Abstract
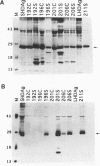
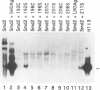
Hepatitis delta antigen (HDAg) consists of two species, large (LHDAg) and small (SHDAg), which are identical in sequence except that the large form contains 19 extra amino acids at the C terminus. The large form is prenylated on the Cxxx motif. The small form can trans activate HDV RNA replication, while the large form inhibits it. To determine the molecular basis for their differential functions, we examined the effects of prenylation on the conformation and function of HDAg. We show that the presence of prenylates masks a conformational epitope which is present in SHDAg but hidden in wild-type LHDAg; this epitope becomes exposed in all of the nonprenylated mutant LHDAgs. Prenylation also plays a major role in conferring the trans-dominant negative inhibitory activity of LHDAg, since the loss of prenylation in LHDAg reduced its inhibitory activity. The primary amino acids of the C-terminal sequence also contributed to the maintenance of the HDAg protein conformation; a prenylated LHDAg mutant with a five-amino-acid deletion had an exposed C-terminal epitope. By examining LHDAg mutants which have deletions of various extents of C-terminal sequence, with or without the prenylation motif, we have further shown that all of the prenylated mutants have much higher levels of trans-dominant suppressor activities than do the corresponding nonprenylated mutants. Surprisingly, a few nonprenylated LHDAg mutants were able to trans activate HDV RNA replication, while all of the prenylated ones lost this function. These results suggest that isoprenylates cause the masking of a conformational epitope of HDAg and that conformational differences between the large and small HDAgs account for the differences in their trans-activating and trans-dominant inhibitory biological activities.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang F. L., Chen P. J., Tu S. J., Wang C. J., Chen D. S. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. F., Baker S. C., Soe L. H., Kamahora T., Keck J. G., Makino S., Govindarajan S., Lai M. M. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988 Jul;62(7):2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M., Hsieh S. Y., Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990 Oct;64(10):5066–5069. doi: 10.1128/jvi.64.10.5066-5069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Glenn J. S., Watson J. A., Havel C. M., White J. M. Identification of a prenylation site in delta virus large antigen. Science. 1992 May 29;256(5061):1331–1333. doi: 10.1126/science.1598578. [DOI] [PubMed] [Google Scholar]
- Glenn J. S., White J. M. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol. 1991 May;65(5):2357–2361. doi: 10.1128/jvi.65.5.2357-2361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Holtz D., Tanaka R. A., Hartwig J., McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989 Dec 22;59(6):969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Lai M. M. A unique conformation at the carboxyl terminus of the small hepatitis delta antigen revealed by a specific monoclonal antibody. Virology. 1993 Apr;193(2):924–931. doi: 10.1006/viro.1993.1201. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Lai M. M. Isoprenylation mediates direct protein-protein interactions between hepatitis large delta antigen and hepatitis B virus surface antigen. J Virol. 1993 Dec;67(12):7659–7662. doi: 10.1128/jvi.67.12.7659-7662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B., Lee C. Z., Lai M. M. Hepatitis delta antigen expressed by recombinant baculoviruses: comparison of biochemical properties and post-translational modifications between the large and small forms. Virology. 1992 Sep;190(1):413–422. doi: 10.1016/0042-6822(92)91227-l. [DOI] [PubMed] [Google Scholar]
- Kos A., Dijkema R., Arnberg A. C., van der Meide P. H., Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986 Oct 9;323(6088):558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Chao M., Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989 May;63(5):1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Z., Chen P. J., Lai M. M., Chen D. S. Isoprenylation of large hepatitis delta antigen is necessary but not sufficient for hepatitis delta virus assembly. Virology. 1994 Feb 15;199(1):169–175. doi: 10.1006/viro.1994.1109. [DOI] [PubMed] [Google Scholar]
- Lee C. Z., Lin J. H., Chao M., McKnight K., Lai M. M. RNA-binding activity of hepatitis delta antigen involves two arginine-rich motifs and is required for hepatitis delta virus RNA replication. J Virol. 1993 Apr;67(4):2221–2227. doi: 10.1128/jvi.67.4.2221-2227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Chao M., Hsieh S. Y., Sureau C., Nishikura K., Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990 Mar;64(3):1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughton T. B., Gowans E. J., McNamara S. P., Burrell C. J. Hepatitis delta antigen is necessary for access of hepatitis delta virus RNA to the cell transcriptional machinery but is not part of the transcriptional complex. Virology. 1991 Sep;184(1):387–390. doi: 10.1016/0042-6822(91)90855-6. [DOI] [PubMed] [Google Scholar]
- Macnaughton T. B., Gowans E. J., Jilbert A. R., Burrell C. J. Hepatitis delta virus RNA, protein synthesis and associated cytotoxicity in a stably transfected cell line. Virology. 1990 Aug;177(2):692–698. doi: 10.1016/0042-6822(90)90535-y. [DOI] [PubMed] [Google Scholar]
- Macnaughton T. B., Wang Y. J., Lai M. M. Replication of hepatitis delta virus RNA: effect of mutations of the autocatalytic cleavage sites. J Virol. 1993 Apr;67(4):2228–2234. doi: 10.1128/jvi.67.4.2228-2234.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Chang M. F., Shieh C. K., Kamahora T., Vannier D. M., Govindarajan S., Lai M. M. Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA. Nature. 1987 Sep 24;329(6137):343–346. doi: 10.1038/329343a0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993 Mar 26;259(5103):1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kaibuchi K., Yamamoto T., Kawamura M., Sakoda T., Fujioka H., Matsuura Y., Takai Y. A stimulatory GDP/GTP exchange protein for smg p21 is active on the post-translationally processed form of c-Ki-ras p21 and rhoA p21. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6442–6446. doi: 10.1073/pnas.88.15.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong O. C., Ota I. M., Clarke S., Fung B. K. The membrane binding domain of rod cGMP phosphodiesterase is posttranslationally modified by methyl esterification at a C-terminal cysteine. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9238–9242. doi: 10.1073/pnas.86.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips M. R., Pillinger M. H., Staud R., Volker C., Rosenfeld M. G., Weissmann G., Stock J. B. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science. 1993 Feb 12;259(5097):977–980. doi: 10.1126/science.8438158. [DOI] [PubMed] [Google Scholar]
- Ryu W. S., Bayer M., Taylor J. Assembly of hepatitis delta virus particles. J Virol. 1992 Apr;66(4):2310–2315. doi: 10.1128/jvi.66.4.2310-2315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Kuo M. Y., Dinter-Gottlieb G., Taylor J. Antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J Virol. 1988 Aug;62(8):2674–2679. doi: 10.1128/jvi.62.8.2674-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Kuo M. Y., Taylor J. Self-ligating RNA sequences on the antigenome of human hepatitis delta virus. J Virol. 1989 Mar;63(3):1428–1430. doi: 10.1128/jvi.63.3.1428-1430.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau C., Guerra B., Lanford R. E. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J Virol. 1993 Jan;67(1):366–372. doi: 10.1128/jvi.67.1.366-372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Choo Q. L., Wang K. S., Govindarajan S., Redeker A. G., Gerin J. L., Houghton M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988 Feb;62(2):594–599. doi: 10.1128/jvi.62.2.594-599.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Lai M. M. Reversible cleavage and ligation of hepatitis delta virus RNA. Science. 1989 Feb 3;243(4891):652–654. doi: 10.1126/science.2492677. [DOI] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. P., Lai M. M. Oligomerization of hepatitis delta antigen is required for both the trans-activating and trans-dominant inhibitory activities of the delta antigen. J Virol. 1992 Nov;66(11):6641–6648. doi: 10.1128/jvi.66.11.6641-6648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. P., Yeh C. T., Ou J. H., Lai M. M. Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex. J Virol. 1992 Feb;66(2):914–921. doi: 10.1128/jvi.66.2.914-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]