Abstract
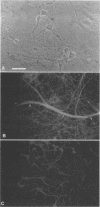
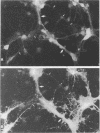
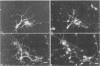
The neurotropic murine coronavirus JHM is capable of inducing various forms of neurologic diseases, including demyelination. Neurons have been shown to act as a repository site at the early stages of the disease process (O. Sorensen and S. Dales, J. Virol. 56:434-438, 1985). JHM virus (JHMV) replication and trafficking of viral proteins and virions in cultured rat hippocampal neurons and a neuronal cell line, OBL-21, were examined, with an emphasis placed on the role of the microtubular network. We show here that JHMV spread within the central nervous system occurs transneuronally and that virus protein trafficking was dependent upon microtubules. Viral trafficking occurred asymmetrically, involving both the somatodendritic and the axonal domains. Thus coronavirus can be disseminated from neurons at either the basolateral or the apical domains. A specific interaction between antibodies derived against the microtubule-associated protein tau and JHMV nucleocapsid protein (N) was observed, which can presumably be explained by an overall amino acid similarity of 44% and an identity of 20% between proteins N and tau, with optimal alignment at the microtubule binding domain of tau. Collectively, our data suggest an important role of the microtubule network in viral protein trafficking and distribution. They also draw attention to protein sequence mimicry of a cell component by this coronavirus as one strategy for making use of the host's functions on behalf of the virus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cowan W. M. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979 Oct 1;187(3):469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Barthold S. W. Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol. 1988;76(5):502–506. doi: 10.1007/BF00686390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Smith A. L. Response of genetically susceptible and resistant mice to intranasal inoculation with mouse hepatitis virus JHM. Virus Res. 1987 May;7(3):225–239. doi: 10.1016/0168-1702(87)90030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett W. P., Banker G. A. An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci. 1984 Aug;4(8):1944–1953. doi: 10.1523/JNEUROSCI.04-08-01944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K. G., Malawista S. E. Microtubular crystals in mammalian cells. J Cell Biol. 1969 Jan;40(1):95–107. doi: 10.1083/jcb.40.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
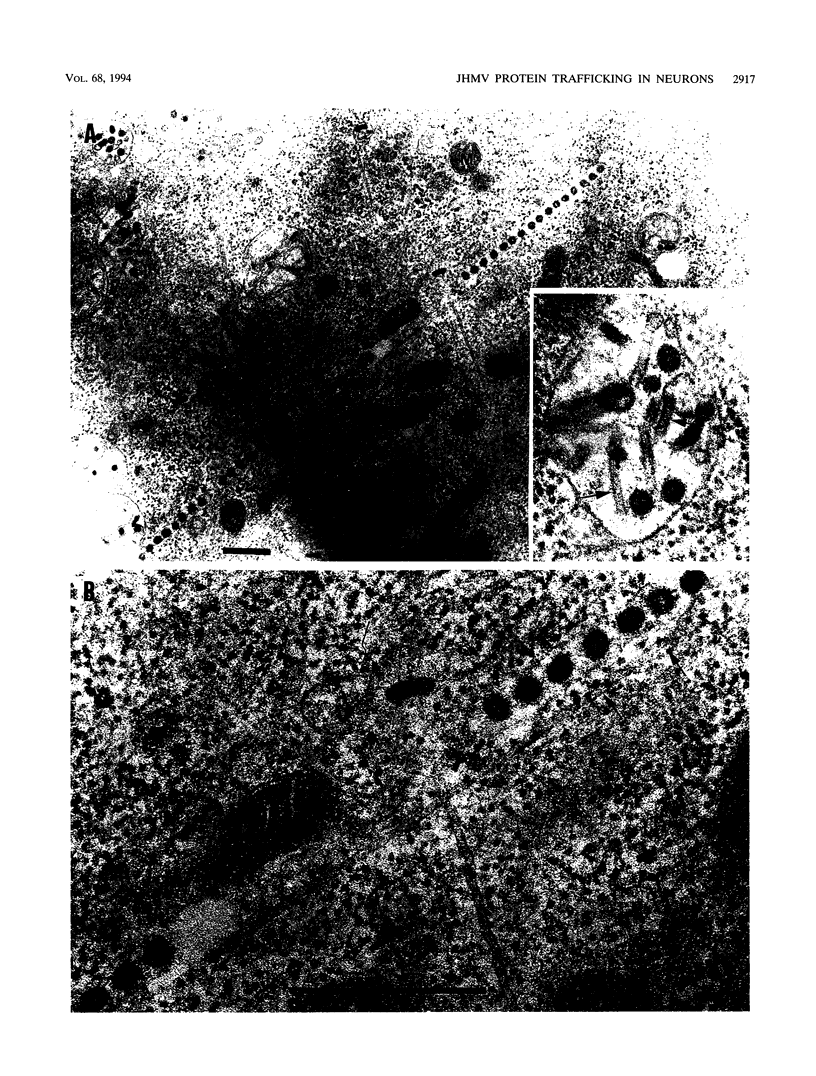
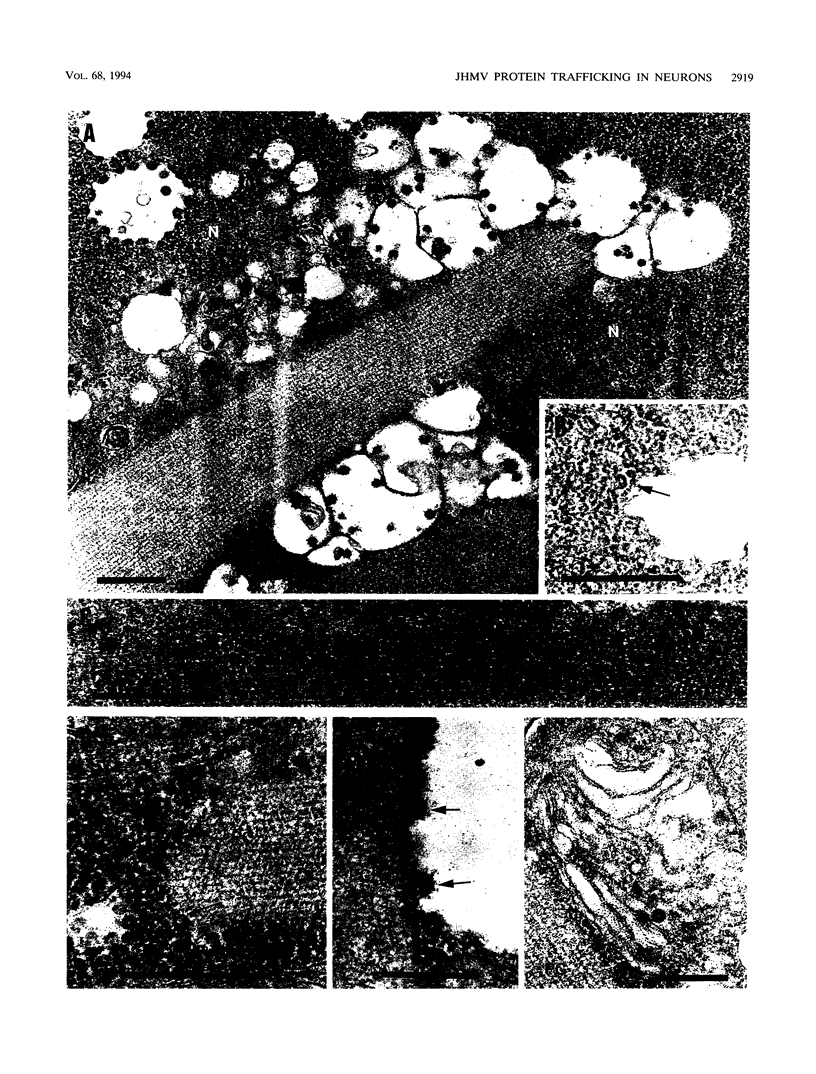
- Beushausen S., Dales S. In vivo and in vitro models of demyelinating disease. XI. Tropism and differentiation regulate the infectious process of coronaviruses in primary explants of the rat CNS. Virology. 1985 Feb;141(1):89–101. doi: 10.1016/0042-6822(85)90185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi P. E., Gillet J. P., Tsiang H. Inhibition of the transport of rabies virus in the central nervous system. J Neuropathol Exp Neurol. 1989 Nov;48(6):620–630. doi: 10.1097/00005072-198911000-00004. [DOI] [PubMed] [Google Scholar]
- Coulon P., Derbin C., Kucera P., Lafay F., Prehaud C., Flamand A. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivative AvO1. J Virol. 1989 Aug;63(8):3550–3554. doi: 10.1128/jvi.63.8.3550-3554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres A., Banker G. A., Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986 Mar;6(3):714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. ASSOCIATION BETWEEN THE SPINDLE APPARATUS AND REOVIRUS. Proc Natl Acad Sci U S A. 1963 Aug;50:268–275. doi: 10.1073/pnas.50.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Hsu K. C., Nagayama A. The fine structure and immunological labeling of the achromatic mitotic apparatus after disruption of cell membranes. J Cell Biol. 1973 Dec;59(3):643–660. doi: 10.1083/jcb.59.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990 Jul 13;62(1):63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988 Apr;8(4):1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. S., Gass J. S., Swoveland P. T., Lavi E., Highkin M. K., Weiss S. R. Infection of the basal ganglia by a murine coronavirus. Science. 1985 Aug 30;229(4716):877–879. doi: 10.1126/science.2992088. [DOI] [PubMed] [Google Scholar]
- Flamand A., Gagner J. P., Morrison L. A., Fields B. N. Penetration of the nervous systems of suckling mice by mammalian reoviruses. J Virol. 1991 Jan;65(1):123–131. doi: 10.1128/jvi.65.1.123-131.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Parker S. E., Buchmeier M. J. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J Virol. 1990 Feb;64(2):731–741. doi: 10.1128/jvi.64.2.731-741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Crowther R. A., Garner C. C. Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci. 1991 May;14(5):193–199. doi: 10.1016/0166-2236(91)90105-4. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Dubois-Dalcq M., Haspel M. V., Claysmith A. P., Lampert P. W., Oldstone M. B. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J Neuroimmunol. 1981 Mar;1(1):81–92. doi: 10.1016/0165-5728(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Finch E. A. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987 Oct;7(10):3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Lundh B., Norrby E., Payne L., Orvell C. Asymmetric budding of viruses in ependymal and choroid plexus epithelial cells. Neuropathol Appl Neurobiol. 1984 May-Jun;10(3):209–219. doi: 10.1111/j.1365-2990.1984.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Kucera P., Dolivo M., Coulon P., Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985 Jul;55(1):158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Monica N., Banner L. R., Morris V. L., Lai M. M. Localization of extensive deletions in the structural genes of two neurotropic variants of murine coronavirus JHM. Virology. 1991 Jun;182(2):883–888. doi: 10.1016/0042-6822(91)90635-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F., Coulon P., Astic L., Saucier D., Riche D., Holley A., Flamand A. Spread of the CVS strain of rabies virus and of the avirulent mutant AvO1 along the olfactory pathways of the mouse after intranasal inoculation. Virology. 1991 Jul;183(1):320–330. doi: 10.1016/0042-6822(91)90145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Fishman P. S., Highkin M. K., Weiss S. R. Limbic encephalitis after inhalation of a murine coronavirus. Lab Invest. 1988 Jan;58(1):31–36. [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lee G., Neve R. L., Kosik K. S. The microtubule binding domain of tau protein. Neuron. 1989 Jun;2(6):1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Massalski A., Coulter-Mackie M., Knobler R. L., Buchmeier M. J., Dales S. In vivo and in vitro models of demyelinating diseases. V. Comparison of the assembly of mouse hepatitis virus, strain JHM, in two murine cell lines. Intervirology. 1982;18(3):135–146. doi: 10.1159/000149316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara Y., Watanabe R., Taguchi F. Neurovirulence of six different murine coronavirus JHMV variants for rats. Virus Res. 1991 Jun;20(1):45–58. doi: 10.1016/0168-1702(91)90060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas D. V., Dales S. Endosomal association of a protein phosphatase with high dephosphorylating activity against a coronavirus nucleocapsid protein. FEBS Lett. 1991 May 6;282(2):419–424. doi: 10.1016/0014-5793(91)80528-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Tieszer C., Mackinnon J., Percy D. Characterization of coronavirus JHM variants isolated from Wistar Furth rats with a viral-induced demyelinating disease. Virology. 1989 Mar;169(1):127–136. doi: 10.1016/0042-6822(89)90048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Sidman R. L., Fields B. N. Direct spread of reovirus from the intestinal lumen to the central nervous system through vagal autonomic nerve fibers. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3852–3856. doi: 10.1073/pnas.88.9.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama A., Dales S. Rapid purification and the immunological specificity of mammalian microtubular paracrystals possessing an ATPase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):464–471. doi: 10.1073/pnas.66.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham D., Tereba A., Talbot P. J., Jackson D. P., Morris V. L. Analysis of JHM central nervous system infections in rats. Arch Neurol. 1986 Jul;43(7):702–708. doi: 10.1001/archneur.1986.00520070058019. [DOI] [PubMed] [Google Scholar]
- Pasick J. M., Dales S. Infection by coronavirus JHM of rat neurons and oligodendrocyte-type-2 astrocyte lineage cells during distinct developmental stages. J Virol. 1991 Sep;65(9):5013–5028. doi: 10.1128/jvi.65.9.5013-5028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Jacobsen G., Afifi A. Spread of a neurotropic murine coronavirus into the CNS via the trigeminal and olfactory nerves. Virology. 1989 Jun;170(2):556–560. doi: 10.1016/0042-6822(89)90446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Jacobsen G., Moore S. Regional localization of virus in the central nervous system of mice persistently infected with murine coronavirus JHM. Virology. 1988 Oct;166(2):328–338. doi: 10.1016/0042-6822(88)90503-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Ryder E. F., Snyder E. Y., Cepko C. L. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol. 1990 Mar;21(2):356–375. doi: 10.1002/neu.480210209. [DOI] [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Siddell S. G. Coronavirus JHM: nucleotide sequence of the mRNA that encodes nucleocapsid protein. Nucleic Acids Res. 1983 Aug 11;11(15):5045–5054. doi: 10.1093/nar/11.15.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Dales S. In vivo and in vitro models of demyelinating disease: JHM virus in the rat central nervous system localized by in situ cDNA hybridization and immunofluorescent microscopy. J Virol. 1985 Nov;56(2):434–438. doi: 10.1128/jvi.56.2.434-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A., Fuller S. D. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol. 1987 Sep;105(3):1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Tooze S. A., Tooze J., Warren G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J Cell Biol. 1988 May;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang H., Lycke E., Ceccaldi P. E., Ermine A., Hirardot X. The anterograde transport of rabies virus in rat sensory dorsal root ganglia neurons. J Gen Virol. 1989 Aug;70(Pt 8):2075–2085. doi: 10.1099/0022-1317-70-8-2075. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., McPhee D. A., Fields B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986 Aug 15;233(4765):770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Ugolini G., Kuypers H. G., Strick P. L. Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science. 1989 Jan 6;243(4887):89–91. doi: 10.1126/science.2536188. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Bloom G. S. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- Walsh E., Ueda Y., Nakanishi H., Yoshida K. Neuronal survival and neurite extension supported by astrocytes co-cultured in transwells. Neurosci Lett. 1992 Apr 13;138(1):103–106. doi: 10.1016/0304-3940(92)90482-m. [DOI] [PubMed] [Google Scholar]
- Wang F. I., Hinton D. R., Gilmore W., Trousdale M. D., Fleming J. O. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab Invest. 1992 Jun;66(6):744–754. [PubMed] [Google Scholar]
- Weclewicz K., Kristensson K., Orvell C. Segregation of viral structural proteins in cultured neurons of rat spinal ganglia and cord. Neuropathol Appl Neurobiol. 1990 Aug;16(4):357–364. doi: 10.1111/j.1365-2990.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]