Abstract
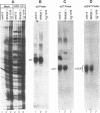
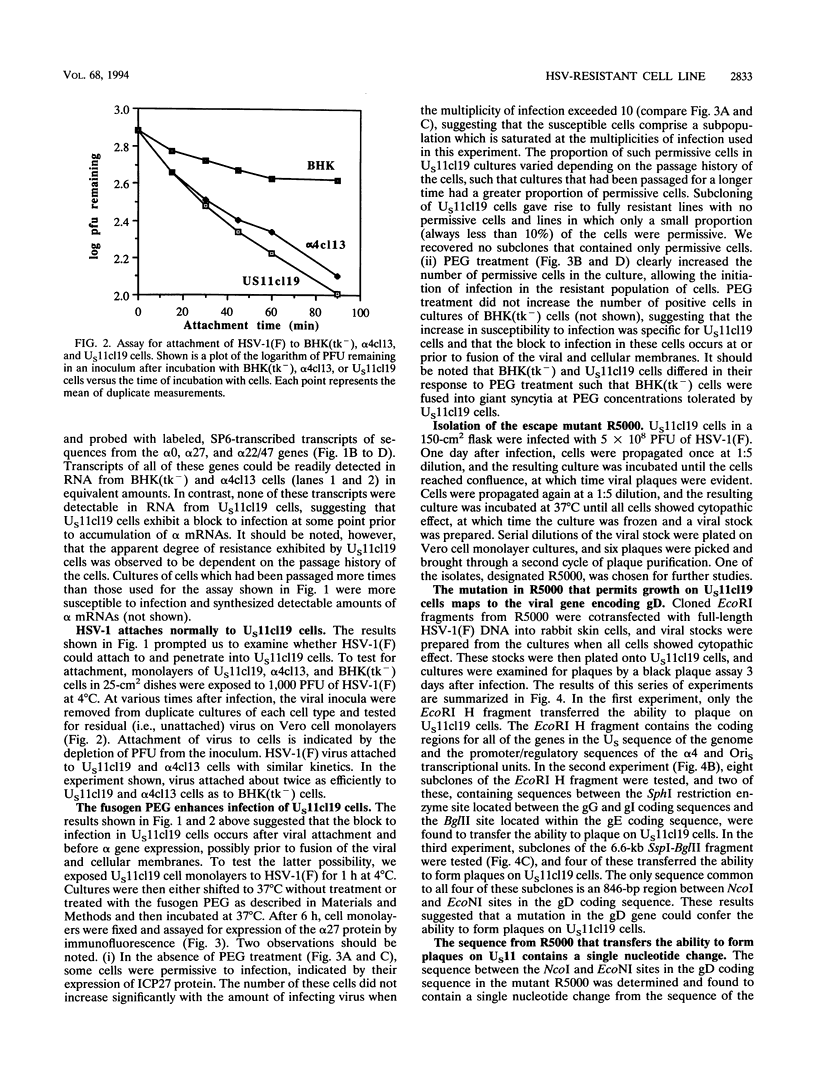
A baby hamster kidney [BHK(tk-)] cell line (US11cl19) which stably expresses the US11 and alpha 4 genes of herpes simplex virus 1 strain F [HSV-1(F)] was found to be resistant to infection with HSV-1. Although wild-type HSV-1(F) attached with normal kinetics to the surface of US11cl19 cells, most cells showed no evidence of infection and failed to accumulate detectable amounts of alpha mRNAs. The relationship between the expression of UL11 and resistance to HSV infection in US11cl19 cells has not been defined, but the block to infection with wild-type HSV-1 was overcome by exposing cells with attached virus on their surface to the fusogen polyethylene glycol, suggesting that the block to infection preceded the fusion of viral and cellular membranes. An escape mutant of HSV-1(F), designated R5000, that forms plaques on US11cl19 cells was selected. This mutant was found to contain a mutation in the glycoprotein D (gD) coding sequence that results in the substitution of the serine at position 140 in the mature protein to asparagine. A recombinant virus, designated R5001, was constructed in which the wild-type gD gene was replaced with the R5000 gD gene. The recombinant formed plaques on US11cl19 cells with an efficiency comparable to that of the escape mutant R5000, suggesting that the mutation in gD determines the ability of the mutant R5000 to grow on US11cl19 cells. The observation that the US11cl19 cells were slightly more resistant to fusion by polyethylene glycol than parental BHK(tk-) cells led to the selection and testing of clonal lines from unselected and polyethylene glycol-selected BHK(tk-) cells. The results were that 16% of unselected to as much as 36% of the clones selected for relative resistance to polyethylene glycol fusion exhibited various degrees of resistance to infection. The exact step at which the infection was blocked is not known, but the results illustrate the ease of selection of cell clones with one or more sites at which infection could be blocked.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arsenakis M., Hubenthal-Voss J., Campadelli-Fiume G., Pereira L., Roizman B. Construction and properties of a cell line constitutively expressing the herpes simplex virus glycoprotein B dependent on functional alpha 4 protein synthesis. J Virol. 1986 Nov;60(2):674–682. doi: 10.1128/jvi.60.2.674-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988 Jan;62(1):159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Avitabile E., Fini S., Stirpe D., Arsenakis M., Roizman B. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology. 1988 Oct;166(2):598–602. doi: 10.1016/0042-6822(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Qi S., Avitabile E., Foà-Tomasi L., Brandimarti R., Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990 Dec;64(12):6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Lee W. C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992 Aug;66(8):5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Burke R. L., Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990 Jun;64(6):2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Ligas M. W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988 Dec;62(12):4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., MacLean C., Marsden H. S., Dalziel R. G., Everett R. D. The product of gene US11 of herpes simplex virus type 1 is expressed as a true late gene. J Gen Virol. 1986 May;67(Pt 5):871–883. doi: 10.1099/0022-1317-67-5-871. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Spear P. G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989 Feb;63(2):819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeland N., Holmsen H., Lillehaug J. R., Haarr L. Evidence that neomycin inhibits binding of herpes simplex virus type 1 to the cellular receptor. J Virol. 1987 Nov;61(11):3388–3393. doi: 10.1128/jvi.61.11.3388-3393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean C. A., Rixon F. J., Marsden H. S. The products of gene US11 of herpes simplex virus type 1 are DNA-binding and localize to the nucleoli of infected cells. J Gen Virol. 1987 Jul;68(Pt 7):1921–1937. doi: 10.1099/0022-1317-68-7-1921. [DOI] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Ackermann M., Roizman B. Construction and properties of a viable herpes simplex virus 1 recombinant lacking coding sequences of the alpha 47 gene. J Virol. 1986 Nov;60(2):807–812. doi: 10.1128/jvi.60.2.807-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Muggeridge M. I., Isola V. J., Byrn R. A., Tucker T. J., Minson A. C., Glorioso J. C., Cohen G. H., Eisenberg R. J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988 Sep;62(9):3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeridge M. I., Wu T. T., Johnson D. C., Glorioso J. C., Eisenberg R. J., Cohen G. H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990 Feb;174(2):375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Pereira L., Dondero D. V., Gallo D., Devlin V., Woodie J. D. Serological analysis of herpes simplex virus types 1 and 2 with monoclonal antibodies. Infect Immun. 1982 Jan;35(1):363–367. doi: 10.1128/iai.35.1.363-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Puvion-Dutilleul F. Localization of viral-specific 21 kDa protein in nucleoli of herpes simplex infected cells. Eur J Cell Biol. 1987 Jun;43(3):487–498. [PubMed] [Google Scholar]
- Rixon F. J., McGeoch D. J. A 3' co-terminal family of mRNAs from the herpes simplex virus type 1 short region: two overlapping reading frames encode unrelated polypeptide one of which has highly reiterated amino acid sequence. Nucleic Acids Res. 1984 Mar 12;12(5):2473–2487. doi: 10.1093/nar/12.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Preparation of herpes simplex virus of high titer. J Virol. 1968 Jan;2(1):83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller R. J., Roizman B. Herpes simplex virus 1 RNA-binding protein US11 negatively regulates the accumulation of a truncated viral mRNA. J Virol. 1991 Nov;65(11):5873–5879. doi: 10.1128/jvi.65.11.5873-5879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller R. J., Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J Virol. 1992 Jun;66(6):3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller R. J., Roizman B. The herpes simplex virus Us11 open reading frame encodes a sequence-specific RNA-binding protein. J Virol. 1990 Jul;64(7):3463–3470. doi: 10.1128/jvi.64.7.3463-3470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Vande Woude G. F. DNA sequence of an immediate-early gene (IEmRNA-5) of herpes simplex virus type I. Nucleic Acids Res. 1982 Feb 11;10(3):979–991. doi: 10.1093/nar/10.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]