Abstract
In vivo functional imaging by means of positron emission tomography (PET) is the sole method for providing a quantitative measurement of μ-, κ and δ-opioid receptor-mediated signalling in the central nervous system. During the last two decades, measurements of changes to the regional brain opioidergic neuronal activation—mediated by endogenously produced opioid peptides, or exogenously administered opioid drugs—have been conducted in numerous chronic pain conditions, in epilepsy, as well as by stimulant- and opioidergic drugs. Although several PET-tracers have been used clinically for depiction and quantification of the opioid receptors changes, the underlying mechanisms for regulation of changes to the availability of opioid receptors are still unclear. After a presentation of the general signalling mechanisms of the opioid receptor system relevant for PET, a critical survey of the pharmacological properties of some currently available PET-tracers is presented. Clinical studies performed with different PET ligands are also reviewed and the compound-dependent findings are summarized. An outlook is given concluding with the tailoring of tracer properties, in order to facilitate for a selective addressment of dynamic changes to the availability of a single subclass, in combination with an optimization of the quantification framework are essentials for further progress in the field of in vivo opioid receptor imaging.
Keywords: PET, opioid receptors, pain, epilepsy, addiction
Introduction
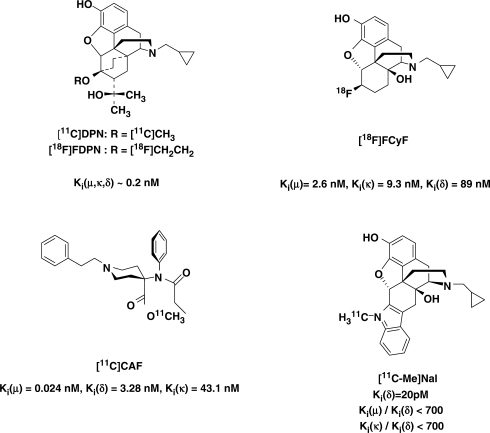
The non-invasive detection of photon pairs within a given body volume affords an opportunity to study the function of opioid receptors (ORs) within the central nervous system (CNS) in vivo with positron emission tomography (PET). Interest also exists in using PET for studying opioid biochemistry and opioidergic neurotransmission in epilepsy, pain processing and neurodegenerative disorders, including its use for evaluation of more selective, improved analgesics, anticonvulsants and neuroprotective agents. In addition, PET may in principle be useful for a qualitative and quantitative assessment of adaptions to the central opoid receptors resulting from opioid and stimulant drug seeking behaviour, development of compulsive drug use, tolerance and withdrawal syndromes. Knowledge of the quantitative relationship between brain biochemistry, receptor occupancy and behavioural as well as therapeutic effects would be helpful in the optimization of therapeutic protocols, including the evaluation and development of new pharmacological treatments. With an appropriate reporter probe (Fig. 1) labelled with positron emitting radionuclides, such as 11C (t½ = 20.3 min), or 18F (t½ = 109.7 min), an in vivo depiction and quantification of CNS-contained receptors may be derived from PET measurements of brain radioactivity.
Fig. 1.
Radiolabelled compounds established for clinical PET-investigations of the opioid receptors.
The design and development of radiolabelled compounds that can be administered intravenously, enter the brain via the blood stream and subsequently bind to specific targets in the CNS constitute the current major challenge in the field of radiopharmaceutical chemistry. Compounds suitable for the in vivo assessment of opioid receptors (ORs) in the CNS can potentially permit investigation of how the functional receptor status in vivo correlates with neurobiological and neuropsychiatric parameters. In addition to providing a review of the published clinical PET-studies on ORs, we review in this article the properties of the tracers available and the kinetic modelling framework used for generating a quantitative measurement of ORs in the brain.
General function of opioid receptors
Opioid-binding sites in the central nervous system were proposed by Beckett and Casy (1954) and Portoghese (1965), and demonstrated in mammalian brain tissue in 1973 by using radioligand-binding assays on isolated brain tissue (Pert and Snyder, 1973; Simon et al., 1973). The extensive pharmacological studies performed during the last decades have uncovered a variety of opioid receptor (OR) subtypes. To date, four ORs have been cloned, the mu-(μ) (MOP-R), kappa-(κ) (KOP-R), delta-(δ) (DOP-R) and the NOP-R, the latter initially referred to as ORL-1 (Mollereau et al., 1994), or nociceptin/orphanin FQ receptor (Meunier et al., 1995). For a definition of the OR terminology see: Dhawan et al. (1996) and http://www.iuphar.org.
The ORs are now known to be distributed widely in the central nervous system (CNS) and in peripheral sensory and autonomic nerves. Activation of ORs by endogenous and exogenous ligands results in a multitude of physiological functions and behaviours. Research has been con-ducted on a wide array of molecular–biochemical effects and neurochemical localization studies of endogenous opioids and their receptors, i.e. attempts have been to clarify the role of OR-mediated signalling mechanisms in pain and analgesia, stress and social status, tolerance and dependence, learning and memory, eating and drinking, alcohol and drugs of abuse, CNS development and endocrinology, mental illness and mood, seizures and neurological disorders, electrical activity and neurophysiology, general activity and locomotion, gastrointestinal, renal and hepatic functions, cardiovascular responses, respiration and thermoregulation and immunological responses [for reviews, see: van Ree et al. (1999); Law et al. (2000); Williams et al. (2001)].
Opioid receptors and their ligands
There are three groups of endogenous opioid peptide ligands; methionine- and leucine-enkephalin (derived from proenkephalin), dynorphins-A and B and neo-endorphin (derived from pro-dynorphin) and β-endorphin (derived from pro-opiomelanocortin). Methionine-enkephalin (met-enk) and leucine-enkephalin (leu-enk) have pronounced affinity for δ- and μ-OR [inhibition constants, Ki,s, in the range of 0.6–4 nM (Raynor et al., 1994)] and very low affinity (>1 μM) for κ-OR. The dynorphins preferentially recognizes κ-ORs [dynorphine A; Ki,κ = 0.5 nM; Ki,μ = 32; Ki,δ > 1000 nM (Raynor et al., 1994)] while β-endorphin displays μ and δ recognition [Kμ = Ki,δ 1 nM; Ki,κ = 52 nM (Raynor et al., 1994)]. Two endogenous peptides, endomorphin-1 and -2, have high and selective affinity for μ-OR (Hackler et al., 1997; Zadina et al., 1997; Ki,μ = 0.34 and 0.69 nM, respectively), are not derived from the above precursors, but via a so far not completely clarified biochemical pathway. For a recent review on endomorphines-1 and -2, see: Fichna et al. (2007). In general, agonists selective for μ-ORs or δ-ORs are analgesic and rewarding, whereas at least some κ-OR-selective agonists produce aversive effects like dysphoria and hallucinations.
Receptor subtypes of μ-, δ and κ-ORs have been proposed from the results of pharmacological in vitro and in vivo studies (Knapp et al., 1995; Connor and Christie, 1999), but at present there is no molecular evidence to account for a further subclassification. Only one example of each of the μ-, δ and κ-ORs has been cloned from a given species (Knapp et al., 1995) although functional splice variants of μ-ORs have been discovered (Abbadie et al., 2004; Pasternak et al., 2004; Pan et al., 2005). A recent explanation for subclasses of μ- δ and κ -OR subtypes has evolved with the identification of OR hetero-dimers or hetero-oligomers that appear to have properties different from the monomeric receptors. Heterodimerization of ORs has been shown to affect receptor trafficking and there are also reports of heterodimerization of the ORs with other classes of guanine nucleotide-binding protein-coupled receptors (GPCRs) (Jordan et al., 2000; Devi, 2001; Rios et al., 2001).
Opioidergic signalling mechanisms
As members of the heterotrimeric GPCRs, ORs are signal transducers anchored to the cell surface plasma membrane; in this manner, they connect receptors to effectors and thus to intracellular signalling pathways [for a general review of G protein signalling pathways see: Neves et al. (2002) and for the opioid receptor system specifically Connor and Christie (1999); Law et al. (2000)]. The GCPRs have seven transmembrane domains, substantial intracellular domains between the fifth and sixth transmembrane segment, an extracellular N-terminal and an intracellular C-terminal domain (Strader et al., 1994). There is evidence for more than 20 types of G protein and different receptor types apparently interacting preferentially with different types of G protein (Gudermann et al., 1996).
G proteins consist of three distinct subunits, Gα, Gβ and Gγ (Neves et al., 2002). μ-, δ- and κ-ORs interact preferentially with the pertussis toxin-(PTX) sensitive G proteins α-subunits of the Gi and Go family (Gi1-3 and Go1-2) as well as two PTX-insensitive subunits [Gz and G16 (Simon et al., 1991; Connor and Christie, 1999 and references cited therein]. As reviewed by others (Connor and Christie, 1999) a differential coupling of the ORs to most types G protein subtypes is in general marginal, but preferential coupling of μ-OR to Gi3 and of δ-OR to G16 relative to that of μ-and κ-OR have been observed.
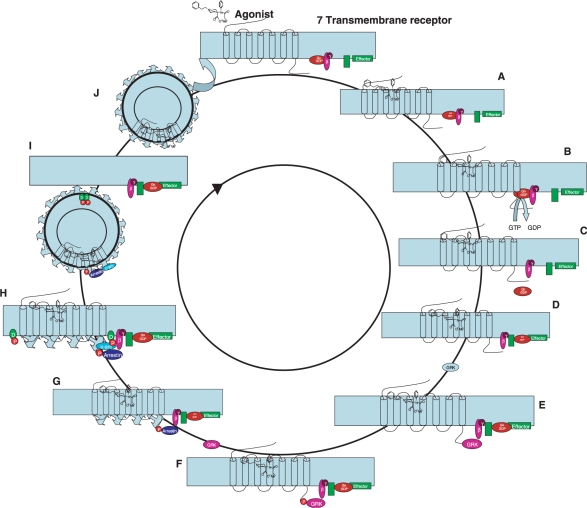
When an agonist binds to an OR, the G protein is split into two subunits, Gα and the Gβγ (Fig. 2). Both subunits activate intracellular second-messenger systems regulating cellular components such as metabolic enzymes, ion channels and the transcriptional machinery. The Gβγ subunit activates neuronal inwardly rectifying K+-channels and inhibits high threshold voltage-activated Ca2+ channels, leading to reduced excitability and inhibition of neurotransmitter release (Clapham and Neer, 1997). The Gi/o α-subunit mediates an inhibition of intracellular adenylyl cyclase and reduction of cyclic adenosine monophosphate (cAMP) that also reduces neuronal membrane excitability and regulates gene expression and the activity of cellular phosphatates and kinases. Opioids can thus inhibit the release of neurotransmitters, such as glutamate and substance P, at spinal and supraspinal level.
Fig. 2.
An opioid agonist binds to an opioid G-protein-coupled opioid receptor (A) activating the G protein complex by a GDP to GTP switch in the Gα subunit (B). Activated Gα and Gβ/Gγ subunits move to regulate effectors (C–E) followed by phosphorylation of the C-terminal end of the receptor by G-protein receptor kinase. Arrestin binds to the phosphorylated C-terminal and binds to clathrin (F) followed by (G) phosphorylation of dynamin (D) by c-src resulting in closing of the endocytotic vesicle (H) which is formed by invagination of a clathrin-coated pit. The receptor is dephosphorylated (I) and subsequently reinserted into the membrane (J).
Through the activation of PTX-sensitive Gi/Go, opioids have predominantly inhibitory effects on cells in the CNS. Functionally, the endogenous opioids act as co-transmitters modulating the effect of fast-acting neurotransmitters (Siggins et al., 1986; Wagner et al., 1993; Simmons and Chavkin 1996). It has been observed that opioids act indirectly to excite neurons through a presynaptic inhibition of GABA release, so-called disinhibition. In addition, opioids cause direct excitatory actions such as increased firing of action potentials, and/or increases in intracellular calcium concentration.
Desensitization, downregulation and cellular counteradaptations
It is currently believed that the intracellular response after activation of a G protein-coupled OR involves a conserved ‘core’ endosomal sorting machinery together (Fig. 2) with additional specialized protein interactions. In this manner, a regulation of response specificity and plasticity beyond the mere ligand–receptor interaction is conferred. It is clear that ORs, as is the case with many G protein-linked receptors, are not static but cycle to and from the plasma membrane (Figs 2 and 3). Receptors found in vesicular membranes can be both newly synthesized or recycled (Shuster et al., 1999). Receptor trafficking initiated by agonist binding and internalization through the endosomal pathway is currently held to be involved in desensitization and/or recycling in a feedback inhibition that is dependent on prior activity in any given terminal. The internalization of the OR is agonist dependent: It appears that all of the endogenous OR agonists and a most alkaloid agonists (except morphine) are potent activators of OR internalization regardless of their ability to induce G protein activation [for review see Williams et al., (2001)].
Fig. 3.
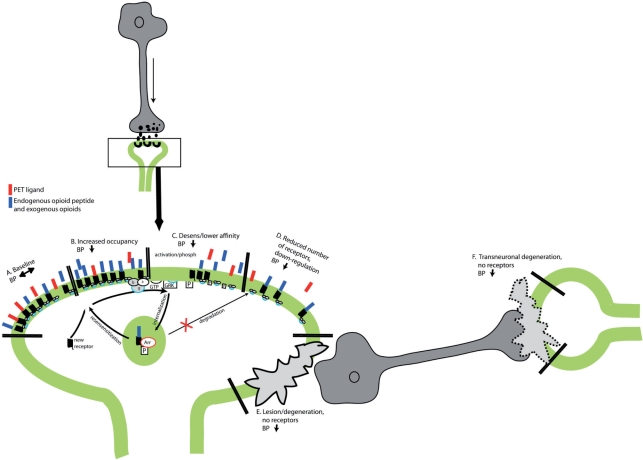
Hypothetical sequence of events leading to changes in the receptor status and thus changes to the baseline (A) receptor binding of a tracer due to increased occupancy (B) of endogenous or exogenous opioid ligands leading potentially also to the induction of lower affinity states of the receptor (C) (decoupling/inactivation) and downregulation and reduced receptor expression (D).
In states of pain, the endogenous OR system is activated as a part of our endogenous analgesic system and is the target of administered opioid analgesic drugs. Morphine and other μ-OR agonists are still the analgesics of choice for patients with cancer pain and the majority of patients with chronic non-malignant pain (Eriksen et al., 2003; Carr et al. 2004). However, a major drawback to a continued use of these drugs is the potential development of tolerance and dependence, the latter in both the psychological and physical context. An increase in the occurrence of direct excitatory effects during chronic opioid treatment or chronic increased opioid neuronal tonus can contribute to the appearance of paradoxical opioid-mediated pain, hyperalgesia and allodynia (Varga et al., 2003). Thus, adaption to chronic OR stimulation may involve tolerance to Gi/o-mediated inhibition (analgesia) coupled with sensitization to excitatory (pronociceptive) opioid actions (Celerier et al., 2001). It is likely that similar counteradaptations take place in other disorders than those reflecting pain and opioid analgesia, which may be very different in a state of disease compared to a healthy brain and should therefore be considered in the interpretation of the findings on OR availability.
Cellular adaptation induced by a compound with agonistic effect at ORs may involve several processes:
Acute desensitization of OR due to effector coupling and receptor internalization that develops during and abates shortly after exposure to agonists (Fig. 2) Unlike the membrane-contained ion channels, the pathway mediating the intracellular signalling part of ORs involves multiple protein–protein interactions, translocations and phosphorylation events. It appears to be at least three general pathways following the activation of Gi/Go-linked receptors that eventually converge on the activation Thus, activation kinetic of ORs is longer than that for other effectors, but occurs over a period of several minutes to 1–2 h (Williams et al., 2001). The significance of OR activation kinetic in PET studies with [11C]carfentanil and [11C]diprenorphine has been addressed recently (Greenwald et al., 2007; Hammers et al., 2007; Scott et al., 2007a).
The signalling pathways that lead to long-term adaptation through an altered genetic expression may be regarded as an effector system. Long-term desensitization of receptor to effector coupling and downregulation of receptors that slowly develops and then persists for many hours to days after removal of agonists. Similarly, long-term treatment with opioid antagonists has shown to upregulate opioid receptors (Lesscher et al., 2003). Desensitization of the receptor (the progressive loss of receptor function), can be a consequence of multiple processes of receptor uncoupling, internalization, degradation and recycling.
Adaptations of intracellular signalling mechanisms in opioid-sensitive neurons (Fig. 2). Phosphorylation of agonist-activated receptors, subsequent arrestin recruitment and uncoupling of the receptors from G proteins are important processes in the modulation of GPCR responsiveness (Ahn et al., 2003; Gainetdinov et al., 2004). GPCRs can be targeted to clathrin-coated pits for endocytosis. Internalized receptors can also be recycled to the plasma membrane or be targeted to lysosomes for degradation (Tsao and von, 2001; Tsao et al., 2001).
Counter-adaptations in neuronal circuitry (Williams et al., 2001) such as μ-agonist-dependent activation of the N-methyl-d-asparate (NMDA)-receptor system and stimulation of the κ-OR system.
Pharmacokinetic and pharmacodynamic considerations of PET-ligands
Modelling of radioligand kinetics
In order to be practically useful for quantification of the target-receptor concentration, the time-dependent count rate information collected by the PET-camera must be converted into figures relating to binding parameters. Since the signal detected represents total radioactivity, it includes specific and non-specific binding as well as free ligand in the tissue and a blood volume component. In order to designate the contributions from each of these parameters, reference has to be made either to the arterial concentration of the ligand (corrected for radioactive metabolites) or to a reference region within the brain with no or negligible specific binding of the tracer in question, hence providing a kinetic estimate of non-specific binding plus free ligand concentration (as well as a blood volume component) which is used as an input. The assumption of no specific binding in a reference region is not valid for all tracers, and especially not for non-selective OR ligands. Only for δ-OR selective tracers there is a valid reference region; the cerebellum (Schadrack et al., 1999). The most frequently used reference region for μ-selective and non-selective ligands, the occipital cortex, has low expression of μ-OR, intermediate of δ- and κ-ORs (Hiller and Fan, 1996). The presence of specific binding in a reference region may underestimate the calculated specific binding.
Traditional models have formal compartmental structures and the rate constants are estimated by using standard non-linear least squares fitting techniques [for reviews see: Ichise et al. (2001); Gunn et al. (2002); Laruelle et al. (2002); Innis et al. (2007))]. Compartments describe the tracer distribution in separate tissue entities (plasma, free, non-specific bound, specific bound) and rate constants are describing the exchange kinetic between these compartments (Innis et al., 2007). Using a single scan, derived parameters such as the volume of distribution (VT) (the ratio of ligand concentration between tissue and plasma) or binding potential (BP) (the equilibrium concentration of specific binding as a ratio to some other reference concentration) can be obtained and both are proportional to the Bavail (concentration of available receptors) and KD (equilibrium dissociation constant of the radioligand). These models have generally been applied to a region of interest, but analysis is prone to errors due to variations in the selection of the image segments to be analysed, which effectively further reduces considerably the spatial resolution of the imaging techniques. These problems can be circumvented by the generation of parametric voxel-by-voxel binding images. This technique, however, is difficult to apply for traditional models as well as being computationally time consuming, prone to errors due to local minima, and, at worst, unidentifiable if a large number of parameters need to be derived.
It is in principle possible to independently estimate Bavail and ligand KD, but these estimates require two imaging studies, at low and high-receptor occupancy, in order to identify specific and non-specific binding (Frost et al., 1989; Smith et al., 1999). Low and high-receptor occupancy can be achieved using a competitive drug (e.g. naloxone) or ligand at high and low-specific activities. However, ligands with agonistic properties at lower specific activity (higher doses) might have unwanted pharmacological effects and therefore not be applicable for PET studies. Approximations have therefore been done using the activity ratio between regions rich in receptors and relative to that obtained in a region devoid (or of very low concentration) of receptors (Frost, 1988) and is referred to as BPND. Assuming the non-specific binding to be uniform, the ratio at binding equilibrium is linearly proportional to the ratio of Bavail/KD (Mintun et al., 1984).
There is a range of parametric imaging analysis techniques, typically based on a compartmental description of the tracer (Gunn et al., 2002). These range from explicitly specified compartmental structures (model driven) to more flexible models derived from a general compartmental description (data driven). Examples of model-driven approaches include a one-tissue compartmental model for the estimation of blood flow (Kety and Schmidt, 1948) and two-tissue compartment models for 2-[18F]fluoro-deoxy-glucose ([18F]FDG) (Sokoloff et al., 1977; Phelps et al. 1979) trapping and receptor ligand binding (Mintun et al., 1984). More complex compartment models have an increasing number of unknown parameters to be estimated and result in higher variability. For data-driven methods, there exist graphical analyses (Gjedde, 1982; Patlak et al., 1983; Patlak and Blasberg 1985; Logan et al., 1990, 1996), spectral analysis (Cunningham and Jones, 1993) and bootstrapped DEPICT (Gunn et al., 2002), among others. Ligands with slow binding kinetics may produce ‘noisy’ standard compartment models because of flow dependency (changes in transport rate will affect a compound that never reaches equilibration) and the uncertainty of calculating the later points in the kinetic curve. Data-driven methods such as spectral analysis may for such ligands with slow kinetics be more suitable (Cunningham and Jones, 1993).
Images and imagination: interpretation of the specific binding
As described earlier, the mechanisms regulating receptor expression and binding status are complex, and a PET-study alone does not reveal the underlying mechanisms responsible for a change in the specific binding of the tracer. A derivation of cellular and subcellular processes directly from a PET-image is speculative and data from complementary methods are required for validation of the PET data.
The interpretation of the receptor affinity-dependent binding is complex and depends, as described earlier, on the G protein-dependent affinity state of the GPCRs (Fig. 3C). This affinity state may further be influenced by endogenous release of opioid peptides and/or administration of opioid receptor modulating drugs. As the agonist/antagonist properties of a PET-tracer may dominate its ability to discriminate between different states of the G protein coupling of the OR. Agonist binding depends on the conformational state of the receptor and agonists bind preferably to the receptors in the high-affinity state, while antagonists will bind to both high and low-affinity states. This dependency implies that interpretation of changes to the receptor presentation in vivo studies would benefit from a consideration of the tracer's agonistic properties in addition to its subtype selectivity. In temporal lobe epilepsy, an asymmetry in receptor binding between the left and right side of the brain was identified using [11C]carfentanil ([11C]CAF) (μ-selective agonist), but no asymmetry was seen using [11C]DPN (non-selective antagonist) (Frost et al., 1988; Mayberg et al., 1991) or [18F]fluoro-cyclofoxy [18F]FCyF (μ- and κ-OR antagonist) (Theodore et al., 1992). These authors discussed the result as an issue of selectivity; however, the agonistic and antagonistic properties of the ligands used may just as much contribute to the differences.
In addition, a reduced receptor binding may be due to receptor down-regulation, internalized (non-available if not immediately recycled) (Fig. 3D) receptors or a pathological change due for example, to neuronal damage or neurodegeneration. For the latter, distant changes may also develop along the neuronal projections or even as an antero- or retrograde transneuronal degeneration (Fig. 3E,F) (Chung et al., 1990). A transneuronal degeneration may at least in part explain changes in binding distant from a neuronal lesion such as stroke (Willoch et al., 2004). In contrast, an increase in receptor binding may be due to an upregulation or activation of a possible receptor reserve (Fan et al., 2003). The above-described mechanisms represent slow changes due to neurodegeneration or chronic activation of the neurotransmitter system.
Acute changes, due to release of neurotransmitter in response to a specific stimulus, can be measured in receptor-activation studies (Fig. 3B). Receptor-activation studies can be performed comparing binding from two separate scans, i.e. one at baseline and one activation/intervention study; this has been the most frequently reported approach. An alternative is the methodologically more challenging scan protocol consisting of a bolus-infusion administration of tracer, performed to achieve equilibrium, where control-binding levels are determined. Subsequently, a physical or mental stimulation is conducted, or pharmacological challenge is administered, while the infusion of radiotracer continues, and the change in specific binding of the tracer is monitored. This approach has been used to measure the difference in amphetamine-induced dopamine release between healthy controls and patients with schizophrenia (Watabe et al., 2000) and recently for measuring release of endogenous opioid peptides during a state of sadness (Zubieta et al., 2003a, b). Again, the change in receptor binding between the two measured states may be dependent on both the number of available receptor-binding sites as well as the affinity state of the receptor. The available binding receptor sites (Bavail) is reduced by both increased occupancy of released endogenous opioid peptides and internalized receptors. The μ-OR agonist [11C]CAF is likely to bind preferentially to receptors in high-affinity states, coupled to G proteins. A lower concentration of high-affinity receptors would be expected after an opioid peptide release.
A pharmacological interaction between a PET ligand and an exogenously administered ligand with an affinity for the target ORs will follow the same biological rules, making the observed changes dependent upon the selectivity and agonistic property of both the ligand and the administered substance. The dependency of the choice of biological model as well as the properties of the radiotracer on the outcome should therefore be carefully considered.
Despite a great development in mathematical modelling and advanced data processing of tracer kinetics, there is no standardized way to publish data from clinical studies. From Table 1 it can be seen that 18 of the 51 published studies have applied the logistically simplest approach, i.e. the ratio method, and 17 of the studies have used a non-invasive Logan plot. Both of these models do not need a generation of an arterial input function. Sixteen of the studies made use of arterial blood sampling to acquire an arterial input function applied for compartmental modelling or spectral analysis. In recent years it has become routine to produce parametric maps of the whole brain data, and not only from drawn volumes of interest (VOIs) (Table 1). The validity of using the whole brain versus VOI was demonstrated by Weeks et al. (1997) in a study of Huntington's disease using [11C]DPN binding. Whole brain parametric maps make it possible to perform further standardized post-processing (e.g. NEUROSTAT and SPM), which may make the statistical analyses more standardized and less dependent on the observer. A practical problem is that the originally calculated parameters of perfusion and receptor binding may be manipulated in the image analyses. Several authors normalize the pixel values, whereas others are more conservative and keep the original parametric values. In principle, parametric data are already normalized to a non-specific region or to blood. A normalization prior to statistical processing (typically to whole brain values, proportional scaling) may reduce variance, but at the same time remove the quantification element inherent to kinetic and graphical models. There is no consensus on the correct approach and it would represent an improvement to report the parametric values and changes in absolute values in addition to statistical maps.
Table 1.
The studies on PET-imaging of ORs in the CNS
| Study | Aim | Ligand | Modelling/statistical analysis/group size | Main findings | Interpretation/comments |
|---|---|---|---|---|---|
| Neurochemical mapping | |||||
| Jones et al., 1991 | Study of OR-binding in relation to the lateral and medial pain system. | [11C]DPN (μ-, κ- and δ- antagonist) | Ratio method, BPND/VOI/n = 2 | High OR binding in medial pain system and low OR binding in lateral pain system (SM1). | The medial pain system is likely to be more susceptible to exogenous and endogenous opioid neuromodulation than the so-called lateral pain system. |
| Baumgartner et al., 2006 | Study of OR-binding in relation to the lateral and medial pain system, focus on secondary sensory cortex. | [18F]FDPN (μ-, κ- and δ- antagonist) | Non-invasive Logan plot, BPND/VOI/n = 11 | All structures of the operculo-insular region (anterior and posterior insula, and parietal and frontal operculum) have high OR binding. Factor analysis revealed high loadings on operculo-insular region, ACC and putamen. | The operculo-insular region as part of the lateral pain system is influenced by opioids and displays a functional unit together with ACC and putamen. |
| Vogt et al., 1995 | Detailed analysis of OR binding in cingulate and adjacent cortex. | [11C]DPN (μ-, κ- and δ- antagonist) | Invasive compartment model, VT /VOI/n = 3 | Highest OR binding in ACC, rostral cingulofrontal transition and frontal cortices. There was a gradient from low to high binding to caudal ACC, PCC and superior frontal cortices. | Variations in binding may reflect functional specializations such as low binding in visuospatial areas and high binding in areas processing affective content. |
| Schadrack et al., 1999 | In vivo and in vitro studies of cerebellum OR binding and mRNA. Comparison of rat and human. | [11C]DPN (μ-, κ- and δ- antagonist) | Invasive SA, IRF60/VOI/n = 10 | No OR in rat cerebellum, and low to medium OR binding in human cerebellum. In human brain, there is a differential OR level in cerebellar cortex, vermis and dentate nuclei, and absence of δ-OR. mRNA mainly observed in granule cells and OR predominantly in the molecular layer. | Presence of opioidergic mechanisms in the human cerebellum in contrast to the rat. |
| Smith et al., 1998 | Regulation of μ-OR during the menstrual cycle. | [11C]CAF (μ-agonist) | Ratio method, BPND/VOI/n = 10 in luteal and in follicular phases. | No significant differences in μ-OR binding between phases of the menstrual cycle. A negative correlation between circulating levels of estradiol during the follicular phase and μ-OR measures in amygdala and hypothalamus, regions regulating GnRH pulsatility. LH pulse amplitude was positively correlated with μ-OR in the amygdala, whereas LH pulse number was negatively correlated with μ-OR in this same region. | These results suggest that amygdalar μ-ORs exert a modulatory effect on GnRH pulsatility, and that circulating levels of estradiol also regulate central μ-OR function. |
| Zubieta et al., 1999 | Examine age- and gender-associated variations in μ-OR binding. | [11C]CAF (μ-agonist) | Ratio method, BPND/VOI/n = 40 females, n = 36 males | μ-OR binding increase with age in neocortical areas and the putamen. Higher μ-OR binding in women was observed in a number of cortical and subcortical areas, but declined in postmenopausal women to levels below those of men. | These data imply that both age and gender are important variables of the OR system. |
| Cohen et al., 2000a | Gender differences in OR binding (healthy controls and AD patients). | [18F]FcyF (μ and κ-antagonist) | Ratio method, BPND/VOI/n = 9 females, n = 15 males | Less combined μ-/κ-OR binding in thalamus in females compared to males. | Consistent with evidence that sexual dimorphism exists with respect to opiate pathways. |
| Epilepsy: focal | |||||
| Frost et al., 1988 | Study role of μ-OR in temporal lobe epilepsy. | [11C]CAF (μ-agonist) and [18F]FDG (glucose metabolism) | Ratio method, BPND/VOI/n = 8 | Inter-ictal increased μ-OR binding in temporal neocortex and hypometabolism ipsilateral to epileptogenic focus. Hippocampus and amygdala had no change in binding or metabolism. | Opioids may represent an anticonvulsant system that limits spread of electrical activity. |
| Mayberg et al., 1991 | Study role of μ-OR (carfentanil) versus non-selective OR (diprenorphine) binding in temporal lobe epilepsy. | [11C]CAF(μ-agonist) and [11C]DPN (μ-, κ- and δ- antagonist) and [18F]FDG (glucose metabolism) | Ratio method, BPND/VOI/n = 11 | Inter-ictal μ-OR was increased in temporal neocortex and reduced in amygdala ipsilateral to epileptogenic focus. Non-selective OR binding exhibited no alteration. FDG showed temporal lobe hypometabolism. | The variation in pattern of carfentanil and diprenorphine binding supports a differential regulation of OR subtypes in unilateral temporal lobe epilepsy. |
| Theodore et al., 1992 | Study role of combined μ-/κ-OR binding in temporal lobe epilepsy. | [18F]FcyF (μ- and κ- antagonist) and H2[15O]O (perfusion) | Invasive compartment model, VT /VOI/n = 14 | Non-significant increased combined μ-/κ-OR binding and significant hypoperfusion in temporal lobe ipsilateral to epileptogenic focus. | Suggest that κ-OR are not involved in temporal lobe epilepsy, and therefore no significant asymmetry in combined μ-/κ-OR binding unlike Mayberg et al., 1991 who used a selective μ-OR agonist. |
| Bartenstein et al., 1994 | Study role of non-selective OR binding in temporal lobe epilepsy before and after surgery. | [11C]DPN (μ-, κ- and δ- antagonist) and [18F]FDG (glucose metabolism) | Ratio method, BPND/VOI/n = 2 patients n = 8 controls | Post-operative there was a reduced diprenorphine binding in the ipsilateral lateral temporal cortex. | Finding is compatible with downregulation of OR in lateral temporal lobe after removal of the epileptic focus. |
| Madar et al., 1997 | Study role of μ-OR (carfentanil) versus δ-OR (naltrindole) binding in temporal lobe epilepsy. | [11C-Me]Nal (δ-antagonist), [11C]CAF (μ-agonist) and [18F]FDG (glucose metabolism) | Ratio method, BPND/VOI/n = 10 | Both μ- and δ-OR binding was increased in the temporal cortex: μ-OR confined to middle aspect of inferior cortex, whereas δ-OR seen in mid-inferior cortex to anterior aspect of middle and superior temporal cortex. Hypometabolism was more widespread than changes in binding to either OR type. | Increase in delta receptors suggests their anticonvulsant action, and the different regional pattern of receptor alterations suggest the distinct roles of different opioid-receptor subtypes in seizure phenomena. |
| Hammers et al., 2007 | Investigate OR availability following spontaneous temporal lobe seizures. | [11C]DPN (μ-, κ- and δ- antagonist) | Invasive SA, VT /SPM + VOI/n = 9 patients × 2 scans (post-ictal and interictal), n = 14 controls × 2 scans | Increase in OR availability in the ipsilateral temporal pole during the postictal scan compared to control and inter-ictal scan. There was no reduced OR binding during the post-ictal scan. | Suggest an association of changes in endogenous opioid transmission with spontaneous seizures in temporal lobe epilepsy. |
| Koepp et al., 1998 | To localize dynamic changes in opioid neurotransmission associated with partial (reading) seizures. | [11C]DPN (μ-, κ- and δ- antagonist) | Invasive SA, VT /SPM/n = 5 patients, n = 6 controls | On activation scans (reading-baseline) OR-binding significantly lower left parieto-temporo-occipital cortex in reading-epilepsy patients compared with controls. | Opioid-like substances are involved in the termination of reading-induced seizures. |
| Epilepsy: absence seizure | |||||
| Bartenstein et al., 1993 | Study role of OR binding during absence seizure (ictal). | [11C]DPN (μ-, κ- and δ- antagonist) and H2[15O]O (perfusion) | Invasive compartment model, simulation/VOI/n = 8 | Increased elimination of diprenorphine from association cortex. | Suggest that endogenous opioids are released in the association cortex at the time of serial absences. |
| Prevett et al., 1994 | Study role of OR binding in absence seizure inter-ictally compared to healthy controls. | [11C]DPN (μ-, κ- and δ- antagonist) | Invasive SA, VT /VOI + SPM/n = 8 patients, n = 8 controls | No statistical difference between patients and healthy controls in diprenorphine binding. | Does not support an overall abnormality of opioid transmission, but does not exclude imbalance of receptor subtypes. |
| Movement disorders/neurodegeneration | |||||
| Burn et al., 1995 | Study differences in OR-binding between akinetic-rigid syndromes. | [11C]DPN (μ-, κ- and δ- antagonist) | Radio method, BPND/VOI/n = 8 PD, n = 7 SND, n = 6 SRO, n = 8 controls | Parkinson's disease was associated with no significant difference in binding compared to controls. Patients with striatonigral degeneration showed a reduced OR binding in putamen. Patients with Steele–Richardson–Olszewski syndrome (SRO) demonstrated reduced OR binding in both putamen and caudate nucleus. In single subject analysis, only in some patients the SRO group showed a reliably reduced binding. | There are differences in the pattern of OR binding in the patient groups that may help to differentiate these akinetic-rigid syndromes in life. |
| Cohen et al., 1998, 1999 | Role of OR in MPTP-lesion model of parkinsonism in monkeys. | [18F]FcyF (μ- and κ-antagonist) | Ratio method, BPND/VOI/n = 4 bilateral lesioned, n = 4 unilateral lesioned, n = 9 controls | Reduced OR-binding in caudate, anterior putamen, thalamus and hypothalamus bilaterally in both uni and bilat lesioned animals. | Endogenous opiates contribute to the phenotype of Parkinson's disease. |
| Piccini et al., 1997 | To determine whether the OR system is involved in levodopa-induced dyskinesias in Parkinson's disease. | [11C]DPN (μ-, κ- and δ- antagonist) | Ratio method, SA, VT, IRF60/VOI + SPM/n = 13 patients, 6 with dyskinesia n = 10 controls | Significantly reduced striatal and thalamic opioid binding in dyskinetic, but not in non-dyskinetic, PD patients. SPM analysis disclosed additionally decreased cingulate and frontal cortical binding in dyskinetic patients. | Confirm that altered opioid transmission is part of the pathophysiology of levodopa-induced dyskinesias in Parkinson's disease. |
| Whone et al., 2004 | Investigate OR binding in DYT1 primary torsion dystonia. | [11C]DPN (μ-, κ- and δ- antagonist) | Invasive SA, VT /SPM/n = 7 patients, n = 15 controls | No difference in diprenorphine binding was found between DYT1 primary torsion dystonia patients and controls, and no correlation between the severity of dystonia and opioid binding. | Aberrant opioid transmission is unlikely to be present in DYT1 primary torsion dystonia. |
| von Spiczak et al., 2005 | Investigate OR binding in relation to sensory and motor symptoms in restless legs syndrome (RLS). | [11C]DPN (μ-, κ- and δ- antagonist)) | Invasive SA, VT /SPM/n = 15 patients, n = 12 controls | No differences in OR binding between patients and controls. Regional negative correlations between OR binding and motor symptoms (thalamus, amygdala, NC, ACC, insula and orbitofrontal cortex) and pain scores (orbitofrontal cortex and ACC). | Suggest a central nervous system involvement of opioids in the pathophysiology of RLS. Pain is an underlying problem in RLS patients and suggested that motor symptoms in RLS are secondary to sensory symptoms. |
| Weeks et al., 1997 | Opioid neuronal loss is involved in the degenerative process of Huntington's disease. | [11C]DPN (μ-, κ- and δ- antagonist) | Ratio method, SA/VOI + SPM/n = 5 HD, n = 9 controls | Decrease in caudate and putamen OR binding compared to controls. SPM revealed additional non-hypothesized changes in cingulate and frontal cortices and thalamic areas. | Confirm that altered opioid transmission is part of the pathophysiology early Huntington's disease. SPM analysis is a viable alternative to conventional VOI analysis. |
| Cohen et al., 2000b | Examine responses in OR binding to neurodegeneration in a lesion model of the visual system in monkeys. | [18F]FcyF (μ- and, κ-antagonist) | Invasive, compartment model, VT /VOI/n = 6 lesioned with (4) and without (2) optical tract involvement. Scanned after 2–3 years n = 9 controls | The animals with the optic tract lesion had significantly higher OR binding in the lateral cortex, cingulate gyrus and posterior putamen. In both lesion groups, OR binding was reduced in the medial cortex. | Reduced OR binding in medial cortex are axonal and transneuronal degeneration. Visual deprivation leads to extensive functional changes of neuronal circuitries involving the OR system. |
| Cohen et al., 1997 | Expected that OR avidity would be lower in patients with Alzheimer's disease than in normal controls. | [18F]FcyF (μ- and κ-antagonist) and H2[15O]O (perfusion) | Invasive, compartment model, VT /VOI/n = 12 patients, n = 12 controls | Global grey combined μ-/κ-OR-binding and global grey perfusion were found to be lower in the Alzheimer's patients compared to controls. A specific hypoperfusion was identified in the parietal cortex, but no significant regional changes in OR binding were found. | Neurodegeneration is the likely underlying process responsible for changes in combined μ-/κ-OR binding. |
| Pain: experimental, acute | |||||
| Zubieta et al., 2001 | Induced muscular (masseter) pain | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 20 × 2 scans (placebo and pain) | Sustained pain induced the regional release of endogenous opioids interacting with μ-OR in a number of cortical and subcortical brain regions. Associated with reductions in the sensory and affective ratings of the pain experience, with distinct neuroanatomical involvements. | Evidence for the role of the μ-OR in the neurotransmission of the individual experience of pain. |
| Bencherif et al., 2002 | Experimental pain induced by topical application of capsaicin. | [11C]CAF (μ-agonist) | Ratio method, BPND/SPM/n = 8 × 2 scans | A pain-related decrease in brain μ-OR binding was observed in the contralateral thalamus. | The supraspinal μ-OR system is activated by acute pain and may play a substantial role in pain processing. |
| Sprenger et al., 2006a | OR binding studied during tonic, heat pain. | [18F]FDPN (μ-, δ- and κ-antagonist) | Non-invasive Logan plot, BPND/SPM/n = 12 × 2 scans (in and out pain) | Reduction of diprenorphine binding in limbic and paralimbic brain areas including the rostral ACC and insula, related to heat pain. | Direct evidence for the involvement of rostral ACC in endogenous opioidergic inhibition of pain. |
| Zubieta et al., 2002 | Study of gender differences. Induced muscular (masseter) pain. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 14 female, n = 14 male. All × 2 scans (placebo and pain) | Men demonstrated larger magnitudes of μ-OR activation than women in the anterior thalamus, ventral basal ganglia and amygdala. Women showed a stronger μ-OR activation during pain in the nucleus accumbens. | Men and women (follicular phase) differ in the magnitude and direction of response of the μ-OR system in distinct brain nuclei. |
| Smith et al., 2006 | Examination of pain-related μ-OR neurotransmission during low and high estrogen states in women. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 10 females × 2 scans (low and high estrogen state), n = 8 males × 1 scan | The high-estrogen state was associated with regional increases in baseline μ-OR binding and greater pain-related activation of opioid neurotransmission. The latter did not differ from that obtained in males. During low-estrogen state, reduced opioid tone was seen in thalamus, NA and amygdala, which was associated with hyperalgesic responses. | Demonstrate a significant role of estrogen in modulating endogenous opioid neurotransmission and associated psychophysical responses to pain. |
| Zubieta et al., 2003a | Study of gene (COMT) polymorphism in relation to pain response and μ-OR activation. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 29. All × 2 scans (placebo and pain) | Met158 allele homozygotes for COMT polymorphism showed diminished μ-OR responses to pain and increased pain ratings compared to heterozygotes. Opposite effects were observed in val158 homozygotes. | COMT val158met polymorphism influences the human experience of pain and may underlie inter-individual differences in the adaptation and responses to pain. |
| Zubieta et al., 2005 | Analgetic placebo and μ-OR neurotransmission. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 14 × 3 scans (baseline, pain and pain + placebo) | Placebo-induced activation of μ-OR-mediated neurotransmission was observed in left dorsolateral frontal cortex, rostral ACC, left NA and right anterior insula, and was paralleled by lower pain ratings. | Demonstrate that cognitive factors are capable of modulating physical and emotional states through the site-specific activation of μ-OR signalling |
| Pain: chronic, pathological | |||||
| Jones et al., 1994 | Investigation of rheumatoid arthritis patients in and out of pain in relation to OR binding. | [11C]DPN (μ-, δ- and κ-antagonist) | Invasive compartment model, VT /VOI/n = 4 × 2 scans (in and out of pain) | Out of pain was related to a general increase in diprenorphine binding and region-specific increases in frontal, cingulate and temporal cortices and straight gyrus. | There are substantial increases in occupancy by endogenous opioid peptides during inflammatory pain. |
| Jones et al., 1999 | Study OR binding in patients with trigeminal neuralgia pain before and after thermocoagulation therapy. | [11C]DPN (μ-, δ- and κ-antagonist) | Invasive SA, VT /SPM/n = 6 × 2 scans (in and out of pain) | In pain condition compared to out of pain the regional OR binding was reduced in frontal, insular, perigenual, mid-cingulate and inferior parietal cortices, basal ganglia and thalamus bilaterally. | Suggest an increased occupancy by endogenous opioid peptides during trigeminal pain. |
| Jones et al., 2004 | Study OR binding in patients with central neuropathic pain. | [11C]DPN (μ-, δ- and κ-antagonist) | Invasive SA, VT /SPM/n = 4 patients, n = 4 controls | Less OR binding in a number of cortical and sub-cortical structures that are mostly, but not exclusively, within the medial pain system. | Demonstration of reduced OR-binding capacity in neurons within the human nociceptive system in patients with central neuropathic pain. This may be a key common factor resulting in undamped nociceptor activity within some of the structures that are predominantly within the medial nociceptive system. |
| Willoch et al., 2004 | Investigate OR binding in patients with central post-stroke pain. | [11C]DPN (μ-, δ- and κ-antagonist) | Ratio method, BPND + SA, IRF60/SPM + VOI/n = 5 patients, n = 12 controls | Independently of localization of lesion there was reduced diprenorphine binding in contralateral thalamus, parietal, secondary somatosensory, insular and lateral frontal cortices, and along the midline in anterior cingulate, posterior cingulate and midbrain grey matter. | A single lesion associated with a characteristic pattern of reduced OR binding within the neural circuitry processing pain. |
| Maarrawi et al., 2007 | Investigate the differences in OR binding between patients with central and peripheral neuropathic pain. | [11C]DPN (μ-, δ- and κ-antagonist) | Ratio method, BPND/SPM/n = 7 + 8 patients with peripheral neuropathic + central post-stroke pain, n = 15 controls | Patients with central post-stroke pain showed predominantly contralateral reductions in OR binding, whereas patients with peripheral neuropathic pain did not show any lateralized decrease in OR binding. | Difference in distribution of brain opioid system changes between peripheral neuropathic and central post-stroke pain suggest an opioid loss or inactivation in the central pain syndrome, and might explain their different sensitivity to opiates. |
| Sprenger et al., 2006b | Study of OR binding in patients with cluster headache, in bout but out of attack. | [11C]DPN (μ-, δ- and κ-antagonist) | Invasive SA, IRF60/SPM/n = 7 patients, n = 8 controls | Decreased overall OR binding in the pineal gland of cluster headache patients compared to controls. Opioid receptor availability in the hypothalamus and ACC depended on the duration of the headache disorder. | The pathophysiology of cluster headache may relate to opioidergic dysfunction in circuitries generating the biological clock. |
| von Spiczak et al., 2005 | Investigate OR binding in relation to sensory and motor symptoms in restless legs syndrome (RLS). | See above Movement disorder/Neurodegeneration. | |||
| Affective states | |||||
| Zubieta et al., 2003b | Dynamic changes in μ-OR binding related to induction of a state of sadness. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 14. All × 2 measurements (neutral and sad state) | Sustained sadness compared to neutral state showed increased μ-OR binding in ACC, pallidum, amygdala and inferior temporal cortex. Changes in μ-OR binding were correlated to increase in negative affect ratings. | The responses confirm the role of the μ-OR system in the physiological regulation of affective experiences in human. |
| Kennedy et al., 2006 | Affection: involvement of μ-OR neurotransmission in major depression and treatment response. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 14 patients, n = 14 controls. All × 2 measurements: sadness and neutral state | Differences in μ-OR availability between women with major depression and control women in neutral state and opposite responses in opioid neurotransmission during induced sadness. | The neurotransmission on μ-OR system, which is implicated in stress responses and emotional regulation, is altered in patients with major depression. |
| Liberzon et al., 2006 | Study of μ-OR after psychological trauma. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/SPM/n = 16 patients, n = 14 (trauma) + 15 (non-trauma) controls. | Trauma groups had lower μ-OR binding in amygdale, nucleus accumbens and dorsal frontal and insular cortices, but higher μ-OR binding in orbitofrontal cortex. Patients had reduced μ-OR in the ACC compared to both control groups. | There are general trauma-related responses and specific post-traumatic changes in the μ-OR system. |
| Addiction | |||||
| Zubieta et al., 1996 | Opioids are involved in the reinforcing actions of cocain. Study of cocain craving. | [11C]CAF (μ-agonist) | Ratio method, BPND/VOI/n = 10 patients × 2 scans, n = 7 controls | Mu opioid binding was increased in several brain regions of the cocaine addicts studied 1–4 days after last use of cocaine and persisted after 4 weeks. Binding was positively correlated with the severity of cocaine craving experienced at the time. | Demonstrate the involvement of the endogenous OR system in cocaine dependence and cocaine craving. |
| Gorelick et al., 2005 | Study μ-OR binding in cocaine abstinence and in relation to craving over an extended time period. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/VOI/n = 17 patients × 3 scans (1 day, 1 week and 12 weeks), n = 16 controls. | After 1 day of abstince μ-OR binding was increased in several areas of the FC and in ACC. FC, ACC and lateral temporal cortex. After 12 weeks of abstinence increased binding was only seen in anterior FC and ACC. Self-reported cocaine craving was associated with μ-OR in several brain regions until after 1 week of abstincence. (No reported craving at 12 weeks) | Mixed pattern of normalizing and persistent increased μ-OR binding suggests that there might be both state and trait relationships. |
| Scott et al., 2007b | Effects of nicotine from smoking cigarettes in humans on μ -opioidergic (and dopaminergic) neurotransmission. | [11C]CAF (μ-agonist) and [11C]raclopride (dopamine-D2 receptor antagonist) | Non-invasive Logan plot, BPND/SPM/n = 6 smokers × 2 measurements in one scan: before and during smoking, n = 6 non-smokers | Smokers have lower μ-OR binding during denicotinized cigarette condition compared to non-smokers in ACC, thalamus, ventral basal ganglia and amygdala. These reductions were reversed during smoking in thalamus, ventral basal ganglia and amygdale. A reduced binding was observed in parts of the ACC during smoking. Dopamine neurotransmission was activated in the ventral basal ganglia. | Smoking is related to changes in μ-OR availability and is paralleled by changes in dopamine neurotransmission. |
| Bencherif et al., 2004 | Study relationship between alcohol craving and μ-OR in alcohol-dependent subjects. | [11C]CAF (μ-agonist) | Ratio method, BPND/SPM/n = 8 patients, n = 8 controls | Alcohol-dependent subjects showed association with higher craving and lower μ-OR binding compared with control subjects in right dorsal lateral frontal cortex, the right anterior frontal cortex, and right parietal cortex. | There is a functional relationship between alcohol craving, mood and μ-OR binding in specific brain regions of recently abstinent, alcohol-dependent men. |
| Heinz et al., 2005 | Study of μ-OR changes after alcholism detoxification and in relation to craving | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/VOI + SPM/n = 25 patients (n = 12 rescanned after 5 weeks), n = 10 controls | After 1–3 weeks of abstinence μ-OR binding was increased in the ventral striatum compared to controls and remained elevated after 5 weeks. Higher μ-OR binding correlated positively with intensity of alcohol craving. | There is increased μ-OR binding in alcohol detoxified patients within a neural network that has been associated with drive states and drug craving. |
| Ingman et al., 2005 | Study of pharmacokinetics and μ-OR occupancy of nalmefene after single and repeated dosing over 7 days. | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/VOI/n = 12 × 3 scans, all before drug and 2 each at 3, 26, 50 or 74 h after single drug intake, and all after 7 days of repeated dosing. | High nalmefene occupancy (83–100%) persisted at 26 h after the dosings, and decline in the occupancy was clearly slower than the decline in the plasma concentration of nalmefene or metabolites. | The slow dissociation of drug from μ-OR suggest that a high receptor occupancy can be maintained when nalmefene is taken once daily. |
| Kling et al., 2000 | Investigation of methadone treatment in heroin addicts. | [18F]FcyF (μ- and κ-antagonist) | Invasive compartment model, VT/VOI/n = 14 patients, n = 14 controls | Combined μ-/κ-OR binding in thalamus, amygdala, caudate, anterior cingulate cortex and putamen was significantly reduced (19–32%). Methadone plasma levels correlated to receptor occupancy in caudate and putamen. | Lower levels of μ-/κ-OR binding may be related to receptor occupancy with methadone and that significant numbers of OR may be available to function in their normal physiological roles. |
| Melichar et al., 2005 | Study relationship between methadone dose and OR in heroin dependent patients | [11C]DPN (μ-, δ- and κ-antagonist) | Invasive SA, VT/VOI/n = 8 patients, n = 8 controls + animal studies | No difference in diprenorphine binding was found between the groups, with no relationship between methadone dose and occupancy either given chronically in humans or acutely in rats. | Suggest high efficacy of methadone at very low levels of OR occupancy. |
| Greenwald et al., 2003 | Study of heroin-dependence and buprenorphine treatment | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/VOI/n = 14 patients. (5 completed all 4 scans at 0, 2, 16 and 32 mg buprenorphine). | μ-OR availability decreases were negatively correlated with BUP plasma level and positively correlated with questionnaire-based opioid withdrawal symptoms. Over 90% receptor occupancy at doses of 32 mg buprenorphine. | Suggest that high-dose buprenorphine maintenance produces near-maximal μ-OR occupation and μ-OR availability correlates well with plasma levels. |
| Greenwald et al., 2007 | Study of buprenorphine duration of action | [11C]CAF (μ-agonist) | Non-invasive Logan plot, BPND/VOI/n = 10 × 4 scans (4, 28, 52 and 76 h after last buprenorphine dose) | Whole-brain μ-OR availability increased from 30%, 54%, 67% to 82% after 4, 28, 52 and 76 h, respectively, of last buprenorphine dose. μ-OR binding correlated with plasma concentration, withdrawal symptoms and hydromorphone blockade. | The μ-OR availability predicts changes in pharmacokinetic and pharmakodynamic measures, and ∼50–60% receptor occupancy of buprenorphine is required for adequate withdrawal symptom suppression. |
| Eating disorder | |||||
| Bencherif et al., 2005 | Study μ-OR in bulimia nervosa as the OR system has been implicated in feeding behaviour. | [11C]CAF (μ-agonist) | Ratio method, BPND/SPM/n = 8 patients, n = 8 controls | μ-OR binding in the left insular cortex was less in bulimic subjects than in controls and correlated negatively with recent fasting behaviour. | μ-OR binding in the insula may be important in the pathogenesis or maintenance of the self-perpetuating behavioural cycle of bulimic subjects. |
The consensus nomenclature for PET receptor binding has been used throughout the table (Innis et al., 2007).
Before calculating a voxel-wise statistical analysis of whole brain, spatial smoothing is routinely applied to reduce the effect of interindividual anatomic variability and to improve statistical power. The spatial smoothing affects the statistical maps (t-maps) and may result in spatial inaccuracy, which can be corrected for (Reimold et al., 2006). These authors suggested use of the mask contrast images (in SPM) to avoid the artifacts and improve the spatial precision. MR-based correction of PET gray matter distribution permits an improved determination of the true radioactivity concentration and reduces partial volume effect mixed tissue sampling errors (Meltzer et al., 1996). The volume of abnormal brains may be different to that of brains of healthy individuals and thus require a correction for partial volume effects. One should also be aware of the possibility that different results between studies may be dependent on the individual scanner systems used, which undoubtedly have different sensitivities and resolutions.
Opioid receptor studies in humans
The first successful PET study with an opioid receptor ligand was performed in 1985 using the μ-OR agonist [11C]CAF (Frost et al., 1985). Two years later, [11C]DPN was introduced as a non-selective OR-antagonist for PET (Lever et al., 1987). Studies of the brain opioid receptor system using PET have been applied for neurochemical mapping and for studies of pain, emotion, drug addiction, movement disorders, neurodegeneration and epilepsy. Based on the last decade of research, [11C]DPN, [11C]CAF and [18F]FCyF are the most widely applied tracers, followed by [18F]FDPN and the δ-OR-selective ligand [11C]MeNTl (Fig. 1). Although being one of the two PET tracers with a pronounced subclass selectivity the quantification of [11C]MeNTl binding is difficult due to a near irreversible binding (Smith et al., 1999).
Most studies use static depiction of the receptor binding in repeated measurements in the same individuals and/or in comparison to healthy volunteers. In later years, dynamic changes in OR availability (opioid activation) have been measured between different states. Table 1 summarizes the clinical studies on PET-imaging of ORs in the CNS reported so far.
Neurochemical mapping
PET offers the possibility to study in vivo anatomy and function of neurochemical systems of the whole brain with information on the regional and the inter-individual characteristics. This makes studies of the receptor systems in healthy individuals appealing. Studies of the opioidergic mechanisms in the brain have been extensively performed in animal models. Although several interspecies differences among mammals such as mouse, rat, guinea pig, rabbit and human have been described for distinct brain areas (Maurer et al., 1983; Zagon et al., 1990), it has been very limited research on the anatomy and function of the opioidergic system of the human brain. An extrapolation to humans of a finding in other mammals may not always be applicable. For example in the rat, which probably represents the most widely used laboratory animal and where the distribution of the three principal opioid receptors has been extensively studied in the brain (e.g. Mansour et al., 1995), the cerebellum typically is devoid of opioid receptors. PET was therefore used in a detailed study of the cerebellum using [11C]DPN and individual MRI in parallel with in vitro receptor autoradiography and mRNA expression in post-mortem human and rat brain (Schadrack et al., 1999). [11C]DPN in PET demonstrated low to intermediate OR binding in the human cerebellum. The human in vitro data showed a differential subtype pattern with pronounced levels of μ-OR and lower levels of κ-OR, but no detectable δ-OR. The specific binding corresponded to the level of the respective OR-expression as measured by quantification of mRNA.
Jones et al. (1991) described the in vivo distribution of [11C]DPN binding in man in relation to the medial (functional subdivision of brain areas mediating the affective-motivational components in pain perception) and lateral pain systems (brain areas mediating sensory-discriminative components in pain perception). In general, high levels of opioid receptor binding was seen in the cortical projections of the medial pain system (cingulate and frontal cortex) and a focal lower binding was observed in the primary sensorimotor cortex, a part of the lateral pain system. A more detailed and complementary study using [18F]FDPN PET and coregistered individual MRI revealed a high opioid receptor binding in the secondary sensory cortex and the posterior part of insula, both parts of the lateral pain system (Baumgartner et al., 2006). The lateral pain system comprises apparently areas with high and low opioid receptor availability. Baumgartner et al. (2006) added in the same study a factor analysis of inter-individual variability in regional OR binding and identified four factors explaining 88% of the variance. Each factor consisted of different sets of brain regions and the authors speculated that each factor may display functional entities.
A detailed study of the OR binding in the human cingulate gyrus combining [11C]DPN PET with individual MRI supported of high binding levels (Vogt et al., 1995). It was also shown that a striking heterogeneity of opioid receptor binding exists on the medial cortical surface with a higher availability in the anterior as compared to midcingulate cortex. The above-described approaches do not only describe a pattern of regional binding, but also suggest a reason for some of the actions of opiate compounds and may provide clues into the functional subdivisions and entities of different brain regions. A constraint of the above studies is the use of non-selective antagonists as PET-ligands, and therefore cannot differentiate between the regional distribution of μ-, κ- and δ-ORs. The differences or lack of differences in receptor binding, Bavail, are a sum of binding to two or more OR subtypes, e.g. increased Bavail for μ-OR and decreased Bavail for δ-OR may result in a net of no differences.
In the first human opioid PET study using the μ-selective agonist [11C]CAF, Frost et al. (1985) described high concentrations of ORs in the basal ganglia and thalamus, intermediate concentrations in the frontal and parietal cerebral cortex, and low concentrations in the cerebellum and occipital cortex. The selective δ-OR antagonist [11C-methyl]naltrindole (11C-MeNal) showed a high binding availability in the frontal cortex and putamen, lower levels in thalamus and an absence of specific binding in the human cerebellum (Smith et al., 1999). Recently, a detailed evaluation of [11C]GR103545 as a PET tracer for κ-OR in non-human primates was reported (Talbot et al., 2005). These investigators found a regional pattern of specific binding of [11C]GR103545 in baboons which agrees with the regional distribution of κ-ORs in humans primates (Hiller and Fan, 1996) with a high binding in cingulate cortex, striatum, frontal cortex, temporal cortex and parietal cortex; intermediate levels were found in thalamus and medial temporal lobe, and low levels in brainstem and occipital cortex. No human studies have been published so far with a PET-tracer with high selectivity for the κ-OR.
Both age and gender may be important variables to consider in the interpretation of investigations of human function in which the opioid system plays a role. μ-OR binding has been shown to increase with age in neocortical areas and caudate putamen, while women show a higher μ-OR binding than that found in men in the thalamus, amygdala and cerebellum (Zubieta et al., 1999). However, in vivo μ-OR binding in thalamus and amygdala declined in postmenopausal women to levels below those of men. The findings of Cohen et al. (2000a) using the μ-/κ-antagonist [18F]FCyF showed in accordance a lower thalamic OR binding in postmenopausal women. These changes observed in postmenopausal women suggest that the hormonal milieu representative of healthy adults is capable of modulating the OR neurochemical system, at least in some brain regions. The sex differences in μ-OR binding during the female reproductive years may reflect early, gender-specific ontogenetic factors. Another study has also shown that there are no significant categorical differences in μ-OR binding between phases of the menstrual cycle (Smith et al., 1998). However, when the receptor-binding data were compared with circulating levels of gonadal steroids separately for each phase of the cycle, several significant correlations emerged. The circulating levels of estradiol in the follicular phase, but not luteal phase, influenced OR binding in amygdala and hypothalamus, presumably reflecting an increased central opioid tone. The μ-OR binding in the amygdala had a modulatory effect on GnRH release pulsatility. This finding is interesting, as the amygdala is associated with emotional responses and memory, providing a neurochemical link between reproductive and emotional function.
There is a strong interest in the discovery of genes that are related to individual differences in the processing of endogenously produced opioid receptor ligands and receptors. The only study so far investigating the association between genotype and μ-OR phenotype was directed towards the polymorphism of catechol-O-methyl-transferase (COMT), the enzyme responsible for the metabolic conversion of catecholamines (dopaminergic and adrenergic/noradrenergic neurotransmission). The efficiency of COMT is dependent on the presence of the combination of valine (val) or methionine (met) in the active site (amino acid 157–158). The three combinations val-val, val-met or met-met allowed identification of an effect of the COMT val158met genotype on baseline binding of the μ-OR selective agonist [11C]CAF in the thalamus and ventral basal ganglia (Zubieta et al., 2003a). The met/met group showed a regional higher μ-receptor binding at baseline in the basal ganglia compared to the met/val group. A lower μ-receptor binding was seen in thalamus, nucleus accumbens and amygdala in the val/val group as compared to the met/val group. The differences in binding are likely secondary compensatory changes within the OR system in response to the COMT enzyme activity. Functional relevancies of genotype to pain perception are discussed below (see ‘Experimental, Acute Pain’ section).
Epilepsy
The first clinical PET study with an OR ligand focussed on epilepsy was reported by Frost et al. (1988), demonstrating an increased μ-OR binding of [11C]CAF in temporal lobe epilepsy. An inverse relationship to the well-known interictal hypometabolism in the epileptogenic temporal lobe was shown. In contrast, no such asymmetry was detectable using the non-selective OR ligand [11C]DPN (Mayberg et al., 1991), indicating that the subtypes (μ, κ and/or δ) are regulated differently or present different availability to the PET ligand in the inter-ictal period. Similarly, there was no overall asymmetry of binding of [18F]FCyF (μ- and κ-OR) in patients with temporal lobe epilepsy (Theodore et al., 1992). Studies using the δ-OR ligand [11C-Me]Nal (Madar et al., 1996, 1997) showed increased δ-OR availability in the ipsilateral temporal lobe, but with a different regional pattern than the μ-OR binding of [11C]CAF. The latter was confined to the middle aspect of the inferior temporal cortex, whereas binding of δ-OR increased in the mid-inferior and anterior aspect of the middle and superior temporal cortex. Both δ- and μ-OR endogenous ligands are thought to play a role in the tonic anticonvulsive mechanism that limits the spread of electrical activity from an epileptogenic focus and the above studies indirectly support such a hypothesis. However, only quantifying the receptor binding at a single time point, as all the above studies do, does not reveal any dynamic changes in neurotransmission. In a longitudinal study, Bartenstein et al. (1994) compared [11C]DPN binding before and after hippocampectomy, and a downregulation of ORs after removal of the epileptic focus was found. No further studies have been conducted to confirm this study, which involved only two patients. OR measurements during a focal epileptic seizure have been performed in patients with reading-induced seizures, using [11C]DPN. During a seizure OR binding was reduced in the left parieto-temporo-occipital cortex, which was related to apparent release of endogenous opioid peptides (Koepp et al., 1998). Although the mechanisms are not clear, this finding supports the hypothesis that opioid substances may be involved in the termination of seizures. A dynamic study with [11C]DPN suggested that endogenous opioids are released in the association cortex during absence seizures (Bartenstein et al., 1993). A follow-up study to identify potential changes interictally did not show any overall differences in [11C]DPN binding between control subjects and patients with childhood and juvenile absence epilepsy (Prevett et al., 1994), suggesting there is no overall abnormality of ORs in this condition. A very recent study investigated the OR binding ([11C]DPN) in the post-ictal period (1.5–21 h) and revealed an increased overall OR availability in the temporal lobe ipsilateral to the ictal focus (Hammers et al., 2007). The OR binding returned to normal levels in the interictal period (6–56 days). In summary, there is an increased availability of μ- and δ-OR at the side of ictus in temporal lobe epilepsy interictally, but there is no identifiable asymmetry seen when using ligands binding to the κ-OR through non-selective μ/δ/κ or μ/κ PET-ligands. Studies with a κ-OR-selective tracer to clearly identify potential changes to the availability of this receptor are still to be performed.
The focal displacement of OR-ligand binding during a seizure in reading-induced seizures (Koepp et al., 1998) and absences (Bartenstein et al., 1993) support the prevailing opinion that endogenous opioids are released following partial and generalized tonic–clonic seizures (Bajorek et al., 1986). It has also been suggested that endogenous opioids contribute to the postictal rise in seizure threshold (Hammers et al., 2007). If this is true, then there must be a significant upregulation of available ORs to explain the increased overall μ/δ/κ-OR binding in the post-ictal period (Hammers et al., 2007) and increased binding to μ- and δ-ORs interictally (Mayberg et al., 1991; Madar et al., 1997; Frost, 1998). An alternative explanation might be that there is a reduced inter-/post-ictal endogenous opioid tone following the phasic release during an ictus. In addition to OR-subtype selectivity, the pharmacological properties of the ligands (agonist, antagonist) may influence the different results of the studies, which all use a limited number of patients (n = 2–14). In general, the opioids are believed to exert anticonvulsive effects, but proconvulsive effects are still debated within specific brain regions (e.g. disinhibition in hippocampus) or as a result of changed cellular function after repetitive opioid release (Tortella, 1988).
Movement disorders/neurodegenerative diseases
Movement disorders
The basal ganglia consist of four main nuclei (the striatum, the globus pallidus, the subthalamic nucleus and the substantia nigra), which provide a major link between the thalamus and the cerebral cortex. These nuclei receive multimodal input from all sensory systems, providing a gating station for continuous sensory information, including pain. Dysfunctions of the basal ganglia result in movement disorders, indicating an important role in motor control. Endogenous opioid peptides are found in high concentrations in the basal ganglia and are thought to play a role in the regulation of motor function (Haber and Watson, 1985). Exogenously administered opioid agonists are associated with an increase in motor activity (Austin and Kalivas, 1990). Abnormal opioid transmission has also been implicated in several movement disorders, including levodopa-induced dyskinesias in Parkinsons's disease, chorea in Huntington's disease, neuroleptic-induced tardive dyskinesia/dystonia and tics in Tourette's syndrome (Brooks et al., 2000).
The clinical differentiation of Parkinson's disease from the striatonigral degeneration type of multiple system atrophy and Steele–Richardson–Olszewski (SRO)-syndrome is challenging. A study concluded that there are differences in the pattern of OR binding in the striatum of Parkinson's disease, striatonigral degeneration and SRO-syndrome patients, as determined by [11C]DPN (Burn et al., 1995). The different binding patterns between the patient groups indicate a potential of opioid-PET to provide a differential diagnosis of these akinetic-rigid syndromes. However, in the single-subject analysis, it was only some patients in the SRO group that showed a reliable difference and revealed a limited potential clinical application. Patients with Parkinson's disease constituted one of the groups that did not show any significant changes in OR binding compared to the healthy controls. These results therefore contradicted those of Cohen et al. (1998, 1999), who used a lesion model in rhesus monkeys that damaged the nigrostriatal system (injecting MPTP intravenously and intraarterially) in order to monitor changes in the OR binding at an early preclinical stage of Parkinson's disease. The availability of μ–κ opioid receptors was shown by means of [18F]FCyF binding, to be reduced by 30–35% in the basal ganglia, thalamus and amygdala. One interpretation might be that, regarding ORs, the MPTP lesion model is not relevant for Parkinson's disease. However, different phenotypes of Parkinson's disease can be reflected at the level of OR transmitters. A significantly reduced binding of [11C]DPN in striatal, thalamic and cingulate cortical regions and increased binding in frontal cortex were found in Parkinson's patients with levodopa-induced dyskinesia but not in non-dyskinetic Parkinson's patients (Piccini et al., 1997). These findings suggest that Parkinson's disease with levodopa-induced dyskinesia is associated with a derangement of basal ganglia opioid transmission that may contribute to the overactivity of basal ganglia-frontal projections. It would be tempting to assign the changes in OR binding to a common mechanism underlying all disorders of dyskinesia. The hypothesis of a common mechanism in dyskinesia, however, was not supported by the finding that primary torsion dystonia (inherited type DYT1) was not associated with any abnormal binding of the non-selective antagonist opioid [11C]DPN compared to healthy controls (Whone et al., 2004).
The pathophysiology of primary restless legs syndrome remains unknown. Dopamine treatment is symptomatically effective (Hening et al., 1999), but imaging studies have not given conclusive results of any consistent functional changes in the nigrostriatal dopaminergic system. A possible role of opioidergic dysfunction in the pathophysiology of this syndrome was investigated using [11C]DPN (von Spiczak et al., 2005) and a relation between symptoms and receptor binding indicated an involvement of opioids. The opioid system may be involved both as part of the aberrant pain perception of the restless legs syndrome and as a disturbed balance of dopamine-opioid inputs to brain regions involved in motor actions.
In the degeneration due to Huntington's disease (HD) an involvement of the opioid system is indicated by a reduced concentration of enkephalin and dynorphin in pallidal, striatal and nigral regions, in addition to a loss of ORs in the basal ganglia (Seizinger et al., 1986; Gulya, 1990). A [11C]DPN-PET study supports these findings and demonstrated a decreased OR binding in the caudate and putamen in HD (Weeks et al., 1997). Atrophy of the caudate and putamen is well known, and number of cells and reduction in volume (neurodegeneration) is a possible explanation for the reduced binding. An exploratory analytical approach in the same study using SPM detected additional OR binding increases in brain areas that were not predicted a priori (cingulate, thalamus and frontal cortex). The involvement of these brain areas is not unlikely, but the non-selective binding of [11C]DPN to different OR subtypes with the resulting lack of information on the contribution of individual receptor subtypes to tracer uptake makes further interpretation difficult.
In an experimental lesion-model of the optical tract in monkeys, changes in the binding of [18F]FCyF (μ- and κ-OR) were studied 2–3 years after performing the lesions (Cohen et al., 2000b). The animals underwent either callosal transection only (split), or callosal and unilateral optic tract transection (tract and split). Cohen et al. (2000b) reported a reduced [18F]FCyF binding in the medial cortex for both groups, suggesting an axonal and transneuronal degeneration. In the tract and split group there was, additionally, a significant bilateral increase in OR binding in the lateral cortex, cingulate and posterior putamen. The increased binding reflects functional changes within the OR system, in response to the visual deprivation, and demonstrates adaptive mechanisms that may take place in neurodegenerative disorders, e.g. non-hypothesized increased binding in the thalamus as well as cingulate and frontal cortices in patients with HD (Weeks et al., 1997).
Dementia
An involvement of the OR system in Alzheimer's disease was shown in a study with [18F]FCyF (μ- and κ-OR) revealing a lower global binding in Alzheimer's cases, but failed to demonstrate regional specificity in the ligand binding, which was interpreted as a general neurodegeneration and no specific involvement of the OR-system (Cohen et al., 1997).
Pain
Experimental, acute pain
A decrease in OR availability related to acute pain has so far been demonstrated by three independent research groups, each using different experimental paradigms (muscle, capsaicin and heat pain) (Zubieta et al., 2001; Bencherif et al., 2002; Sprenger et al., 2006a). Changes in OR binding in the insular cortex, the amygdala, the thalamus and the nucleus accumbens were confirmed using both the μ-OR selective ligand [11C]CAF (Zubieta et al., 2001; Bencherif et al., 2002) and the non-selective ligand [11C]DPN (Sprenger et al., 2006a). Unlike the two first studies, which activated the dorsal part of the anterior cingulate cortex (ACC), the study of Sprenger et al. included an opioid activation of the rostral/perigenual part of the ACC. The rostral part of the ACC is frequently activated in modulation of pain and analgesia (Willoch et al., 2003). The experimental setup of stimulus/activation and the choice of tracer may explain the divergency. Interestingly, the effect of placebo on μ-OR-mediated neurotransmission was seen in the perigenual part of the ACC together with significant effects in the dorsolateral frontal cortex, anterior insula and nucleus accumbens (Zubieta et al., 2005). The study of opioid involvement in the effect of placebo demonstrates that cognitive factors are capable of modulating physical and emotional states through a site-specific activation of the μ-signalling in the brain. It has also been shown that the activation of the μ-OR system was associated with reductions in the sensory and affective ratings of the pain experience, with distinct neuroanatomical involvements (Zubieta et al., 2001; Bencherif et al., 2002). These data demonstrate the central role of μ-ORs and their endogenous ligands in the regulation of sensory and affective components of the pain experience.
As mentioned earlier, men and women have region-specific differences in OR binding at rest, but also differ in their OR-mediated responses in distinct brain areas at matched levels of perceived pain intensity (Zubieta et al., 2002). Recently, the specific effect of estradiol on opioid neurotransmission in women was investigated (Smith et al., 2006). The authors concluded that a low-estradiol, low-progesterone condition is associated with a pain vulnerability state by a reduction in endogenous opioid system function, possibly through β-endorphin-mediated mechanisms. Genotype influences the opioid neurotransmission to pain stressors as well. As referred to above in section ‘Neurochemical mapping’ heterozygote and homozygote individuals have significant different regional OR binding at baseline (Zubieta et al., 2003a). In the same study, it was shown that the COMT polymorphism (met/met, met/val or val/val) influenced the μ-opioid responses to pain stimulation. The met/met individuals had lower magnitudes of μ-opioid system activation and higher pain ratings, and opposite, the val/val individuals had the higher magnitude of opioid activation and lower pain ratings.
Chronic, pathological pain
Opioid analgesics are used extensively in the management of pain, and the endogenous opioid receptor system takes part in the pain processing in the CNS. The first study investigating changes to the OR-availability in relation to pain was published by Jones et al. (1994). Patients with rheumatoid arthritis showed, during a period of inflammatory pain, lower binding compared to a pain-free period, particularly in orbitofrontal cortex, anterior insula, amygdala and anterior putamen, but also in periventricular grey, thalamus, and temporal, frontal and anterior cingulate cortices. These findings were interpreted as an increased occupancy by endogenous opioid peptides during inflammatory pain (Jones et al., 1994). The change in receptor binding did not take place acutely, but after a substantial interval between first and second PET scan. Therefore, the changes observed could just as well have been due to slower regulating mechanisms, e.g. down-regulation.
Studies on central neuropathic pain (Willoch et al., 1999, 2004; Jones et al., 2004) demonstrated reductions in [11C]DPN binding predominantly within the medial pain system, but also the lateral pain system. These changes in binding could not be accounted for by the cerebral lesions shown by CT or MRI, and may instead reflect an undamped nociceptor activity within the pain-processing network. The information of a single focal lesion inducing widespread changes may represent an important step towards an understanding of the pathophysiology of central pain (Casey, 2004; Willloch et al., 2004). Maarrawi et al. (2007) demonstrated that patients with central neuropathic pain have predominantly contralateral reductions in ORs, whereas patients with peripheral neuropathic pain do not show lateralized OR binding. The study supports the idea that pain syndromes may have, if not archetypical binding patterns, then at least different neurochemical mechanisms. The difference between the patients with central and peripheral neuropathic pain is reflected in their different sensitivity to opioid analgesia. Maladaptive plasticity within the opioidergic neurotransmission system may be a part of the pathology of central pain. An increase in the occurrence of direct excitatory effects in response to chronic increased opioid neuronal tonus could contribute to the appearance of paradoxical opioid-mediated pain, hyperalgesia and allodynia (Varga et al., 2003).
In a recent study, it was demonstrated that a focal decrease in OR binding occurred in the pineal gland in patients with cluster headache during a headache period, but not during an acute attack (Sprenger et al., 2006b). The OR availability in the hypothalamus and cingulate cortex was found to be dependent on the duration of the patient's cluster headache. Overall, the findings indicate that opioidergic mechanisms are involved in the pathophysiology of cluster headache. Interestingly, the changes in opioidergic neurotransmission had another pattern than in neuropathic and inflammatory pain. This may imply that a different regulation/dysregulation of opioidergic signalling may take place according to each specific pain disease, and that there is not merely an unspecific recruitment of the opioid system within the pain-processing network in all pain pictures.
These PET studies confirm the central role of the OR system in pain and analgesia and show that there are specific differences due to genetic variation and gender, and the participation of the OR system in the processing and perception of and coping with pain may be dependent on individual constitution. Finally, the OR system does not seem to respond with a common neurochemical activation pattern to all kinds of pain, but may express or take part in the adaption and/or pathophysiology of pain specific for each pain disease/entity.
Affective states
It has been suggested that activation of the μ-OR has a suppressive effect on emotional reactivity, with the opposite effect being found for the δ-OR (Filliol et al., 2000). This notion is in accordance with an involvement of the μ-OR-mediated neurotransmission in the suppression of the affective qualities of a pain stimulus. Using a similar scanning paradigm as in the studies of acute experimental pain, the effect of sustained sadness versus neutral emotional states on [11C]CAF binding has been investigated (Zubieta et al., 2003b). The self-induction of a sustained sadness state was associated with significant reductions in μ-OR-mediated neurotransmission (increased OR availability) in the rostral ACC, ventral pallidum, amygdala and inferior temporal cortex. These brain areas are part of the neural circuits representing and integrating emotional information. A more recent study demonstrated that opioid neurotransmission during sadness was altered in patients with major depression disorder compared to healthy controls (Kennedy et al., 2006). Larger reductions in OR binding during sadness were obtained in the patient group in the anterior insula, thalamus, ventral basal ganglia and amygdala, and larger increases in OR binding was observed in the control group in the ACC, ventral basal ganglia, hypothalamus and amygdala.
In a study of psychological trauma, war veterans exposed to trauma both with and without posttraumatic stress disorder had lower OR binding in the insula, ventral basal ganglia, dorsal frontal cortex and amygdala as well as increased binding in the orbitofrontal cortex, compared to healthy controls. This response may have a general adaptive role in trauma with changes in endogenous opioid peptide release and a subsequent up- or downregulation of the OR. The group with posttraumatic stress disorder had additionally reduced OR binding in the ACC compared to the other groups, which may represent specific changes associated with the stress disorder.
Eating disorders
Disorders related to eating behaviour include bulimia nervosa. This is characterized by a behavioural cycle characterized in restricted food consumption, bingeing and vomiting, thus sharing phenomenological similarity with the addiction disorders. The sole study on this topic carried out so far demonstrated a relative decrease in μ-OR binding of [11C]CAF in the left temporoinsular cortex, and the changes in binding correlated inversely with the frequency of fasting behaviour in the prior month (Bencherif et al., 2005). The insula is the primary part of the cortex processing gustatory sensory information (Frey and Petrides, 1999) and anticipation and reward of eating, and is most consistently activated in anxiety neuroimaging studies (Malizia, 1999).
Addiction
Stimulation of the μ-OR is mainly responsible for the reinforcing effects of opioid drugs, although δ-ORs may also contribute (Self and Stein, 1992). The κ-OR is also involved in the responses to addictive drugs, particularly cocaine, but also to opiates (for recent reviews see Prisinzano et al., 2005; Henriksen et al., 2006). Different drugs of abuse stimulate dopamine release in the ventral striatum, which includes the nucleus accumbens. Striatal dopamine release is stimulated by μ-OR activation and inhibited by striatal κ-OR activation. It has been hypothesized the latter may thus represent a homeostatic mechanism that limits drug-induced dopamine release in the ventral striatum (Prisinzano et al., 2005 and references cited therein). An elevated κ-OR-mediated signalling may persist during the phase of withdrawal from the drug and thus contribute to dysphoric mood states and thus increase the relapse risk. In vivo brain imaging with κ-OR-selective radioligands will allow the assessment of the opioidergic system in drug-dependent humans, to examine the psychological correlates of its functional state, and to develop new strategies to target drug effects and alleviate drug addiction.
An involvement of the endogenous opioid system after cocaine use was shown in subjects with current cocaine abuse/dependence (Zubieta et al., 1996; Gorelick et al., 2005). After 1–4 days of cocaine abstinence, μ-OR binding of [11C]CAF was increased in several brain regions, including frontal cortex, lateral temporal cortex and ACC. The increased binding correlated positively with self-reported cocaine craving. Twelve weeks after abstinence, the binding remained increased in the anterior frontal and anterior cingulate cortex, but returned to normal in the other brain regions. The mixed pattern suggests that there might be both state- and trait mechanisms, the state-dependent changes reflected in OR-binding returning to normal levels, and apparent trait-dependent OR-binding differences identified in the ACC. It would be interesting to examine the relationship between the trait-dependent, elevated μ-OR binding in the ACC and long-term clinical phenomena, such as vulnerability to craving and relapse.
Some of the effects of tobacco consumption are mediated through nicotine, which activates opioid neurotransmission (Scott et al., 2007b). At baseline, cigarette smokers had a reduced μ-OR binding in the ACC, thalamus, ventral basal ganglia and amygdala, which were (except in the ACC) reversed to normal during smoking. The observed reduced binding in the de-nicotined state was unexpected, but may represent a state of stress-related increased opioid tonus.
Several studies provide support for an association between alcohol dependence and CNS dopaminergic function (for reviews see: Noble, 1996; Soderpalm et al., 2000) as well as opioidergic function (Herz, 1997). PET studies of OR binding have shown very small group differences between alcoholic patients and healthy controls (Bencherif et al., 2004; Heinz et al., 2005). Heinz et al. (2005) identified an increased availability of μ-ORs in the ventral striatum (including the nucleus accumbens), in alcoholic patients (compared with healthy controls) after 1–3 weeks of abstinence, which persisted 5 weeks later. Interestingly, alcohol craving correlated positively with μ-OR-binding levels in striatum and frontal cortex. In another study, Bencherif et al. (2004) also found a positive correlation between craving and μ-OR binding in lateral frontal cortices 4 days after abstinence. We have identified slightly elevated cortical binding of the non-selective opioid receptor ligand [11C]DPN in alcoholics while still on alcohol, and the binding levels decreased over the time course of 4 weeks of abstinence (Willoch et al., unpublished data). All these studies are consistent with an influence of alcohol on OR status.
Receptor occupancy
The PET ligands and administered drugs can compete for the same binding site on the receptors. The competitional binding makes it possible to measure the degree of receptor occupancy of a given drug. Nalmefene is a non-selective OR antagonist with a stronger binding to δ-OR than that of naltrexone (DeHaven-Hudkins et al., 1990) and with a longer plasma half-life (Dixon et al., 1987). Because of its pharmacological properties, nalmefene has been proposed to offer an advantage in the treatment of alcoholism. Recently, a study was undertaken to determine the receptor occupancy of nalmefene at the μ-OR in the brain in healthy volunteers using the μ-agonist [11C]CAF (Ingman et al., 2005). Both single and repeated nalmefene oral dosing led to a very high μ-OR occupancy and 50 h after administration, the occupancy was still ∼50% while plasma concentrations were negligible. This discrepancy is probably due to a slow dissociation rate of nalmefene from μ-ORs. These results support an earlier study of receptor occupancy of a single intravenous bolus applying a simple dual-detector system and [11C]CAF as ligand (Kim et al., 1997).
Abuse of μ-opioids is increasingly manifested as a major health issue due to the large potential for a strong physical addiction developed to this drug. From early work that led to the development of methadone maintenance treatment for heroin addiction in the 1960s, it is known that μ-selective agonists with long-acting pharmacokinetics such as methadone or partial agonists (buprenorphine) are the most effective treatments for this disorder (Dole et al., 1966; Kreek et al., 2002). A successful pharmacotherapeutic treatment is dependent on a sustained and steady effect on the ORs and requires a tailored dose regimen. The use of PET molecular imaging with opioid-selective radioligands will be important for evaluating substitution therapy regimens and developing improved medications by correlating the dose and efficacy of the pharmacological agent with the resulting receptor occupancy (Kling et al., 2000; Zubieta et al., 2000; Greenwald et al., 2003). Methadone is a potent μ-selective agonist (Quock et al., 1999) and a study of its receptor occupancy when given to heroin addicts was performed using [18F]FCyF (μ- and κ-OR antagonist). Relative to healthy non-treated controls a reduction in receptor availability of 19–32% was found. The degree of receptor occupancy correlated with methadone plasma levels (Kling et al., 2000). In contrast, no difference in [11C]DPN (μ-, δ- and κ-antagonist) binding was seen in a study between opioid-dependent subjects on methadone compared to drug-free healthy volunteers (Melichar et al., 2005). The lack of change in binding was verified in an animal study. The high efficacy of methadone expressed at a low fraction of receptor occupancy, was suggested by Melichar et al. as the most likely explanation for the missing displacement of PET ligand binding. Some effects must be related to the dissimilar properties of the two tracers used, and to what degree these are displaced from the OR. Hume et al. (2007) studied the receptor occupancy at clinical doses of oxycodone (μ- and κ-receptor agonist), morphine (μ-receptor agonist) and buprenorphine (partial agonist at the μ-receptor and antagonist at the δ- and κ-receptors) in rats. In vivo binding of [11C]DPN was not significantly reduced after treatment with the full agonists, but was reduced by ∼90% by buprenorphine. Thus the study supports the minimal competition between highly potent agonists and [11C]DPN.
Buprenorphine does effectively displace the μ-agonist tracer [11C]CAF (Greenwald et al., 2003, 2007). Up to 90% receptor occupancy has been measured at stabilized clinical doses of buprenorphine. The latter study (Greenwald et al., 2007) investigated the duration of action and receptor occupancy of buprenorphine in relation to withdrawal symptoms; an occupancy of 50–60% of the receptors was required for adequate suppression of withdrawal symptoms.
Conclusion and perspectives
Imaging of opioid receptors in the CNS has contributed to delineate the in vivo neurochemistry of healthy humans. Changes in regional OR function have been identified in epilepsy, movement disorders, neurodegeneration, pain and addiction and the neurobiological correlates of affective and eating disorders have been disclosed. Furthermore, OR imaging has proven valuable in drug administration and dosage studies.
The field of non-invasive imaging is facing continued efforts in the design of subclass selective tracers with faster binding kinetics, as well as enhanced emphasis on combining the knowledge from chemistry and pharmacology subdisciplines in the design of better imaging protocols in order to achieve more accurate determinations of the imaging data. In general, PET data are available only to the few centres having access to these techniques. The scientific community would certainly profit not only from a detailed description of the regional binding profiles of more selective OR ligands, but also the presentation of such data in available databases as presently done for fMRI (Barinaga, 2003).
Apparently, the tracer properties in terms of OR subclass selectivity and agonistic/antagonistic character dominate the information that can be drawn from in vivo studies. Currently, only two of the ligands available for application in humans have pronounced selectivity for a single subclass of OR; the δ-OR-selective [11C-Me]naltrindole and the μ-OR-selective [11C]carfentanil of which only the latter has been demonstrated to be applicable for quantitative measurements. We conclude that the current major obstacle in the field is presented by the inherent limitations of the currently available tracers in reporting dynamic changes in the availability of a single OR subclass in the CNS. Upon solving these methodological challenges and limitations, opioid receptor imagings should be applicable for further and more detailed in vivo studies of brain pathology involving the opioid signalling.
Acknowledgements
The authors thank Dr Erik Arstad and Dr Andy Constanti for their critical reading of the manuscript. Funding to pay the Open Access publication charges for this article was provided by Aker University Hospital.
Glossary
Abbreviations:
- ACC
anterior cingulate cortex
- BP
binding potential
- BPND
BP using reference tissue method
- Bavail
concentration of available receptor
- CAF
carfentanil
- DPN
diprenorphine
- FC
frontal cortex
- FDPN
6-O-desmethyl-2-fluoroethyl-diprenorphine
- FCyF
fluoro-cyclofoxy
- fMRI
functional magnetic resonance imaging
- GPCR
guanine nucleotide-binding protein coupled receptor
- KD
radioligand equilibrium dissociation constant
- MeNTl
methyl-naltrindole
- NA
nucleus accumbens
- NMDA
N-methyl-d-asparate
- DV
volume of distribution
- DVR
volume of distribution ratio
- VOI
volume of interest
- SM1
primary sensorimotor cortex
- SA
spectral analysis
- SPM
statistical parametric mapping
- VT
volume of distribution.
References
- Abbadie C, Pan YX, Pasternak GW. Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in mouse brain. Neuroscience. 2004;127:419–30. doi: 10.1016/j.neuroscience.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–4. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum. J Pharmacol Exp Ther. 1990;252:1370–7. [PubMed] [Google Scholar]
- Bajorek JG, Lee RJ, Lomax P. Neuropeptides: anticonvulsant and convulsant mechanisms in epileptic model systems and in humans. Adv Neurol. 1986;44:489–500. [PubMed] [Google Scholar]
- Barinaga M. Neuroimaging. Still debated, brain image archives are catching on. Science. 2003;300:43–5. doi: 10.1126/science.300.5616.43. [DOI] [PubMed] [Google Scholar]
- Bartenstein PA, Duncan JS, Prevett MC, Cunningham VJ, Fish DR, Jones AK, et al. Investigation of the opioid system in absence seizures with positron emission tomography. J Neurol Neurosurg Psychiatry. 1993;56:1295–302. doi: 10.1136/jnnp.56.12.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenstein PA, Prevett MC, Duncan JS, Hajek M, Wieser HG. Quantification of opiate receptors in two patients with mesiobasal temporal lobe epilepsy, before and after selective amygdalohippocampectomy, using positron emission tomography. Epilepsy Res. 1994;18:119–25. doi: 10.1016/0920-1211(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–9. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Beckett AH, Casy AF. Synthetic analgesics: stereochemical considerations. J Pharm Pharmacol. 1954;6:986–1001. doi: 10.1111/j.2042-7158.1954.tb11033.x. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]carfentanil and positron emission tomography (PET) Pain. 2002;99:589–98. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–51. [PubMed] [Google Scholar]
- Bencherif B, Wand GS, McCaul ME, Kim YK, Ilgin N, Dannals RF, et al. Mu-opioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004;55:255–62. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Piccini P, Turjanski N, Samuel M. Neuroimaging of dyskinesia. Ann Neurol. 2000;47:S154–8. [PubMed] [Google Scholar]
- Burn DJ, Rinne JO, Quinn NP, Lees AJ, Marsden CD, Brooks DJ. Striatal opioid receptor binding in Parkinson's disease, striatonigral degeneration and Steele-Richardson-Olszewski syndrome, a [11C]diprenorphine PET study. Brain. 1995;118:951–8. doi: 10.1093/brain/118.4.951. [DOI] [PubMed] [Google Scholar]
- Carr DB, Goudas LC, Balk EM, Bloch R, Ioannidis JP, Lau J. Evidence report on the treatment of pain in cancer patients. J Natl Cancer Inst Monogr. 2004;32:23–31. doi: 10.1093/jncimonographs/lgh012. [DOI] [PubMed] [Google Scholar]
- Casey KL. Central pain: distributed effects of focal lesions. Pain. 2004;108:205–6. doi: 10.1016/j.pain.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le MM, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–80. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SK, Cohen RS, Pfaff DW. Transneuronal degeneration in the midbrain central gray following chemical lesions in the ventromedial nucleus: a qualitative and quantitative analysis. Neuroscience. 1990;38:409–26. doi: 10.1016/0306-4522(90)90038-6. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. G protein beta gamma subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Andreason PJ, Doudet DJ, Carson RE, Sunderland T. Opiate receptor avidity and cerebral blood flow in Alzheimer's disease. J Neurol Sci. 1997;148:171–80. doi: 10.1016/s0022-510x(96)05315-4. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Carson RE, Aigner TG, Doudet DJ. Opiate receptor avidity is reduced in non-motor impaired MPTP-lesioned rhesus monkeys. Brain Res. 1998;806:292–6. doi: 10.1016/s0006-8993(98)00777-x. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Carson RE, Saunders RC, Doudet DJ. Opiate receptor avidity is increased in rhesus monkeys following unilateral optic tract lesion combined with transections of corpus callosum and hippocampal and anterior commissures. Brain Res. 2000b;879:1–6. doi: 10.1016/s0006-8993(00)02528-2. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Carson RE, Sunderland T. Opiate receptor avidity in the thalamus is sexually dimorphic in the elderly. Synapse. 2000a;38:226–9. doi: 10.1002/1098-2396(200011)38:2<226::AID-SYN13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Carson RE, Wyatt RJ, Doudet DJ. Opiate receptor avidity is reduced bilaterally in rhesus monkeys unilaterally lesioned with MPTP. Synapse. 1999;33:282–8. doi: 10.1002/(SICI)1098-2396(19990915)33:4<282::AID-SYN5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MDJ. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–9. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Contreras PC, Tam L, Drower E, Rafferty MF. [3H]naltrindole: a potent and selective ligand for labeling delta-opioid receptors. Brain Res. 1993;604:160–4. doi: 10.1016/0006-8993(93)90363-r. [DOI] [PubMed] [Google Scholar]
- Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. doi: 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Brostrom PA, Allen JT, Lesko LJ, Ferkany JW, Kaplita PV, et al. Pharmacologic profile of NPC 168 (naltrexone phenyl oxime), a novel compound with activity at opioid receptors. Pharmacol Biochem Behav. 1990;37:497–504. doi: 10.1016/0091-3057(90)90019-e. [DOI] [PubMed] [Google Scholar]
- Devi LA. Heterodimerization of G protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–7. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, et al. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–92. [PubMed] [Google Scholar]
- Dixon R, Gentile J, Hsu HB, Hsiao J, Howes J, Garg D, et al. Nalmefene: safety and kinetics after single and multiple oral doses of a new opioid antagonist. J Clin Pharmacol. 1987;27:233–9. doi: 10.1002/j.1552-4604.1987.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Jensen MK, Sjogren P, Ekholm O, Rasmussen NK. Epidemiology of chronic non-malignant pain in Denmark. Pain. 2003;106:221–8. doi: 10.1016/S0304-3959(03)00225-2. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Tanaka S, Ma T, Chudapongse N, Sinchaisuk S, et al. Changes in the brain kappa-opioid receptor levels of rats in withdrawal from physical dependence upon butorphanol. Neuroscience. 2003;121:1063–74. doi: 10.1016/s0306-4522(03)00299-9. [DOI] [PubMed] [Google Scholar]
- Fichna J, Janecka A, Costentin J, Do Rego JC. The endomorphin system and its evolving neurophysiological role. Pharmacol Rev. 2007;59:88–123. doi: 10.1124/pr.59.1.3. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Re-examination of the human taste region: a positron emission tomography study. Eur J Neurosci. 1999;11:2985–8. doi: 10.1046/j.1460-9568.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- Frost JJ. Receptor localization and quantification with PET. Radiology. 1988;169:273–4. doi: 10.1148/radiology.169.1.3262227. [DOI] [PubMed] [Google Scholar]
- Frost JJ, Douglass KH, Mayberg HS, Dannals RF, Links JM, Wilson AA, et al. Multicompartmental analysis of [11C]carfentanil binding to opiate receptors in humans measured by positron emission tomography. J Cereb Blood Flow Metab. 1989;9:398–409. doi: 10.1038/jcbfm.1989.59. [DOI] [PubMed] [Google Scholar]
- Frost JJ, Mayberg HS, Fisher RS, Douglass KH, Dannals RF, Links JM, et al. Mu-opiate receptors measured by positron emission tomography are increased in temporal lobe epilepsy. Ann Neurol. 1988;23:231–7. doi: 10.1002/ana.410230304. [DOI] [PubMed] [Google Scholar]
- Frost JJ, Douglass KH, Mayberg HS, Dannals RF, Links JM, Wilson AA, et al. Imaging opiate receptors in the human brain by positron tomography. J Comput Assist Tomogr. 1985;9:231–6. doi: 10.1097/00004728-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Calculation of cerebral glucose phosphorylation from brai uptake of glucose analogs in vivo: a re-examination. Brain Res Rev. 1982;4:237–74. doi: 10.1016/0165-0173(82)90018-2. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, et al. maging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–82. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Greenwald M, Johanson CE, Bueller J, Chang Y, Moody DE, Kilbourn M, et al. Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry. 2007;61:101–10. doi: 10.1016/j.biopsych.2006.04.043. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28:2000–9. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- Guderman T, Kalkbrenner F, Schultz G. Diversity and selectivity of receptor-G protein interaction. Annu Rev Pharmacol Toxicol. 1996;36:429–59. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- Gulya K. The opioid system in neurologic and psychiatric disorders and in their experimental models. Pharmacol Ther. 1990;46:395–428. doi: 10.1016/0163-7258(90)90026-x. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Gunn SR, Turkheimer FE, Aston JA, Cunningham VJ. Positron emission tomography compartmental models: a basis pursuit strategy for kinetic modeling. J Cereb Blood Flow Metab. 2002;22:1425–39. doi: 10.1097/01.wcb.0000045042.03034.42. [DOI] [PubMed] [Google Scholar]
- Haber SN, Watson SJ. The comparative distribution of enkephalin, dynorphin and substance P in the human globus pallidus and basal forebrain. Neuroscience. 1985;14:1011–24. doi: 10.1016/0306-4522(85)90272-6. [DOI] [PubMed] [Google Scholar]
- Hackler L, Zadina JE, Ge L-J, Kastin AJ. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–9. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- Hammers A, Asselin MC, Hinz R, Kitchen I, Brooks DJ, Duncan JS, et al. Upregulation of opioid receptor binding following spontaneous epileptic seizures. Brain. 2007;30:1009–16. doi: 10.1093/brain/awm012. [DOI] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, et al. Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psych. 2005;62:57–64. doi: 10.1001/archpsyc.62.1.57. [DOI] [PubMed] [Google Scholar]
- Hening W, Allen R, Earley C, Kushida C, Picchietti D, Silber M. The treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Review. Sleep. 1999;22:970–99. [PubMed] [Google Scholar]
- Henriksen G, Willoch F, Talbot PS, Wester HJ. Recent development and potential use of μ- and κ-opioid receptor ligands in positron emission tomography. Drug Dev Rev. 2006;67:890–904. [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacol (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hiller JM, Fan LQ. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochemical Research. 1996;21:1333–45. doi: 10.1007/BF02532374. [DOI] [PubMed] [Google Scholar]
- Hume SP, Lingford-Hughes AR, Nataf V, Hirani E, Ahmad R, Davies AN, et al. Low sensitivity of the positron emission tomography ligand [11C]diprenorphine to agonist opiates. J Pharmacol Exp Ther. 2007;322:661–67. doi: 10.1124/jpet.107.121749. [DOI] [PubMed] [Google Scholar]
- Ichise M, Meyer JH, Yonekura Y. An introduction to PET and SPECT neuroreceptor quantification models. J Nucl Med. 2001;42:755–63. [PubMed] [Google Scholar]
- Ingman K, Hagelberg N, Aalto S, Nagren K, Juhakoski A, Karhuvaara S, et al. Prolonged central mu-opioid receptor occupancy after single and repeated nalmefene dosing. Neuropsychopharmacology. 2005;30:2245–53. doi: 10.1038/sj.npp.1300790. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, et al. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33:909–16. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- Jones AK, Kitchen ND, Watabe H, Cunningham VJ, Jones T, Luthra SK, et al. Measurement of changes in opioid receptor binding in vivo during trigeminal neuralgic pain using [11C]diprenorphine and positron emission tomography. J Cereb Blood Flow Metab. 1999;19:803–8. doi: 10.1097/00004647-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett. 1991;126:25–8. doi: 10.1016/0304-3940(91)90362-w. [DOI] [PubMed] [Google Scholar]
- Jones AK, Watabe H, Cunningham VJ, Jones T. Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur J Pain. 2004;8:479–85. doi: 10.1016/j.ejpain.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Cvejic S, Devi LA. Opioids and their complicated receptor complexes. Neuropsychopharmacology. 2000;23:S5–18. doi: 10.1016/S0893-133X(00)00143-3. [DOI] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. Clin Invest. 1948;27:476–83. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Wagner HN, Jr, Villemagne VL, Kao PF, Dannals RF, Ravert HT, et al. Longer occupancy of opioid receptors by nalmefene compared to naloxone as measured in vivo by a dual-detector system. J Nucl Med. 1997;38:1726–31. [PubMed] [Google Scholar]
- Kling MA, Carson RE, Borg L, Zametkin A, Matochik JA, Schluger J, et al. Opioid receptor imaging with positron emission tomography and [18F]cyclofoxy in long-term, methadone-treated former heroin addicts. J Pharmacol Exp Ther. 2000;295:1070–6. [PubMed] [Google Scholar]
- Knapp RJ, Malatynska E, Collins N, Fang L, Wang JY, Hruby VJ, et al. Molecular biology and pharmacology of cloned opioid receptors. FASEB J. 1995;9:516–25. doi: 10.1096/fasebj.9.7.7737460. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Richardson MP, Brooks DJ, Duncan JS. Focal cortical release of endogenous opioids during reading-induced seizures. Lancet. 1998;352:952–5. doi: 10.1016/s0140-6736(97)09077-6. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–26. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Slifstein M, Huang Y. Positron emission tomography: imaging and quantification of neurotransporter availability. Methods. 2002;27:287–99. doi: 10.1016/s1046-2023(02)00085-3. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Lesscher HMB, Bailey A, Burbach JPH, van Ree JM, Kitchen I, Gerrits MAFM. Receptor-selective changes in μ-, δ- and κ-opioid receptors after chronic naltrexone treatment in mice. Eur J Neurosci. 2003;17:1006–12. doi: 10.1046/j.1460-9568.2003.02502.x. [DOI] [PubMed] [Google Scholar]
- Lever JR, Dannals RF, Wilson AA, Ravert HT, Frost JJ, Wagner HN., Jr Synthesis of carbon-11 labeled diprenorphine: a radioligand for positron emission tomography studies of opiate receptors. Tetrahedon Lett. 1987;28:4015–8. [Google Scholar]
- Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, et al. Altered central mu-opioid receptor binding after psychological trauma. Biol Psychiatry. 2007;6:1030–8. doi: 10.1016/j.biopsych.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to (-)-N-[11C-methyl]cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007;127:183–9. doi: 10.1016/j.pain.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Madar I, Lesser RP, Krauss G, Zubieta JK, Lever JR, Kinter CM, et al. Imaging of delta- and mu-opioid receptors in temporal lobe epilepsy by positron emission tomography. Ann Neurol. 1997;41:358–67. doi: 10.1002/ana.410410311. [DOI] [PubMed] [Google Scholar]
- Madar I, Lever JR, Kinter CM, et al. Imaging of delta opioid receptors in human brain by N1′-([11C]methyl)naltrindole and PET. Synapse. 1996;24:19–28. doi: 10.1002/(SICI)1098-2396(199609)24:1<19::AID-SYN3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13:372–8. doi: 10.1177/026988119901300418. [DOI] [PubMed] [Google Scholar]
- Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995;18:69–70. [PubMed] [Google Scholar]
- Maurer R, Cortes R, Probst A, Palacios JM. Multiple opiate receptor in human brain: an autoradiographic investigation. Life Sci. 1983;33(Suppl 1):231–4. doi: 10.1016/0024-3205(83)90485-x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Sadzot B, Meltzer CC, Fisher RS, Lesser RP, Dannals RF, et al. Quantification of mu and non-mu opiate receptors in temporal lobe epilepsy using positron emission tomography. Ann Neurol. 1991;30:3–11. doi: 10.1002/ana.410300103. [DOI] [PubMed] [Google Scholar]
- Melichar JK, Hume SP, Williams TM, Daglish MR, Taylor LG, Ahmad R, et al. Using [11C]diprenorphine to image opioid receptor occupancy by methadone in opioid addiction: clinical and preclinical studies. J Pharm Exp Ther. 2005;312:309–15. doi: 10.1124/jpet.104.072686. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Zubieta JK, Links JM, Brakeman P, Stumpf MJ, Frost JJ. MR-based correction of brain PET measurements for heterogeneous gray matter radioactivity distribution. J Cereb Blood Flow Metab. 1996;16:650–8. doi: 10.1097/00004647-199607000-00016. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–5. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–27. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, et al. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–8. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Noble EP. Alcoholism and the dopaminergic system: a review. Addict Biol. 1996;1:333–48. doi: 10.1080/1355621961000124956. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Hatano K, Kawasumi Y, Wichmann J, Ito K. Synthesis and in vivo evaluation of [11C]methyl-Ro 64-6198 as an ORL1 receptor imaging agent. Nucl Med Biol. 2001;28:941–7. doi: 10.1016/s0969-8051(01)00260-8. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol. 2005;68:866–75. doi: 10.1124/mol.105.011858. [DOI] [PubMed] [Google Scholar]
- Pasternak DA, Pan L, Xu J, et al. Identification of three new alternatively spliced variants of the rat mu opioid receptor gene: dissociation of affinity and efficacy. J Neurochem. 2004;91:881–90. doi: 10.1111/j.1471-4159.2004.02767.x. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–4. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with 2-[F-18]fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol. 1979;6:371–88. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Piccini P, Weeks RA, Brooks DJ. Alterations in opioid receptor binding in Parkinson's disease patients with levodopa-induced dyskinesias. Ann Neurol. 1997;42:720–6. doi: 10.1002/ana.410420508. [DOI] [PubMed] [Google Scholar]
- Portoghese PS. A new concept on the mode of interaction of narcotic analgesics with receptors. J Med Chem. 1965;8:609–16. doi: 10.1021/jm00329a013. [DOI] [PubMed] [Google Scholar]
- Prevett MC, Cunningham VJ, Brooks DJ, Fish DR, Duncan JS. Opiate receptors in idiopathic generalised epilepsy measured with [11C]diprenorphine and positron emission tomography. Epilepsy Res. 1994;19:71–7. doi: 10.1016/0920-1211(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Quock RM, Burkey TH, Varga E, et al. The δ-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol Rev. 1999;51:503–32. [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, et al. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–4. [PubMed] [Google Scholar]
- Reimold M, Slifstein M, Heinz A, Mueller-Schauenburg W, Bares R. Effect of spatial smoothing on t-maps: arguments for going back from t-maps to masked contrast images. J Cereb Blood Flow Metab. 2006;26:751–9. doi: 10.1038/sj.jcbfm.9600231. [DOI] [PubMed] [Google Scholar]
- Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Schadrack J, Willoch F, Platzer S, Bartenstein P, Mahal B, Dworzak D, et al. Opioid receptors in the human cerebellum: evidence from [11C]diprenorphine PET, mRNA expression and autoradiography. Neuroreport. 1999;10:619–24. doi: 10.1097/00001756-199902250-00032. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, et al. Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology. 2007b;32:450–7. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Koeppe RA, Zubieta JK. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse. 2007a;61:707–14. doi: 10.1002/syn.20404. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Liebisch DC, Kish SJ, Arendt RM, Hornykiewicz O, Herz A. Opioid peptides in Huntington's disease: alterations in prodynorphin and proenkephalin system. Brain Res. 1986;378:405–8. doi: 10.1016/0006-8993(86)90946-7. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. Receptor subtypes in opioid and stimulant reward. Pharmacol Toxicol. 1992;70:87–94. doi: 10.1111/j.1600-0773.1992.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. Stimulus-dependent translocation of kappa opioid receptors to the plasma membrane. J Neurosci. 1999;19:2658–64. doi: 10.1523/JNEUROSCI.19-07-02658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, Henriksen SJ, Chavkin C, Gruol D. Opioid peptides and epileptogenesis in the limbic system: cellular mechanisms. Adv Neurol. 1986;44:501–12. [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–96. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Simon EJ, Hiller JM, Edelman I. Stereospecific binding of the potent narcotic analgesic [3H]Etorphine to rat-brain homogenate. Proc Natl Acad Sci USA. 1973;70:1947–9. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Smith JS, Zubieta JK, Price JC, Flesher JE, Madar I, Lever JR, et al. Quantification of delta-opioid receptors in human brain with N1′-([11C]methyl) naltrindole and positron emission tomography. J Cereb Blood Flow Metab. 1999;19:956–66. doi: 10.1097/00004647-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–85. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, et al. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metabol. 1998;83:4498–505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, et al. Opioidergic activation in the medial pain system after heat pain. Pain. 2006a;122:63–7. doi: 10.1016/j.pain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Willoch F, Miederer M, Schindler F, Valet M, Berthele A, et al. Opioidergic changes in the pineal gland and hypothalamus in cluster headache: a ligand PET study. Neurology. 2006b;66:1108–10. doi: 10.1212/01.wnl.0000204225.15947.f8. [DOI] [PubMed] [Google Scholar]
- Strader CD, Fong TM, Tota MR, Underwood D, Dixon RAF. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–32. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Narendran R, Butelman ER, Huang Y, Ngo K, Slifstein M, et al. [11C] GR103545, a radiotracer for imaging kappa-opioid receptors in vivo with PET: synthesis and evaluation in baboons. J Nucl Med. 2005;46:484–94. [PubMed] [Google Scholar]
- Theodore WH, Carson RE, Andreasen P, Zametkin A, Blasberg R, Leiderman DB. PET imaging of opiate receptor binding in human epilepsy using [18F]cyclofoxy. Epilepsy Res. 1992;13:129–39. doi: 10.1016/0920-1211(92)90068-5. [DOI] [PubMed] [Google Scholar]
- Tortella FC. Endogenous opioid peptides and epilepsy: quieting the seizing brain? Trends Pharmacol Sci. 1988;9:366–72. doi: 10.1016/0165-6147(88)90256-8. [DOI] [PubMed] [Google Scholar]
- Tsao P, Cao T, von ZM. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:91–6. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- Tsao PI, von Zastrow M. Diversity and specificity in the regulated endocytic membrane trafficking of G-protein-coupled receptors. Pharmacol Ther. 2001;89:139–47. doi: 10.1016/s0163-7258(00)00107-8. [DOI] [PubMed] [Google Scholar]
- Varga EV, Yamamura HI, Rubenzik MK, Stropova D, Navratilova E, Roeske WR. Molecular mechanisms of excitatory signaling upon chronic opioid agonist treatment. Life Sci. 2003;74:299–311. doi: 10.1016/j.lfs.2003.09.017. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51:341–96. [PubMed] [Google Scholar]
- Vogt BA, Watanabe H, Gootoonk S, Jones AKP. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from coregistered PET and MR images. Human Brain Mapp. 1995;3:1–12. [Google Scholar]
- von Spiczak S, Whone AL, Hammers A, Asselin MC, Turkheimer F, Tings T, et al. The role of opioids in restless legs syndrome: an [11C]diprenorphine PET study. Brain. 2005;128:906–17. doi: 10.1093/brain/awh441. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–4. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–30. [PubMed] [Google Scholar]
- Weeks RA, Cunningham VJ, Piccini P, Waters S, Harding AE, Brooks DJ. [11C]Diprenorphine binding in Huntington's disease: a comparison of region of interest analysis with statistical parametric mapping. J Cereb Blood Flow Metab. 1997;17:943–9. doi: 10.1097/00004647-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Whone AL, Von Spiczak S, Edwards M, Valente EM, Hammers A, Bhatia KP, et al. Opioid binding in DYT1 primary torsion dystonia: a [11C]diprenorphine PET study. Mov Disord. 2004;19:1498–503. doi: 10.1002/mds.20238. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Willoch F, Tölle TR, Wester HJ, Munz F, Petzold A, Schwaiger M, et al. Central pain after pontine infarction is associated with changes in opioid receptor binding: a PET study with 11C-diprenorphine. AJNR Am J Neuroradiol. 1999;20:686–90. [PMC free article] [PubMed] [Google Scholar]
- Willoch F, Gamringer U, Medele R, Steude U, Tolle TR. Analgesia by electrostimulation of the trigeminal ganglion in patients with trigeminopathic pain: a PET activation study. Pain. 2003;103:119–30. doi: 10.1016/s0304-3959(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, et al. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–20. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Gibo DM, McLaughlin PJ. Adult and developing human cerebella exhibit different profiles of opioid binding sites. Brain Res. 1990;523:62–8. doi: 10.1016/0006-8993(90)91635-t. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Greenwald MK, Lombardi U, Woods JH, Kilbourn MR, Jewett DM, et al. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: a preliminary study. Neuropsychopharmacology. 2000;23:326–34. doi: 10.1016/S0893-133X(00)00110-X. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psyschiatry. 1999;156:842–8. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2:1225–9. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003a;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003b;60:1145–53. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–7. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]