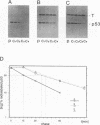
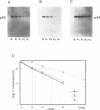
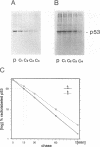
Abstract
To understand the process and biological significance of metabolic stabilization of p53 during simian virus 40 (SV40)-induced cellular transformation, we analyzed cellular and viral parameters involved in this process. We demonstrate that neither large T expression as such nor the cellular phenotype (normal versus transformed) markedly influence the stability of p53 complexed to large T in SV40 abortively infected BALB/c mouse fibroblasts. In contrast, metabolic stabilization of p53 is an active cellular event, specifically induced by SV40. The ability of SV40 to induce a cellular response leading to stabilization of p53 complexed to large T is independent from the cellular phenotype and greatly varies between different cells. However, metabolic stability was conferred only to p53 in complex with large T, whereas the free p53 in these cells remained metabolically unstable. Comparative analyses of cellular transformation in various cells differing in stability of p53 complexed to large T upon abortive infection with SV40 revealed a strong correlation between the ability of SV40 to induce metabolic stabilization and its transformation efficiency. Our data suggest that metabolic stabilization and the ensuing enhanced levels of p53 are important for initiation and/or maintenance of SV40 transformation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chellappan S., Kraus V. B., Kroger B., Munger K., Howley P. M., Phelps W. C., Nevins J. R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherington V., Brown M., Paucha E., St Louis J., Spiegelman B. M., Roberts T. M. Separation of simian virus 40 large-T-antigen-transforming and origin-binding functions from the ability to block differentiation. Mol Cell Biol. 1988 Mar;8(3):1380–1384. doi: 10.1128/mcb.8.3.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Deppert W., Buschhausen-Denker G., Patschinsky T., Steinmeyer K. Cell cycle control of p53 in normal (3T3) and chemically transformed (Meth A) mouse cells. II. Requirement for cell cycle progression. Oncogene. 1990 Nov;5(11):1701–1706. [PubMed] [Google Scholar]
- Deppert W., Haug M. Evidence for free and metabolically stable p53 protein in nuclear subfractions of simian virus 40-transformed cells. Mol Cell Biol. 1986 Jun;6(6):2233–2240. doi: 10.1128/mcb.6.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Haug M., Steinmayer T. Modulation of p53 protein expression during cellular transformation with simian virus 40. Mol Cell Biol. 1987 Dec;7(12):4453–4463. doi: 10.1128/mcb.7.12.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Steinmayer T., Richter W. Cooperation of SV40 large T antigen and the cellular protein p53 in maintenance of cell transformation. Oncogene. 1989 Sep;4(9):1103–1110. [PubMed] [Google Scholar]
- Dittmer D., Pati S., Zambetti G., Chu S., Teresky A. K., Moore M., Finlay C., Levine A. J. Gain of function mutations in p53. Nat Genet. 1993 May;4(1):42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- Duthu A., Ehrhart J. C., Benchimol S., Chandrasekaran K., May P. P53-transformation-related protein: kinetics of synthesis and accumulation in SV40-infected primary mouse kidney cell cultures. Virology. 1985 Dec;147(2):275–286. doi: 10.1016/0042-6822(85)90130-8. [DOI] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Fanning E., Burger C., Gurney E. G. Comparison of T antigen-associated host phosphoproteins from SV40-infected and -transformed cells of different species. J Gen Virol. 1981 Aug;55(Pt 2):367–378. doi: 10.1099/0022-1317-55-2-367. [DOI] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Igel H. J., Price P. J. I. In vitrol transformation of rat embryo cells: correlations with the known tumorigenic activities of chemicals in rodents. In Vitro. 1975 Mar-Apr;11(2):107–116. doi: 10.1007/BF02624083. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudray P., Rassoulzadegan M., Cuzin F. Expression of simian virus 40 early genes in transformed rat cells is correlated with maintenance of the transformed phenotype. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4987–4991. doi: 10.1073/pnas.75.10.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Hall A., Oren M. Stabilization of the p53 transformation-related protein in mouse fibrosarcoma cell lines: effects of protein sequence and intracellular environment. Mol Cell Biol. 1989 Aug;9(8):3385–3392. doi: 10.1128/mcb.9.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Pim D. C., Crawford L. V. Complex of simian virus 40 large-T antigen and host 53,000-molecular-weight protein in monkey cells. J Virol. 1981 Feb;37(2):564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M., Levine A. J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991 Dec;5(12B):2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Shin E., Sheppard K. A., Chokroverty L., Shan B., Qian Y. W., Lee E. Y., Yee A. S. The retinoblastoma protein region required for interaction with the E2F transcription factor includes the T/E1A binding and carboxy-terminal sequences. DNA Cell Biol. 1992 Sep;11(7):539–548. doi: 10.1089/dna.1992.11.539. [DOI] [PubMed] [Google Scholar]
- Huang S., Wang N. P., Tseng B. Y., Lee W. H., Lee E. H. Two distinct and frequently mutated regions of retinoblastoma protein are required for binding to SV40 T antigen. EMBO J. 1990 Jun;9(6):1815–1822. doi: 10.1002/j.1460-2075.1990.tb08306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Chumakov P., Addison C., Stürzbecher H. W., Wade-Evans A. Two distinct regions of the murine p53 primary amino acid sequence are implicated in stable complex formation with simian virus 40 T antigen. J Virol. 1988 Oct;62(10):3903–3906. doi: 10.1128/jvi.62.10.3903-3906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Ewen M. E., Livingston D. M. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol. 1990 Jul;10(7):3761–3769. doi: 10.1128/mcb.10.7.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J. Tumor suppressor genes: the p53 and retinoblastoma sensitivity genes and gene products. Biochim Biophys Acta. 1990 Jun 1;1032(1):119–136. doi: 10.1016/0304-419x(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Levine A. J. Oncogenes of DNA tumor viruses. Cancer Res. 1988 Feb 1;48(3):493–496. [PubMed] [Google Scholar]
- Levine A. J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990 Aug;177(2):419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 tumor-suppressor gene. N Engl J Med. 1992 May 14;326(20):1350–1352. doi: 10.1056/NEJM199205143262008. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- Lin J. Y., Simmons D. T. Transformation by simian virus 40 does not involve the mutational activation of p53 to an oncogenic form. Virology. 1990 May;176(1):302–305. doi: 10.1016/0042-6822(90)90258-s. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993 Apr;7(4):535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Müller E., Boldyreff B., Scheidtmann K. H. Characterization of protein kinase activities associated with p53-large-T immune complexes from SV40-transformed rat cells. Oncogene. 1993 Aug;8(8):2193–2205. [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Patschinsky T., Knippschild U., Deppert W. Species-specific phosphorylation of mouse and rat p53 in simian virus 40-transformed cells. J Virol. 1992 Jun;66(6):3846–3859. doi: 10.1128/jvi.66.6.3846-3859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Srinivasan A., Farber J. M., Pipas J. M. Mutants with changes within or near a hydrophobic region of simian virus 40 large tumor antigen are defective for binding cellular protein p53. Virology. 1989 Jan;168(1):13–21. doi: 10.1016/0042-6822(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Prives C., Bargonetti J., Friedman P. N., Manfredi J. J., Wang E. H. Functional consequences of the interactions of the p53 tumor suppressor protein and SV40 large tumor antigen. Cold Spring Harb Symp Quant Biol. 1991;56:227–235. doi: 10.1101/sqb.1991.056.01.028. [DOI] [PubMed] [Google Scholar]
- Prives C., Manfredi J. J. The p53 tumor suppressor protein: meeting review. Genes Dev. 1993 Apr;7(4):529–534. doi: 10.1101/gad.7.4.529. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cuzin F. Transformation of rat fibroblast cells with early mutants of polyoma (tsa) and simian virus 40 (tsA30): occurrence of either A or N transformants depends on the multiplicity of infection. J Virol. 1980 Feb;33(2):909–911. doi: 10.1128/jvi.33.2.909-911.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Rotter V., Witte O. N., Coffman R., Baltimore D. Abelson murine leukemia virus-induced tumors elicit antibodies against a host cell protein, P50. J Virol. 1980 Nov;36(2):547–555. doi: 10.1128/jvi.36.2.547-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Takahashi T., Huibregtse J. M., Minna J. D., Howley P. M. Interaction of the human papillomavirus type 16 E6 oncoprotein with wild-type and mutant human p53 proteins. J Virol. 1992 Aug;66(8):5100–5105. doi: 10.1128/jvi.66.8.5100-5105.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Haber A. Simian virus 40 large T antigen induces or activates a protein kinase which phosphorylates the transformation-associated protein p53. J Virol. 1990 Feb;64(2):672–679. doi: 10.1128/jvi.64.2.672-679.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Deppert W. Specific interaction of simian virus 40 large T antigen with cellular chromatin and nuclear matrix during the course of infection. J Virol. 1987 Nov;61(11):3561–3569. doi: 10.1128/jvi.61.11.3561-3569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T., Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Different structural systems of the nucleus are targets for SV40 large T antigen. Cell. 1983 May;33(1):173–181. doi: 10.1016/0092-8674(83)90346-x. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Maacke H., Deppert W. Cell cycle control by p53 in normal (3T3) and chemically transformed (Meth A) mouse cells. I. Regulation of p53 expression. Oncogene. 1990 Nov;5(11):1691–1699. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B. Cancer. A deadly inheritance. Nature. 1990 Dec 20;348(6303):681–682. doi: 10.1038/348681a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Walser A., Deppert W. The kinase activity of SV40 large T antigen is mediated by a cellular kinase. EMBO J. 1986 May;5(5):883–889. doi: 10.1002/j.1460-2075.1986.tb04299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Wolf D., Harris N., Rotter V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell. 1984 Aug;38(1):119–126. doi: 10.1016/0092-8674(84)90532-4. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerrahn J., Deppert W., Weidemann D., Patschinsky T., Richards F., Milner J. Correlation between the conformational phenotype of p53 and its subcellular location. Oncogene. 1992 Jul;7(7):1371–1381. [PubMed] [Google Scholar]
- Zhu L., van den Heuvel S., Helin K., Fattaey A., Ewen M., Livingston D., Dyson N., Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]
- von der Weth A., Deppert W. Wild-type p53 is not a negative regulator of simian virus 40 DNA replication in infected monkey cells. J Virol. 1993 Feb;67(2):886–893. doi: 10.1128/jvi.67.2.886-893.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]