Abstract
In neurodegenerative disorders associated with primary or secondary mitochondrial defects such as Huntington's disease (HD), cells of the striatum are particularly vulnerable to cell death, although the mechanisms by which this cell death is induced are unclear. Dopamine, found in high concentrations in the striatum, may play a role in striatal cell death. We show that in primary striatal cultures, dopamine increases the toxicity of an N-terminal fragment of mutated huntingtin (Htt-171-82Q). Mitochondrial complex II protein (mCII) levels are reduced in HD striatum, indicating that this protein may be important for dopamine-mediated striatal cell death. We found that dopamine enhances the toxicity of the selective mCII inhibitor, 3-nitropropionic acid. We also demonstrated that dopamine doses that are insufficient to produce cell loss regulate mCII expression at the mRNA, protein and catalytic activity level. We also show that dopamine-induced down-regulation of mCII levels can be blocked by several dopamine D2 receptor antagonists. Sustained overexpression of mCII subunits using lentiviral vectors abrogated the effects of dopamine, both by high dopamine concentrations alone and neuronal death induced by low dopamine concentrations together with Htt-171-82Q. This novel pathway links dopamine signaling and regulation of mCII activity and could play a key role in oxidative energy metabolism and explain the vulnerability of the striatum in neurodegenerative diseases.
INTRODUCTION
The striatum is preferentially damaged in a number of acute and chronic neurological conditions, for reasons that are still unclear. One hypothesis is that the striatum is inherently sensitive to impairment of energy metabolism. Indeed, primary genetic mitochondrial defects, the accidental ingestion of mitochondrial toxins, perinatal hypoxia/ischemia and focal stroke in adults are all associated with striatal degeneration (1).
Among the chronic neurological disorders that affect the striatum, one of the best studied is Huntington's disease (HD). HD is an inherited progressive neurodegenerative disorder associated with abnormal movements (chorea), cognitive deficits and psychiatric disturbances (2). The most striking neuropathological change in HD is the preferential loss of medium spiny GABAergic neurons from the striatum (3). At a genetic level, the disease is caused by an abnormal expansion of a CAG repeat located in exon 1 of the gene encoding huntingtin protein (Htt) (4). This mutation confers a new toxic function on the protein, at least in part through the production of short N-terminal fragments carrying the poly-glutamine tract. A causal role for these fragments is strongly suggested by the finding that mutagenesis of cleavage sites in full-length mutant Htt inhibits disease progression in mice (5). There is also compelling evidence that the Huntington phenotype involves a loss of Htt function (6). Indeed, wild-type Htt has a pro-survival function, at least in part through the direct regulation of cell death pathways (7–9), and indirectly through the regulation of the expression (10) and secretion (11) of brain-derived neurotrophic factor (BDNF).
The expression of wild-type and mutant Htt is virtually ubiquitous in the brain, so the mechanisms underlying the preferential vulnerability of the striatum in HD remain unknown. One hypothesis is that the toxic effects of mutant Htt are aggravated by environmental factors that are specific to the striatum (12). Among these potential factors, dopamine (DA), which is found at high concentrations in the striatum, may render striatal neurons highly sensitive to mutant Htt (13). Elevation of extracellular dopamine concentration can be neurotoxic to striatal neurons both in vitro (14,15) and in vivo (16,17). DA also renders striatal cells highly vulnerable to degeneration induced by an inhibitor of mitochondrial complex II (mCII), 3-nitropropionic acid (3NP) (15,18,19). Direct support for a ‘protoxic’ role for DA in the toxicity of mutated Htt comes from the recent demonstration that the toxicity of the N-terminal fragments of mutated Htt is potentiated by DA in striatal neurons in primary culture, an effect at least partly due to D2 receptor-mediated mechanisms (20). In addition, in vivo experiments using DAT (dopamine transporter) knock-out (KO) mice crossed with a knock-in transgenic mouse model of HD showed that the resulting elevated DA concentration enhances motor symptoms and striatal degeneration induced by mutant Htt (21). Tang et al. also recently demonstrated that striatal dysfunction in yeast artificial chromosome (YAC 128) HD transgenic mice was exacerbated by the elevation of DA signaling using L-DOPA treatment (22). However, the mechanisms by which DA enhances mutated Htt toxicity have not been elucidated.
One important aspect of HD pathogenesis is mitochondrial dysfunction. A reduction in mitochondrial membrane potential is present in various genetic models of HD, as well as in lymphoblasts isolated from HD patients (23–25). The activity of mitochondrial complex II (mCII), an enzyme that plays a major role in oxidative energy metabolism, is reduced in the striatum of HD patients (26,27). Additionally, we recently showed that the loss of mCII activity in the striatum of HD patients is due to the reduced expression of mCII subunits (28). A similar loss of mCII subunits and activity was found in striatal neurons in primary culture, following transduction with lentiviral vectors encoding the N-terminal fragment of mutated Htt carrying the polyglutamine tract (28).
In the present study, we explored the possibility that mCII regulation constitutes the missing link that explains the synergy between the effects of DA and mutant Htt toxicity. We showed that degeneration of striatal neurons induced by either the mitochondrial toxin 3NP or lentiviral vectors encoding the first 171 amino acids of mutated Htt with 82 polyglutamine repeats (lenti-Htt171-82Q) was dramatically augmented by the presence of sub-toxic concentrations of DA. We therefore asked whether DA could down-regulate mCII expression, and thus increase the vulnerability of striatal cells to the short fragment of mutant Htt. To this purpose, we characterized the cellular and biochemical changes that occur during neurodegeneration produced by dopamine and mutated Htt fragment, and performed rescue experiments using lentiviral vectors encoding mCII subunits. The results show that dopamine increases the vulnerability of striatal neurons to mutant Htt toxicity via a down-regulation of mCII expression that involves an upstream activation of D2 pharmacological subtype of dopamine receptors.
RESULTS
Dopamine enhances the toxicity of the N-terminal fragment of mutated huntingtin
DA (100 µm) was shown previously to increase the toxicity of the N-terminal fragment of mutated huntingtin (exon 1 of Htt with 123 polyglutamine repeats), expressed acutely after transfection (20). In the present study, we examined whether DA similarly affected the progressive toxicity at low levels of a shorter N-terminal fragment of mutated Htt (171 first amino acids with 82 glutamines) expressed using lentiviral vectors in a model where neuronal cell loss is only detected at 6–8 weeks post-infection (28,29).
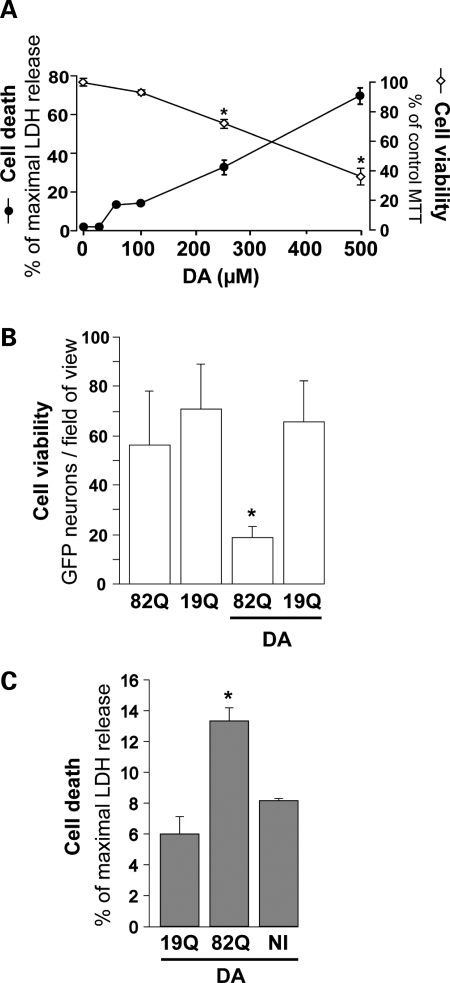
The dose-dependent neurotoxic effects of DA were re-examined under our experimental conditions using two cell death/viability assays: reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), an index of the integrity of dehydrogenases and detection of lactate dehydrogenase (LDH) that is released from the cytosol to the extracellular space during cell death. Both assays indicated an absence of major degeneration of striatal cell at or below 100 µm DA (Fig. 1A), while significant degeneration was seen at DA concentrations ≥250 µm. Thus, to study the ‘protoxic’ effect of DA, we treated striatal neurons with 100 µm DA for 24 h, after initial co-transduction with lentiviral vectors encoding GFP and either Htt171-82Q or Htt171-19Q (19 polyglutamine repeats; control). The simultaneous exposure of neurons to both vectors resulted in the co-expression of the two transgenes (GFP and one form of Htt) by nearly all cells (97%; 29, Supplementary Material, Fig. S1), which allowed direct counts of neurons that survived DA treatment (Fig. 1B). In agreement with our previous work (28), Htt171-82Q alone induced low levels of cell death at 6 weeks post-infection. DA treatment did not result in the degeneration of cells expressing Htt171-19Q, whereas in cultures expressing Htt171-82Q, DA induced a 3-fold drop in the number of surviving GFP-positive cells. We also studied cell death after a 24 h treatment with 100 µm DA, using the LDH assay (Fig. 1C). The addition of DA augmented neuronal degeneration in cultures expressing Htt171-82Q, but did not affect striatal cells expressing Htt171-19Q or non-infected cells, confirming that DA treatment increased the toxicity of Htt171-82Q under our experimental conditions.
Figure 1.
Synergistic effects of mutated huntingtin and dopamine on striatal neuron degeneration. (A) Dose-dependent toxicity of DA (24 h treatment) assessed in striatal neurons (21 DIV) using the LDH and MTT cell death/viability assays. Note the clear-cut toxicity of DA above 250 µm. (B) Effect of 100 µm DA on the survival of striatal neurons 4 weeks after simultaneous transduction with a lentiviral vector encoding Htt171-82Q (mutated) or Htt171-19Q (wild-type), and a lentiviral vector encoding the reporter GFP. Neither DA alone nor Htt171-82Q alone is toxic to GFP-labeled neurons at this time point, but a combination of the two results in a 3-fold reduction in the number of neurons compared with Htt171-82Q controls. (C) Cell death assessed using the LDH assay in an experiment similar to that in (B), in the absence of lentiviral GFP. In the presence of DA, LDH release is significantly increased in cultures expressing Htt171-82Q. No LDH activity was observed without DA (data not shown). *P < 0.05; ANOVA and Fisher's post-hoc PLSD test.
The sensitization of striatal cells by dopamine is associated with mCII down-regulation
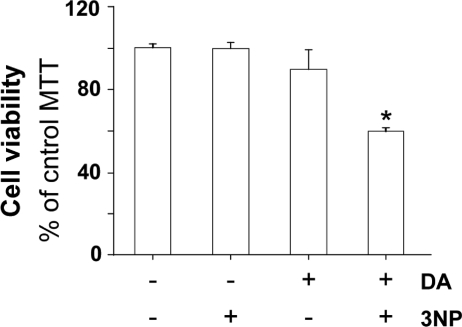
We next wanted to determine whether mCII depletion is implicated in the DA-induced increase of Htt171-82Q-mediated toxicity. We therefore explored whether striatal cells with mCII deficits induced by 3NP were more vulnerable to DA than untreated cells, as was previously suggested by in vitro (15) and in vivo (18) experiments. A combination of 100 µm DA and a non-toxic concentration of 3NP (30) induced the degeneration of striatal cells (Fig. 2), while either DA or 3NP alone had no effect. This suggests that under our cell culture conditions, minimal/sub-acute mCII deficits rendered striatal neurons highly vulnerable to DA treatment.
Figure 2.
Synergistic effects of mitochondrial complex II deficits and dopamine on striatal neuron degeneration. Cell viability assessed by the MTT assay after treatment for 24 h with 3-NP (75 µm), an irreversible inhibitor of mCII, and DA (100 µm). Note that when applied alone, 3-NP is non-toxic and DA is very mildly toxic. Treatment with both agents synergistically induces cell death. *P < 0.0001; ANOVA and Fisher's post-hoc PLSD test.
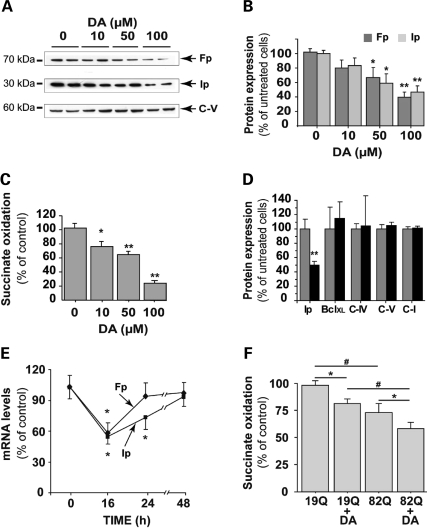
We next examined whether DA could regulate mCII subunit levels. Western blot analysis was used to evaluate the protein levels of the two largest mCII subunits, namely Fp (flavoprotein, SDH-A, 70 kDa) and Ip (Iron-sulfur protein, SDH-B; 30 kDa); each are components of succinate dehydrogenase (SDH). Both Fp and Ip levels were significantly reduced after a 24 h DA treatment, in a concentration-dependent manner (Fig. 3A and B). Biochemical analysis of succinate oxidation, a direct index of SDH/mCII catalytic activity, also demonstrated a dose-dependent reduction in the enzymatic function of the complex (Fig. 3C). In order to determine the selectivity of these alterations, we analyzed the expression of other mitochondrial proteins. Our results show that, in contrast to mCII subunits, the levels of the four other proteins tested (BclXL, subunit 4 of complex IV, alpha subunit of complex V and the 39 kDa subunit of complex I) remained unchanged (Fig. 3D). This indicates that the changes in mCII subunit expression were not due to a global reduction of mitochondrial proteins.
Figure 3.
Cumulative effects of dopamine and mutated huntingtin on mCII expression and catalytic activity. Measurement of Ip and Fp protein expression levels as indicated by western blotting, and mCII activity in striatal cultures treated for 24 h with increasing concentrations of DA. (A) Representative western blot showing the reduction of Ip and Fp expression, while levels of the alpha-subunit of complex V (C-V) remain essentially unchanged. (B) Quantification of protein levels for Ip and Fp after western blotting. (C) Dose-dependent effect of a 24 h DA treatment on the catalytic activity (succinate dehydrogenase) of mCII. (D) Quantification of protein levels for BclXL, the alpha-subunit of complex V (C-V), subunit 4 of complex IV (C-IV) and the 39 kDa subunit of complex I (C-I) after 100 µm DA treatment. (E) Changes in Ip and Fp mRNA levels over time during a 100 µm DA treatment, using quantitative RT–PCR. Note the transient down-regulation of both transcripts. (F) mCII activity in striatal neurons transduced with lentiviral Htt171-82Q (mutant) or Htt171-19Q (wild-type), before treatment with 100 µm DA or vehicle. The effects of DA and mutated Htt on the reduction of mCII activity are cumulative, and correspond to the synergistic effects on neuronal degeneration seen in Figure 1. *P < 0.05; **P < 0.001; #P < 0.01; ANOVA and Fisher's post-hoc PLSD test.
We studied whether the dopamine-induced alterations of mCII expression could result from loss of expression in neurons and/or astrocytes. First we further characterized our cultures at 4 weeks in vitro. Qualitative immunofluorescence and quantitative FACS analyses confirmed that striatal cultures consisted of a relatively low proportion of astrocytes (∼12%) whereas cells with a neuronal phenotype (∼80%) predominated (Supplementary Material, Fig. S2). We also examined the potential effect of dopamine on astrocytes. We treated pure astrocyte cultures with increasing concentrations (100–300 µm) of dopamine and found no effect of the neurotransmitter on SDH/mCII expression in these glial cells (Supplementary Material, Fig. S3). These results suggested that the dopamine-induced loss of mCII subunits in striatal cultures most likely corresponded to an alteration of expression in neurons.
The effect of DA on transcription of Fp and Ip genes was explored in primary culture of striatal neurons. Levels of SDH-A (Fp) and SDH-B (Ip) gene transcripts were evaluated by quantitative RT–PCR at different times after DA treatment. Levels of both SDH-A and SDH-B transcripts were significantly decreased 16 h after DA treatment (Fig. 3E). At 24 h, SDH-A mRNA levels returned to original values, whereas the expression of SDH-B transcripts remained down-regulated. At 48 h after DA treatment, the levels of both SDH-A and SDH-B transcripts were similar to those of control cells. The depletion of mCII catalytic subunits and activity seen at 24 h could thus result, at least in part, from the reduction in the transcription of SDH genes 8 h earlier.
We next studied whether the increase in vulnerability of striatal neurons to mutated Htt after treatment with DA could be related to a cumulative depletion in mCII levels. We measured the enzymatic activity of mCII after a 24 h treatment with DA in uninfected cells, and neurons transduced with either Htt171-19Q or Htt171-82Q. Our results showed that the expression of Htt171-82Q induced a significant (20%) reduction in mCII activity when compared with Htt-171-19Q transduced controls (Fig. 3F), in agreement with previous findings (28). Interestingly, treatment with 100 µm DA also induced a reduction (∼23%) in complex II activity in neurons transduced with lenti-Htt171-19Q. DA treatment of cells expressing Htt171-82Q resulted in a cumulative reduction (40%) of mCII activity (Fig. 3F), suggesting that SDH/mCII depletion could be a critical event underlying the ‘sensitization’ of striatal cells in the combination of Htt171-82Q and DA.
Overexpression of mCII subunits rescues striatal cells from DA and mutated Htt toxicity
We asked whether the loss of mCII could be instrumental to the toxicity of DA in normal cells and to the ‘protoxic’ effect in striatal cells expressing the mutated Htt fragment. To this end, lentiviral vectors expressing SDH-A (lenti-Fp) and SDH-B (lenti-Ip) were used (28).
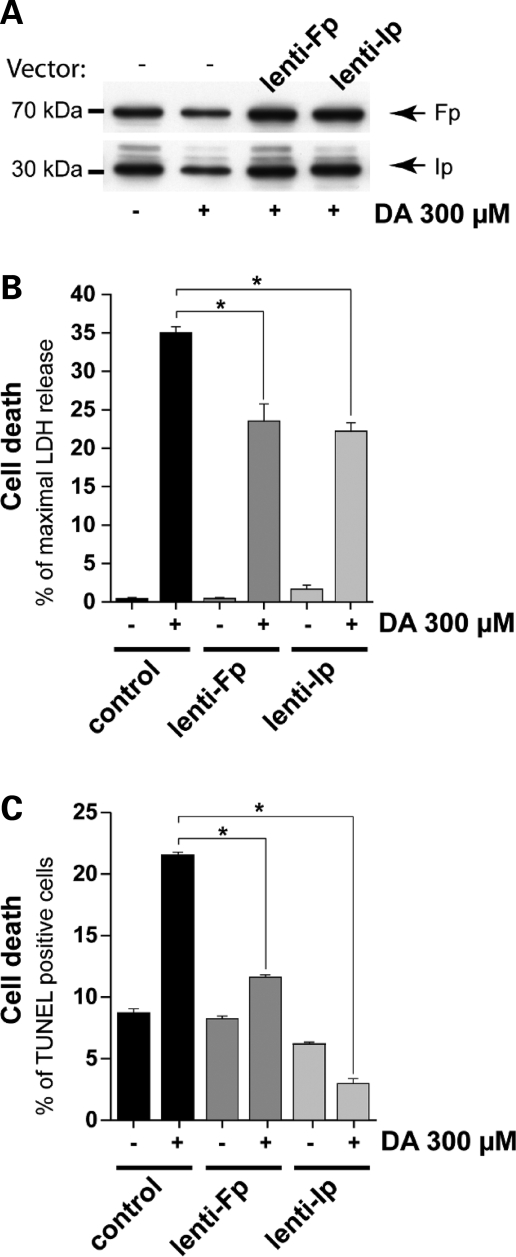
We used western blots to examine whether the overexpression of mCII subunits could block the effects of DA in normal cells. A profound loss of mCII was seen in DA-treated cells, whereas transduction with lenti-Ip and lenti-Fp reversed this loss (Fig. 4A). We then assessed whether the maintenance of mCII levels correlated with a neuroprotective effect. LDH assays and FACS analysis of TUNEL-positive cells revealed that the toxicity of DA was markedly reduced by the overexpression of Ip and Fp mCII subunits (Fig. 4B and C). Quantitation of GFP-positive cells further confirmed that lenti-Ip rescued neurons from DA toxicity (Fig. 5). The loss of mCII thus appears to play a causal role in DA-induced toxicity in striatal neurons that do not express mutated Htt.
Figure 4.
Rescue of striatal neurons from DA toxicity by lentiviral mCII components, Fp and Ip. Effects of DA on mCII Ip and Fp subunit expression in neurons plated for 3 weeks in culture. Cultures were exposed to 300 µm DA for 24 h, 2 weeks after transduction with lentiviral vectors coding Ip (lenti-Ip) or Fp (lenti-Fp) proteins or vehicle alone. (A) Levels of Ip and Fp assessed by western blot analysis reveal that the overexpression of mCII subunits compensates for the loss induced by DA. (B and C) Evaluation of the protective effects of Ip and Fp overexpression using the LDH assay (B) and counts of TUNEL-positive cells by FACS (C). Note that the overexpression of Ip and Fp mCII subunits is neuroprotective. *P < 0.0001; ANOVA and Fisher's post-hoc PLSD test.
Figure 5.
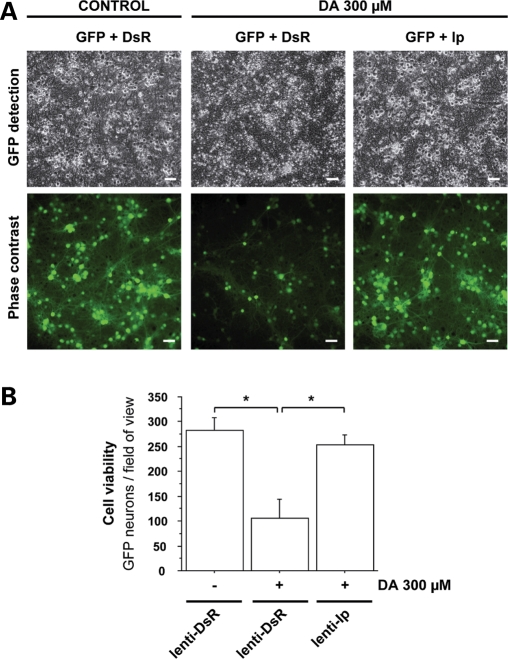
Microscopic analysis of the effects of Ip mCII subunit overexpression on DA-induced neuronal death. Cultures of striatal neurons expressing the reporter gene GFP, were transduced with a lentiviral vector coding the Ip subunit of mCII (lenti-Ip) or the DsRed reporter protein (lenti-DsR) as a control of transduction. (A) Representative field of view using phase contrast imaging (upper images) and fluorescence imaging of GFP (lower images) (Scale bar, 20 µm). (B) Histogram of GFP-positive cell counts in cultures transduced with lenti-Ds or lenti-Ip after a 24 h treatment with DA. Note the severity of neurodegeneration in cultures treated with DA, and the rescuing effect of Ip overexpression. *P < 0.005; ANOVA and Fisher's post-hoc PLSD test.
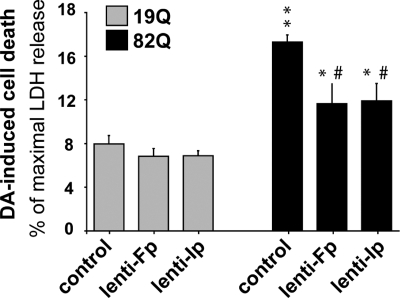
To test whether mCII loss is similarly involved in the exacerbation of DA toxicity in striatal neurons expressing mutated Htt, the cells were transduced with lentiviral vectors encoding Htt171-82Q or Htt171-19Q as described earlier, and re-infected with either lenti-Fp or lenti-Ip 1 week later. After 5 weeks, the cells were treated with 100 µm DA. Ip and Fp overexpression significantly protected neurons against death induced by the combination of DA and Htt171-82Q (Fig. 6). Thus, mCII loss is, at least in part, involved in the toxicity of DA in cells expressing the mutated Htt fragment.
Figure 6.
Rescue of striatal neurons by Ip and Fp overexpression from the synergistic degeneration induced by DA and mutated Htt. Striatal cell cultures transduced with either Ip or Fp subunits of mCII in addition to Htt171-82Q are less vulnerable than mock-infected controls to neurodegeneration induced by 100 µm DA treatment 6 weeks later (i.e. before the bulk of cell death produced by Htt171-82Q alone). Note that neurons expressing Htt171-82Q are more vulnerable to DA than those expressing Htt171-19Q. *P < 0.05 and **P < 0.0001 (Htt171-82Q versus htt171-19Q); #P < 0.005 (Fp or Ip transduction versus mock-infection, in htt171-82Q expressing cells). ANOVA and Fisher's post-hoc PLSD test.
The dopamine-induced down-regulation of mCII expression depends on the activation of D2 receptors
In order to further investigate the mechanisms underlying the DA-mediated depletion of mCII subunits, we focused on the interaction of DA with its membrane receptors, D2 and D1.
We measured the activity of mCII in neurons treated with 100 µm DA in the presence or absence of D1 and D2 receptor antagonists. Spiperone and haloperidol, two D2-receptor antagonists, totally abolished the effects of DA on mCII activity (Fig. 7A). In contrast, the D1-receptor antagonist SCH 23390 did not alter the DA-induced reduction of mCII/SDH activity. Similar experiments were performed to assess mCII subunit expression levels. Western blot analysis demonstrates that the loss of mCII Ip and Fp induced by DA was blocked by the D2 receptor antagonists (Fig. 7B). In contrast, the D1 receptor antagonist was ineffective. Another selective antagonist of D2 receptors, raclopride, also blocked the effect of DA on mCII subunit expression, whereas the direct activation of D2 receptors by the DA agonist quinpirole significantly reduced mCII expression in striatal cells, replicating the effect of DA (Fig. 8).
Figure 7.
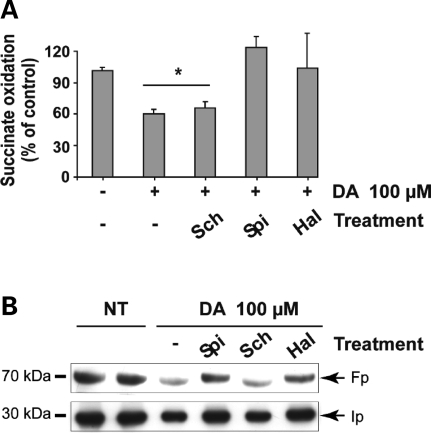
D2 receptor inhibition reduces the DA-induced loss of mCII subunit expression and activity. Neurons were treated with 100 µm DA for 24 h in the presence or absence of the D2 receptor antagonists spiperone (SPI) and haloperidol (HAL), and the D1 receptor antagonist SCH23390 (SCH). (A) Quantification of the catalytic activity of mCII (succinate dehydrogenase). (B) Western blot analysis of Fp and Ip corresponding to the culture wells analyzed for mCII activity above. In both cases, the blockade of D2 receptors abolishes the loss of mCII triggered by DA, while D1 receptor blockade has no effect. *P < 0.05; ANOVA and Fisher's post-hoc PLSD test.
Figure 8.
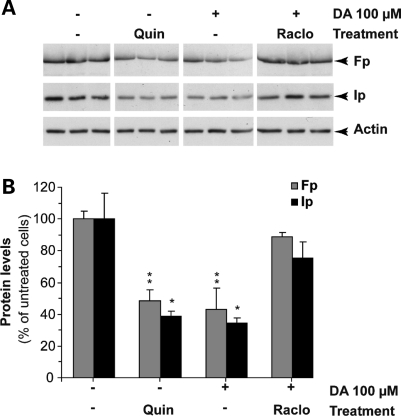
Activation of D2 receptors down-regulates the level of mCII subunits. Striatal cultures were treated for 24 h with either the D2 agonist quinpirole (QUIN) or with 100µm DA in the presence or absence of the D2 antagonist raclopride (RACLO). (A) Representative western blot showing the levels of Ip and Fp under the various experimental conditions (all images are from the same blot). (B) Quantification of western blots for Ip and Fp. Note that quinpirole mimics the effect of DA on Ip and Fp expression, while raclopride has a restorative effect similar to that of spiperone and haloperidol (see Fig. 7). *P < 0.05; **P < 0.01 (Untreated and raclopride+DA groups compared with quinpirole and DA only groups). ANOVA and Fisher's post-hoc PLSD test.
These data suggest that DA regulates the expression of mCII activity by decreasing Ip and Fp subunit expression, an effect at least partly mediated by D2 receptor activation.
DISCUSSION
The present study shows that the treatment of primary cultures of striatal neurons with DA induces neurodegeneration that is accompanied by the decreased levels of an important mitochondrial enzyme, mCII. This is in agreement with previous findings, including studies of primary neuronal cultures, in which DA was consistently found to be cytotoxic at the concentration range used in our study (reviewed in 15). These in vitro results are highly relevant to the vulnerability of striatal cells in vivo. Indeed, an acute elevation of DA levels in the striatum by intrastriatal stereotaxic injections induces neuronal degeneration (17). A sustained increase in extracellular concentrations of DA in DAT KO mice also leads progressively to striatal cell loss (16). However, the mechanisms involved in DA toxicity have not yet been fully elucidated (13).
In the present study, we hypothesized that DA induces neurodegeneration through the reduction of mCII expression. In support of this hypothesis, we found that DA reduces mCII catalytic activity. This effect is associated with a reduction in the expression of the Ip and Fp subunits of mCII, as indicated by western blotting and quantitative RT–PCR assays. Importantly, when the expression of these subunits is artificially increased using lentiviral vectors, DA appears to be significantly less toxic, indicating that the loss of mCII is instrumental in DA-induced neurodegeneration. Since the lentiviral vectors we use in the present study preferentially transduce neurons (28,29,31), these results suggest that the phenomena we observe mainly involve neurons and not astrocytes. In line with this, our striatal cultures mostly contain neurons with a low proportion of astrocytes. In addition, we showed that mCII expression in astrocytes is insensitive to dopamine treatment, even at concentrations shown to kill neurons.
Interestingly, we demonstrated that mCII depletion after DA treatment is linked to D2 activation, as D2 (but not D1) antagonists block the DA-induced reduction in mCII subunit expression. Further supporting the potential role of D2 receptors, the agonist quinpirole mimics the effects of DA on mCII expression. To our knowledge, this is the first evidence that DA might directly regulate mitochondrial physiology through a specific signaling pathway involving DA membrane receptors.
The underlying mechanisms linking D2 receptor activation and the down-regulation of mCII remain to be elucidated. A careful analysis of the effects of DA on neuronal survival indicates that the reduction in mCII levels occurs in the absence of detectable cell loss, and is highly selective. In addition, the mRNA levels of mCII subunits are depleted transiently. These observations rule out any possibility that the loss of mCII is proportional to the extent of cell death, and indicate the existence of a relatively specific mechanism that controls mCII levels. DA signaling through the D2 receptor subfamily is complex and involves multiple factors, including heterotrimeric G proteins, adenylyl cyclase, cAMP, PKA and intracellular Ca2+ (reviewed in 32). Other signaling pathways have recently been unraveled. Of particular interest is the finding that D2-like receptors signal by down-regulating Akt via a beta-arrestin-2 and protein phosphate 2A interaction (33). The activation of ERK has also been reported after D2 receptor stimulation (34). Ongoing experiments in our lab aim to discover which of the pathways activated by D2-like receptors might regulate mCII.
Our study identifies a novel pathway linking DA signaling to mitochondrial function. There is a growing body of evidence indicating that mitochondria can be ‘linked’ to the external environment through a variety of mechanisms/pathways. Mitochondria receive a variety of signals influencing the death/survival balance, and play a central role in apoptosis (35). The integration of signals at the mitochondrial level could also participate in other physiological processes not directly associated with cell death. For example, during neuronal activation at the glutamatergic synapse, post-synaptic mitochondria are sensors of synaptic activity through their capacity to rapidly uptake the Ca2+ that enters via activated cation channels (36). Ca2+ changes within the mitochondria have important functional consequences, including the modulation of respiratory chain activity. In line with this, a new type of mechanism linking an extracellular signal to mitochondrial regulation through the intervention of transmembrane proteins, the integrins, has been described (37). Similarly, the link between D2 receptor signaling and mCII might constitute an original mechanism allowing post-synaptic regulation in response to a sustained elevation of dopamine concentrations. The physiology of this pathway remains to be defined, but might represent an integrated response for controlling energy demand. Consistent with this speculation, the stimulation of D2 receptors is generally considered to inhibit striatal medium spiny neuron firing. Thus, a D2-induced reduction in energy consumption associated with reduced firing might be coordinated on a long-term basis with a reduction of energy production through the regulation of mCII expression.
The present results indicate that the effect of DA on mCII expression may play a role in pathological conditions associated with striatal degeneration, especially in HD. The striatum is a brain region that plays an important role in the control of movement and cognitive function. For unknown reasons, the striatum is damaged in several neurodegenerative disorders associated with a chronic impairment of energy metabolism (1). Intoxication with mitochondrial toxins often induces striatal necrosis. This exquisite sensitivity of the striatum to impairments in energy metabolism remains a mystery, but convergent lines of evidence indicate that the nigro-striatal dopaminergic pathway may play a ‘permissive’ or ‘facilitating’ role (reviewed in 12). Our present results further substantiate this hypothesis and demonstrate that DA, via an interaction with D2 receptors, directly regulates the levels of mCII in striatal neurons. mCII has an important role at the intersection of the TCA cycle and the respiratory chain, since the reduction of succinate to fumarate results in electron feeding to ubiquinone and complex III (ubiquinone–cytochrome c oxido-reductase). Thus mCII plays a key role in oxidative energy metabolism. In pathological situations where DA metabolism or levels are increased, as during hypoxia, ischemia and intoxication with mitochondrial poisons, the down-regulation of mCII would further increase the vulnerability of striatal cells to cell death.
We observed that DA in association with mutated Htt expression cumulatively reduces mCII expression. Under our experimental conditions, this enhanced reduction synergistically triggers neurodegeneration, whereas neither DA nor mutated Htt alone were toxic. We also demonstrate that the overexpression of mCII using lentiviral vector-mediated gene transfer protects striatal neurons against the toxicity induced by the combination of mutated Htt and DA. This further supports the hypothesis that mCII deficits play a role in striatal degeneration in HD (28). We previously showed that mCII/SDH expression is preferentially lowered both in the striatum of HD patients and in primary striatal neurons expressing the N-terminal segment of mutated Htt (171 amino acid with 82 glutamine repeats), while the expression of other mitochondrial proteins remains essentially unchanged (28). Interestingly, the reduction in Fp and Ip proteins was not seen in the cerebral cortex or cerebellum of HD patients, suggesting that the preferential loss of mCII in the striatum of HD patients (26–28) might involve, at least in part, a factor highly enriched in the striatum. The present study suggests that DA, present at high concentrations in the striatum, could augment the mCII deficits mediated by mutated Htt.
No evidence exists for the increased release of DA in HD. The concentrations of DA and its metabolites in the HD striatum are relatively conserved or even slightly reduced in grade II/III patients (13,38). PET scan studies with 11C-raclopride have demonstrated a reduction in the density of D2 antagonist binding sites at the onset of the disease (39). DA signaling dysfunctions have also been reported in transgenic mouse models of HD (40). This apparent ‘slow-down’ of DA signaling in HD does not contradict our hypothesis that DA could play a role in HD pathogenesis. First, the effect of DA signaling on mCII regulation might occur at a relatively early stage of the disease. Another important aspect is that if an apparent ‘slow-down’ of DA signaling does take place, a sustained down-regulating effect of residual DA on mCII expression in the HD striatum could be maintained and be larger than in regions devoid of major DA afferents, such as the cerebral cortex. Therefore, residual DA signaling might still be able to enhance the effects of mutant Htt on mCII in the striatum of HD patients, supporting the idea that the DA signaling pathway is a therapeutic target for slowing disease progression. Further supporting this hypothesis, the recent work of Tang et al. (22) demonstrated that in mice expressing the full length mutant Htt and treated with L-DOPA to elevate dopamine concentrations and accelerate striatal degeneration, the neuroleptic tetrabenazine was neuroprotective. In addition, we recently reported that chronic treatment with the D2 antagonist haloperidol significantly reduces the striatal toxicity Htt171-82Q in vivo (41). In line with this, Stack et al. (42) showed that 6OHDA-induced degeneration of the nigro-striatal dopaminergic pathway in R6/2 HD mice improves the pathology, in particular extends survival.
In conclusion, the present study demonstrates that DA, through a D2-mediated mechanism, regulates the expression levels and catalytic activity of mCII. Our results indicate that through this novel pathway, DA may act synergistically with mutant Htt to trigger the preferential degeneration of the striatum in HD.
MATERIALS AND METHODS
Primary embryonic striatal neurons and primary culture of astrocytes
Primary striatal neurons from E14 to E15 rat embryos were cultured as previously described (28,29). Timed-pregnant Sprague-Dawley rats (Janvier, Le Genest-St-Isle, France) were killed with a lethal dose of pentobarbital, and embryos were quickly removed and dissected in cooled Hank's balanced salt solution (without Ca2+ and Mg2+, Sigma). Ganglionic eminences were isolated and incubated for 15 min at 37°C in 0.3 mg/ml DNAse I (Sigma, St Louis). The tissue was mechanically dissociated with a fire-polished Pasteur pipette and the debris removed by decantation of the suspension. The cells were then concentrated by centrifugation (20°C, 5 min, 1500 g) and resuspended in serum-free Neurobasal medium supplemented with 2% B27 supplement (Gibco Brand, InvitroGen, Carlsbad, CA), 1% antibiotic–antifungal mixture (Gibco), and 0.5 mm l-Glutamine (Sigma). The cells were plated at a density of 400 000 cells/well in 24-well Costar® plates coated with 50 µg/ml 30–70 kDa Poly-d-lysine (Sigma). The cultures were kept in a humid incubator (5% CO2, 37°C) and half the medium was changed once per week.
Primary cultures of astrocytes were prepared from striatum of E19 embryos of Sprague-Dawley rats according to the method of Vega et al. (43) with modification. Striatum were dissected in cooled Hank's balanced salt solution (without Ca2+ and Mg2+, Sigma) and placed in a medium containing 50% Dulbecco's modified Eagle's medium (DMEM; Gibco), 50% F12 (Gibco), 10% SVF and 1% antibiotic–antifungal mixture (Gibco). Cells were dissociated through needles of decreasing gauge sizes (1.1 × 40 mm, 0.9 × 25 mm, 0.6 × 20 mm). Then cells were plated at 200 000 cell/cm2. Culture medium was replaced 4–5 days after seeding and subsequently every 2–3 days. Before changing medium, plates were gently tapped to remove the less adherent oligodendrocytes, oligodendrocyte-type 2 astrocyte progenitor cells and microglia, but not the astrocytes. Three weeks after plating, cultures were treated with increasing doses of dopamine (100–300 µm) for 24 h.
Lentivirus construction, production and infection
The cDNA coding for the first 171 amino acids of human huntingtin, containing 19 or 82 CAG repeats (htt171-19Q/82Q), GFP, DsRed, human SDH-A and SDH-B were cloned into the SIN-W-PGK transfer vector as previously described (28,29). Viral production was carried out in 293T cells with a four plasmid system (44). The viruses were re-suspended in PBS with 1% BSA and matched for particle concentration after measurement of p24 antigen content. Twenty-four hours after plating (1 day in vitro; DIV), the cell cultures described earlier were exposed to the lentiviral vectors, each at a concentration of 10 ng p24/105 cells. At 2 DIV, half of the medium was replaced with freshly prepared culture medium. For rescue experiments, Htt-encoding vectors alone or mixed with GFP encoding vectors (in a 2/1 lenti-Htt/lenti-GFP ratio) were used as described earlier and a second transduction was performed 1 week later with one of the SDH-encoding vectors or with the DsRed-encoding vector used as a control. Previous quantitative RT–PCR analyses at 3 weeks have shown that the transcriptional levels of Htt171-82Q and Htt171-19Q are similar when lentiviral vectors are matched for p24 antigen levels (29). The transduction efficiency, as demonstrated by the increased expression of SDH-A (Fp subunit) and SDH-B (Ip subunit), was re-tested by western blot analysis at 21 DIV, or 2 weeks after treating cells with the lentiviral vectors (28).
Dopamine treatment, cell death evaluation and immunofluorescence
DA (Sigma) was freshly prepared as a 100× solution in a Neurobasal medium and directly added to the culture medium of 4 week in vitro cultures, at least 5 days after changing half the medium. Cell death was evaluated by different approaches. The level of LDH that is released upon cell death was measured in culture medium samples (20 µl) collected at 24 h, using the LDH colorimetric assay kit (Roche Pharma, Switzerland) following the manufacturer's instructions (30). Cell viability was also assessed after DA treatment by colorimetric measurement of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) reduction as previously described (45). In one experiment, FACS analysis of the number of cells positive for TUNEL (an indicator of death related to DNA fragmentation) was performed as described earlier (28,29).
Cells were directly counted after co-transduction with lentiviral vectors encoding GFP. For this, we took advantage of lentiviral vectors that preferentially transduce neurons (29). GFP-positive cells were counted using an inverted epifluorescence microscope (CKX41, Olympus, France) equipped with an image acquisition and analysis system (Explora Nova, La Rochelle, France). Five-to-ten fields of view randomly positioned throughout the coverslips were acquired. The number of GFP-positive cells in the field of view was automatically determined by thresholding the image. The average number of GFP cells per field of view was calculated for each coverslip. Between 4 and 8 cell-culture wells/coverslips per experimental condition, in triplicate, were used for statistical analysis.
For determination of the proportion of astrocytes versus neurons immunofluorescence labeling of cultured cells was performed on coverslips at 3 and 4 week in culture. After fixation with PFA 4%, and blocking with normal goat serum, cells were incubated with a monoclonal mouse anti-TUJ-1 (Co ance, 1:500), a mouse monoclonal anti-MAP2 (Chemicon, 1:500) or a mouse monoclonal anti-GFAP (Sigma, 1:200). Fluorescent secondary antibody was a goat anti-mouse Alexa-555 (1:1000). For FACS analysis of cell markers, the secondary antibody was a goat anti-mouse Alexa-488 (1:1000).
Western blot analysis
Cultures were trypsinized and collected by centrifugation (1000 g, 10 min). The pellets were incubated on ice in lysis buffer [150 mm NaCl, 50 mm Tris pH 8.0, 5 mm EDTA, 0.5% Triton, 1% NP 40 and 0.5% protease inhibitor cocktail (Sigma)] for 30 min with vortexing every 10 min. Homogenates were centrifuged at 13 000g for 30 min at 4°C and supernatants were stored at −80°C. Protein concentrations were determined with the BCA protein assay (Pierce, Rockford, USA). Protein (10 µg) was resolved on a 12% SDS–PAGE gel and transferred onto polyvinylidene difluoride membranes. Membranes were incubated in 5% nonfat dry milk in TBS containing 0.1% Tween 20 (T-TBS) for 2 h at room temperature with the following primary mouse monoclonal antibodies (Molecular Probes): anti-70 kDa (SDH-A or Fp) subunit of complex II, 1/3000; anti-30 kDa (SDH-B or Ip) subunit of complex II, 1/3000; anti- cytochrome oxidase (complex IV) subunit IV, 1/500; anti-39 kDa subunit of complex I (1/1000). The membranes were also probed with a polyclonal anti-BclXL antibody (1/1000, Burnham Institute). After a 1 h-incubation with anti-mouse or anti-rabbit IgG HRP-coupled secondary antibodies (1/5000, Amersham Pharmacia Biotech., Les Ulis, France), the antigens were revealed using Enhanced Chemiluminescence Reaction (ECL, Amersham Pharmacia Biotech.). Blots were routinely stripped in a denaturing solution (Tris–HCl 0.5 M pH 6.8, SDS 10%, beta-mercaptoethanol 0.8%), and reprobed with a monoclonal anti-alpha-subunit of mitochondrial complex V (F1-ATPase, Molecular Probes) and an anti-actin (monoclonal/polyclonal—which species) (1/10 000, Sigma). Coomassie blue was used as an absolute control for total protein loading. Signal intensity was quantified using TotaLab software (Amersham) and normalized to coomassie values (28).
SDH activity assay
SDH activity was measured using Iodonitrotetrazolium chloride (INT, Sigma) as previously described (46). Typically, 1.5 × 106 cells were homogenized using a glass dounce homogenizer in a mitochondrial buffer containing 20 mm Tris–HCl pH 7.2, 250 mm saccharose, 2 mm EGTA, 40 mm KCl and 1 mg/ml BSA. Homogenates were centrifuged at 1500g for 5 min at 4°C and the supernatant processed immediately for SDH activity, or stored at −80°C until further use. The homogenate (20 µl) was diluted in 2× activity buffer (100 mm Tris–HCL pH 8.3, 1 mm EDTA) containing 20 mm succinate. The reaction was started by adding 2 mm of INT, and samples incubated for 90 min at 37°C. The formation of red formazan produced by INT reduction was measured at 490 nm with a spectrometer (Biorad). OD values were normalized to the quantity of protein used; background OD was measured by omitting succinate from the reaction medium. For each experimental condition, background OD was subtracted from the total OD in the presence of succinate to obtain INT reduction due specifically to SDH activity.
Real-time RT–PCR
Total RNA was extracted from 1.5 × 106 cells using guanidium thiocyanate/phenol followed by digestion with RQ1 DNase (Promega, France). The RT reaction was performed with 200 ng of total RNA using Superscript II reverse transcriptase (Invitrogen) followed by treatment with RNase H. Real-time quantitative PCR was carried out with 1% of the RT product, using an ABI 7000 Sequence Detection System (Applied Biosystems), and PCR products were quantified by measuring SYBR Green fluorescent dye incorporation. Values obtained for SDH mRNA were normalized to the corresponding value of the reference mRNA for cyclophilin A. Primers were designed using Oligo 6.3 software and are available from the authors upon request.
Statistical analysis
Data are expressed as mean±SEM. One way ANOVA was performed to compare differences between groups, followed by a Fisher's post-hoc PLSD test. A P-value <0.05 was considered significant.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the CEA, CNRS and Hereditary Disease Foundation. This study also benefited from support of the HighQ Foundation and NIH (NS 036821 for S.K.).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Nathalie Lefort for her help with the FACS analyses and Dr Jocelyne Caboche and Delphine Charvin for their fruitful discussions related to dopamine. We also thank Mathilde Faideau, Angélique Colin and Gilles Bonvento for helping in setting up astrocyte cultures. Dr S. Rasika of Gap Junction (www.gap-junction.com) assisted with English editing. We are also grateful to Dr Sharon Schendel and Tara Mabry for English editing. A.B. is the recipient of a post-doctoral fellowship from the CEA. Y.T. is the recipient of a post-doctoral fellowship from the Hereditary Disease Foundation. Funding to pay the Open Access publication charges for this article was provide by … .
Conflict of Interest statement. None declared.
REFERENCES
- 1.Beal M.F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann. Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- 2.Harper P.S. Huntington's Disease. London: WB Saunders Company Ltd; 1991. [Google Scholar]
- 3.Ferrante R.J., Kowall N.W., Beal M.F., Martin J.B., Bird E.D., Richardson E.P., Jr Morphologic and histochemical characteristics of a spared subset of striatal neurons in Huntington's disease. J. Neuropathol. Exp. Neurol. 1987;46:12–27. doi: 10.1097/00005072-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 4.The Huntington Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 5.Graham R.K., Deng Y., Slow E.J., Haigh B., Bissada N., Lu G., Pearson J., Shehadeh J., Bertram L., Murphy Z., et al. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125:1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo E., Zuccato C., Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat. Rev. Neurosci. 2005;6:919–930. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- 7.Gervais F.G., Singaraja R., Xanthoudakis S., Gutekunst C.A., Leavitt B.R., Metzler M., Hackam A.S., Tam J., Vaillancourt J.P., Houtzager V., et al. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat. Cell Biol. 2002;4:95–105. doi: 10.1038/ncb735. [DOI] [PubMed] [Google Scholar]
- 8.Rigamonti D., Bauer J.H., De-Fraja C., Conti L., Sipione S., Sciorati C., Clementi E., Hackam A., Hayden M.R., Li Y., et al. Wild-type huntingtin protects from apoptosis upstream of caspase-3. J. Neurosci. 2000;20:3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigamonti D., Sipione S., Goffredo D., Zuccato C., Fossale E., Cattaneo E. Huntingtin's neuroprotective activity occurs via inhibition of procaspase-9 processing. J. Biol. Chem. 2001;276:14545–14548. doi: 10.1074/jbc.C100044200. [DOI] [PubMed] [Google Scholar]
- 10.Zuccato C., Ciammola A., Rigamonti D., Leavitt B.R., Goffredo D., Conti L., MacDonald M.E., Friedlander R.M., Silani V., Hayden M.R., et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier L.R., Charrin B.C., Borrell-Pages M., Dompierre J.P., Rangone H., Cordelieres F.P., De Mey J., MacDonald M.E., Lessmann V., Humbert S., et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Brouillet E., Jacquard C., Bizat N., Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington's disease. J. Neurochem. 2005;95:1521–1540. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- 13.Jakel R.J., Maragos W.F. Neuronal cell death in Huntington's disease: a potential role for dopamine. Trends Neurosci. 2000;23:239–245. doi: 10.1016/s0166-2236(00)01568-x. [DOI] [PubMed] [Google Scholar]
- 14.Hoyt K.R., Reynolds I.J., Hastings T.G. Mechanisms of dopamine-induced cell death in cultured rat forebrain neurons: interactions with and differences from glutamate-induced cell death. Exp. Neurol. 1997;143:269–281. doi: 10.1006/exnr.1996.6374. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin B.A., Nelson D., Erecinska M., Chesselet M.F. Toxicity of dopamine to striatal neurons in vitro and potentiation of cell death by a mitochondrial inhibitor. J. Neurochem. 1998;70:2406–2415. doi: 10.1046/j.1471-4159.1998.70062406.x. [DOI] [PubMed] [Google Scholar]
- 16.Cyr M., Beaulieu J.M., Laakso A., Sotnikova T.D., Yao W.D., Bohn L.M., Gainetdinov R.R., Caron M.G. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl Acad. Sci. USA. 2003;100:11035–11040. doi: 10.1073/pnas.1831768100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filloux F., Townsend J.J. Pre- and postsynaptic neurotoxic effects of dopamine demonstrated by intrastriatal injection. Exp. Neurol. 1993;119:79–88. doi: 10.1006/exnr.1993.1008. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds D.S., Carter R.J., Morton A.J. Dopamine modulates the susceptibility of striatal neurons to 3-nitropropionic acid in the rat model of Huntington's disease. J. Neurosci. 1998;18:10116–10127. doi: 10.1523/JNEUROSCI.18-23-10116.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowyer J.F., Clausing P., Schmued L., Davies D.L., Binienda Z., Newport G.D., Scallet A.C., Slikker W., Jr Parenterally administered 3-nitropropionic acid and amphetamine can combine to produce damage to terminals and cell bodies in the striatum. Brain Res. 1996;712:221–229. doi: 10.1016/0006-8993(95)01417-9. [DOI] [PubMed] [Google Scholar]
- 20.Charvin D., Vanhoutte P., Pages C., Borrelli E., Caboche J. Unraveling a role for dopamine in Huntington's disease: the dual role of reactive oxygen species and D2 receptor stimulation. Proc. Natl Acad. Sci. USA. 2005;102:12218–12223. doi: 10.1073/pnas.0502698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cyr M., Sotnikova T.D., Gainetdinov R.R., Caron M.G. Dopamine enhances motor and neuropathological consequences of polyglutamine expanded huntingtin. FASEB J. 2006;20:2541–2543. doi: 10.1096/fj.06-6533fje. [DOI] [PubMed] [Google Scholar]
- 22.Tang T.S., Chen X., Liu J., Bezprozvanny I. Dopaminergic signaling and striatal neurodegeneration in Huntington's disease. J. Neurosci. 2007;27:7899–7910. doi: 10.1523/JNEUROSCI.1396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae B.I., Xu H., Igarashi S., Fujimuro M., Agrawal N., Taya Y., Hayward S.D., Moran T.H., Montell C., Ross C.A., et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Sawa A., Wiegand G.W., Cooper J., Margolis R.L., Sharp A.H., Lawler J.F., Jr, Greenamyre J.T., Snyder S.H., Ross C.A. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 1999;5:1194–1198. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- 25.Panov A.V., Gutekunst C.A., Leavitt B.R., Hayden M.R., Burke J.R., Strittmatter W.J., Greenamyre J.T. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 26.Browne S.E., Bowling A.C., MacGarvey U., Baik M.J., Berger S.C., Muqit M.M., Bird E.D., Beal M.F. Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann. Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 27.Gu M., Gash M.T., Mann V.M., Javoy-Agid F., Cooper J.M., Schapira A.H. Mitochondrial defect in Huntington's disease caudate nucleus. Ann. Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 28.Benchoua A., Trioulier Y., Zala D., Gaillard M.C., Lefort N., Dufour N., Saudou F., Elalouf J.M., Hirsch E., Hantraye P., et al. Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol. Biol. Cell. 2006;17:1652–1663. doi: 10.1091/mbc.E05-07-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zala D., Benchoua A., Brouillet E., Perrin V., Gaillard M.C., Zurn A.D., Aebischer P., Deglon N. Progressive and selective striatal degeneration in primary neuronal cultures using lentiviral vector coding for a mutant huntingtin fragment. Neurobiol. Dis. 2005;20:785–798. doi: 10.1016/j.nbd.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Jacquard C., Trioulier Y., Cosker F., Escartin C., Bizat N., Hantraye P., Cancela J.M., Bonvento G., Brouillet E. Brain mitochondrial defects amplify intracellular [Ca2+] rise and neurodegeneration but not Ca2+ entry during NMDA receptor activation. FASEB J. 2006;20:1021–1023. doi: 10.1096/fj.05-5085fje. [DOI] [PubMed] [Google Scholar]
- 31.Arango M., Holbert S., Zala D., Brouillet E., Pearson J., Regulier E., Thakur A.K., Aebischer P., Wetzel R., Deglon N., et al. CA150 expression delays striatal cell death in overexpression and knock-in conditions for mutant huntingtin neurotoxicity. J. Neurosci. 2006;26:4649–4659. doi: 10.1523/JNEUROSCI.5409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Missale C., Nash S.R., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 33.Beaulieu J.M., Sotnikova T.D., Marion S., Lefkowitz R.J., Gainetdinov R.R., Caron M.G. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Brami-Cherrier K., Valjent E., Garcia M., Pages C., Hipskind R.A., Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J. Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroemer G., Reed J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 36.Toescu E.C., Verkhratsky A., Landfield P.W. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Werner E., Werb Z. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. J. Cell. Biol. 2002;158:357–368. doi: 10.1083/jcb.200111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brouillet E., Conde F., Beal M.F., Hantraye P. Replicating Huntington's disease phenotype in experimental animals. Prog. Neurobiol. 1999;59:427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 39.Antonini A., Leenders K.L., Spiegel R., Meier D., Vontobel P., Weigell-Weber M., Sanchez-Pernaute R., de Yebenez J.G., Boesiger P., Weindl A., et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington's disease. Brain. 1996;119:2085–2095. doi: 10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 40.Bibb J.A., Yan Z., Svenningsson P., Snyder G.L., Pieribone V.A., Horiuchi A., Nairn A.C., Messer A., Greengard P. Severe deficiencies in dopamine signaling in presymptomatic Huntington's disease mice. Proc. Natl Acad. Sci. USA. 2000;97:6809–6814. doi: 10.1073/pnas.120166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charvin D., Roze E., Perrin V., Deyts C., Betuing S., Pagès C., Régulier E., Luthi-Carter R., Brouillet E., Deglon E., et al. Haloperidol protects striatal neurons from dysfunction induced by mutated huntingtin in vivo. Neurobiol. Dis. 2007 doi: 10.1016/j.nbd.2007.07.028. in press. [DOI] [PubMed] [Google Scholar]
- 42.Stack E.C., Dedeoglu A., Smith K.M., Cormier K., Kubilus J.K., Bogdanov M., Matson W.R., Yang L., Jenkins B.G., Luthi-Carter R., et al. Neuroprotective effects of synaptic modulation in Huntington's disease R6/2 mice. J. Neurosci. 2007;27:12908–12915. doi: 10.1523/JNEUROSCI.4318-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vega C., Pellerin L., Dantzer R., Magistretti P.J. Long-term modulation of glucose utilization by IL-1 alpha and TNF-alpha in astrocytes: Na+ pump activity as a potential target via distinct signaling mechanisms. Glia. 2002;39:10–18. doi: 10.1002/glia.10080. [DOI] [PubMed] [Google Scholar]
- 44.Hottinger A.F., Azzouz M., Deglon N., Aebischer P., Zurn A.D. Complete and long-term rescue of lesioned adult motoneurons by lentiviral-mediated expression of glial cell line-derived neurotrophic factor in the facial nucleus. J. Neurosci. 2000;20:5587–5593. doi: 10.1523/JNEUROSCI.20-15-05587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galas M.C., Bizat N., Cuvelier L., Bantubungi K., Brouillet E., Schiffmann S.N., Blum D. Death of cortical and striatal neurons induced by mitochondrial defect involves differential molecular mechanisms. Neurobiol. Dis. 2004;15:152–159. doi: 10.1016/j.nbd.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Munujos P., Coll-Canti J., Gonzalez-Sastre F., Gella F.J. Assay of succinate dehydrogenase activity by a colorimetric-continuous method using iodonitrotetrazolium chloride as electron acceptor. Anal. Biochem. 1993;212:506–509. doi: 10.1006/abio.1993.1360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.