatocytomegaly, acidophilic cytoplasm, binucleated hepatocytes, and eosinophilic bodies (Hardy et al. 2002; Son et al. 2007). Although we did not analyze the tissue histologically, others have shown that oral administration of DE-71 to rats for up to 90 days resulted in increased liver weights, with concomitant histologic changes (International Programme on Chemical Safety 1994). In BALB/c mice treated for 3 days, DE-71 increased liver weight in a dose-dependent manner only when administered po. Interestingly, EB potentiated the DE-71–induced increase in liver weight in BALB/c but not in C57BL/6 mice. Previous studies have shown that an estrogen-induced increase in liver weight in mice is strain dependent (Buchanan et al. 2002; Duffel et al. 1981; Goodin et al. 2002; Wang et al. 2004).
Because DE-71 treatment increased liver weight while at the same time diminishing the action of administered EB on the uterine epithelium (34-day treated mice only), it is possible that DE-71 can alter liver metabolic pathways that regulate systemic estrogen activity. A major pathway responsible for E2 deactivation is catalyzed by CYP enzymes, and of those CYP1A have the highest activity for E2 hydroxylation (Lee et al. 2003). A less active enzyme, CYP2B, also may be involved in E2 hydroxylation (Acevedo et al. 2005). Induction of hepatic EROD (CYP1A activity) and PROD (CYP2B activity) has been shown in rats (Stoker et al. 2004; Zhou et al. 2001, 2002) and mink (Martin et al. 2007) treated with DE-71 and mice treated with Bromkal 70-5 DE, a PBDE mixture with congener composition similar to DE-71 (Lundgren et al. 2007). In the present study, EROD (CYP1A) was increased by DE-71 but only when administered orally. PROD (CYP2B) was increased by DE-71 regardless of the route of administration, but the increase occurred to a much greater extent when the po route was used. Because the small antiestrogenic effect observed in the uterine epithelium occurred only when DE-71 was administered sc, it does not appear that this effect can be attributed to altered estrogen metabolism through increased CYP1A or CYP2B.
Individual PBDE congeners are known to induce expression of CYP2B, but the induction of CYP1A activity by PBDEs is most likely due to contamination with polybrominated dibenzo-p-furans (Hanari et al. 2006; Kuiper et al. 2006), which are known to induce aryl hydrocarbon receptor signaling in mammals (Olsman et al. 2007). Sanders et al. (2005) found that both DE-71 and its three main component congeners (BDEs 47, 99, and 153) increased CYP2B gene expression in rats, but that only DE-71 and not the individual congeners strongly up-regulated CYP1A, thus suggesting that the CYP1A increase was due to furan contamination of the DE-71 mixture. Because furans do not induce CYP2B, PROD induction is most likely mediated by PBDE activation of the constitutive androstane receptor, CAR (reviewed by Yamada et al. 2006).
In summary, the PBDE mixture DE-71 behaves as a weak estrogen in both MCF-7 breast cancer cell proliferation and the ovariectomized adult mouse models. The anti-estrogenic activity may be due to an interaction with ERs, not to a metabolic depletion of coadministered estrogens. In animal studies, treatment route and duration determined whether DE-71 was estrogenic or not. BALB/c mice are more susceptible to DE-71 effects in estrogen target tissues and in liver than C57BL/6 mice. DE-71 also increased liver weight in both mouse strains tested, and this effect was not dependent on ER-α. It still remains to be seen if the above listed effects of DE-71 are due to the original BDE congeners or to their hydroxylated metabolites.
Footnotes
We thank G. Eckert for assistance with the statistical analyses.
This work was supported by grants ES013341 and ES014367 from the National Institute of Environmental Health Sciences. The authors declare they have no competing financial interests.
References
- Acevedo R, Parnell PG, Villanueva H, Chapman LM, Gimenez T, Gray SL, et al. The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated MMTVneu mice and its potential effects on breast cancer incidence and latency. J Appl Toxicol. 2005;25(5):339–353. doi: 10.1002/jat.1078. [DOI] [PubMed] [Google Scholar]
- ATSDR. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2004. [[accessed 19 December 2007]]. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs) Available: http://www.atsdr.cdc.gov/toxprofiles/tp68.html. [Google Scholar]
- Ashby J, Tinwell H, Odum J, Lefevre P. Natural variability and the influence of concurrent control values on the detection and interpretation of low-dose or weak endocrine toxicities. Environ Health Perspect. 2004;112:847–853. doi: 10.1289/ehp.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (PBDEs) induce altered characteristics in MCF-7 cells. Mutagenesis. 2006;21(5):351–360. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol. 2007;23(2):138–144. doi: 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Bigsby RM, Mercado-Feliciano M, Mubiru J. Molecular mechanisms of endocrine disruption in estrogen dependent processes. In: Naz RK, editor. Endocrine Disruptors: Effects on Male and Female Reproductive Systems. Boca Raton, FL: CRC Press; 2005. pp. 217–247. [Google Scholar]
- Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, et al. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54(1):138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Ohsako S, Tohyama C, Cooke PS, Iguchi T. Dioxin inhibition of estrogen-induced mouse uterine epithelial mitogenesis involves changes in cyclin and transforming growth factor-beta expression. Toxicol Sci. 2002;66(1):62–68. doi: 10.1093/toxsci/66.1.62. [DOI] [PubMed] [Google Scholar]
- Cassidy MK, Houston JB. In vivo capacity of hepatic and extrahepatic enzymes to conjugate phenol. Drug Metab Dispos. 1984;12(5):619–624. [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220(2–3):104–116. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Clode SA. Assessment of in vivo assays for endocrine disruption. Best Pract Res Clin Endocrinol Metab. 2006;20(1):35–43. doi: 10.1016/j.beem.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Dang VH, Choi KC, Jeung EB. Tetrabromodiphenyl ether (BDE 47) evokes estrogenicity and calbindin-D9k expression through an estrogen receptor-mediated pathway in the uterus of immature rats. Toxicol Sci. 2007;97(2):504–511. doi: 10.1093/toxsci/kfm051. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, Hallgren S. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere. 2007;67(9):S386–S392. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- Diliberto JJ, Staskal DF, Hakk H, Birnbaum LS. Differential urinary protein binding of PBDEs in mice [Abstract] Toxicologist. 2007;96(S-1) Abstract ID 2012. [Google Scholar]
- Domingo JL. Human exposure to polybrominated diphenyl ethers through the diet. J Chromatogr A. 2004;1054(1–2):321–326. doi: 10.1016/j.chroma.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Duffel MW, Graham JM, Ziegler DM. Changes in dimethyl-aniline N-oxidase activity of mouse liver and kidney induced by steroid sex hormones. Mol Pharmacol. 1981;19(1):134–139. [PubMed] [Google Scholar]
- Evans JS, Varney RF, Koch FC. The mouse uterine wet weight method for the assay of estrogens. Endocrinology. 1941;28:747–752. [Google Scholar]
- Goodin MG, Fertuck KC, Zacharewski TR, Rosengren RJ. Estrogen receptor-mediated actions of polyphenolic cate-chins in vivo and in vitro. Toxicol Sci. 2002;69(2):354–361. doi: 10.1093/toxsci/69.2.354. [DOI] [PubMed] [Google Scholar]
- Gordon MN, Osterburg HH, May PC, Finch CE. Effective oral administration of 17β-estradiol to female C57BL/6J mice through the drinking water. Biol Reprod. 1986;35(5):1088–1095. doi: 10.1095/biolreprod35.5.1088. [DOI] [PubMed] [Google Scholar]
- Great Lakes Chemical Corp. West Lafayette, IN: Great Lakes Chemical Corporation; 2006. [[accessed 3 March 2008]]. Material Safety Data Sheet, Great Lakes DE-71™. Available: http://www.e1.greatlakes.com/common/msdspdf/00043.pdf. [Google Scholar]
- Hahnel R, Twaddle E. Estrogen receptors in human breast cancer. 1. Methodology and characterization of receptors. Steroids. 1971;18(6):653–680. doi: 10.1016/0039-128x(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92(1):157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hanari N, Kannan K, Miyake Y, Okazawa T, Kodavanti PR, Aldous KM, et al. Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and poly-brominated dibenzofurans as impurities in commercial polybrominated diphenyl ether mixtures. Environ Sci Technol. 2006;40(14):4400–4405. doi: 10.1021/es060559k. [DOI] [PubMed] [Google Scholar]
- Hardy ML, Margitich D, Ackerman L, Smith RL. The sub-chronic oral toxicity of ethane, 1,2-bis(pentabromophenyl) (Saytex 8010) in rats. Int J Toxicol. 2002;21(3):165–170. doi: 10.1080/10915810290096298. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Zou E, Matsumura F. Comparison of the characteristic of estrogenic action patterns of beta-HCH and heregulin beta1 in MCF-7 human breast cancer cells. J Biochem Mol Toxicol. 2002;16(5):209–219. doi: 10.1002/jbt.10047. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol 15. 2004;38(4):945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hooper K, McDonald TA. The PBDEs: an emerging environmental challenge and another reason for breast-milk monitoring programs. Environ Health Perspect. 2000;108:387–392. doi: 10.1289/ehp.00108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Programme on Chemical Safety. Environmental Health Criteria 162. Geneva: World Health Organization; 1994. Brominated Diphenyl Ethers. [Google Scholar]
- Kuiper RV, Murk AJ, Leonards PE, Grinwis GC, van den Berg M, Vos JG. In vivo and in vitro Ah-receptor activation by commercial and fractionated pentabromodiphenylether using zebrafish (Danio rerio) and the DR-CALUX assay. Aquat Toxicol. 2006;79(4):366–375. doi: 10.1016/j.aquatox.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low-dose PBDE-99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–154. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, et al. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64(2):187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17β-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144(8):3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- Legler J, van den Brink CE, Brouwer A, Murk AJ, van der Saag PT, Vethaak AD, et al. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicol Sci. 1999;48(1):55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: Eighth Cain Memorial Award lecture. Cancer Res. 1990;50(14):4177–4189. [PubMed] [Google Scholar]
- Li XD, Xia SQ, Lv Y, He P, Han J, Wu MC. Conjugation metabolism of acetaminophen and bilirubin in extrahepatic tissues of rats. Life Sci. 2004;74(10):1307–1315. doi: 10.1016/j.lfs.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Hack A, Roth-Harer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M, Darnerud PO, Molin Y, Lilienthal H, Blomberg J, Ilback NG. Viral infection and PBDE exposure interact on CYP gene expression and enzyme activities in the mouse liver. Toxicology. 2007;242(1–3):100–108. doi: 10.1016/j.tox.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Martin PA, Mayne GJ, Bursian FS, Tomy G, Palace V, Pekarik C, et al. Immunotoxicity of the commercial polybrominated diphenyl ether mixture DE-71 in ranch mink (Mustela vison) Environ Toxicol Chem. 2007;26(5):988–997. doi: 10.1897/06-246r.1. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, et al. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immuno Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of PBDE-47 in primary cultures of rat neo-cortical cells. Toxicol Sci. 2004;82(1):164–169. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Olsman H, Engwall M, Kammann U, Klempt M, Otte J, Bavel B, et al. Relative differences in aryl hydrocarbon receptor-mediated response for 18 polybrominated and mixed halogenated dibenzo-p-dioxins and -furans in cell lines from four different species. Environ Toxicol Chem. 2007;26(11):2448–2454. doi: 10.1897/07-004R.1. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45(2):584–590. [PubMed] [Google Scholar]
- Peters AK, van Londen K, Bergman A, Bohonowych J, Denison MS, van den Berg M, et al. Effects of polybrominated diphenyl ethers on basal and TCDD-induced ethoxy-resorufin activity and cytochrome P450-1A1 expression in MCF-7, HepG2, and H4IIE cells. Toxicol Sci. 2004;82(2):488–496. doi: 10.1093/toxsci/kfh284. [DOI] [PubMed] [Google Scholar]
- Pijnenburg AM, Everts JW, de Boer J, Boon JP. Polybrominated biphenyl and diphenylether flame retardants: analysis, toxicity, and environmental occurrence. Rev Environ Contam Toxicol. 1995;141:1–26. doi: 10.1007/978-1-4612-2530-0_1. [DOI] [PubMed] [Google Scholar]
- Qiu X, Mercado-Feliciano M, Bigsby RM, Hites RA. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromo diphenyl ether mixture. Environ Health Perspect. 2007;115:1054–1057. doi: 10.1289/ehp.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JM, Burka LT, Smith CS, Black W, James R, Cunningham ML. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol Sci. 2005;88(1):127–133. doi: 10.1093/toxsci/kfi288. [DOI] [PubMed] [Google Scholar]
- Savvas M, Studd JW, Norman S, Leather AT, Garnett TJ, Fogelman I. Increase in bone mass after one year of percutaneous oestradiol and testosterone implants in post-menopausal women who have previously received long-term oral oestrogens. Br J Obstet Gynaecol. 1992;99(9):757–760. doi: 10.1111/j.1471-0528.1992.tb13879.x. [DOI] [PubMed] [Google Scholar]
- Shimada T, Sugie A, Shindo M, Nakajima T, Azuma E, Hashimoto M, et al. Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene. Toxicol Appl Pharmacol. 2003;187(1):1–10. doi: 10.1016/s0041-008x(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants-old diseases. Clin Med Res. 2003;1(4):281–290. doi: 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg M, Silberberg R. Susceptibility to estrogen of breast, vagina, and endometrium of various strains of mice. Proc Soc Exp Biol Med. 1951;76(1):161–164. doi: 10.3181/00379727-76-18423. [DOI] [PubMed] [Google Scholar]
- Son HY, Kim SH, Shin HI, Bae HI, Yang JH. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch Toxicol. 2007 doi: 10.1007/s00204-007-0246-x. [Online 14 September 2007] [DOI] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995;103(suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285(5431):1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- Spearow JL, O’Henley P, Doemeny P, Sera R, Leffler R, Sofos T, et al. Genetic variation in physiological sensitivity to estrogen in mice. APMIS. 2001;109(5):356–364. doi: 10.1034/j.1600-0463.2001.090504.x. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Birnbaum LS. Impact of repeated exposure on the toxicokinetics of BDE 47 in mice. Toxicol Sci. 2006;89(2):380–385. doi: 10.1093/toxsci/kfj038. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Devito MJ, Birnbaum LS. Inhibition of human and rat CYP1A2 by TCDD and dioxin-like chemicals. Toxicol Sci. 2005;84(2):225–231. doi: 10.1093/toxsci/kfi090. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Young PC, Caperell-Grant A, Gize EA, Madhukar BV, Ben-Jonathan N, et al. Novel estrogenic action of the pesticide residue beta-hexachlorocyclohexane in human breast cancer cells. Cancer Res. 1996;56(23):5403–5409. [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78(1):144–155. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Enari M, Abe Y, Ohta Y, Iguchi T. Effect of ovariectomy on histological change and protein expression in female mouse reproductive tracts. In Vivo. 1996;10(1):103–110. [PubMed] [Google Scholar]
- Thigpen JE, Haseman JK, Saunders H, Locklear J, Caviness G, Grant M, et al. Dietary factors affecting uterine weights of immature CD-1 mice used in uterotrophic bioassays. Cancer Detect Prev. 2002;26(5):381–393. doi: 10.1016/s0361-090x(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Tinwell H, Joiner R, Pate I, Soames A, Foster J, Ashby J. Uterotrophic activity of bisphenol A in the immature mouse. Regul Toxicol Pharmacol. 2000;32(1):118–126. doi: 10.1006/rtph.2000.1412. [DOI] [PubMed] [Google Scholar]
- Ulrich EM, Caperell-Grant A, Jung SH, Hites RA, Bigsby RM. Environmentally relevant xenoestrogen tissue concentrations correlated to biological responses in mice. Environ Health Perspect. 2000;108:973–977. doi: 10.1289/ehp.00108973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2’,4,4’,5-pentabromo-diphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol Sci. 2002;67(1):104–107. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192(2):95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Wang HH, Afdhal NH, Wang DQ. Estrogen receptor alpha, but not beta, plays a major role in 17beta-estradiol-induced murine cholesterol gallstones. Gastroenterology. 2004;127(1):239–249. doi: 10.1053/j.gastro.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Weichselbaum RR, Hellman S, Piro AJ, Nove JJ, Little JB. Proliferation kinetics of a human breast cancer line in vitro following treatment with 17beta-estradiol and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1978;38(8):2339–2342. [PubMed] [Google Scholar]
- Welsch CW, Swim EL, McManus MJ, White AC, McGrath CM. Estrogen induced growth of human breast cancer cells (MCF-7) in athymic nude mice is enhanced by secretions from a transplantable pituitary tumor. Cancer Lett. 1981;14(3):309–316. doi: 10.1016/0304-3835(81)90160-9. [DOI] [PubMed] [Google Scholar]
- Yamada H, Ishii Y, Yamamoto M, Oguri K. Induction of the hepatic cytochrome P450 2B subfamily by xenobiotics: research history, evolutionary aspect, relation to tumorigenesis, and mechanism. Curr Drug Metab. 2006;7(4):397–409. doi: 10.2174/138920006776873508. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Okino N, Sato T, Sugatani J, Miwa M. Induction of detoxifying enzymes in rodent white adipose tissue by aryl hydrocarbon receptor agonists and anti-oxidants. Drug Metab Dispos. 2006;34(7):1081–1089. doi: 10.1124/dmd.105.007286. [DOI] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61(1):76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66(1):105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]