Abstract
Distinct interrelationships between inflammation and β-amyloid-associated degeneration, the two major hallmarks of the skeletal muscle pathology in sporadic inclusion body myositis (sIBM), have remained elusive. Expression of markers relevant for these pathomechanisms were analysed in biopsies of sIBM, polymyositis (PM), dermatomyositis (DM), dystrophic and non-myopathic muscle as controls, and cultured human myotubes. By quantitative PCR, a higher upregulation was noted for the mRNA-expression of CXCL-9, CCL-3, CCL-4, IFN-γ, TNF-α and IL-1β in sIBM muscle compared to PM, DM and controls. All inflammatory myopathies displayed overexpression of degeneration-associated markers, yet only in sIBM, expression of the mRNA of amyloid precursor protein (APP) significantly and consistently correlated with inflammation in the muscle and mRNA-levels of chemokines and IFN-γ. Only in sIBM, immunohistochemical analysis revealed that inflammatory mediators including IL-1β co-localized to β-amyloid depositions within myofibres. In human myotubes, exposure to IL-1β caused upregulation of APP with subsequent intracellular aggregation of β-amyloid. Our data suggest that, in sIBM muscle, production of high amounts of pro-inflammatory mediators specifically induces β-amyloid-associated degeneration. The observations may help to design targeted treatment strategies for chronic inflammatory disorders of the skeletal muscle.
Keywords: muscle inflammation, protein aggregation, autoimmunity, β-amyloid, chemokines and cytokines
Introduction
Sporadic inclusion body myositis (sIBM) is the most prevalent myopathy acquired by individuals above 50 years of age. It is a severely disabling disorder of the skeletal muscle of unknown cause and no treatment is available (Askanas and Engel, 2006; Dalakas, 2006). The pathology consists of inflammation that includes upregulation of proinflammatory chemokines (De Paepe et al., 2008), most importantly CC- or CXC-chemokine ligands (CXCL)-9 (synonym: monokine induced by interferon-γ, MIG) and CXCL-10 (Raju et al., 2003; De Paepe et al., 2007), CCL-3 (macrophage inflammatory protein, MIP-1α) (Confalonieri et al., 2000; Civatte et al., 2005) and CCL-4 (MIP-1β) (Civatte et al., 2005), and cytokines (Tournadre and Miossec, 2007) such as interleukin (IL)-1β (Tews and Goebel, 1996; Lundberg et al., 1997) and tumour necrosis factor (TNF)-α (Tews and Goebel, 1996; Lundberg et al., 1997; De Bleecker et al., 1999), and transforming growth factor (TGF)-β (Lundberg et al., 1997). Other inflammatory mediators including CCL-2 have also been demonstrated to be crucial during the pathogenesis of inflammatory myopathies (De Bleecker et al., 2002). In this inflammatory environment, cytotoxic CD8+ T-cells are attracted to the muscle, where they clonally expand and attack myofibres that overexpress major histocompatibility complex (MHC)-class I (Schmidt et al., 2004; Wiendl et al., 2005). Moreover, the chronic inflammatory environment directly contributes to the damage of muscle cells as previously demonstrated for IL-1β and TNF-α (Broussard et al., 2004; Li et al., 2005).
The second hallmark of sIBM is accumulation of aberrant molecules, most of all β-amyloid, within the myofibres (Askanas and Engel, 2006). The source of β-amyloid is amyloid precursor protein (APP) that, when overexpressed in the muscle of mice, causes muscle weakness and atrophy (Fukuchi et al., 1998; Jin et al., 1998; Kitazawa et al., 2006; Sugarman et al., 2006). In vitro, overexpression of APP and accumulation of β-amyloid and/or tau protein functionally impairs muscle cells and induces features similar to myofibres in sIBM, including formation of vacuoles and intracellular protein aggregates (Askanas et al., 1996; Christensen et al., 2004). Accumulations of β-amyloid and even APP are toxic to the muscle as well as other cells in vivo and in vitro (Querfurth et al., 2001; Lu et al., 2003). Other cell-stress- and degeneration-associated molecules such as the small heat-shock molecule αB-crystallin and ubiquitin, a tagging molecule for abnormal proteins, are also overexpressed in sIBM (Banwell and Engel, 2000). As one possible link between inflammation and β-amyloid-associated degeneration, IL-1β has been proposed several years ago (Dalakas, 1998; Sondag and Combs, 2004).
To address whether pro-inflammatory and degeneration-associated molecules are unrelated events or trigger each other in sIBM, we quantified the expression of relevant molecules in sIBM muscle, arbitrarily selected based on previous publications and own data (Raju and Dalakas, 2005), and compared their mRNA levels and cellular localization of the respective protein with those in polymyositis (PM), dermatomyositis (DM) and non-myopathic control muscles. Using cultures of human myotubes, we also investigated the expression of inflammatory chemokines, cytokines and accumulation of β-amyloid under pro-inflammatory conditions.
Methods
Patients
We investigated the muscle biopsies of patients with sIBM (n = 12), PM (n = 12), DM (n = 11) or granulomatous myositis (n = 2), who each fulfilled the clinical, electrophysiological and histopathological criteria of the respective disease (Dalakas and Hohlfeld, 2003; Dalakas, 2006); the diagnostic criteria for PM included a myopathic muscle weakness and electromyogram, elevated creatine kinase and, on biopsy, a primary endomysial inflammation with a CD8/MHC-I complex and no rimmed vacuoles (Dalakas and Hohlfeld, 2003). Control muscle specimens were obtained from patients with amyotrophic lateral sclerosis (n = 3), myopathy due to mutations in the β-myosin chain gene (n = 2), one patient with a genetically defined myofibrillar myopathy, and six patients with morphologically normal muscle. As another control group, six patients with a dystrophic myopathy, where some secondary inflammation may also exist, were used. The patients were admitted to the National Institutes of Health (NIH) Clinical Centre (sIBM, DM, controls), the University of Göttingen, Germany, (sIBM, PM, controls) and the Friedrich-Baur-Institute, Muscle Tissue Culture Collection (muscle dystrophy [MD]-NET, partner of Eurobiobank), in München, Germany (PM) and studied under Institutional Review Board (IRB)-approved clinical protocols.
Muscle biopsies and immunohistochemistry
Five-micrometer frozen sections of muscle biopsy specimens were fixed in 4% paraformaldehyde at room temperature (for β-amyloid, IFN-γ) or acetone at −20°C (all other stainings) for 10 min. Unspecific binding was reduced by 30 min incubation with 5% bovine serum albumin (BSA) and 3% goat or chicken serum (all from Jackson ImmunoResearch, West Grove, PA) in Tris–buffered saline (TBS, 0.05 M, pH 7.4, 0.15 M saline). All primary and secondary reagents were diluted in 1% BSA in TBS. The primary anti-human antibodies are listed in Table 1. Immunoreactivity was detected using Alexa-488, Alexa-594 or Alexa-350-conjugated highly pre-adsorbed secondary goat or chicken antibodies against mouse, rat, goat or rabbit IgG (all from Molecular Probes/Invitrogen, Carlsbad, CA). To avoid cross-reactions in double and triple labelings with primary goat antibodies, sections were blocked with chicken serum and the other secondary antibodies were preadsorbed with chicken serum as previously described (Schmidt et al., 2004). Negative controls were performed by omission of one of each primary antibody in every staining. Nuclear counterstaining was performed by DAPI (Molecular probes/Invitrogen) at 1:50 000 for 1 min, followed by mounting in Fluoromount G (Electron Microscopy Sciences, Hatfield, PA, USA). Immunofluorescent microscopy and digital photography was performed on a Zeiss Axiophot microscope (Zeiss, Göttingen, Germany), using appropriate filters for green (488 nm), red (594 nm) and blue (350 nm) fluorescence and a cooled CCD digital camera (Retiga 1300, Qimaging, Burnaby, BC, Canada) using the Qcapture software.
Table 1.
List of primary antibodies used for immunohistochemical staining of skeletal muscle biopsy tissue and cultured muscle cells
| Specificity | Host species and clone | Incubation and dilution or concentration | Supplier |
|---|---|---|---|
| αB-crystallin | Rabbit polyclonal | 1 h; 1 : 1000 dilution | Serotec, Oxford, UK |
| APP | Goat polyclonal | 1 h; 10 μg/ml | R&D, Minneapolis, MN |
| β-amyloid | Mouse clone 6E10 | 24 h/4°C; 10 μg/ml | Signet, Dedham, MA, USA |
| CCL-3 | Goat polyclonal | 24 h/4°C; 20 μg/ml | R&D |
| CCL-4 | Goat polyclonal | 24 h/4°C; 5 μg/ml | Abcam, Cambridge, MA |
| CXCL-9 | Goat polyclonal | 24 h/4°C; 10 μg/ml | R&D |
| Desmin | Rabbit polyclonal | 1 h; 30 μg/ml | Abcam |
| IFN-γ | Mouse clone 25723 | 24 h/4°C; 5.6 μg/ml | R&D |
| IL-1β | Rabbit polyclonal | 24 h/4°C; 10 µg/ml | Abcam |
| MHC-I | Rat clone YTH 862.2 | 1 h; 5 μg/ml | Serotec |
| NCAM | Mouse clone Eric-1 | 1 h; 2 μg/ml | Labvision/Neomarkers, Fremont, CA |
| TGF-β | Mouse clone MAB1032 | 1 h; 0.5 μg/ml | Chemicon, Temecula, CA |
| Ubiquitin | Rabbit polyclonal | 30 min; 15 μg/ml | DAKO, Carpinteria, CA |
For quantitative assessment of immunohistochemical stainings, grey-scale analysis was performed using the Scion image software (Scion Image software, Scioncorp., MD, USA). In coded hematoxylin/eosin sections from all IBM, PM, DM and control patients, the degree of endomysial inflammation was evaluated in the entire area of two cross-sections from one biopsy at 100× magnification. Similar to a recent report (Schmidt et al., 2004), a semi-quantitative grading system ranging from 0 to 3 has been applied for this purpose with no inflammation (grade 0); mild inflammation (up to ∼25% of the biopsy showed invasion by inflammatory cells: grade 1); moderate inflammation (<50% of the biopsy was invaded by inflammatory cells: grade 2); severe inflammation (inflammatory cells were present in an area of 50% and above: grade 3). Each biopsy was evaluated by at least two independent investigators and a mean grade was calculated.
To quantify stainings for β-amyloid and MHC-I, a mean of 145 muscle fibres per patient were analysed by microscopy and digital photography by K.B. and Ingrid Müller (University of Göttingen).
Extraction of mRNA and RT-PCR
RT–PCR was performed as previously described (Schmidt et al., 2004). Total RNA was extracted from muscle biopsies using a kit (RNeasy from Quiagen, Valencia, CA, USA), following the supplier's instructions. In brief, the tissue was homogenized with a plastic tissue grinder and pestle (Kontes Glass Company, Vineland, NJ, USA) in 350 µl lysis buffer. RNA was eluted in 30 µl water and stored at −80°C. cDNA synthesis was performed with the SuperScript II (Invitrogen) reverse transcriptase, following the supplier's instructions. The resulting cDNA was stored at −20°C. For amplification, 5 ng cDNA was used in a 20 µl ready-to-use master mix for quantitative (real-time) PCR (Eurogentec, Serainc, Belgium). Using 6-carboxy-fluorescein (FAM)-labelled probes and specific primers (Applied Biosystems, Foster City, CA, USA): Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Hs99999905_m1); APP (Hs00169098_m1), TGFβ1 (Hs00171257_m); IL-1β (Hs00174097_m1); CCL-3 (Hs00234142_m1); UBB (Hs00430290_m1); CXCL-9 (Hs00171065_m1); αB-crystallin (Hs00157107_m1); NCAM (Hs00169851_m1); desmin (Hs00157258_m1); CCL-4 (Hs00605740_g1); IFN-γ (Hs00174143_m1); TNF-α (Hs00174128_m1); IL-6 (Hs00174131_m1). The reactions were run in duplicates on an Opticon 2 DNA engine (MJ research/Applied Biosystems), following the standard cycle protocol and instructions given by the supplier. Target mRNA-expression was quantified using the Δc(t) method in relation to expression of GAPDH mRNA. For all targets with a difference in the duplicate expression of greater than one c(t), a re-analysis in triplicates was performed. The quantitative expression analysis of all targets remained within the linear part of the amplification by PCR.
Cell culture stimulation studies
From diagnostic biopsies of patients without myopathic changes, muscle cell progenitors (satellite cells) were grown using a modified protocol (Askanas et al., 1996; Sugiura et al., 2002): a 3 × 3 × 3 mm3 sized piece of muscle was thoroughly minced using a scissor. After washing in PBS, the muscle was trypsinized in three subsequent fractions of 15 min. each at 37°C. All fragments were collected and seeded in a 25 cm2 flask in DMEM with pyruvate, high glucose and glutamine (Gibco/Invitrogen), supplemented with 10% FCS (Cambrex Bio Science, Walkersville, MD, USA), penicillin, streptomycin (all from Gibco/Invitrogen) and 0.5% chick embryo extract (Accurate chemicals, Westbury, NY, USA). After two to three subcultures over 2–3 weeks, myoblasts were labelled with NCAM (anti-CD56, mouse clone Eric-1, Neomarkers/Labvision, Fremont, CA, USA), magnetic bead-labelled secondary antibodies and separated by magnets, following the supplier's protocol (Dynal/Invitrogen). After expansion during two to three subcultures over 2–3 weeks, myoblasts were seeded in 8-chamber slides (LabTek II from Nunc, Rochester, NY, USA) and 24-well plates (Nunc). At 80% confluence, fusion was induced by switching to DMEM supplemented with 2% horse serum (Gibco/Invitrogen), penicillin and streptomycin. After 3–5 days, multinucleated myotubes had formed that typically reached 95% purity as revealed by immunohistochemical staining for the muscle marker desmin. Chamber slides and culture wells in duplicates were exposed to the cytokines IFN-γ (100–1000 U/ml), TNF-α (1–10 ng/ml), IL-1β (1–20 ng/ml) or TGF-β (1–30 ng/ml) (all from R&D), alone or in combinations, for 4–72 h in serum free medium X-vivo 15 (Cambrex Bio Science). Controls were kept in X-vivo 15 only. After washing in prewarmed PBS, the chamber slides were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) at room temperature or in acetone at −20°C and stored at −80°C until staining (see above). Culture wells were used for RNA-extraction with subsequent quantitative (real-time) PCR analysis as described above or protein-extraction with subsequent Western blot as described below. Secretion of selected cytokines and chemokines (CCL-3, CXCL-9, IL-1β, TNF-α) was determined by analysis of the supernatant by multiplexed sandwich ELISA (Endogen- Searchlight™ proteome array by Pierce Biotechnology, Woburn, MA, USA).
Western blot
Cells were washed once with cold PBS and lysed in lysis buffer (20 mM Hepes, 150 mM NaCl, 2 mM EDTA, 1% NP40, pH 7.9) containing protease inhibitors (complete protease inhibitor cocktail tablets; Roche, Mannheim, Germany). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). After blocking with 5% skimmed milk in TBS for 1 h, membranes were incubated over night at 4°C with the primary antibodies anti-APP (rabbit polyclonal antibody C9, diluted 1:2000, kindly provided by Dennis J. Selkoe, Centre for Neurologic Diseases, Harvard Medical School and Brigham and Women's Hospital, Boston, MA, USA) and anti-β-actin (mouse monoclonal, diluted 1:1000, Sigma, St Louis, MO, USA). Horseradish peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse antibodies (GE Healthcare, Buckinghamshire, UK) were used as secondary reagents. Blots were developed with the enhanced chemiluminescence technique (ChemiGlow West; Alpha Innotech, San Leandro, CA, USA) following the supplier's protocol and visualization on X-ray films (Amersham Hyperfilm™ECL; GE Healthcare).
Statistics
For statistical analysis of non-Gaussian distributed mRNA-expression results, the non-parametric Mann–Whitney U-test and Spearman r-correlation were used. An unpaired t-test was used for the analysis of all normally distributed results and Grubb's test for detection of outliers. All statistics were calculated using GraphPad Prism V4.0 (San Diego, CA, USA) and *P < 0.05, **P < 0.01, ***P < 0.001 as statistically significant values.
Results
Higher expression of inflammatory mediators in sIBM compared to PM and DM muscle
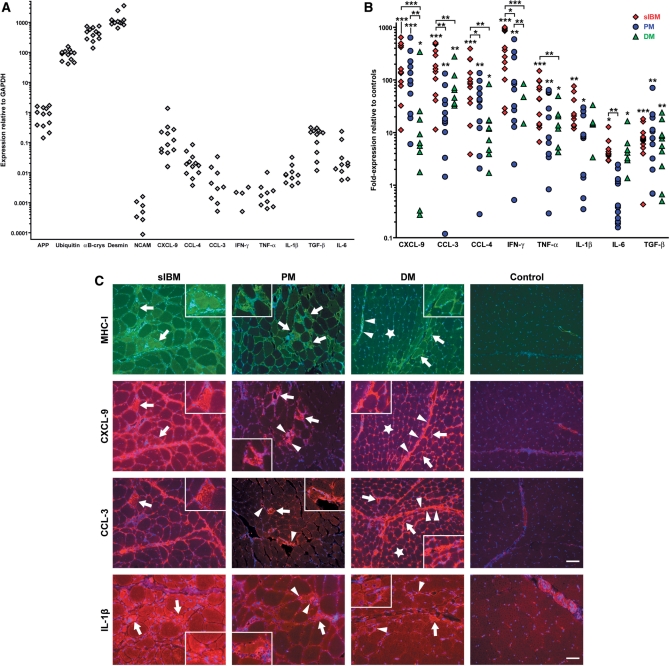
By quantitative (real-time) PCR, the mRNA expression of key cytokines and chemokines in the muscle biopsies was compared between non-myopathic controls, sIBM, PM and DM. Except for IFN-γ, present in 4 out 12 samples, a basal expression of all markers was noted in most non-myopathic control patients (expression relative to GAPDH; Fig. 1A). All three chemokines CXCL-9, CCL-4 and CCL-3 were significantly upregulated in sIBM versus controls by 123–235-fold (Fig. 1B: data indicate the fold- expression relative to values of controls from Fig. 1A). Their expression remained clearly lower in PM (31–152-fold; all significant versus controls; CXCL-9 significant versus DM; CCL-3 and CCL-4 significant versus sIBM) and DM (14- to 62-fold; all significant versus controls and sIBM; CXCL-9 significant versus PM). By immunohistochemistry, CXCL-9 was widely expressed on the surface of myofibres in sIBM and, as revealed by double labelling, co-localized and corresponded well with MHC-I as the standard upregulated inflammatory marker in myofibres (Fig. 1C). In contrast, in PM, the signal on myofibres was lower and restricted to areas of severe inflammation. Immune cells were positive for CXCL-9 in PM as well as in sIBM. In DM, the signal for CXCL-9 was much more prevalent in capillaries, immune cells and connective tissue; only some perifascicular myofibres, which were also positive for MHC-I, stained positive for CXCL-9. In non-myopathic control tissue, staining for CXCL-9 displayed only little signal in some blood vessels, immune cells and connective tissue (Fig. 1C). In all three inflammatory myopathies compared to controls, staining for CCL-3 revealed a very similar pattern as for CXCL-9, but remained at a lower level (Fig. 1C). Immunohistochemical labelling for CCL-4 displayed a pattern that was essentially identical to CCL-3 (data not shown).
Fig. 1.
Quantification and localization of pro-inflammatory markers in sIBM compared to PM, DM and non-myopathic control muscle. (A) GAPDH-normalized mRNA expression of pro-inflammatory and degeneration-associated markers amplified by quantitative (real-time) PCR from skeletal muscle tissue of non-myopathic control patients (n = 12). Each dot represents one patient with arbitrary units multiplied by 1000. (B) GAPDH-normalized mRNA expression of pro-inflammatory markers amplified by quantitative PCR from skeletal muscle tissue of sIBM patients (n = 12, red spades), PM (n = 12, blue circles) and DM (n = 11, green triangles). Data are expressed as fold-expression in relation to values from (A) in non-myopathic control muscle. Each dot represents one patient. Statistics were performed as detailed in the Methods section using *P < 0.05, **P < 0.01, ***P < 0.001 as significant values. Undetectable values are not plotted on the logarithmic scale of the y-axis. (C) Fluorescent double-labelling (MHC-I and CXCL-9) and single-labelling (CCL-3, IL-1β) immunohistochemistry in representative muscle biopsy specimens of sIBM versus PM, DM and non-myopathic muscle, using antibodies for MHC-I (top row), CXCL-9 (second row), CCL-3 (third row) or IL-1β (bottom row) with Alexa-594 (red) or Alexa-488 (green)-labelled secondary antibodies and DAPI-counterstain (blue). Arrows in sIBM denote myofibres that display overexpression of IL-1β or co-expression of MHC-I, CXCL-9 and CCL-3. In PM, arrows indicate myofibres that are localized in areas of inflammation and show an enhanced staining signal of MHC-I, CXCL-9, CCL-3 or IL-1β; arrowheads point to mononuclear cells that express CXCL-9, CCL-3 or IL-1β. In DM, arrows indicate muscle fibres that are located in the perifascicular area and display an enhanced staining signal of MHC-I, CXCL-9, CCL-3 or IL-1β; stars indicate upregulation of MHC-I, CXCL-9 and CCL-3 in capillaries; arrowheads denote connective tissue positive for MHC-I, CXCL-9 or CCL-3 as well as mononuclear cells positive for IL-1β. Photos taken by a CCD-camera using a conventional fluorescent microscope with a 10× objective. Scale bars represent 100 µm in rows 1–3 and 50 µm in the last row.
Expression of mRNA of IFN-γ was low in non-myopathic muscle and drastically upregulated in sIBM by 441-fold, which was significant compared to PM and DM (Fig. 1B). TNF-α-mRNA was upregulated in PM (26-fold) and DM (12-fold) compared to controls and much higher in sIBM (53-fold), which was significant compared to DM. The mRNA-expression of IL-1β, a cytokine associated with APP and β-amyloid (Dalakas, 1998; Sondag and Combs, 2004), was significantly upregulated in sIBM versus controls by 23-fold and remained clearly lower in PM (9.5-fold) and DM (7.4-fold). By immunohistochemistry in sIBM muscle, IL-1β was mostly localized to myofibres (Fig. 1C) and colocalized to MHC-I; some inflammatory cells and the connective tissue were also positive. In contrast, in PM, the staining intensity of IL-1β in muscle fibres remained much lower except for areas of severe inflammation. Unlike in sIBM, a major part of the signal of IL-1β in PM localized to immune cells. In DM, IL-1β was almost absent in muscle fibres and basically restricted to mononuclear cells and connective tissue (Fig. 1C).
The mRNA-expression of TGF-β was similarly overexpressed in all three inflammatory myopathies by 7–14-fold (Fig. 1B). By immunohistochemistry, TGF-β was expressed in connective tissue and endomysial fibroblasts or capillaries in sIBM, PM, DM and dystrophic myopathies (data not shown), confirming previous observations (Confalonieri et al., 1997; Amemiya et al., 2000). The mRNA expression of IL-6 was significantly augmented in sIBM versus controls by 5-fold, and DM (4-fold), whereas in PM no change was observed (Fig. 1B).
Two patients with a granulomatous myositis displayed a similar mRNA-expression of inflammatory markers compared to PM (data not shown). In dystrophic myopathies, TGF-β was the only inflammatory mediator that was significantly upregulated (7-fold, P < 0.01 versus controls), but its expression remained significantly lower compared to sIBM (P < 0.05) (data not shown).
Collectively, the data show that in sIBM muscle, there is a much higher upregulation of the mRNA- and protein-expression of relevant pro-inflammatory chemokines and cytokines compared to PM and DM. Most importantly, in sIBM, the pattern of distribution of the chemokines and IL-1β in the muscle was fundamentally different because the majority of the inflammatory mediators were abundantly found in the myofibres, whereas in PM, a lower expression was observed with a restriction to areas of severe inflammation. In DM, the pro-inflammatory molecules were mainly localized to the connective tissue and capillaries and only to a minor degree in some perifascicular areas.
Upregulation of degeneration-associated molecules in sIBM, PM and DM muscle
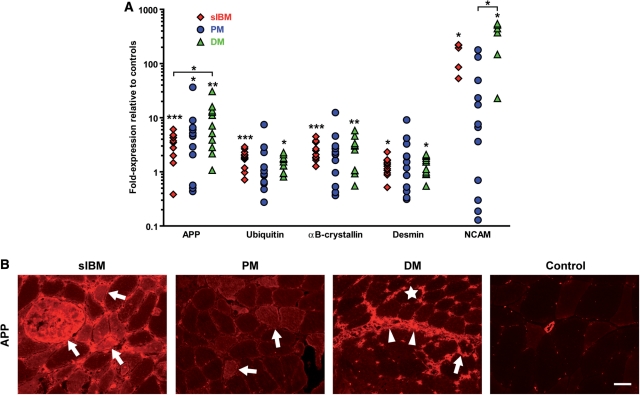
The mRNA levels of degeneration-associated molecules were analysed by quantitative (real-time) PCR in the muscle biopsies of sIBM, PM, DM and controls. Except for NCAM, which was detectable in 5 out of 12 samples, a baseline expression of all markers was present in all non-myopathic control patients (expression relative to GAPDH; Fig. 1A). In sIBM versus controls, there was a significant upregulation of APP, ubiquitin and the heat-shock protein αB-crystallin (Fig. 2A: data indicate the fold- expression relative to values in controls from Fig. 1A). These molecules were similarly upregulated in DM and PM, although—due to a higher variability—not statistically significant in PM, except for APP. Surprisingly, APP was expressed even higher in DM than in sIBM. Desmin was similarly and significantly upregulated in sIBM and DM; the expression level in PM was similar, yet remained statistically insignificant due to a higher variability. The mRNA-expression of NCAM was elevated in sIBM, which was significant compared to controls, and DM, which was significant compared to controls and PM. These data suggest that the noted changes were not related to major differential effects on degenerative/regenerative fibres. None of the degeneration-associated markers was significantly upregulated in a group of six patients with dystrophic myopathies (data not shown).
Fig. 2.
Quantification and localization of degeneration-associated markers in sIBM compared to PM, DM and non-myopathic control muscle. (A) By quantitative (real-time) PCR from muscle tissue, an overexpression of the mRNA of the degenerative markers APP, desmin, NCAM, ubiquitin and αB-crystallin was observed in sIBM, PM and DM compared to non-myopathic controls. Symbols, analysis, statistics and plotting same as in Fig. 1B. (B) Fluorescent immunohistochemistry in representative muscle biopsy specimens of sIBM versus PM, DM and non-myopathic muscle, using an antibody for APP and an Alexa-594 (red)-labelled secondary antibody. Arrows point to muscle fibres that display overexpression of APP in sIBM, PM or DM (perifascicularly). Arrowheads denote an enhanced staining signal for APP in connective tissue in DM. The star indicates an upregulated expression of APP in capillaries of DM muscle. Photos taken by a CCD-camera using a conventional fluorescent microscope with a 20× objective. Scale bar represents 50 µm.
Despite a similar upregulation of the mRNA of all the aforementioned molecules in all three inflammatory myopathies, immunohistochemical analysis revealed striking differences of the staining pattern of APP (Fig. 2B). In sIBM, the vast majority of the staining signal of APP derived from a remarkable overexpression in a large fraction of muscle fibres. By contrast, in PM, a much lower expression was observed, which was more pronounced in myofibres of areas with severe inflammation. In DM, most of the signal localized to inflammatory cells, connective tissue, vessels and only some myofibres in the perifascicular regions (Fig. 2B). In some fibres of sIBM but not PM and DM muscle, ubiquitin was co-expressed with β-amyloid (data not shown).
Many muscle fibres in sIBM were double positive for NCAM and desmin, many of which were also positive for αB-crystallin (data not shown). In PM, a similar distribution of these markers was observed only in some of the cases. In contrast, in DM, muscle fibres that overexpressed NCAM, αB-crystallin, MHC-I or desmin were restricted to the perifascicular region (data not shown).
Collectively, these data show that, in sIBM, degeneration-associated molecules were overexpressed at the level of mRNA and protein. A co-localization of several of these molecules could be demonstrated by immunohistochemistry. To the contrary, in PM the mRNA-expression of degeneration-associated molecules was much more variable and the protein expression lower compared to sIBM. In DM, most of the signal of APP was localized to the connective tissue and capillaries, but only some myofibres in the perifascicular areas.
β-amyloid and APP correlate with expression of inflammatory molecules in sIBM in contrast to PM and DM
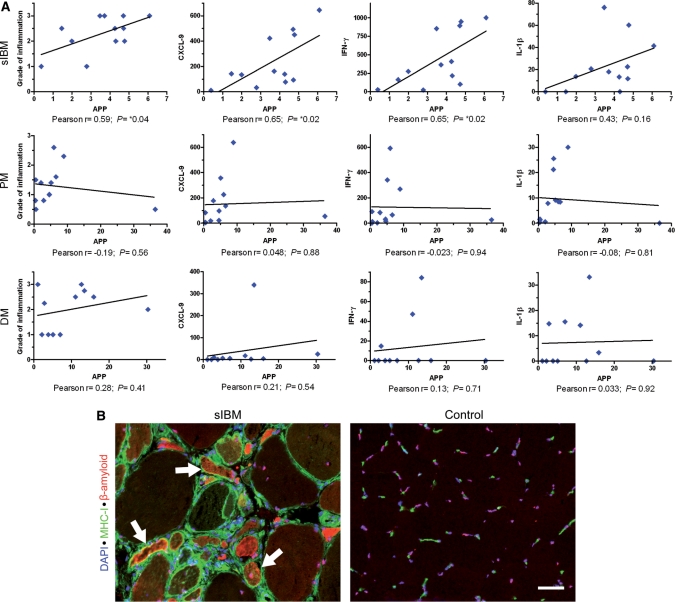
As expected, the mRNA expression of ubiquitin correlated with the expression of APP in sIBM (data not shown). Strikingly, in sIBM, but not in PM and DM, the mRNA expression of APP was significantly associated with the grade of the cellular infiltration as assessed by semiquantitative grading of H&E staining (Fig. 3A). Furthermore, in sIBM but not in DM or PM, the mRNA-expression of APP significantly correlated with the mRNA-expression of CXCL-9 and IFN-γ (Fig. 3A) as well as CCL-3 (Pearson r = 0.6; P < 0.05) and CCL-4 (Pearson r = 0.7; P < 0.05) (data not shown). In sIBM compared to PM and DM, a closer association between the mRNA-expression of IL-1β and APP was observed (Fig. 3A). The other cytokines TNF-α and IL-6 did not correlate with the mRNA-expression of APP in sIBM (data not shown). By Grubb's test of mRNA-expression levels, outliers in the groups of PM and DM were statistically identified, while in the IBM group no outliers were detected. Whereas the majority of PM and DM patients had a short history of clinical symptoms for less than one year, three patients in each group had a longer disease course: 4, 7 and 10 years in the PM group and >5, 2 and 3 years in DM. Outlier patients with a high expression of APP had a relatively long disease course (7 years in PM and 3 years in DM). In contrast, patients with a high grade of inflammation—and a relatively low expression of APP—had presented with a short, but very severe course of the disease. After removal of outliers, there was a significant correlation in the group of PM-patients between the mRNA-expression of APP and the grade of inflammation and the mRNA-expression of IL-1β. All other correlations in PM as well as in DM remained insignificant (Supplementary Fig. 1). In line with the lack of overexpression of pro-inflammatory mediators except TGF-β, there was no detectable cellular inflammation in the group of patients with a dystrophic myopathy.
Fig. 3.
Correlation of the mRNA- and protein-expression (same mRNA-data as shown in Figs 1 and 2) of β-amyloid-associated and inflammatory markers in the muscle of sIBM, PM and DM patients. (A) Correlation of the mRNA-expression of APP with the grade of cellular infiltration as assessed by H&E staining (first column), the mRNA-expression of CXCL-9 (second column), IFN-γ (third column) as well as with IL-1β (last column). One dot per patient. Due to similar values, two patients in the PM group are plotted with a high degree of overlap. (B) Immunohistochemical double labelling for β-amyloid (Alexa-594; red) and MHC-I (Alexa-488; green) reveals a frequent co-localization (arrows) of these markers in myofibres in a representative muscle biopsy specimen of a patient with sIBM. Nuclear counterstain with DAPI. Images taken by a CCD-camera using a conventional fluorescent microscope with a 20× objective. Scale bar represents 50 µm.
Double-labelling fluorescent immunohistochemistry further corroborated these associations on a cellular level. In sIBM, β-amyloid was present in 15.3 ± 3.7% (mean ± SEM) of the muscle fibres. Almost all β-amyloid-positive fibres expressed MHC-I and often represented the population of the brightest MHC-I expressing fibres (Fig. 3B). In PM and DM, no such accumulation of β-amyloid was observed (data not shown).
Collectively, these findings indicate that a distinct relationship between β-amyloid-associated markers and the expression of inflammatory mediators was mainly observed in sIBM muscle compared to PM and absent in DM.
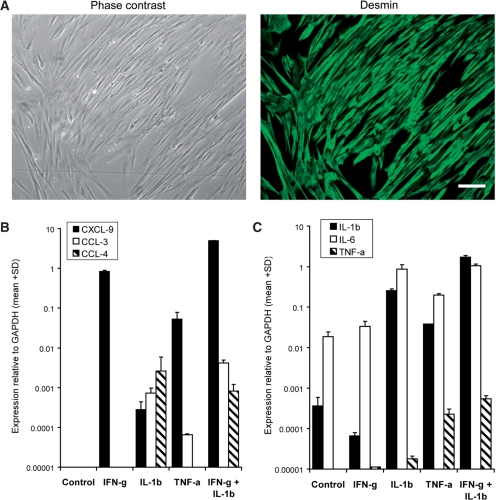
Human muscle cells in vitro display molecular mechanisms of chronic muscle inflammation
To address a possible interrelationship between inflammation and β-amyloid-associated degeneration in normal skeletal muscle in vitro, we established cultures of 95% pure myotubes from biopsies of non-myopathic individuals (Fig. 4A). Under normal cell culture conditions, myotubes did not express detectable levels of mRNA-expression of CXCL-9, CCL-3, CCL-4 or TNF-α, but baseline levels of IL-6 and IL-1β (Fig. 4B and C). In pilot experiments, the response to cytokine-stimulation was evaluated by comparing effects of IFN-γ (100–1000 U/ml), TNF-α (1–10 ng/ml), IL-1β (1–20 ng/ml) and TGF-β (1–30 ng/ml) for 4 h up to 48 h (data not shown). After 24 h of exposure to IFN-γ (300 U/ml), TNF-α (5 ng/ml), IL-1β (10 ng/ml), alone or in combination, there was a striking upregulation of the mRNA expression of CXCL-9, CCL-3, CCL-4, IL-6 and IL-1β, particularly after combinations of IFN-γ with IL-1β (Fig. 4B and C). TNF-α expression in myotubes was upregulated after TNF-α alone or in combination with IFN-γ or IL-1β as well as by IFN-γ and IL-1β, which displayed a synergistic effect on the TNF-α expression. Most importantly, the striking upregulation of IL-1β in response to various pro-inflammatory stimuli, including a feed-forward amplification, is explained by an upregulation of the IL-1β receptor (Adams et al., 2002) and is consistent with previous reports (Nagaraju et al., 1998). Particularly this synergism between IFN-γ and IL-1β may be crucial for a cell-stress response inflicted by conditions of a severe inflammation that correlates with accumulation of β-amyloid in sIBM as shown above and previously suggested (Dalakas, 1998; Sondag and Combs, 2004). Respective protein expression of CCL-3, CXCL-9, IL-1β and TNF-α by myotubes upon exposure to IL-1β, IFN-γ, TNF-α, alone or in combination, was confirmed by analysis of the supernatant by ELISA (data not shown).
Fig. 4.
In vitro analysis of expression of pro-inflammatory mediators in primary cultures of differentiated human myotubes. (A) Purity of primary myotube cultures was assessed by immunohistochemical staining of the muscle cell marker desmin (Alexa-488; green) with a typical purity of 95% as revealed by comparison to phase contrast photos of the same culture well. Scale bar represents 100 µm. (B and C) mRNA-expression relative to GAPDH of chemokines (B) and cytokines (C) as assessed by quantitative (real-time) PCR in myotubes exposed 24 h to IFN-γ (300 U/ml), TNF-α (5 ng/ml) or IL-1β (10 ng/ml)—alone or in combinations. Data are given as mean + SD of two wells per condition from one representative experiment of four with similar results.
Taken together, we showed that, upon stimulation with high concentrations of cytokines, particularly a combination of IFN-γ and IL-1β, differentiated human myotubes displayed a striking expression of chemokines and cytokines including an auto-amplificatory overexpression of IL-1β. The pattern of expression of the pro-inflammatory mediators appeared similar to what was observed in myofibres of sIBM muscle and confirmed the inherent ability of the muscle to produce inflammatory cytokines under pro-inflammatory conditions.
IL-1β augments accumulation of β-amyloid in human skeletal muscle cell cultures and co-localizes to β-amyloid in sIBM muscle
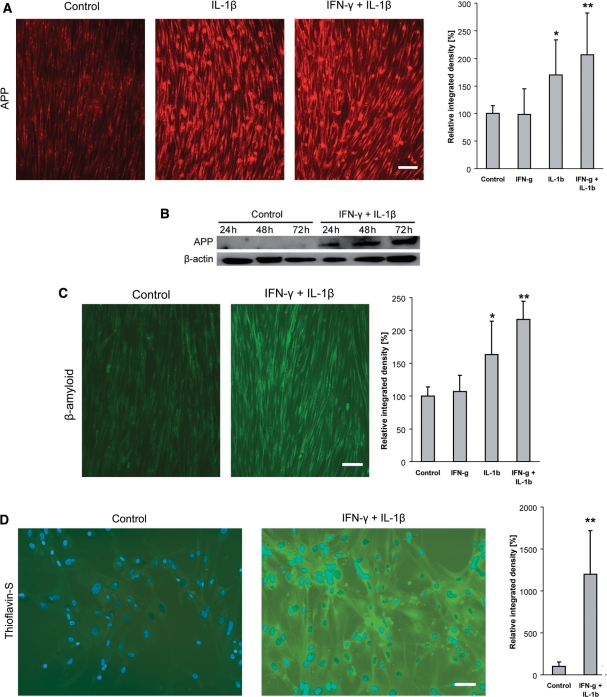
To test the hypothesis that a severe inflammation would trigger or augment accumulation of β-amyloid, myotubes were exposed to the amyloidogenic cytokine IL-1β, alone or in combination with IFN-γ. After 24 h, IL-1β, alone or in combination with IFN-γ, significantly upregulated the protein expression of APP as revealed by immunocytochemistry (Fig. 5A). Consistent with this, an upregulation of APP was observed by immunoblot analysis after exposure to IL-1β and IFN-γ for 24–72 h (Fig. 5B). After 48 h, IL-β, and more so IL-1β in combination with IFN-γ, significantly augmented intracellular staining for β-amyloid (Fig. 5C). Forty-eight hours after cytokine exposure, the enhanced immuno-labelling for β-amyloid was accompanied by significant protein aggregation as indicated by thioflavin-S cytochemistry (Fig. 5D). This intracellular accumulation of β-amyloid further increased after 72 h and was associated with degeneration of the myotubes (data not shown).
Fig. 5.
Overexpression of APP and accumulation of β-amyloid in human primary skeletal myotube cultures exposed to IL-1β (10 ng/ml) and/or IFN-γ (300 U/ml) for 24–72 h. (A) A 24 h exposure to IL-1β, alone or in combination with IFN-γ, induced enhanced staining for APP (Alexa-594, red). Grey-scale analysis of the same experiment demonstrates a significant increase of the staining intensity upon IL-1β, and even more in combination with IFN-γ. (B) Immunoblot analysis of APP demonstrates an increased level of protein expression upon 24–72 h of exposure to IL-1β in combination with IFN-γ. Protein loading in each lane is confirmed by detection of β-actin. (C) Staining for β-amyloid reveals an enhanced intracellular accumulation upon 48 h of exposure to IL-1β, particularly in combination with IFN-γ (Alexa-488, green). Grey-scale analysis of the same experiment demonstrates a significant increase of the staining intensity upon IL-1β, and even more in combination with IFN-γ. (D) After 48 h of exposure to IL-1β and IFN-γ, intracellular aggregation of β-amyloid is evidenced by thioflavin-S, which is statistically significant as revealed by grey-scale analysis of the same experiment. Photos taken by a CCD-camera using a conventional fluorescent microscope with a 10× (A and C) or 20× (D) objective. All photomicrographs for the analyses in this Figure have been acquired with the same settings of camera and microscope; all data are representative of at least three experiments with similar results. Scale bars represent 100 µm in A, C and 60 µm in D.
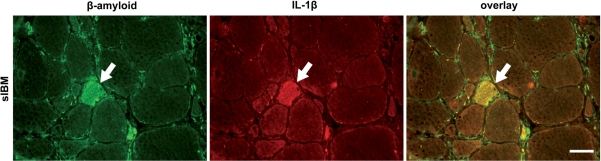
The in vitro association between inflammatory cytokines and β-amyloid was further substantiated by a co-localization of β-amyloid and IL-1β only in sIBM muscle (Fig. 6), which is in line with a previous observation (Dalakas, 1998) and was not present in DM or PM as reconfirmed in the present study.
Fig. 6.
Fluorescent immunohistochemical double labelling for β-amyloid and IL-1β. Representative muscle biopsy specimen of a patient with sIBM stained with anti-β-amyloid (Alexa-488, green; left) and anti-IL-1β (Alexa-594, red; middle). A degenerating muscle fibre in the centre is double positive (arrows; yellow in merged image, right). Photos taken by a CCD camera using a conventional fluorescent microscope with a 40× objective. Scale bar represents 40 µm.
Collectively, IL-1β, particularly in combination with IFN-γ, induced an overexpression of APP at the protein level in human myotubes. Subsequently, an enhanced β-amyloid staining and thioflavin-S positive aggregations of protein were observed. This suggests a direct contribution of IL-1β to the generation of intracellular accumulation of β-amyloid in human muscle cell culture.
Discussion
We here demonstrate that highly upregulated proinflammatory cytokines and chemokines in sIBM muscle correlate and co-localize with the expression of β-amyloid-associated proteins. In contrast to PM and DM, the other two inflammatory myopathies used as comparative controls, in sIBM, the majority of the signal for inflammatory and degenerative markers localized strongly to muscle fibres. In PM, the expression level remained lower and limited to chemokines and IL-1β, which mainly localized to myofibres next to areas of severe inflammation; in contrast, these markers were widespread in myofibres of sIBM muscle. In DM, the expression remained much lower and was predominantly observed in blood vessels and connective tissue. Under inflammatory conditions, purified human myotube cultures displayed a similar pattern of expression of pro-inflammatory cytokines and chemokines, including an auto-amplificatory upregulation of IL-1β. Exposure of myotubes to IL-1β, particularly in combination with IFN-γ, induced an overexpression of APP with subsequent intracellular accumulation of β-amyloid and protein aggregation.
Overexpression of the mRNA or protein of pro-inflammatory chemokines and cytokines in sIBM muscle was in line with previous observations for CXCL-9 (Raju et al., 2003), CCL-3 (Confalonieri et al., 2000) and CCL-4 (Civatte et al., 2005), and the cytokines IFN-γ, TNF-α and IL-1β (Tews and Goebel, 1996); moreover, the chemokines and IL-1β localized to muscle fibres. In contrast, in PM, the expression was lower and restricted to areas of severe inflammation. In DM, the expression was much less and mostly confined to the connective tissue. This is consistent with previous observations that myofibres in sIBM as well as muscle cells—upon pro-inflammatory stimulation in vitro—produce large amounts of these mediators (Nagaraju et al., 1998; Raju et al., 2003), which contribute to the infiltration of muscle fibres by immune cells or even to their local activation (Schmidt et al., 2004; Wiendl et al., 2005). Given the similar magnitude of upregulation of the degeneration-associated molecules including APP, ubiquitin, desmin and αB-crystallin that we observed in all three inflammatory myopathies, one could argue that a chronic inflammation in muscle always results in overexpression of pathways associated with degenerative pathomechanisms. In line with this, a high expression of the mRNA of APP was noted in patients with a chronic disease course of DM or PM. However, only in sIBM, there was a significant and consistent correlation between the mRNA expression of β-amyloid-associated molecules and the major inflammatory markers. Moreover, only in sIBM, the inflammatory molecules CXCL-9, MHC-I and IL-1β co-localized with APP and β-amyloid within the fibres, which suggests some association between inflammation and degeneration in sIBM muscle. It is conceivable that a so far unknown underlying condition in sIBM may cause a higher baseline expression of APP as well as its extensive upregulation under inflammatory conditions. An increased generation of β-amyloid may be due to alterations of the processing of APP via β-site of APP cleaving enzyme (BACE)-1 (Vattemi et al., 2001) or macroautophagy (Lunemann et al., 2007). Moreover, muscle fibres in sIBM may be susceptible even to weak inflammatory stimuli and readily respond with a chronic auto-amplificatory production of chemokines and cytokines. Lastly, mechanisms that protect from intracellular cell-stress inflicted by inflammation or accumulation of β-amyloid may be insufficiently present in sIBM.
It is conceivable that a particularly low grade of inflammation and an unusual disease course in some PM patients might have been responsible for the lower expression levels of inflammatory mediators and an outlier with a high APP-mRNA expression compared to IBM and, thus, biased parts of the results. However, in patients with low as well as a more severe inflammation, immunohistochemical analysis revealed a fundamentally different pattern of distribution of these markers: In PM, only some muscle fibres next to the areas of intense inflammation displayed a focal staining signal, while in DM the expression predominantly localized to blood vessels, capillaries and connective tissue. In contrast, independent of the grade of cellular inflammation in sIBM muscle, there was a widespread labelling of the muscle fibres and some minor staining of connective tissue. Collectively, we surmise that the most likely reasons for inflammation to cause chronic degenerative damage of muscle fibres in sIBM—as opposed to PM and DM—are due to (i) the magnitude of the production of pro-inflammatory mediators and their chronic, auto-amplificatory augmentation and (ii) the predominant localization of the inflammatory stressors within the muscle fibres themselves. Particularly in DM, inflammatory mediators localized to small vessels, connective tissue and slightly the perifascicular regions (areas with the most significant cell-stress response), but not to the muscle fibres as seen in sIBM. The grade of expression of the mRNA of APP was even higher in DM than in sIBM. Since APP is considered to be among the acute phase proteins (Gitter et al., 2000) and is upregulated in vitro upon stimulation by inflammatory cytokines as demonstrated by our results, it is conceivable that a similar mechanism of induction of overexpression of APP is active on the level of blood vessels and connective tissue. The differential response by muscle fibres in sIBM compared to blood vessels in DM may be explained by a higher local concentration of inflammatory mediators produced by the muscle fibres themselves in sIBM instead of blood vessels and connective tissue in DM. In PM, some myofibres expressed chemokines and IL-1β, but the levels remained much lower compared to sIBM and restricted to fibres of areas with severe inflammation. Consistent with this interpretation, our data showed that a prolonged exposure of muscle cells to high concentrations of pro-inflammatory cytokines induced β-amyloid-associated degeneration.
High concentrations of cytokines and chemokines can be directly detrimental to muscle cells as previously demonstrated for IL-1β (Broussard et al., 2004) and TNF-α (Li et al., 2005). Furthermore, direct toxicity of APP or accumulation of β-amyloid may contribute to muscle cell damage in vitro and in vivo (Askanas et al., 1996; Fukuchi et al., 1998; Jin et al., 1998; Querfurth et al., 2001; Christensen et al., 2004; Kitazawa et al., 2006; Sugarman et al., 2006). However, inflammation could also augment processing of APP through upregulation of BACE-1, as recently observed in astrocytes upon IFN-γ (Hong et al., 2003). On the other hand, β-amyloid induces upregulation of various cytokines in vitro (Franciosi et al., 2005) and an association between β-amyloid and inflammation has been noted in human AD and in its mouse model (Fonseca et al., 2004). Such a bi-directional augmentation between APP and IL-1β has recently been demonstrated (Sondag and Combs, 2004) and suggested almost a decade ago (Dalakas, 1998). As another inflammatory-degenerative association in muscle cells, an increased production of nitric oxide has been demonstrated after combined exposure to β-amyloid and IFN-γ (Baron et al., 2000). Collectively, it appears that in sIBM, inflammatory and degenerative mechanisms act in concert to exert toxicity. But in spite of the evidence that cytokines and APP/β-amyloid augment each other, it remains unclear if the triggering factor in sIBM is the inflammation or the accumulation of β-amyloid.
In summary, our data demonstrate a striking upregulation of inflammatory chemokines and cytokines in sIBM muscle and a specific interplay between inflammatory and β-amyloid-associated pathomechanisms. Ubiquitous upregulation of IL-1β in muscle fibres may aggravate an overexpression of APP and lead to an accumulation of β-amyloid. The data further our understanding of the pathology not only of sIBM, but also of other neuroinflammatory and neurodegenerative conditions where interactions between inflammation and accumulation of β-amyloid contribute to the pathology.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The study was supported by the intramural program of NINDS (to M.C.D.). Additional funding was provided by Deutsche Forschungsgemeinschaft (DFG; Schm 1669/1-1 and Schm 1669/2-1 to J.S.); Association Française contre les Myopathies (AFM; AM/NM/2006.1377/12087 to J.S.); University of Göttingen (Rückkehrer-Freistellung to J.S.). We thank Goran Rakocevic (NIH), Rhaghavan Raju (NIH) and Ingrid Müller (University of Göttingen) for analysis of parts of the data. The technical assistance of Rebekah Granger (NIH) and Nicole Tasch (University of Göttingen) is gratefully acknowledged. We appreciate Ellen Gerhardt's (University of Göttingen) expert advice on protein detection by Western blot. The authors thank Hanns Lochmüller for providing tissue specimen of patients with PM from the Muscle Tissue Culture Collection, which is part of the German network on muscular dystrophies (MD-NET, service structure S1, 01GM0601) funded by the German ministry of education and research (BMBF, Bonn, Germany) and a partner of Eurobiobank (www.eurobiobank.org). We thank Dennis J. Selkoe (Centre for Neurologic Diseases, Harvard Medical School and Brigham and Women's Hospital, Boston, MA) for kindly providing an anti-APP antibody. Funding to pay the Open Access publication charges for this article was provided by the Intramural program of the National Institutes of Health, NINDS.
Glossary
Abbreviations:
- APP
amyloid precursor protein
- BACE-1
β-site of APP cleaving enzyme 1
- DM
dermatomyositis
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- MHC class-I
major histocompatibility complex class I
- NCAM
neural cell adhesion molecule
- PM
polymyositis
- sIBM
sporadic inclusion body myositis
- TGF-β
transforming growth factor-β
- TNF-α
tumour necrosis factor-α
References
- Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, et al. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Amemiya K, Semino-Mora C, Granger RP, Dalakas MC. Downregulation of TGF-beta1 mRNA and protein in the muscles of patients with inflammatory myopathies after treatment with high-dose intravenous immunoglobulin. Clin Immunol. 2000;94:99–104. doi: 10.1006/clim.1999.4823. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology. 2006;66:S39–48. doi: 10.1212/01.wnl.0000192128.13875.1e. [DOI] [PubMed] [Google Scholar]
- Askanas V, McFerrin J, Baque S, Alvarez RB, Sarkozi E, Engel WK. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci USA. 1996;93:1314–9. doi: 10.1073/pnas.93.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwell BL, Engel AG. Alpha B-crystallin immunolocalization yields new insights into inclusion body myositis. Neurology. 2000;54:1033–41. doi: 10.1212/wnl.54.5.1033. [DOI] [PubMed] [Google Scholar]
- Baron P, Galimberti D, Meda L, Prat E, Scarpini E, Conti G, et al. Synergistic effect of beta-amyloid protein and interferon gamma on nitric oxide production by C2C12 muscle cells. Brain. 2000;123:374–9. doi: 10.1093/brain/123.2.374. [DOI] [PubMed] [Google Scholar]
- Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, et al. IL-1 beta impairs insulin-like growth factor I-induced differentiation and downstream activation signals of the insulin-like growth factor I receptor in myoblasts. J Immunol. 2004;172:7713–20. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- Christensen RA, Shtifman A, Allen PD, Lopez JR, Querfurth HW. Calcium dyshomeostasis in beta-amyloid and Tau-bearing skeletal myotubes. J Biol Chem. 2004;279:53524–32. doi: 10.1074/jbc.M408473200. [DOI] [PubMed] [Google Scholar]
- Civatte M, Bartoli C, Schleinitz N, Chetaille B, Pellissier JF, Figarella-Branger D. Expression of the beta chemokines CCL3, CCL4, CCL5 and their receptors in idiopathic inflammatory myopathies. Neuropathol Appl Neurobiol. 2005;31:70–9. doi: 10.1111/j.1365-2990.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- Confalonieri P, Bernasconi P, Cornelio F, Mantegazza R. Transforming growth factor-beta 1 in polymyositis and dermatomyositis correlates with fibrosis but not with mononuclear cell infiltrate. J Neuropathol Exp Neurol. 1997;56:479–84. doi: 10.1097/00005072-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Confalonieri P, Bernasconi P, Megna P, Galbiati S, Cornelio F, Mantegazza R. Increased expression of beta-chemokines in muscle of patients with inflammatory myopathies. J Neuropathol Exp Neurol. 2000;59:164–9. doi: 10.1093/jnen/59.2.164. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Molecular immunology and genetics of inflammatory muscle diseases. Arch Neurol. 1998;55:1509–12. doi: 10.1001/archneur.55.12.1509. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Sporadic inclusion body myositis-diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006;2:437–47. doi: 10.1038/ncpneuro0261. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–82. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- De Bleecker JL, De Paepe B, Vanwalleghem IE, Schroder JM. Differential expression of chemokines in inflammatory myopathies. Neurology. 2002;58:1779–85. doi: 10.1212/wnl.58.12.1779. [DOI] [PubMed] [Google Scholar]
- De Bleecker JL, Meire VI, Declercq W, Van Aken EH. Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromuscul Disord. 1999;9:239–46. doi: 10.1016/s0960-8966(98)00126-6. [DOI] [PubMed] [Google Scholar]
- De Paepe B, Creus KK, De Bleecker JL. Chemokine profile of different inflammatory myopathies reflects humoral versus cytotoxic immune responses. Ann N Y Acad Sci. 2007;1109:441–53. doi: 10.1196/annals.1398.050. [DOI] [PubMed] [Google Scholar]
- De Paepe B, Creus KK, De Bleecker JL. Chemokines in idiopathic inflammatory myopathies. Front Biosci. 2008;13:2548–77. doi: 10.2741/2866. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J Neurosci. 2004;24:6457–65. doi: 10.1523/JNEUROSCI.0901-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. IL-8 enhancement of amyloid-beta (A beta(1-42))-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol. 2005;159:66–74. doi: 10.1016/j.jneuroim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Pham D, Hart M, Li L, Lindsey JR. Amyloid-beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol. 1998;153:1687–93. doi: 10.1016/s0002-9440(10)65682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter BD, Boggs LN, May PC, Czilli DL, Carlson CD. Regulation of cytokine secretion and amyloid precursor protein processing by proinflammatory amyloid beta (A beta) Ann N Y Acad Sci. 2000;917:154–64. doi: 10.1111/j.1749-6632.2000.tb05379.x. [DOI] [PubMed] [Google Scholar]
- Hong HS, Hwang EM, Sim HJ, Cho HJ, Boo JH, Oh SS, et al. Interferon gamma stimulates beta-secretase expression and sAPPbeta production in astrocytes. Biochem Biophys Res Commun. 2003;307:922–7. doi: 10.1016/s0006-291x(03)01270-1. [DOI] [PubMed] [Google Scholar]
- Jin LW, Hearn MG, Ogburn CE, Dang N, Nochlin D, Ladiges WC, et al. Transgenic mice over-expressing the C-99 fragment of betaPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol. 1998;153:1679–86. doi: 10.1016/s0002-9440(10)65681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Green KN, Caccamo A, LaFerla FM. Genetically augmenting Abeta42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am J Pathol. 2006;168:1986–97. doi: 10.2353/ajpath.2006.051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–70. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Shaked GM, Masliah E, Bredesen DE, Koo EH. Amyloid beta protein toxicity mediated by the formation of amyloid-beta protein precursor complexes. Ann Neurol. 2003;54:781–9. doi: 10.1002/ana.10761. [DOI] [PubMed] [Google Scholar]
- Lundberg I, Ulfgren AK, Nyberg P, Andersson U, Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997;40:865–74. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]
- Lunemann JD, Schmidt J, Schmid D, Barthel K, Wrede A, Dalakas MC, et al. Beta-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann Neurol. 2007;61:476–83. doi: 10.1002/ana.21115. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Raben N, Merritt G, Loeffler L, Kirk K, Plotz P. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 1998;113:407–14. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, Suhara T, Rosen KM, Mcphie DL, Fujio Y, Tejada G, et al. Beta-amyloid peptide expression is sufficient for myotube death: implications for human inclusion body myopathy. Mol Cell Neurosci. 2001;17:793–810. doi: 10.1006/mcne.2001.0972. [DOI] [PubMed] [Google Scholar]
- Raju R, Dalakas MC. Gene expression profile in the muscles of patients with inflammatory myopathies: effect of therapy with IVIg and biological validation of clinically relevant genes. Brain. 2005;128:1887–96. doi: 10.1093/brain/awh518. [DOI] [PubMed] [Google Scholar]
- Raju R, Vasconcelos O, Granger R, Dalakas MC. Expression of IFN-gamma-inducible chemokines in inclusion body myositis. J Neuroimmunol. 2003;141:125–31. doi: 10.1016/s0165-5728(03)00218-2. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Rakocevic G, Raju R, Dalakas MC. Upregulated inducible co-stimulator (ICOS) and ICOS-ligand in inclusion body myositis muscle: significance for CD8(+) T cell cytotoxicity. Brain. 2004;127:1182–90. doi: 10.1093/brain/awh148. [DOI] [PubMed] [Google Scholar]
- Sondag CM, Combs CK. Amyloid precursor protein mediates proinflammatory activation of monocytic lineage cells. J Biol Chem. 2004;279:14456–63. doi: 10.1074/jbc.M313747200. [DOI] [PubMed] [Google Scholar]
- Sugarman MC, Kitazawa M, Baker M, Caiozzo VJ, Querfurth HW, LaFerla FM. Pathogenic accumulation of APP in fast twitch muscle of IBM patients and a transgenic model. Neurobiol Aging. 2006;27:423–32. doi: 10.1016/j.neurobiolaging.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Harigai M, Kawaguchi Y, Takagi K, Fukasawa C, Ohsako-Higami S, et al. Increased IL-15 production of muscle cells in polymyositis and dermatomyositis. Int Immunol. 2002;14:917–24. doi: 10.1093/intimm/dxf062. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. Cytokine expression profile in idiopathic inflammatory myopathies. J Neuropathol Exp Neurol. 1996;55:342–7. doi: 10.1097/00005072-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Tournadre A, Miossec P. Cytokine response in inflammatory myopathies. Curr Rheumatol Rep. 2007;9:286–90. doi: 10.1007/s11926-007-0046-6. [DOI] [PubMed] [Google Scholar]
- Vattemi G, Engel WK, McFerrin J, Buxbaum JD, Pastorino L, Askanas V. Presence of BACE1 and BACE2 in muscle fibres of patients with sporadic inclusion-body myositis. Lancet. 2001;358:1962–4. doi: 10.1016/S0140-6736(01)06969-0. [DOI] [PubMed] [Google Scholar]
- Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol. 2005;26:373–80. doi: 10.1016/j.it.2005.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.