Abstract
Methylprednisolone (MP) has been shown to reduce acute inflammation resulting from a secondary damage cascade initiated by the primary physical injury to the spinal cord. The current clinical practice for delivering systemic MP is inefficient, and high doses are required resulting in adverse, undesired dose-related side effects in patients. Here, we report a novel, minimally invasive, localized drug delivery system for delivering MP to the contused adult rat spinal cord that potentially side-steps the deleterious consequences of systemic cortico-steroid therapy. MP was encapsulated in biodegradable PLGA based nanoparticles (NP), and these nanoparticles were embedded in an agarose hydrogel for localization to the site of contusion injury. To visualize and quantify its spatial distribution within the injured spinal cord, MP was conjugated to Texas-red cadaverine prior to encapsulation in nanoparticles. When delivered via the hydrogel-nanoparticle system, MP entered the injured spinal cord and diffused up to 1.5mm deep and up to 3mm laterally into the injured spinal cord within 2 days. Furthermore, topically delivered MP significantly decreased early inflammation inside the contusion injured spinal cord as evidenced by a significant reduction in the number of ED-1+ macrophages/activated microglia. This decreased early inflammation was accompanied by a significantly diminished expression of pro-inflammatory proteins including Calpain and iNOS. Additionally, topically delivered MP significantly reduced lesion volume 7 days after contusion injury. The minimally invasive, MP delivery system reported in this study has the potential to enhance the effectiveness of MP therapy after contusion injured spinal cord and avoid the side-effects arising from high dose cortico-steroid therapy.
INTRODUCTION
There are approximately 200,000 individuals living with a spinal cord injury (SCI) in the U.S. alone, with nearly 11,000 new cases each year [1]. Damage to the spinal cord results both from the initial trauma and then from secondary processes that occur in the hours following the initial trauma. These secondary injury processes cause oxidative damage, calcium-mediated damage, excitotoxicity, immune reactions, and apoptosis [2]. While the extent of primary injury is determined by the initial trauma which is beyond control, the severity of secondary injury can potentially be modulated through the use of pharmacological agents such as Methylprednisolone.
Methylprednisolone (MP) is a synthetic corticosteroid that is clinically used for the treatment of acute SCI. The National Acute Spinal Cord Injury Study II (NASCIS II) study demonstrated that MP given within 8 hours following SCI can improve neurologic recovery in humans [3]. Typically MP is administered in high doses systemically (30mg/kg bolus injection followed by a 5.4 mg/kg/h infusion over 23 hours), and the high dose MP causes adverse side effects including wound infections, pneumonia, and acute corticosteroid myopathy accompanied by only modest improvements in neurological recovery [4, 5]. Thus, there is a need for localized delivery of MP to the lesion site to minimize the systemic delivery related side effects. To achieve this local delivery, MP could be directly injected into the spinal cord at the site of injury. However this may require multiple injections (for maintaining the appropriate dose) which may further damage the spinal cord. Also, MP can be intrathecally delivered into the lesion [6], but an implantable MP depot such as an osmotic pump or a catheter is required to achieve the sustained delivery of MP into the lesion site. The implantation of the MP depots may further damage (e.g., compress) the injured spinal cord and cause implant-related complications such as an occlusion, inflammatory mass, or infection [7, 8].
A contusion injury best mimics the most common form of human spinal cord injury. In this study, we have developed a minimally invasive drug delivery system that allows for local delivery of MP to a contused spinal cord. It consists of a thermoreversible agarose gel that acts as a carrier for MP that is encapsulated in biodegradable poly(lactic-co-glycolic acid) (PLGA) based nanoparticles (MP-NPs). The MP-NP embedded gel is placed directly onto the injury site, on top of the dura, after contusion injury for sustained release of MP. A second layer of agarose gel is then injected on top of the MP-NP embedded gel and quickly cooled to secure the MP-NP embedded gel at the lesion site. The objective of this study was to determine if this topical delivery system is efficacious in terms of a) facilitating MP diffusion into the injured spinal cord, b) retaining MP’s anti-inflammatory effects within the acutely injured cord, and c) sparing injured spinal cord tissue at 7 days after injury.
To visualize and quantify the acute spatial distribution of diffused MP in the spinal cord within two days after contusion injury, MP was conjugated to Texas-red cadaverine (Tx-MP). Tx-MP was then encapsulated in biodegradable PLGA based nanoparticles (Tx-MP-NP). Saline encapsulated nanoparticles (Saline-NP) were used as a control. The animals were perfused after either Tx-MP-NP or Saline-NP topical delivery into the contusion injured site, and the distribution of Tx-MP was quantified. The expression of acute injury related proteins including Calpain and iNOS, and the number of ED-1+ cells (e.g., macrophages and activated microglia) at the lesion site were also quantified and compared between experimental groups. Since the expression level of these proteins and ED-1+ cells is at its peak at the lesion site within 0 to 3 days after injury, we chose to examine anti-inflammatory effects of topically delivered MPs on the contused spinal cord tissue two days after injury. Additional animals were perfused at 7 days after contusion injury/treatment to study the long-term consequences of this type of release (i.e., formation of cavity at the lesion site).
MATERIALS AND METHODS
Preparation of Tx-MP conjugate
Methylprednisolone sodium succinate (MP, Pharmacia) and Texas-red cadaverine (Tx, Biotium) were conjugated to allow for visualization of the MP in and around an injured spinal cord. Texas red cadaverine was selected due to its low molecular weight (690.87 Da), since our goal was to determine if MP could diffuse into the contusion lesion site while minimizing the increased molecular weight due to conjugating MP to Texas-red. MP (18mg, 0.036mmol) and Tx (5mg, 0.0072mmol) were dissolved in deionized (DI) water (this corresponds to a mole ratio of MP to Tx of 5:1), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (11mg, 0.071mmol, Pierce Biotechnology) was added as a coupling agent for conjugation of Tx to MP (Tx-MP). This reaction occurred for 24 hours at room temperature, protected from light. The solution was dialyzed against DI water for 2 hours (using a dialysis membrane with molecular weight cutoff of 1000 Da) to remove unreacted MP, Tx, and EDC. The conjugates are referred to henceforth as Tx-MP. The absence of free Tx in the solution was verified using electrospray ionization (ESI) mass spectroscopy.
Preparation of Tx-MP encapsulated PLGA based nanoparticles (Tx-MP-NP)
Tx-MP used in the in vitro studies and delivered to the animals via the gel release method were first encapsulated in poly(lactic-co-glycolic acid) (PLGA) based nanoparticles (Tx-MP-NP) by the double emulsion method. Briefly, 2% PLGA (w/v, 50:50, Polysciences) was first dissolved in methylene chloride (2.5ml, Fisher Scientifics). Tx-MP solution (250µl) was then added into the PLGA solution and emulsified three times in PLGA solution by homogenization at 4000 rpm. This emulsion was then added into 0.4% poly(vinyl alcohol)(PVA, Aldrich) solution and homogenized three times at 7000 rpm. This double emulsion was diluted in 0.1% PVA solution and the methylene chloride was removed by evaporation for 3 hours. The Tx-MP-NPs were collected by centrifugation (8500g) and washed twice with sterilized DI water before lyophilization. All described procedures were done under sterilized conditions. Saline encapsulated NPs (Saline-NP) were fabricated as an experimental control. The fabrication procedure was the exactly same as the Tx-MP-NPs, except that sterilized saline was encapsulated instead of Tx-MP. Also, MP encapsulated NPs (MP-NP) were used as a positive control to examine if the Tx conjugation to MP affects the pharmacological functions of the MP. All lyophilized NPs were stored at −20°C until use.
Preparation of agarose gels
The agarose gels that embed the NPs were made of 0.6% (w/v) agarose gel (SeaPlaque, Cambrex) in 1x PBS. This solution was mixed well with the desired quantity of NPs and allowed to cool at 4°C for 30 minutes. The more dense upper layer of gel that was used to hold the NP embedded gel in place in vivo was a solution of 0.7% (w/v) agarose gel (SeaKem, Cambrex) in 1x PBS. This was maintained around 45°C so as to maintain the liquid state and prevent gelling until after the surgery was complete and the gel containing the NPs was satisfactorily in place (Figure 1C).
Figure 1.
Tx-MP encapsulated NPs and topical delivery. A) Mass spectroscopy results, verifying the purity of the Methylprednisolone-Texas red cadaverine conjugates (Tx-MP). The arrow indicates Tx-MP. B) A fluorescence image of Tx-MP-NPs in saline. Inset figure is magnified Tx-MP-NPs. Scale bar = 1µm. C) Schematic showing the Tx-MP-NP embedded gel is placed directly onto the injury site, on top of the dura. The denser gel is injected on top and quickly cooled to hold the NP embedded gel in place and minimize outward diffusion of Tx-MP.
In vitro Tx-MP release profile
To examine how much of the Tx-MP was released from the NPs embedded within the gel a release profile study was conducted measuring released Tx-MP at different incubation times. Gels (n=3 for each condition) were prepared containing either Tx-MP-NP or Saline-NP (as a control) by mixing 2mg of NPs (containing approximately 156µg MP; calculated based upon 27% of Tx conjugation efficiency and 65% of encapsulation efficiency) with 500µL 0.6% SeaPlaque agarose as described above and allowed to cool for 30 minutes. Once the NP embedded gels solidified, 500µL of 1xPBS was placed on top and the gels were incubated at 37°C. The supernatant was collected every 24 hours up to 6 days after incubation and the amount of released Tx-MP was determined through UV spectroscopy (at 247nm, BIO-TEK).
In vitro bioactivity of Tx-MP released from NPs
A bioassay was designed in which primary microglia were harvested from postnatal day 3 rat pups as described in Giulian et al. [9]. The microglia were cultured with astrocytes, then mechanically harvested (through vigorous agitation overnight on an orbital shaker) and seeded on poly-L-lysine (0.1mg/ml, Sigma) pre-coated tissue culture plates. Four types of gels were prepared: three of which (MP-NPs, Tx-MP-NPs, and Saline-NPs) were prepared as described above each containing 2mg of NPs, and one gel that contained no NPs. The microglia were activated (to release nitric oxide) by lipopolysaccharide (LPS, Sigma, 5ng/ml) and then each type of gel was placed on top of microglial cultures. The assay used measures the amount of nitrite in the media, a byproduct when nitric oxide (NO) breaks down, indirectly quantifying NO production (Griess Reagent System, Promega). Gels were placed on top of the activated microglia to determine whether MP and Tx-MP were both able to reduce NO production. Samples of the media were collected every 24 hours for four days and the amount of nitrite generated in the microglial wells was quantified. The concentration of nitrite (µM) in each condition was plotted and statistical differences between conditions were determined (Minitab).
Topical delivery of Tx-MP-NPs into the contusion injured spinal cord
All procedures were conducted according to IACUC (Institutional Animal Care and Use Committee at Georgia Institute of Technology) approved protocols. Adult male rats (n=7 for Tx-MP-NP with visibly damaged dura mater after injury; n=3 for Tx-MP-NP without visibly damaged dura mater after injury; and n=7 for Saline-NP, 200–230g, Sprague-Dawley, Harlan) were induced to anesthetic depth with intraperitoneal injection of pentobarbital sodium (Nembutal, 50mg/ml). These animals were perfused at two days after contusion injury. Before surgery, the surgical area was shaved and the incision site cleansed with a chlorohexaderm scrub. The ninth or tenth thoracic spinal cord segments (T9-10) were exposed by laminectomy. The contusion injury was performed using an Infinite Horizon spinal cord injury device (150 kilodynes, Precision Systems & Instrumentation, Lexington, KY). Five minutes after injury, either Tx-MP-NP (containing approximately 156µg MP) or Saline-NP embedded gel was placed directly onto the injury site, on top of the dura. The NP embedded gel was secured by applying a liquid state of the denser agarose gel (0.7% SeaKem), which was quickly solidified by local cooling as described in Jain et al. [10]. After delivering the Tx-MP-NPs or Saline-NPs, the muscle and skin were closed in layers, and the animals recovered on a heating pad. Animals were monitored regularly and their bladders were manually expressed.
Additional animals received only laminectomy and the Tx-MP-NP embedded gel was placed on the top of the dura of the uninjured spinal cord (n=4). This experiment was conducted to determine whether the Tx-MP can diffuse through intact, uninjured dura mater and enter into the spinal cord. To examine the impact of Tx-MP-NP treatment on spinal cord tissue sparing/cell survival (e.g., neurons or astrocytes), the contusion injury was performed as described above on additional animals (n=4 for Tx-MP-NP with visibly damaged dura mater after injury and n=4 for Saline-NP). These animals were perfused at 7 days after contusion injury.
Tissue preparation and histological analysis of spinal cord tissue reactivity
At two days after contusion injury or sham surgery, the animals (Tx-MP-NP with visibly damaged dura mater after injury (n=7), Tx-MP-NP without visibly damaged dura mater after injury (n=3), Saline-NP (n=7), and Tx-MP-NP on uninjured spinal cord (n=4)) were perfused transcardially with PBS followed by 4% paraformaldehyde in PBS. The spinal cord was removed and post-fixed overnight in the same fixative. Longitudinal 18µm thick tissue sections were cryosectioned and immunostained. Tissue sections were incubated with the following primary antibody solutions overnight at 4°C: ED-1 (1:1000, mouse IgG1, Serotec) to identify macrophage/reactive microglia, Calpain (1:500, mouse IgG1, Sigma) and inducible nitric oxide synthase (iNOS, 1:500, rabbit IgG, Sigma) to identify the reactivity of these injured related proteins. Secondary antibodies were diluted 1:220 in 0.5% triton in PBS and included goat anti-mouse IgG1 Alexa 488 (Invitrogen) for ED-1 and Calpain, and goat anti-rabbit IgG(H+L) Alexa 488 for iNOS. Sections treated only with secondary antibody but with no primary antibody were used to determine non-specific binding. Some of the samples were stained with haematoxylin and eosin stain (H&E) to visualize the overall structure of the contusion injured spinal cord.
At seven days after contusion injury, additional animals (Tx-MP-NP with visibly damaged dura mater after injury (n=4) and Saline-NP (n=4)) were perfused as described above. Longitudinal 25µm thick tissue sections were cryosectioned and double immunostained with the following primary antibodies: GFAP (1:1000, rabbit IgG(H+L), Dako) to identify astrocytes and NF160 (1:500, mouse IgG1, Sigma) to identify axons.
Quantitative immunofluorescence analysis for assessing spinal cord tissue reactivity
For a quantitative comparison of the tissue reactivity at the injured spinal cord between experimental groups, the immunostained images at the lesion site (i.e., 1.5mm anterior and posterior to the lesion center) were captured with an Olympus digital camera and their immunostaining intensity was quantified, averaged and compared using a custom MATLAB (Mathworks) based image analysis program as described in Jain et al. [10]. The contusion injury site was easily distinguished from intact spinal cord tissue because of its obstructed tissue structure. Briefly, for Calpain and iNOS quantification, the image analysis program generated line profiles (40 line profiles/image), extracted relative fluorescent intensity, and averaged each one to create an average intensity profile. A minimum of 126 images (i.e., 126 representative quantitative intensities; 3 images/spinal cord section × 6 spinal cord sections per animal × 7 animals) for each experimental group was used to obtain the overall average intensity for each staining, and this overall average intensity was compared between experimental groups. Background subtraction using secondary antibody alone on tissue sections was performed.
For ED-1+ cell counting, the ED-1+ immunostained images (a minimum of 84 images for each experimental group) were captured at the lesion site, and the number of ED-1+ cells/mm2 was determined by Image-pro plus (MediaCybernetics, Silver Spring, MD) software and compared between experimental groups.
Quantification of spatial distribution of Tx-MP in the contusion injured spinal cord
The extent of Tx-MP diffusion through the spinal cord was quantified using Image-pro plus area analysis. A grid was superimposed onto the fluorescent images (n=37 images; images acquired from Tx-MP-NP with visibly damaged dura mater at 2 days after injury (n=7 animals)) with 1mm lateral (i.e., rostrocaudal) divisions and 500µm vertical (i.e., dorsoventral depth) divisions to quantitatively determine both the lateral and vertical diffusion of MP in the spinal cord from the site of delivery (Figure 4E). For lateral diffusion examination, the zero points on the grid correspond to the center of the delivered gel. For vertical diffusion examination, the zero points on the grid correspond to the dorsal spinal cord surface where the Tx-MP-NP embedded gel was placed (Figure 4E). The area of tissue with positive Tx-MP signal in each grid section was obtained by manually tracing the area filled with Tx-MP signal using Image-pro plus software. The data was normalized by dividing the Tx-MP positive area in the grid section by the total Tx-MP positive area to give the percentage diffused either laterally or vertically. In order to confirm positive Tx-MP signal was not due to autofluorescence, some of the samples were treated with CuSO4 to eliminate potential autofluorescent signal as described in Schnell et al. [11]. Briefly, the Tx-MP diffused spinal cord samples (18µm thick and longitudinally sectioned) were incubated with 1mM CuSO4 in ammonium acetate buffer (50mM CH3COONH4, pH 5.0) for 1 hour prior to imaging. The Texas-red signals in CuSO4 treated samples and untreated samples were compared under fluorescent microscopy.
Figure 4.

Tx-MP diffused spinal cord tissue 2 days after injury. A, B) Show representative H&E stained montage (longitudinally sectioned) of Tx-MP-NP treated spinal cord tissue (A) and Saline-NP treated spinal cord tissue (B). Abbreviation: r = rostral and c = caudal. The arrow indicates the contusion injured site. C, D) Show representative Texas-red fluorescent image of Tx-MP-NP treated spinal cord tissue (C) and Saline-NP treated spinal cord tissue (D). The arrow indicates the contusion injury site and the arrow heads indicate the dorsal surface where the Tx-MP-NP embedded gel was placed. Numbered images are magnified from figure (C), showing the diffused Tx-MP. A and C (Tx-MP-NP) and B and D (Saline-NP) are consecutive images. E) Shows the representative Tx-MP-NP diffused spinal cord with a grid used for quantifying the distribution of Tx-MP in the spinal cord tissue. Two yellow lines are reference lines for distance. The grid was formed every 1mm laterally and every 500µm vertically. Black arrow indicates the contusion injured site. F and G) Show the quantification (n=37) of the distribution of the diffused Tx-MP either laterally (i.e., rostrocaudal) (F) and vertically (i.e., dorsoventral depth) (G). In lateral diffusion, 0 mm is corresponding to vertical yellow reference line (i.e., center of Tx-MP delivered spinal cord) in (E). In vertical diffusion, 0 mm is corresponding to horizontal yellow reference line (i.e., dorsal surface) in (E). Error bars represent s.e.m. H) Shows the representative Texas-red fluorescent image of Tx-MP-NP embedded gel topically placed on the uninjured animal (i.e., sham surgery). The arrow head indicates the dorsal surface where the Tx-MP-NP embedded gel was placed on the intact dura mater.
Quantification of lesion volume at seven days after contusion injury
For lesion volume quantification, the GFAP immunostained images were captured (a minimum of 24 tissue sections per each animal; n=4 animals for Tx-MP-NP with visibly damaged dura mater after injury and n=4 for Saline-NP). The lesion area (i.e., neuron and astrocyte depleted cavity) was delineated by GFAP+ glial scar (Figure 6A). The lesion area/image was measured by Image-pro plus software, multiplied by tissue section thickness, and then added to generate the lesion volume. The measured lesion volume was averaged and compared between experimental groups at 7 days after contusion injury.
Figure 6.
Significantly reduced lesion volume after Tx-MP-NP local treatment. A) Shows GFAP (a marker for astrocytes, red) and NF160 (a marker for axons, green) double immunostained (longitudinally sectioned) spinal cord tissue at the lesion site 7 days after contusion injury. The arrow indicates the contusion injury site, and the arrow heads indicate the lesion (i.e., neuron and astrocyte depleted cavity). B) Shows a quantitative comparison of the lesion volume (delineated by GFAP+ astroglial scar, see Methods section) between Tx-MP-NP and saline-NP treated animals at 7 days after contusion injury. Error bars represent s.e.m. *P< 0.05.
Statistical analysis
The Student’s t-test was used to determine the significant differences between experimental conditions. A p value less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
In this study we examined whether a novel hydrogel-nanoparticle system can topically deliver MP into the contusion injured spinal cord in a minimally invasive manner. We have shown that MP can be topically delivered into the lesion site through diffusion, and furthermore that the delivered MP is pharmacologically active by being anti-inflammatory, as confirmed by the significant reduction of ED-1+ macrophages/reactive microglia and both Calpain and iNOS protein expression. Furthermore, topically delivered MP significantly reduced lesion volume at 7 days after contusion injury.
Formation of Tx-MP encapsulated PLGA based nanoparticle and in vitro characterization
To visualize the distribution of delivered MP, we conjugated MP to a fluorescent tag, Texas Red cadaverine. Electrospray ionization (ESI) mass spectroscopy was used to verify that we had successfully purified the conjugates. Figure 1A shows a large peak at 1147.4, representing the Tx-MP conjugates. There was no peak around 690 (or any logical values less than this that might represent Texas-red cadaverine without certain functional groups), which indicates that there was no free Texas-red in the Tx-MP solution (data not shown). Therefore, the Texas-red fluorescent signal confirms the presence of MP. After conjugates were successfully prepared and verified, they were encapsulated in PLGA to form nanoparticles (NPs). Figure 1B shows Tx-MP-NPs suspended in saline. As evident, Texas-red fluorescence allows for easy identification of the location of the Tx-MP. Figure 1C shows the Tx-MP-NP embedded gel topically placed on top of the dura in a contusion injured spinal cord.
The in vitro studies verify that the Tx-MP was released slowly and continuously over a period of at least 6 days (Figure 2A). The release profile study was performed to determine how much Tx-MP is released from the gel embedded NPs at different incubation times. The amount of Tx-MP released during each 24 hour period for 6 days following the preparation of the gel is shown in Figure 2A. As shown in this figure, though the release amount gradually decreases, Tx-MP is continuously delivered from the NPs for the entire 6 days of the experiment. We speculate that the continual release over time could be due to the gradual breakdown of the biodegradable PLGA particles. This sustained release of MP allows the maintenance of the pharmacological effects of MP such as anti-inflammation with a single administration and the prolongation of the effects through the initial injury period (i.e., acute phase of secondary injury processes).
Figure 2.
In vitro characterization of Tx-MP-NPs. A) Release profile of Tx-MP over 6 days in vitro. Plotted is the amount of MP released every 24 hours. Error bars represent s.e.m. B) Bioassay showing the amount of nitrite in the media surrounding LPS activated microglia that had been treated with gels containing either MP-NP, Tx-MP-NP, Saline-NP, or nothing. This represents the amount of NO produced by microglia in each of these conditions for 4 days. Error bars represent s.e.m. *P< 0.05
The in vitro bioactivity of the released Tx-MP from NPs embedded in agarose hydrogels was examined by co-incubation with LPS-stimulated primary rat microglia and by quantification of their nitric oxide (NO) release. The bioassay measures nitrite, a byproduct of NO breakdown, that can quantify NO release from LPS-activated microglia under the various conditions. Figure 2B shows the nitrite concentration in the media under each of the various treatment conditions at four different time points. After 24, 72, and 96 hours, the cells treated with Tx-MP-NPs had significantly less nitrite (which indicates a reduction in NO production) than did the cells treated with Saline-NPs (as a negative control). At all timepoints, there was no significant difference in the amount of NO produced by the cells treated with MP-NP and those treated with Tx-MP-NP, indicating that the Texas-red conjugation to MP did not affect the anti-inflammatory activity of the MP. This result demonstrates that the addition of a fluorescent tag to MP did not interfere with the anti-inflammatory function of the MP for at least 4 days and released MP is bioactive. Additionally, this result shows that the hydrogel-based delivery system (i.e., NPs embedded agarose gel) can be used as a MP depot.
Topical MP delivery enables minimally invasive delivery to contusion injured spinal cord
Figure 3 shows the steps involved in delivering Tx-MP embedded gel to the injured spinal cord. The laminectomy at T9-10 was typically clean with minimal blood loss, exposing a segment of the spinal cord large enough for the contusion impact device (Figure 3A). Immediately following contusion (within 5 minutes), visible swelling of the spinal cord was observed as well as additional bleeding (Figure 3B). In some animals, a slight but visible peeling of the dura mater occurred after contusion injury. In other animals, however, no visible damage to the dura was observed after contusion injury. In this study, we separated Tx-MP treated animals after contusion injury into two groups - animals with visibly damaged dura (i.e., a slight but visible peeling of the dura mater) and animals without visibly damaged dura to examine whether Tx-MP can be delivered into the spinal cord tissue through the contusion injured dura which was not visibly damaged.
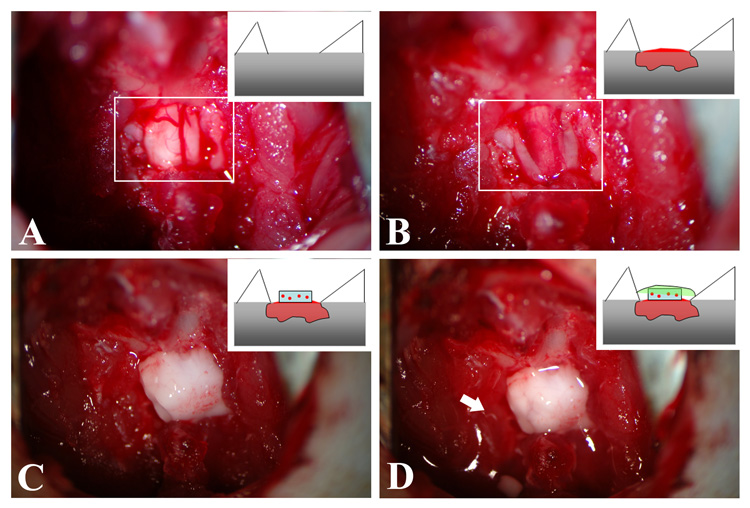
Figure 3.
Surgical procedure of topically delivering Tx-MP-NPs embedded gel. A) Shows the exposed T10 segment by laminectomy. Photograph is a top-view and cartoon is a longitudinal-view. White box indicates exposed spinal cord. B) Five minutes after contusion injury by impactor. White box indicates the injured spinal cord. C) The Tx-MP-NP embedded gel was topically placed on the lesion site. D) The denser agarose gel (liquid state) was applied onto the gel and quickly solidified by local cooling (in situ gelling). The arrow indicates the solidified denser agarose gel.
The injury area was cleaned, and the gel containing NPs (either Tx-MP-NPs or Saline-NPs) was placed directly on top of the spinal cord at the site of injury, as seen in Figure 3C. Finally the denser gel (0.7% SeaKem agarose gel) was applied on top in liquid form and quickly cooled (in situ gelling, within 2–3 seconds using a custom designed local cooling device) to hold the NP embedded gel in place (Figure 3D). Two days after surgery when the animals were sacrificed and the spinal cords removed, it was observed that the gels had not been disturbed from their position above the injury site.
The in situ gelling technique-based delivery method we have developed and described in Figure 3 allows one to topically delivery MP (or other molecules) to the contusion injured spinal cord in a minimally invasive manner. Specifically, a quick transition from liquid to solid gel form by local cooling enables one to create a tight seal between these two gels, resulting in the fixation of the NP embedded gel at the lesion site, which localizes MP delivery to the site of injury and minimizes its diffusion to surrounding tissues. Additionally, since the entire in situ gelling procedure is conducted under sterilized conditions (by syringe filtered gel) and at low temperature (by cooling), it may significantly minimize degradation of high-temperature related molecules (e.g., proteins). Any effects of lower temperature on inflammation in the spinal cord were controlled for by the use of saline-NP hydrogels which were introduced in a manner identical to Tx-MP-NP.
Only Tx-MP that was placed directly on visibly damaged dura diffused into the contusion injured spinal cord
PLGA-NP encapsulated Tx-MP that was placed directly on visibly damaged dura (i.e., a slight but visible peeling of the dura mater) via this agarose gel based delivery system entered into the contusion injured spinal cord and diffused either laterally (i.e., rostrocaudal) or vertically (i.e., dorsoventral). However, Tx-MP that was placed directly on non-visibly damaged dura did not enter into the spinal cord tissue (data not shown). Figure 4A and B show the representative contusion injured spinal cord, either Tx-MP-NP treated (A) or Saline-NP treated (B). These H&E stained spinal cord tissues show that the injured tissue is loosely distributed in both conditions. Figure 4C and D show the representative spinal cord tissues visualized under a Texas-red fluorescent filter. The Tx-MP diffused through the spinal cord tissue and was easily detected due to its bright intensity. In the Saline-NP treated spinal cord, there was no positive signal for Texas red (Figure 4D), and the comparison of CuSO4 treated and untreated tissue samples confirmed that the Texas-red fluorescent signal observed only in Tx-MP-NP treated spinal cord was positive signal for Tx-MP, not from autofluorescence of native tissue (data not shown).
The diffusion profile of Tx-MP was quantified by measuring the percentage of the area filled with a positive signal laterally and vertically (Figures 4F and G). In lateral (i.e., rostrocaudal) diffusion, approximately 60% of Tx-MP was detected within 1mm from the center line (both rostral and caudal stump) and it diffused over 3mm from the center line within two days after delivery. In vertical (i.e., dorsoventral depth) diffusion, the Tx-MP was detected over 1.5mm from the dorsal surface where the Tx-MP-NPs were placed even though it was a very low percentage of total MP delivered (less than 2.6% of Tx-MP). These results demonstrated that the Tx-MP placed on visibly damaged dura was able to enter the spinal cord and diffuse through and around the lesion site within 2 days after delivery.
To examine whether Tx-MP could cross an intact, unaltered dura mater, we applied the gel containing Tx-MP-NP to animals that had a laminectomy but no contusion injury. The Tx-MP positive signal was not observed in the spinal cord, rather it was only detected at the intact dura mater where the Tx-MP-NP embedded agarose gel was placed after laminectory (Figure 4H). This result indicates that it may not be possible to topically deliver Tx-MP through intact dura mater.
Only the dura mater visibly damaged by the contusion injury allowed the Tx-MP to enter into the spinal cord tissue and diffuse through the tissue. To maximize the delivery of MP into the contusion injured spinal cord tissue using our delivery system, one might need to use intrathecal injection of MP-NP embedded gel just underneath the dura mater using a method described in Jimenez Hamann, et al. [12], or one might also consider physically peeling off the dura mater before placing the MP-NP embedded gel on the lesion site.
In vivo bioactivity of Tx-MP on the contusion injured spinal cord
Tx-MP-NPs that were placed directly on visibly damaged dura significantly reduced the number of ED-1+ cells such as macrophages/activated microglia and the expression of pro-inflammatory related proteins, including Calpain and iNOS, as compared to Saline-NP treatment 2 days after injury (Figure 5). Tx-MP-NPs that were placed directly on non-visibly damaged dura, however, did not decrease the number of ED-1+ cells and the expression of pro-inflammatory related proteins (no statistical difference from Saline-NP, data not shown). Figure 5A and B show the representative distribution of ED-1+ cells in the injured spinal cord following treatment with Saline-NP or Tx-MP-NP, respectively. There were significantly fewer ED-1+ cells in the Tx-MP treated spinal cord than in the Saline treated cord (Figure 5D). Moreover, Figure 5C demonstrates that the significantly reduced number of ED-1+ cells is correlated with the diffused Tx-MP, indicating that the topically delivered MP was able to diffuse through the injured spinal cord and reduce ED-1+ macrophage/reactive microglia which are major inflammation related cells after injury. This significant reduction in macrophage/reactive microglia was accompanied by diminished expression of pro-inflammatory related proteins including Calpain and iNOS (Figures 5E–J). Calpain (a ubiquitous Ca2+ dependent cystein protease that cleaves cytoskeletal and myelin proteins [13]) immunostaining (Figures 5E and F) and iNOS (a major mediator of inflammation and neurotoxic effects after injury [14]) immunostaining (Figures 5H and I) were significantly reduced in the animals treated with Tx-MP-NPs as compared to those treated with Saline-NPs, indicating that topical Tx-MP-NP treatment is a promising therapeutic intervention in the acute phase of contusion spinal cord injury because of its multifaceted anti-secondary injury properties.
Figure 5.

In vivo bioactivity of topically delivered Tx-MP on contusion injured spinal cord (2 days after treatment). A–D) Show representative (longitudinally sectioned) ED-1 immunostained (marker for macrophages/reactive microglia) spinal cord tissue at two days after injury: A) Saline-NP and B) Tx-MP-NP treated animal. The contusion impacted site is indicated by the arrow. Numbered images are magnified and either from (A) or (B). C) Shows the diffused Tx-MP (red) and ED-1 (green) overlapped image. D) Shows a quantitative comparison of the ED-1+ cells/mm2 (a minimum of 84 images for each experimental group (n=7 animals)). Abbreviation: r = rostral and c = caudal. E–G) Show representative Calpain immunostained spinal cord tissue: (E) Saline-NP and (F) Tx-MP-NP treated spinal cord. Number images are magnified and either from (E) or (F). G) Shows a quantitative comparison of the fluorescence intensity of Calpain expression at the lesion site (a minimum of 126 images for each experimental group (n=7 animals)). Background subtraction using secondary antibody alone on tissue sections was performed. H-J) Show representative iNOS immunostained spinal cord tissue: (H) Saline-NP and (I) Tx-MP-NP treated spinal cord. Number images are magnified and either from (H) or (I). J) Shows a quantitative comparison of the fluorescence intensity of iNOS at the lesion site (a minimum of 126 images for each experimental group (n=7 animals)). Background subtraction using secondary antibody alone on tissue sections was performed. Error bars represent s.e.m. *P<0.05.
Tx-MP-NP local treatment significantly reduces lesion volume at 7 days after injury
Tx-MP-NPs that were placed directly on visibly damaged dura significantly decreased lesion volume as compared to Saline-NP treatment at 7 days after contusion injury (Figure 6B). In both experiments, the contusion injury created a cavity at the middle of the spinal cord tissue (Figure 6A). The cavity is an area depleted of both neurons and astrocytes, and fragmented neurons are observed. These results suggest that the significant reduction in lesion volume at 7 days after injury may be the long-term consequences of the significant decrease in the acute inflammatory response by Tx-MP-NP local treatment.
CONCLUSIONS
In this study, we report that the novel hydrogel-nanoparticle system enables effective delivery of MP to contusion injured spinal cord in a minimally invasive manner. By using this system, not only can MP be delivered into contusion injured spinal cord tissue, but it remains bioactive in vivo and significantly decreases secondary injury-related inflammation and protein expression, resulting in significantly reduced lesion volume at 7 days after injury. Furthermore, the hydrogel-nanoparticle system described can be used as a tool for sustained, controlled, and localized delivery of several other therapeutic molecules from pharmacological agents to neurotrophic factors to effectively manage the site of spinal cord injury with local and effective delivery avoiding the complications associated with systemic delivery.
ACKNOWLEDGEMENTS
This work was supported by NSF IGERT DGE-0333411, NIH NS43486 (to RVB), and GTEC, an NSF funded Engineering Research Center based at Georgia Institute of Technology and Emory University. We also thank Prof. Michelle LaPlaca and Crystal Simon for assistance with use of the Kentucky Infinite Horizon contusion injury device.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.C.D.C. Report and Recommendations to the Advisory Committee for Injury Prevention and Control on the Interagency Meeting on Spinal Cord Injury. 2006 [cited; Available from: http://www.cdc.gov/ncipc/factsheets/scifacts.htm.
- 2.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001 Dec 15;26(24 suppl):S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990 May 17;322(20):1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 4.Gerndt SJ, Rodriguez JL, Pawlik JW, Taheri PA, Wahl WL, Micheals AJ, et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma. 1997 Feb;42(2):279–284. doi: 10.1097/00005373-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Qian T, Guo X, Levi AD, Vanni S, Shebert RT, Sipski ML. High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005 Apr;43(4):199–203. doi: 10.1038/sj.sc.3101681. [DOI] [PubMed] [Google Scholar]
- 6.Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, et al. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N Engl J Med. 2000 Nov 23;343(21):1514–1519. doi: 10.1056/NEJM200011233432102. [DOI] [PubMed] [Google Scholar]
- 7.Deer TR, Raso LJ, Garten TG. Inflammatory mass of an intrathecal catheter in patients receiving baclofen as a sole agent: a report of two cases and a review of the identification and treatment of the complication. Pain medicine (Malden, Mass. 2007 May–Jun;8(3):259–262. doi: 10.1111/j.1526-4637.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Piper NA, Flack SH, Loeser JD, Lynn AM. Epidural analgesia in a patient with an intrathecal catheter and subcutaneous pump to deliver baclofen. Paediatric anaesthesia. 2006 Sep;16(9):989–992. doi: 10.1111/j.1460-9592.2006.01908.x. [DOI] [PubMed] [Google Scholar]
- 9.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Kim YT, McKeon RJ, Bellamkonda RV. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006 Jan;27(3):497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999 Jun;47(6):719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez Hamann MC, Tsai EC, Tator CH, Shoichet MS. Novel intrathecal delivery system for treatment of spinal cord injury. Exp Neurol. 2003 Aug;182(2):300–309. doi: 10.1016/s0014-4886(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 13.Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999 Oct 1;58(1):167–190. [PubMed] [Google Scholar]
- 14.Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, et al. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996;20(1):1–9. doi: 10.1016/0891-5849(95)02017-9. [DOI] [PubMed] [Google Scholar]